Abstract
The objectives of this study were to examine cadmium (Cd) levels and relationships to demographics in an observational, prospective pregnancy cohort study in Durham County, North Carolina. Multivariable models were used to compare blood Cd levels across demographic characteristics. The relative risk of having a blood Cd level that exceeds the US national median (0.32 μg/l) was estimated. Overall, >60% of the women had an elevated (>0.32 μg/l) blood Cd level. Controlling for confounding variables, smoking was associated with 21% (95% CI: 15–28%) increased risk for an elevated blood Cd level. High Cd levels were also observed in non-smokers and motivated smoking status-stratified models. Race, age, education, relationship status, insurance status and cotinine level were not associated with risk of elevated Cd levels among smokers; however, older age and higher cotinine levels were associated with elevated Cd levels among non-smokers. Taken together, more than half of pregnant women in this cohort had elevated blood Cd levels. Additionally, among non-smokers, 53% of the women had elevated levels of Cd, highlighting other potential sources of exposure. This study expands on the limited data describing Cd levels in pregnant populations and highlights the importance of understanding Cd exposures among non-smokers. Given the latent health risks of both smoking and Cd exposure, this study further highlights the need to biomonitor for exposure to toxic metals during pregnancy among all women of child-bearing age.
Keywords: cadmium, pregnancy, risk
INTRODUCTION
Cadmium (Cd) is a developmental toxicant and a ubiquitous heavy metal present in the environment. Cd exposure during pregnancy is associated with adverse pregnancy outcomes, including increased risk for miscarriage and fetal undernourishment.1–3 Prenatal exposure to Cd may also have long-term implications on child development.4,5 In adults, chronic Cd exposure is associated with various health effects, including elevated risks for cancer and heart and lung disease.6
Smoking cigarettes accounts for a significant portion of human exposure to Cd,7 while diet is the major source of Cd exposure among non-smokers.8 Cd in the blood has a half-life of approximately 3–4 months and can remain in the body for up to 10 years.8 Cotinine, the primary metabolite of nicotine and biomarker of cigarette smoke exposure, has a half-life of <1 day.9,10 Thus cotinine is a general measure of recent exposure, whereas Cd can represent long-term and/or historic tobacco exposure, as well as other exposure sources in the environment.
National surveys, such as the National Health and Nutrition Examination Survey (NHANES),11 provide data on Cd levels in the general population. However, little data are available on Cd levels among pregnant women. The NHANES 2003–2004 subsample demonstrate that the geometric mean Cd level in the blood of non-pregnant women of childrearing age is 0.33 μg/l (n = 1396) and among pregnant women is lower with a level of 0.22 μg/l (n = 253).12 Recognizing the utility of additional data on Cd during pregnancy, a recent pilot study examined Cd levels in blood samples from 211 pregnant women residing in six North Carolina counties.13 Notably, 57.3% of the women tested had detectable levels of Cd in their blood, with a geometric mean of 0.181 μg/l and a range of <0.11–2.79 μg/l.13
In the present study, we utilize data from a much larger study population to examine Cd levels during pregnancy and factors that influence these levels. We leverage data from a cohort of pregnant women in an urban population in North Carolina to examine blood Cd levels generally and relate these levels not only to smoking behavior but to other demographic factors as well. This builds upon our previous work assessing other toxic metals (e.g., lead and mercury) in a separate subset of this cohort.14,15 Here we set out to: (i) establish baseline data on Cd levels among pregnant women; and (ii) assess the extent to which non-smokers were at risk for elevated Cd levels, as well as factors influencing such risk. These two objectives have implications for appropriate ways to biomonitor heavy metals among pregnant women and components of intervention programs to prevent exposure. For example, intervention programs could focus on smoking cessation only or the establishment of a broader intervention addressing the multiple sources of Cd exposure. As both Cd and cotinine were assessed in the cohort, there is a unique opportunity to compare and contrast these two measures to establish demographic predictors of elevated Cd among non-smokers. Understanding the distribution of blood Cd levels among non-smokers may help focus efforts to identify those at risk and highlight other important sources of Cd exposure. With this work, we aim to provide information that can improve understanding of prenatal Cd exposure as a public health issue and assist in the development of targeted public health initiatives, including intervention programs.
MATERIALS AND METHODS
Study Population
The Healthy Pregnancy, Healthy Baby Study is a prospective cohort study that enrolled pregnant women living in Durham County, North Carolina, USA, during the period of 2005 through 2010. Of the 2306 women approached, 1897 women enrolled in the study (82.3%), and 43 were subsequently withdrawn either by participant’s request or by investigators after determination of ineligibility (e.g., duplicate enrollment or congenital anomalies identified soon after enrollment), resulting in 1854 subjects. This study is a key component of the Southern Center on Environmentally-Driven Disparities in Birth Outcomes, an interdisciplinary center aimed at understanding how environmental, social, and host factors jointly contribute to health disparities in pregnancy outcomes.
Women receiving prenatal care at either the Duke University Obstetrics Clinic or the Durham County Public Health’s Prenatal Clinic were eligible to participate if they planned to deliver at Duke University Medical Center, were between 18 and 28 weeks of gestation, were at least 18 years of age, were English literate, lived in Durham County, and did not have a multi-fetal gestation or any known congenital anomalies. The Healthy Pregnancy, Healthy Baby Study and all associated analysis were conducted according to a human subjects’ research protocol approved by the Duke University’s Institutional Review Board.
Blood Cd and Cotinine Levels
At the time of hospital admission for delivery, maternal blood samples were collected in a Monoject trace element blood collection tube containing EDTA as an anticoagulant. To measure Cd, whole blood samples were analyzed by Inductively Coupled Plasma-Mass Spectrometry at the Mayo Clinical Laboratories (MCL) or the Dwyer (Duke) labs. To measure cotinine, plasma samples were analyzed using Inductively Coupled Plasma-Mass Spectrometry at the MCL or University of California, San Francisco (UCSF) Clinical Pharmacology Laboratory. Aqueous acidic calibrating standards and blanks are diluted with an aqueous acidic diluent containing three internal standards. Quality control specimens were diluted in an identical manner. The detection limits for Cd were 0.2 μg/l and 0.08 μg/l at the MCL and Duke laboratories, respectively. The detection limits for cotinine were 2.0 μg/l and 0.02 μg/l at the MCL and UCSF laboratories, respectively. For Cd, 29.9% and 3.5% of the samples were below the limit of detection at the MCL and Duke laboratories, respectively. For cotinine, 75.4% and 16.4% of the samples were below the limit of detection at the MCL and UCSF laboratories, respectively. Because women vary in timing of delivery, blood samples were collected from 23 weeks to 42 weeks of gestation, with a median gestational age of 39 weeks and an interquartile range of 37–39 weeks. As the labs used to analyze samples for both Cd and cotinine levels changed over the course of the study, results were normalized across labs, and values were imputed below detection limits. For each chemical (i.e., Cd and cotinine), the rank permutation method as proposed in Burgette and Reiter 16 was used to flexibly transform the lab measurements into a normalized scale.
Data Restrictions
Imputed and normalized Cd values were not available for participants who were lost to follow-up before delivery or who were not of non-Hispanic white (NHW) or non-Hispanic black (NHB) race/ethnicity. Thus the full study cohort (n = 1854) was restricted to participants with an imputed and normalized blood Cd level per the transformation described above (excluded n = 617). Four participants delivering after the imputations and normalization were conducted were excluded, and one participant with an outlier value for blood Cd level was excluded. An additional three women were removed owing to missing covariate data. These restrictions resulted in a working sample size of 1229 women.
Statistical Analysis
Exploratory analyses were conducted in order to describe blood Cd levels within the study population of pregnant women overall, by demographic characteristics, and by smoking status. Demographic characteristics included race, age, education, insurance status, and relationship status. For this exploratory step, age was trichotomized as <20 years (i.e., 18–19 years given eligibility criteria), 20–34 years, and ≥35 years. Educational attainment was trichotomized as less than a high school degree, completed high school, and beyond high school. Participant relationship status was dichotomized as whether or not the participant was in a committed relationship. Insurance status was dichotomized as having private insurance or not having private insurance. The classification of “no private insurance” includes subjects who were either uninsured or covered by public health insurance services, such as Medicaid. Lack of private insurance is considered a proxy for low socioeconomic status (SES). Blood cotinine levels >10 μg/l are indicative of active smoking, 17,18 thus this cutoff was used to define participants as smokers or non-smokers. Cohen’s κ was used to assess agreement between reported and blood cotinine-defined smoking status.
The 2009–2010 NHANES reported the geometric mean blood Cd level among US adults was 0.36 μg/l and the median to be 0.32 μg/l.11 For the purposes of this study, a blood Cd level greater than the national median was considered “elevated”. Log-binomial models were used to calculate the relative risk (RR) of an elevated blood Cd level in all participants and stratified by smoking status. Models controlled for blood cotinine level, race, age, education, relationship status, and insurance status. Age and cotinine were entered as continuous linear predictors in the models, while all other variables were categorized as described above. All analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
Characteristics of the Study Population
The demographic composition of the study population is presented in Table 1. Of the 1229 participants meeting all restrictions, most were NHB (76.7%), 20–34 years old (73.4%), in a committed relationship (71.2%), and lacked private insurance (73.7%). Roughly 86% had a minimum of a high school education, with 49.5% having received at least some additional education beyond the high school level. Compared with cotinine-defined non-smokers where blood cotinine levels exceeded 10 ng/ml, smokers were more likely to be NHB, had lower educational attainment, were less likely to be in a committed relationship, and were less likely to have private insurance.
Table 1.
Study population characteristics.
|
All
|
Non-smokers
|
Smokers
|
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Race | ||||||
| Non-Hispanic white | 287 | 23.4 | 238 | 25.7 | 49 | 16.2 |
| Non-Hispanic black | 942 | 76.7 | 688 | 74.3 | 254 | 83.8 |
| Age (years) | ||||||
| 18—19 | 169 | 13.8 | 129 | 13.9 | 40 | 13.2 |
| 20—34 | 902 | 73.4 | 665 | 71.8 | 237 | 78.2 |
| 35 + | 158 | 12.9 | 132 | 14.3 | 26 | 8.6 |
| Educational attainment | ||||||
| Less than high school | 170 | 13.8 | 87 | 9.4 | 83 | 27.4 |
| Completed high school | 451 | 36.7 | 315 | 34.0 | 136 | 44.9 |
| More than high school | 608 | 49.5 | 524 | 56.6 | 84 | 27.7 |
| Relationship status | ||||||
| In a committed relationship | 875 | 71.2 | 681 | 73.5 | 194 | 64.0 |
| Not in a committed relationship | 354 | 28.8 | 245 | 26.5 | 109 | 36.0 |
| Insurance status | ||||||
| Private insurance | 323 | 26.3 | 304 | 32.8 | 19 | 6.3 |
| No private insurance | 906 | 73.7 | 622 | 67.2 | 284 | 93.7 |
| Cotinine-defined smoking status | ||||||
| Non-smoker | 926 | 75.4 | — | — | — | — |
| Smoker | 303 | 24.7 | — | — | — | — |
| Reported smoking status | ||||||
| Non-smoker | 1025 | 83.4 | — | — | — | — |
| Smoker | 204 | 16.6 | — | — | — | — |
| Blood Cd level | ||||||
| ≤Median for US adults | 477 | 38.8 | 434 | 46.9 | 43 | 14.2 |
| >Median for US adults | 752 | 61.2 | 492 | 53.1 | 260 | 85.8 |
Based on cotinine levels, 24.7% of participants were active smokers during pregnancy. Self-reported and medical record data only identified 16.6% of the participants as smokers. Agreement between the two measures of smoking status was substantial (κ = 0.62), and all subsequent analyses presented here utilize cotinine-defined smoking status.
Cd Levels in the Study Population
The geometric mean and standard deviation (SD) of blood Cd levels overall and by demographic categories are presented in Table 2. In the study population, the geometric mean blood Cd level was 0.33 μg/l, the 50th percentile was 0.40 μg/l, and the 75th percentile was 0.56 μg/l. Over 60% of the study population had a blood Cd level >0.32 μg/l (the median for US adults as reported in NHANES 2009–2010),11 indicating that our Durham County study population had higher levels of Cd exposure than might be expected given national data, especially given their pregnancy status.
Table 2.
Geometric mean and SD of blood Cd levels (μg/l) by demographic and socioeconomic characteristics.
| N | Mean | SD | P-valuea | Minimum Cd level | Maximum Cd level | |
|---|---|---|---|---|---|---|
| All | 1229 | 0.33 | 3.24 | 0 | 4.02 | |
| Race | 0.11 | |||||
| Non-Hispanic white | 287 | 0.30 | 3.41 | 0 | 4.02 | |
| Non-Hispanic black | 942 | 0.34 | 3.19 | 0 | 2.52 | |
| Age (years) | 0.37 | |||||
| 18–19 | 169 | 0.29 | 3.28 | 0 | 1.7 | |
| 20–34 | 902 | 0.33 | 3.15 | 0 | 4.02 | |
| 35 + | 158 | 0.32 | 3.73 | 0 | 1.28 | |
| Educational attainment | <0.01 | |||||
| Less than high school | 170 | 0.42 | 3.22 | 0 | 2.52 | |
| Completed high school | 451 | 0.35 | 2.92 | 0 | 4.02 | |
| More than high school | 608 | 0.29 | 3.45 | 0 | 2.46 | |
| Relationship status | <0.01 | |||||
| In a committed relationship | 875 | 0.31 | 3.51 | 0 | 4.02 | |
| Not in a committed relationship | 354 | 0.38 | 2.55 | 0 | 2.50 | |
| Insurance status | <0.01 | |||||
| Private insurance | 323 | 0.27 | 3.37 | 0 | 1.48 | |
| No private insurance | 906 | 0.35 | 3.17 | 0 | 4.02 | |
| Cotinine-defined smoking status | <0.01 | |||||
| Non-smoker | 926 | 0.27 | 3.26 | 0 | 2.26 | |
| Smoker | 303 | 0.59 | 2.56 | 0 | 4.02 |
P-value for tests for differences in geometric means across levels of each demographic characteristic.
Differences in the geometric mean blood Cd levels were found by educational attainment, relationship status, and insurance status (Table 2). Mean blood Cd levels decreased as educational attainment increased (P<0.01). Participants not in a committed relationship had, on an average, higher blood Cd levels than those in a committed relationship (P<0.01). A lack of private insurance was also associated with higher mean blood Cd levels (P<0.01). In the present study, race and age were not significantly associated with blood Cd levels.
Cd Levels and Smoking Status
Smoking status was highly related to Cd level, with a crude RR of an elevated blood Cd level for smokers versus non-smokers of 1.62 (95% confidence interval (CI): 1.50–1.74). This association remained significant after controlling for demographic characteristics, with a RR of an elevated blood Cd level for smokers versus non-smokers of 1.21 (95% CI: 1.15–1.28).
The distribution of blood Cd levels by cotinine-defined or reported smoking status are presented in Figures 1a and b. The geometric mean blood Cd level among cotinine-defined smokers was twice as high as the geometric mean blood Cd level among non-smokers (0.59 μg/l versus 0.27 μg/l, respectively; P<0.01). Among cotinine-defined smokers, 260 women (85.8%) had an elevated Cd level, with a maximum Cd level of 4.02 μg/l. Non-smokers also experienced Cd exposures, with 492 non-smoking women (53.1%) having a blood Cd level above the US median and Cd levels reaching as high as 2.26 μg/l (Tables 1 and 2).
Figure 1.
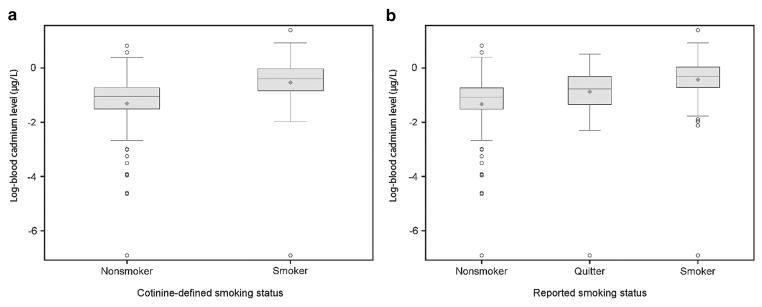
Distribution of log-transformed blood Cd level (μg/l) on cotinine-defined smoking status (a) and reported smoking status (b).
RR of Elevated Blood Cd Level
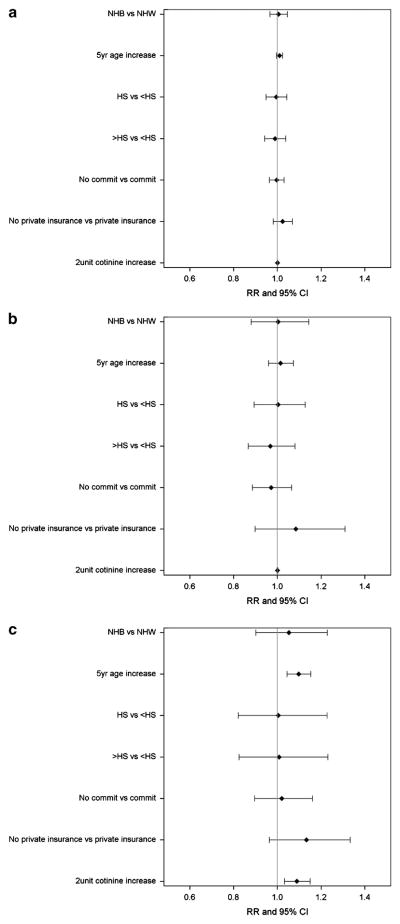
Log-binomial models estimated the RR of elevated blood Cd levels based on demographics and cotinine levels. Figure 2 displays the RRs and 95% CIs of an elevated blood Cd level among all participants (Figure 2a) and in smoking-stratified models (Figure 2b and c). Among all participants, race, age, education, relationship status, insurance status, and cotinine level were not associated with risk of elevated Cd level (all P>0.05). Similar results were observed in the model based on smoker-only data (see Figure 2b).
Figure 2.
Relative risks and 95% confidence intervals for high blood Cd level from a model controlling for demographic characteristics and cotinine level among all participants (a), smokers (b), and non-smokers (c).
Among non-smokers (see Figure 2c), age and cotinine level were associated with the risk of elevated Cd (P<0.05). A 5-year increase in age was associated with a 10% increase in risk of elevated Cd level (RR = 1.10, 95% CI = 1.04–1.15). A 2-μg/l increase in cotinine level was associated with a 9% increase in risk of elevated Cd level (RR = 1.09, 95% CI = 1.03–1.15). Race, educational attainment, insurance status, and relationship status were not significantly associated with blood Cd level among non-smokers.
DISCUSSION
Exposure to Cd during the prenatal period is associated with various detrimental health effects, including adverse pregnancy outcomes such as preterm labor, decreased birth weight, placental calcification, and early pregnancy loss.19–22 Although national biomonitoring efforts measure blood Cd levels in the general population, data on exposure to Cd during pregnancy are not widely available. In fact, very few states in the United States perform any sort of prenatal screening for environmental toxicants.23 In this paper, we described Cd levels among a cohort of urban pregnant women, demonstrating significant Cd exposures even among non-smokers. We did not observe associations between elevated Cd levels and either demographic covariates or blood cotinine levels in smokers; however, increasing age and cotinine levels were associated with higher risk of having a Cd level above the national median among non-smokers.
In a recent pilot study, we assessed blood Cd levels of 211 pregnant women residing in six counties across North Carolina and identified women with elevated Cd levels.13 In the present study, we expand upon this previous work to describe Cd levels among 1229 participants in the Healthy Pregnancy, Healthy Baby Study cohort. We observed a geometric mean blood Cd level of 0.33 μg/l. The levels of Cd observed in women in this study are relatively high, with roughly 60% of participants having a Cd level above the US median. We note that this over-representation of pregnant women above the US median in this study’s cohort runs counter to the intuitive belief that behavioral choice among pregnant women keeps adverse environmental exposures at or below national medians.
We found that blood Cd levels among smokers were twice as high as levels among non-smokers (0.59 μg/l versus 0.27 μg/l). We also showed that smoking was associated with a 21% higher risk of an elevated blood Cd level. These data are in line with previous research that demonstrated that smokers had 1.4 times the levels of blood Cd than never smokers and a 2% (95% CI: 0.5–3%) increase in blood Cd levels per pack-year.24 The association between smoking and Cd levels may help in understanding the relatively high blood Cd levels observed in this cohort. It is not surprising to find that smoking was associated with Cd levels as this is a known contributor to Cd levels.7 Our results, however, demonstrate the surprising results that smoking remained a major contributor to Cd levels even during pregnancy, a time when women have additional incentive to cease or reduce smoking. Thus this study highlights the need for continued efforts to promote smoking cessation particularly during pregnancy. In the United States, 17.1% of women of child-bearing age report smoking, and 12.8% of women report smoking during the last 3 months of pregnancy.25 The self-reported smoking rate in this cohort was 16.6% and the cotinine-based smoking rate in this cohort is 24.6%, almost twice the CDC-reported smoking rate during pregnancy, and highlights how smoking rates can vary dramatically across communities. This represents a critical public health issue that warrants further effort to educate women about the dangers of smoking during pregnancy.
Non-smokers in this cohort also experienced significant Cd exposures. As a result, cessation programs may not be sufficient to protect children from the impact of prenatal Cd exposure. Blood Cd levels above the US median were observed among 53% of the non-smokers in our cohort. This is an unexpectedly high proportion of non-smokers with elevated Cd levels. Among non-smokers, Cd levels reached as high as 2.26 μg/l. This indicates the importance of understanding factors that may impact Cd levels among women without the most common source of Cd exposure, smoking. We observed associations between age and cotinine levels and risk of elevated Cd level among non-smokers. These results may indicate cumulative burden of Cd exposure across the lifetime. We note, however, that, unexpectedly, age was not predictive of Cd levels among smokers; so it is unclear whether the cumulative exposure argument is a reasonable explanation of the Cd–age relationship among non-smokers.
In addition to smoking history and passive smoke/environmental tobacco smoke, other possible sources of Cd exposure among non-smokers may include diet, house dust, and proximity to industrial sources.24,26–28 Further exploration of sources of exposure among non-smokers may help in the design of public health interventions aimed at reducing Cd levels among women of child-bearing age. We note that standard demographic data do not predict Cd levels well among either smokers or non-smokers. This elevates the importance of developing a deeper understanding of non-smoking sources of Cd exposure, as well as better correlates of Cd levels. This would allow for the development of more effective interventions for minimizing Cd exposure during pregnancy.
This work is not without limitations. First, we do not directly measure SES here due to high levels of missingness in the available SES data. However, lack of private insurance is a good proxy for SES. In the study sample, NHBs and low-income women were intentionally oversampled, and small sample sizes required exclusion of Hispanic or Asians women from the analysis. Thus it will be important to evaluate blood Cd levels in other demographic groups in future studies. Finally, related to Cd levels, we were not able to identify/establish other potential sources of exposure that could include dietary intake. Our results inform future study design to better identify sources of Cd exposure.
CONCLUSIONS
This work highlights elevated levels of Cd in pregnant women, suggesting a clear need to collect more data on Cd exposure during pregnancy, ideally from demographically and geographically diverse communities. No doubt, such a change in public health biomonitoring strategy would be a massive undertaking, with high costs of sample collection and analysis, difficulties in interpreting biomonitoring data, potential legal and ethical issues of consent, and privacy issues.29 However, such biomonitoring efforts would be worthwhile given the documented health implications of prenatal Cd exposure.21,22,30 Perhaps not surprisingly, in the present study, smoking was consistently associated with Cd levels and remains a clear public health concern. It is important to note that we also demonstrate that non-smokers were also at risk for elevated Cd levels, suggesting other potential exposure sources of concern for future study. It is the hope that by prioritizing pregnant women and women of child-bearing age for biomonitoring efforts, those at risk for elevated levels of toxic metals will be identified.
Acknowledgments
We gratefully acknowledge Amber Ingram, Cheyenne Beach, Marteh Bowen, Anne Giguere, Jerrie Kumalah, Mollie Oudenhoven, Caroline Paulsen, and Nancy Schneider for their clinical recruitment of study participants. We also acknowledge Dr. Jill Johnston, Sloane Miller, and Rachel Kauffman for their help with the manuscript. This research was supported by the US Environmental Protection Agency (RD-83329301) and in part by the National Institute of Environmental Health Sciences (ES005948, ES010126, ES019315, and ES007018).
ABBREVIATIONS
- Cd
Cadmium
- NHB
non-Hispanic black
- NHW
non-Hispanic white
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Larsen LG, Clausen HV, Jonsson L. Stereologic examination of placentas from mothers who smoke during pregnancy. Am J Obstet Gynecol. 2002;186:531–537. doi: 10.1067/mob.2002.120481. [DOI] [PubMed] [Google Scholar]
- 2.Milnerowicz H, Zalewski J, Milnerowicz-Nabzdyk E, Zaslawski R, Woyton J. Effects of exposure to tobacco smoke in pregnancies complicted by oligohydramnios and premature rupture of the membranes. II. Activity of brush border enzymes in human amniotic fluid. Int J Occup Med Environ Health. 2001;14:275–285. [PubMed] [Google Scholar]
- 3.Shiverick KT, Salafia C. Cigarette smoking and pregnancy I: ovarian, uterine and placental effects. Placenta. 1999;20:265–272. doi: 10.1053/plac.1998.0377. [DOI] [PubMed] [Google Scholar]
- 4.Tian LL, Zhao YC, Wang XC, Gu JL, Sun ZJ, Zhang YL, et al. Effects of gestational cadmium exposure on pregnancy outcome and development in the offspring at age 4. 5 years. Biol Trace Elem Res. 2009;132:51–59. doi: 10.1007/s12011-009-8391-0. [DOI] [PubMed] [Google Scholar]
- 5.Kippler M, Tofail F, Hamadani JD, Gardner RM, Grantham-McGregor SM, Bottai M, et al. Early-life cadmium exposure and child development in 5-year-old girls and boys: a cohort study in rural Bangladesh. Environ Health Perspect. 2012;120:1462–1468. doi: 10.1289/ehp.1104431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pappas RS, Polzin GM, Watson CH, Ashley DL. Cadmium, lead, and thallium in smoke particulate from counterfeit cigarettes compared to authentic US brands. Food Chem Toxicol. 2007;45:202–209. doi: 10.1016/j.fct.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Ashraf MW. Levels of heavy metals in popular cigarette brands and exposure to these metals via smoking. Scientific World Journal. 2012;2012:729430. doi: 10.1100/2012/729430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238:201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Klebanoff MA, Levine RJ, Clemens JD, DerSimonian R, Wilkins DG. Serum cotinine concentration and self-reported smoking during pregnancy. Am J Epidemiol. 1998;148:259–262. doi: 10.1093/oxfordjournals.aje.a009633. [DOI] [PubMed] [Google Scholar]
- 10.Jarvis MJ, Russell MA, Benowitz NL, Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health. 1988;78:696–698. doi: 10.2105/ajph.78.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. Services USDoHaH; Atlanta, GA, USA: 2013. [Google Scholar]
- 12.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders AP, Flood K, Chiang S, Herring AH, Wolf L, Fry RC. Towards prenatal biomonitoring in North Carolina: assessing arsenic, cadmium, mercury, and lead levels in pregnant women. PLoS One. 2012;7:e31354. doi: 10.1371/journal.pone.0031354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miranda ML, Edwards SE, Swamy GK, Paul CJ, Neelon B. Blood lead levels among pregnant women: historical versus contemporaneous exposures. Int J Environ Res Public Health. 2010;7:1508–1519. doi: 10.3390/ijerph7041508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miranda ML, Edwards S, Maxson PJ. Mercury levels in an urban pregnant population in Durham County, North Carolina. Int J Environ Res Public Health. 2011;8:698–712. doi: 10.3390/ijerph8030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgette LF, Reiter JP. Nonparametric Bayesian multiple imputation for missing data due to mid-study switching of measurement methods. J Am Stat Assoc. 2012;107:439–449. [Google Scholar]
- 17.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 18.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA. 1996;275:1233–1240. [PubMed] [Google Scholar]
- 19.Nishijo M, Nakagawa H, Honda R, Tanebe K, Saito S, Teranishi H, et al. Effects of maternal exposure to cadmium on pregnancy outcome and breast milk. Occup Environ Med. 2002;59:394–396. doi: 10.1136/oem.59.6.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishijo M, Tawara K, Honda R, Nakagawa H, Tanebe K, Saito S. Relationship between newborn size and mother’s blood cadmium levels, Toyama, Japan. Arch Environ Health. 2004;59:22–25. doi: 10.3200/AEOH.59.1.22-25. [DOI] [PubMed] [Google Scholar]
- 21.Kippler M, Tofail F, Gardner R, Rahman A, Hamadani JD, Bottai M, et al. Maternal cadmium exposure during pregnancy and size at birth: a prospective cohort study. Environ Health Perspect. 2012;120:284–289. doi: 10.1289/ehp.1103711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirai S, Suzuki Y, Yoshinaga J, Mizumoto Y. Maternal exposure to low-level heavy metals during pregnancy and birth size. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2010;45:1468–1474. doi: 10.1080/10934529.2010.500942. [DOI] [PubMed] [Google Scholar]
- 23.CDC EA, Wengrovitz AG, Portier C, Brown MJ. Guidelines for the Identification and Management of Lead Exposure in Pregnant and Lactating Women. Services DoHaH; Atlanta, GA, USA: 2010. [Google Scholar]
- 24.Adams SV, Newcomb PA, Shafer MM, Atkinson C, Bowles EJA, Newton KM, et al. Sources of cadmium exposure among healthy premenopausal women. Sci Total Environ. 2011;409:1632–1637. doi: 10.1016/j.scitotenv.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC; Division of Reproductive Health NCfCDPaHP, editor. PRAMS and smoking. Center for Disease Control and Prevention; Atlanta, GA, USA: 2012. [Google Scholar]
- 26.Hogervorst J, Plusquin M, Vangronsveld J, Nawrot T, Cuypers A, Van Hecke E, et al. House dust as possible route of environmental exposure to cadmium and lead in the adult general population. Environ Res. 2007;103:30–37. doi: 10.1016/j.envres.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Oyoo-Okoth E, Admiraal W, Osano O, Ngure V, Kraak MHS, Omutange ES. Monitoring exposure to heavy metals among children in Lake Victoria, Kenya: environmental and fish matrix. Ecotox Environ Safe. 2010;73:1797–1803. doi: 10.1016/j.ecoenv.2010.07.040. [DOI] [PubMed] [Google Scholar]
- 28.Banza CLN, Nawrot TS, Haufroid V, Decree S, De Putter T, Smolders E, et al. High human exposure to cobalt and other metals in Katanga, a mining area of the Democratic Republic of Congo. Environ Res. 2009;109:745–752. doi: 10.1016/j.envres.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Arbuckle TE. Maternal-infant biomonitoring of environmental chemicals: the epidemiologic challenges. Birth Defects Res A Clin Mol Teratol. 2010;88:931–937. doi: 10.1002/bdra.20694. [DOI] [PubMed] [Google Scholar]
- 30.Lin CM, Doyle P, Wang D, Hwang YH, Chen PC. Does prenatal cadmium exposure affect fetal and child growth? Occup Environ Med. 2011;68:641–646. doi: 10.1136/oem.2010.059758. [DOI] [PubMed] [Google Scholar]