Abstract
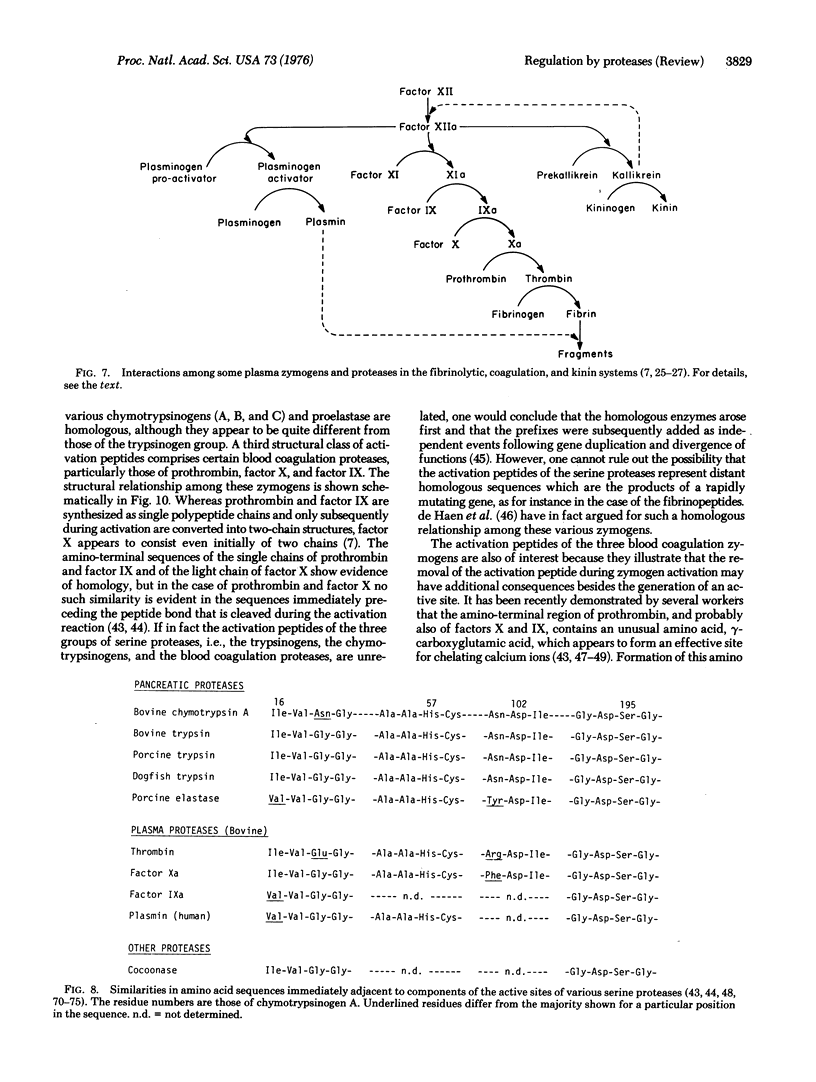
Many enzymes, hormones, and other physiologically active proteins are synthesized as inactive precursors (zymogens) that are subsequently converted to the active form by the selective enzymatic cleavage (limited proteolysis) of peptide bonds. The ultimate agency of activating enzymatic function is limited proteolysis, either in a single activation step or in a consecutive series (cascade). The specificity of each activation reaction is determined by the complementarity of the zymogen substrate and the active site of the attacking protease. The sequence of consecutive activation reactions is regulated by the specificity of each enzyme, whereas the degree of amplification of the initial stimulus is determined by the efficiency of each activating step. Zymogen activation produces a prompt and irreversible response to a physiological stimulus, and is capable of initiating new physiological functions. Typical examples are the precesses of blood coagulation, fibrinolysis, complement activation, hormone production, metamorphosis, fertilazation, supra-molecular assembly, and digestion. The zymogens of the pancreatic serine proteases, in particular, have served as models for detailed studies of the nature of the molecular changes that are involved in the dramatic increase in enzymatic activity that ensues upon limited proteolysis of the zymogen.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. The structure and assembly of procollagen - a review. J Supramol Struct. 1974;2(2-4):108–120. doi: 10.1002/jss.400020206. [DOI] [PubMed] [Google Scholar]
- Cabib E., Farkas V. The control of morphogenesis: an enzymatic mechanism for the initiation of septum formation in yeast. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2052–2056. doi: 10.1073/pnas.68.9.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
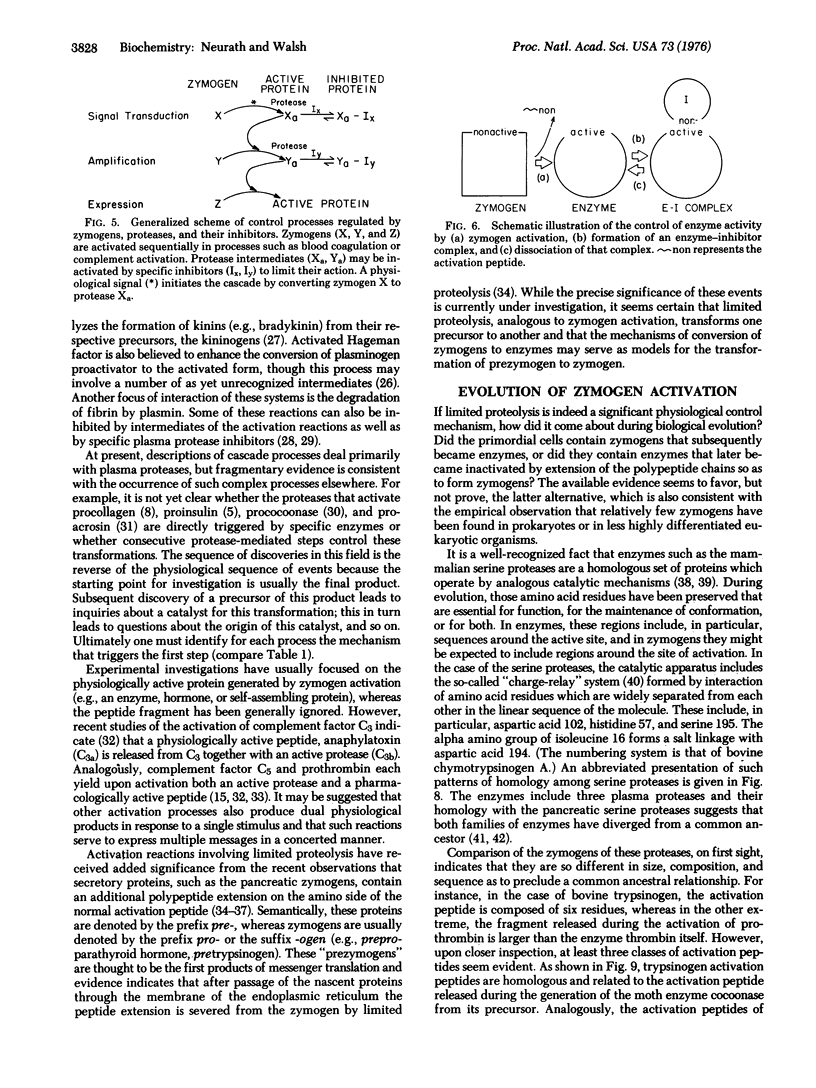
- DAVIE E. W., RATNOFF O. D. WATERFALL SEQUENCE FOR INTRINSIC BLOOD CLOTTING. Science. 1964 Sep 18;145(3638):1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- Davie E. W., Fujikawa K. Basic mechanisms in blood coagulation. Annu Rev Biochem. 1975;44:799–829. doi: 10.1146/annurev.bi.44.070175.004055. [DOI] [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohke K. Studies on prephenoloxidase-activating enzyme from cuticle of the silkworm Bombyx mori. I. Activation reaction by the enzyme. Arch Biochem Biophys. 1973 Jul;157(1):203–209. doi: 10.1016/0003-9861(73)90406-2. [DOI] [PubMed] [Google Scholar]
- Enfield D. L., Ericsson L. H., Fujikawa K., Titani K., Walsh K. A., Neurath H. Bovine factor IX (Christmas factor). Further evidence of homology with factor X (Stuart factor) and prothrombin. FEBS Lett. 1974 Oct 1;47(1):132–135. doi: 10.1016/0014-5793(74)80442-4. [DOI] [PubMed] [Google Scholar]
- Enfield D. L., Ericsson L. H., Walsh K. A., Neurath H., Titani K. Bovine factor X1 (Stuart factor). Primary structure of the light chain. Proc Natl Acad Sci U S A. 1975 Jan;72(1):16–19. doi: 10.1073/pnas.72.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsted R. L., Kramer K. J., Law J. H., Berger E., Kafatos F. C. Cocoonase. IV. Mechanism of activation of prococoonase from Antheraea polyphemus. J Biol Chem. 1973 May 10;248(9):3012–3020. [PubMed] [Google Scholar]
- Freer S. T., Kraut J., Robertus J. D., Wright H. T., Xuong N. H. Chymotrypsinogen: 2.5-angstrom crystal structure, comparison with alpha-chymotrypsin, and implications for zymogen activation. Biochemistry. 1970 Apr 28;9(9):1997–2009. doi: 10.1021/bi00811a022. [DOI] [PubMed] [Google Scholar]
- Gertler A., Walsh K. A., Neurath H. Catalysis by chymotrypsinogen. Demonstration of an acyl-zymogen intermediate. Biochemistry. 1974 Mar 12;13(6):1302–1310. doi: 10.1021/bi00703a038. [DOI] [PubMed] [Google Scholar]
- Harper E., Bloch K. J., Gross J. The zymogen of tadpole collagenase. Biochemistry. 1971 Aug 3;10(16):3035–3041. doi: 10.1021/bi00792a008. [DOI] [PubMed] [Google Scholar]
- Hass G. M., Ako H., Grahn D. T., Neurath H. Carboxypeptidase inhibitor from potatoes. The effects of chemical modifications on inhibitory activity. Biochemistry. 1976 Jan 13;15(1):93–100. doi: 10.1021/bi00646a015. [DOI] [PubMed] [Google Scholar]
- Henderson R., Wright C. S., Hess G. P., Blow D. M. -Chymotrypsin: what can we learn about catalysis from x-ray diffraction? Cold Spring Harb Symp Quant Biol. 1972;36:63–70. doi: 10.1101/sqb.1972.036.01.011. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Neurath H., Walsh K. A. Determination of the amino acid sequence of porcine trypsin by sequenator aalysis. Biochemistry. 1973 Aug 14;12(17):3146–3153. doi: 10.1021/bi00741a002. [DOI] [PubMed] [Google Scholar]
- Holstein T. J., Stowell C. P., Quevedo W. C., Jr, Zarcaro R. M., Bienieki T. C. Peroxidase, "protyrosinase," and the multiple forms of tyrosinase in mice. Yale J Biol Med. 1973 Dec;46(5):560–571. [PMC free article] [PubMed] [Google Scholar]
- Jesty J., Esnouf M. P. The preparation of activated factor X and its action on prothrombin. Biochem J. 1973 Apr;131(4):791–799. doi: 10.1042/bj1310791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassell B., Kay J. Zymogens of proteolytic enzymes. Science. 1973 Jun 8;180(4090):1022–1027. doi: 10.1126/science.180.4090.1022. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Ernst M. D., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone: analysis of radioactive tryptic peptides and amino acid sequence. Biochemistry. 1976 Jan 13;15(1):15–19. doi: 10.1021/bi00646a003. [DOI] [PubMed] [Google Scholar]
- Kerr M. A., Walsh K. A., Neurath H. Catalysis by serine proteases and their zymogens. A study of acyl intermediates by circular dichroism. Biochemistry. 1975 Nov 18;14(23):5088–5094. doi: 10.1021/bi00694a010. [DOI] [PubMed] [Google Scholar]
- Kramer K. J., Felsted R. L., Law J. H. Cocoonase. V. Structural studies on an insect serine protease. J Biol Chem. 1973 May 10;248(9):3021–3028. [PubMed] [Google Scholar]
- Kreil G. Biosynthesis of melittin, a toxic peptide from bee venom. Amino-acid sequence of the precursor. Eur J Biochem. 1973 Mar 15;33(3):558–566. doi: 10.1111/j.1432-1033.1973.tb02716.x. [DOI] [PubMed] [Google Scholar]
- Leckie B. The activation of a possible zymogen of renin in rabbit kidney. Clin Sci. 1973 Mar;44(3):301–304. [PubMed] [Google Scholar]
- MACFARLANE R. G. AN ENZYME CASCADE IN THE BLOOD CLOTTING MECHANISM, AND ITS FUNCTION AS A BIOCHEMICAL AMPLIFIER. Nature. 1964 May 2;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- Magnusson S., Sottrup-Jensen L., Petersen T. E., Morris H. R., Dell A. Primary structure of the vitamin K-dependent part of prothrombin. FEBS Lett. 1974 Aug 25;44(2):189–193. doi: 10.1016/0014-5793(74)80723-4. [DOI] [PubMed] [Google Scholar]
- Maroux S., Baratti J., Desnuelle P. Purification and specificity of porcine enterokinase. J Biol Chem. 1971 Aug 25;246(16):5031–5039. [PubMed] [Google Scholar]
- McGuire J., Newman D., Barisas G. The tyrosinase-protyrosinase system in frog epidermis. Yale J Biol Med. 1973 Dec;46(5):572–582. [PMC free article] [PubMed] [Google Scholar]
- Meizel S., Mukerji S. K. Proacrosin from rabbit epididymal spermatozoa: partial purification and initial biochemical characterization. Biol Reprod. 1975 Aug;13(1):83–93. doi: 10.1095/biolreprod13.1.83. [DOI] [PubMed] [Google Scholar]
- Morgan P. H., Robinson N. C., Walsh K. A., Neurath H. Inactivation of bovine trypsinogen and chymotrypsinogen by diisopropylphosphorofluoridate. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3312–3316. doi: 10.1073/pnas.69.11.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan P. H., Walsh K. A., Neurath H. Inactivation of trypsinogen by methane sulfonyl fluoride. FEBS Lett. 1974 Apr 15;41(1):108–110. doi: 10.1016/0014-5793(74)80965-8. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Takahashi H., Koida M., Suzuki T., Schoenmakers J. G. Partial purification of bovine plasma kallikreinogen, its activation by the Hageman factor. Biochem Biophys Res Commun. 1968 Aug 21;32(4):644–649. doi: 10.1016/0006-291x(68)90287-8. [DOI] [PubMed] [Google Scholar]
- Neurath H., Walsh K. A., Winter W. P. Evolution of structure and function of proteases. Science. 1967 Dec 29;158(3809):1638–1644. doi: 10.1126/science.158.3809.1638. [DOI] [PubMed] [Google Scholar]
- RICHARDS F. M., VITHAYATHIL P. J. The preparation of subtilisn-modified ribonuclease and the separation of the peptide and protein components. J Biol Chem. 1959 Jun;234(6):1459–1465. [PubMed] [Google Scholar]
- Reddy K. N., Markus G. Mechanism of activation of human plasminogen by streptokinase. Presence of active center in streptokinase-plasminogen complex. J Biol Chem. 1972 Mar 25;247(6):1683–1691. [PubMed] [Google Scholar]
- Robbins K. C., Bernabe P., Arzadon L., Summaria L. The primary structure of human plasminogen. I. The NH 2 -terminal sequences of human plasminogen and the S-carboxymethyl heavy (A) and light (B) chain derivatives of plasmin. J Biol Chem. 1972 Nov 10;247(21):6757–6762. [PubMed] [Google Scholar]
- Robbins K. C., Bernabe P., Arzadon L., Summaria L. The primary structure of human plasminogen. II. The histidine loop of human plasmin: light (B) chain active center histidine sequence. J Biol Chem. 1973 Mar 10;248(5):1631–1633. [PubMed] [Google Scholar]
- Robertus J. D., Kraut J., Alden R. A., Birktoft J. J. Subtilisin; a stereochemical mechanism involving transition-state stabilization. Biochemistry. 1972 Nov 7;11(23):4293–4303. doi: 10.1021/bi00773a016. [DOI] [PubMed] [Google Scholar]
- Robinson N. C., Neurath H., Walsh K. A. The relation of the -amino group of trypsin to enzyme function and zymogen activation. Biochemistry. 1973 Jan 30;12(3):420–426. doi: 10.1021/bi00727a010. [DOI] [PubMed] [Google Scholar]
- SKEGGS L. T., Jr, KAHN J. R., LENTZ K., SHUMWAY N. P. The preparation, purification, and amino acid sequence of a polypeptide renin substrate. J Exp Med. 1957 Sep 1;106(3):439–453. doi: 10.1084/jem.106.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Burstein Y. Partial sequence of the precursors of immunoglobulin light-chains of different subgroups: evidence that the immunoglobulin variable-region gene is larger than hitherto known. Biochem Biophys Res Commun. 1976 Jan 26;68(2):489–496. doi: 10.1016/0006-291x(76)91172-4. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Stenflo J. Vitamin K and the biosynthesis of prothrombin. IV. Isolation of peptides containing prosthetic groups from normal prothrombin and the corresponding peptides from dicoumarol-induced prothrombin. J Biol Chem. 1974 Sep 10;249(17):5527–5535. [PubMed] [Google Scholar]
- Summaria L., Arzadon L., Bernabe P., Robbins K. C. The interaction of streptokinase with human, cat, dog, and rabbit plasminogens. The fragmentation of streptokinase in the equimolar plasminogen-streptokinase complexes. J Biol Chem. 1974 Aug 10;249(15):4760–4769. [PubMed] [Google Scholar]
- Sweet R. M., Wright H. T., Janin J., Chothia C. H., Blow D. M. Crystal structure of the complex of porcine trypsin with soybean trypsin inhibitor (Kunitz) at 2.6-A resolution. Biochemistry. 1974 Sep 24;13(20):4212–4228. doi: 10.1021/bi00717a024. [DOI] [PubMed] [Google Scholar]
- Tager H. S., Steiner D. F. Isolation of a glucagon-containing peptide: primary structure of a possible fragment of proglucagon. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2321–2325. doi: 10.1073/pnas.70.8.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Ericsson L. H., Neurath H., Walsh K. A. Amino acid sequence of dogfish trypsin. Biochemistry. 1975 Apr 8;14(7):1358–1366. doi: 10.1021/bi00678a003. [DOI] [PubMed] [Google Scholar]
- Titani K., Fujikawa K., Enfield D. L., Ericsson L. H., Walsh K. A., Neurath H. Bovine factor X1 (Stuart factor): amino-acid sequence of heavey chain. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3082–3086. doi: 10.1073/pnas.72.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Hermodson M. A., Fujikawa K., Ericsson L. H., Walsh K. A., Neurath H., Davie E. W. Bovine factor X 1a (activated Stuart factor). Evidence of homology with mammalian serine proteases. Biochemistry. 1972 Dec 19;11(26):4899–4903. doi: 10.1021/bi00776a004. [DOI] [PubMed] [Google Scholar]
- de Haas G. H., Slotboom A. J., Bonsen P. P., van Deenen L. L. Studies on phospholipase A and its zymogen from porcine pancreas. I. The complete amino acid sequence. Biochim Biophys Acta. 1970 Oct 20;221(1):31–53. doi: 10.1016/0005-2795(70)90195-9. [DOI] [PubMed] [Google Scholar]
- de Haën C., Neurath H., Teller D. C. The phylogeny of trypsin-related serine proteases and their zymogens. New methods for the investigation of distant evolutionary relationships. J Mol Biol. 1975 Feb 25;92(2):225–259. doi: 10.1016/0022-2836(75)90225-9. [DOI] [PubMed] [Google Scholar]


