Abstract
Background:
Habitual physical activity (PA) may help to optimize bariatric surgery outcomes; however objective PA measures show that most patients have low PA preoperatively and make only modest PA changes postoperatively. Patients require additional support to adopt habitual PA.
Objectives:
Test the efficacy of a preoperative PA intervention (PAI) versus standard pre-surgical care (SC) for increasing daily moderate-to-vigorous PA (MVPA) in bariatric surgery patients.
Setting:
University Hospital, United States.
Methods:
Outcomes analysis included 75 participants (86.7% women; 46.0±8.9 years; Body Mass Index [BMI]=45.0±6.5 kg/m2) who were randomly assigned preoperatively to 6 weeks of PAI (n=40) or SC (n=35). PAI received weekly individual face-to-face sessions with tailored instruction in behavioral strategies (e.g., self-monitoring, goal-setting) to increase home-based walking exercise. The primary outcome, pre- to post-intervention change in daily bout-related (≥10-min bouts) and total (≥1-minute bouts) MVPA minutes, was assessed objectively via a multi-sensor monitor worn for 7 days at baseline- and post-intervention.
Results:
Retention was 84% at the post-intervention primary end point. In intent-to-treat analyses with baseline value carried forward for missing data and adjusted for baseline MVPA, PAI achieved a mean increase of 16.6±20.6 minutes/day in bout-related MVPA (baseline: 4.4±5.5 to post-intervention: 21.0±21.4 minutes/day) compared to no change (−0.3±12.7 minutes/day; baseline: 7.9±16.6 to post-intervention: 7.6±11.5 minutes/day) for SC (p=0.001). Similarly, PAI achieved a mean increase of 21.0±26.9 minutes/day in total MVPA (baseline: 30.9±21.2 to post-intervention: 51.9±30.0 minutes/day), whereas SC demonstrated no change (− 0.1±16.3 minutes/day; baseline: 33.7±33.2 to post-intervention: 33.6±28.5 minutes/day) (p=0.001).
Conclusions:
With behavioral intervention, patients can significantly increase MVPA before bariatric surgery compared to SC. Future studies should determine whether preoperative increases in PA can be maintained postoperatively and contribute to improved surgical outcomes.
Keywords: physical activity, objective measurement, severe obesity, intervention, behavior, bariatric, randomized controlled trial
INTRODUCTION
Bariatric surgery patients are typically advised to adopt an active lifestyle that includes walking exercise and other similar moderate-intensity activities given that habitual physical activity (PA) may help to optimize weight loss and other surgical outcomes (1). Previous research using subjective methods to assess PA in bariatric surgery patients suggests that many patients heed this advice, reporting large pre- to post-operative increases in PA (1). However, recent studies using objective measures to obtain more valid estimates of patients’ PA patterns, duration, and intensity do not corroborate these self-reported findings (2-4). Most patients demonstrate an insufficient level of PA preoperatively, accumulating little if any moderate-to-vigorous intensity PA (MVPA) in sustained bouts of ≥10 minutes (5,6), and remain insufficiently active postoperatively, showing only modest changes in MVPA that are markedly smaller than those based on self-report (2-4).
While the above research suggests that becoming habitually active represents a significant challenge for many bariatric surgery patients, few interventions to assist patients in meeting this challenge have been conducted. Moreover, despite recommendations that patients initiate habitual PA preoperatively (1,7,8), only one intervention study has focused solely on increasing PA during this period (9). However, this feasibility study lacked a control group, provided bi-weekly supervised exercise training but no behavioral strategies to increase the level of PA performed by participants in their own environment (i.e. free-living PA), and did not assess changes in daily time spent in PA.
The current Bari-Active study used a randomized controlled design to compare the efficacy of a 6-week preoperative behavioral physical activity intervention (PAI) versus a standard pre-surgical care control condition (SC) for increasing daily MVPA performed in sustained bouts ≥10-minutes (i.e. bout-related MVPA). Accumulating MVPA in sustained bouts of this duration is emphasized by national PA guidelines (10) and may be important for exercise adherence and weight control (11). The primary outcome was pre to post-intervention changes in minutes/day of both bout-related MVPA and total MVPA (i.e. MVPA accumulated in ≥1-minute bouts). These changes were assessed objectively via a multi-sensor armband monitor worn for 7 days before and after the 6-week intervention period. We hypothesized that participants randomly assigned to the PAI condition would demonstrate significantly greater increases in daily bout-related and total MVPA minutes at post-intervention follow-up than those assigned to the SC condition. A secondary aim was to examine pre- to post-intervention changes in daily steps, using the same objective measure.
METHODS
Design
Participants were referred to the Bari-Active Trial by surgeons from 3 different hospital clinics in Rhode Island, USA between April 2010 and January 2014. Potential participants were identified during an initial surgery consultation. Prior to meeting the surgeon, a member of the surgical team reviewed a printed study flyer with the patient. If interested, the patient informed the surgeon who signed the flyer along with the patient to verify that the patient could safely engage in walking exercise. The flyer was then faxed to research staff who contacted the patient to conduct a telephone eligibility screening and schedule an orientation/baseline assessment visit. During this visit, participants received more detailed study information, provided informed consent, underwent height and weight measurements, and completed questionnaires. At the conclusion of the visit, participants were provided with a multi-sensor armband activity monitor to wear for seven consecutive days during waking hours, which served as a baseline objective PA assessment for post-intervention comparisons. Approximately one week later, participants returned with the monitor and were randomized.
Participants were randomly assigned preoperatively to 6 weeks of either: PA intervention (PAI) or standard pre-surgical care control (SC). The PAI consisted of weekly individual face-to-face counseling sessions involving instruction in behavioral strategies to increase home-based walking exercise performed at a moderate intensity and in bouts ≥10-min by 30 minutes/day. Participants assigned to SC attended routine clinical appointments and had contact with members of the surgical team, but received no intervention.
Participants in both groups were assessed again after the 6-week intervention and wore the objective PA monitor for another 7-day period. Upon returning the monitor 1 week later, participants were provided with a $50 honorarium. Study procedures were approved by the Institutional Review Board of XXX, Rhode Island USA. This trial was registered at clinicaltrials.gov.
Participants
Participants were 80 ambulatory severely obese individuals with a body mass index (BMI) of ≥35 kg/m2, aged 18-70 years old, who were seeking bariatric surgery, had obtained written consent from a surgeon to participate, and reported insufficient PA (i.e., <150 weekly minutes of MVPA) and ability to walk ≥2 blocks unassisted. Exclusion criteria included being scheduled for a bariatric operation within 10 weeks of initial study screening or during the intervention period, current participation in another PA or weight loss program, intention to move to another geographic location during the course of the study, and having medical, psychiatric, or language barriers that would interfere with ability to participate in and follow the study protocol.
Randomization
Using a computer-generated random sequence, participants were randomly assigned 1:1 to either the PAI or SC conditions between April 2010 and January 2014. The allocation sequence was revealed to the patient after completion of the baseline assessment by a research staff member.
Physical Activity Intervention (PAI)
The intervention consisted of 6 consecutive weekly individual face-to-face counseling sessions delivered by the primary author at a single site. The intervention was implemented using a counselor manual and lesson handouts for participants. Intervention sessions lasted 30-45 minutes and were structured to complete the following objectives: 1) review participants’ PA self-monitoring records and progress towards intervention goals; 2) problem-solve barriers to achieving goals; 3) teach behavioral and cognitive strategies to achieve goals; 4) set bout-related walking exercise and step goals; 5) develop a daily action plan detailing when, where, and how the bout-related walking exercise goal would be achieved; and 6) discuss homework assignment involving application of behavioral and cognitive strategies to achieve goals. Intervention behavioral and cognitive strategies were based on: 1) the PA component of the lifestyle intervention employed in the Diabetes and Prevention Program (DPP) (12) and Look AHEAD (13) trials; and 2) theoretical constructs from the Transtheoretical Model (14), Theory of Planned Behavior (15), Social Cognitive Theory (16), and Self-Determination Theory (17) shown to be important for increasing PA motivation, self-efficacy and behavior among obese individuals (18,19). Table 1 provides a detailed overview of the topics, content, and strategies, beyond self-monitoring, goal-setting, action planning, and problem-solving, employed in each session.
Table 1.
Physical activity intervention content and strategies
| Week | Session Topic and Content | Behavioral and Cognitive Strategies |
|---|---|---|
| 1 | Welcome to the Bari-Active Program
|
|
| 2 | Building a Preoperative Walking Program
|
|
| 3 | Creating an Active Environment: Making Physical and Social Cues Work for You
|
|
| 4 | Setting Goals
|
|
| 5 | Problem Solving
|
|
| 6 | Putting it all Together and Establishing Commitment
|
|
The primary aim of the intervention was to increase minutes/day spent in bout-related and total MVPA. To accomplish this, two goals were prescribed. The main goal was to increase walking exercise performed at a moderate intensity in bouts ≥10 minutes by 30 minutes/day. This goal was intentionally set higher than the national guideline of ≥150 minutes/week (22 minutes/day) of bout-related MVPA (10) in an effort to increase the likelihood that participants would meet this national guideline. The talk test was used to help participants gauge whether they were engaging in moderate-intensity activity (20). Participants were taught that they were performing walking exercise at a moderate or appropriate pace if they could talk comfortably, too slow of a pace if they could sing, and too fast of a pace if they were breathless and could not talk. A secondary goal was to increase number of steps taken by 5,000/day. To track and motivate progress toward these goals, participants received a monitoring log and pedometer at the first weekly session. They were asked to record the number of bout-related walking exercise minutes performed and steps taken in the log at the end of each day throughout the 6-week intervention. For the first week, participants were instructed not to alter their usual activity patterns to establish baseline daily averages for bout-related walking exercise minutes and steps. These data were then used to determine daily bout-related walking exercise and step goals and the rate of progression in these behaviors over the remaining weeks of the intervention. For the second intervention week, participants were given the goals to add 10 minutes of bout-related walking exercise minutes/day and 1,000 steps/day to baseline. It was estimated that participants could accumulate 1,000 steps during 10 minutes of moderate-intensity walking exercise, with additional steps being derived from activities of daily living. For each of the subsequent weeks, participants added 5 minutes of daily bout-related walking exercise and 1,000 daily steps to the totals achieved during the previous week. To further help participants meet the goals, intervention sessions focused on identifying other enjoyable forms of moderate-intensity exercise to perform in addition to walking exercise, and making more active choices (e.g., parking farther away from the store, avoiding drive-through conveniences, taking the stairs instead of the elevator, etc.) throughout the day to accumulate more steps.
Standard Pre-Surgical Care Control (SC)
Participants assigned to the SC condition attended routine preoperative clinic visits and received the standard of preoperative care, but did not receive a PA intervention. Surgeons and members of the surgical team advised participants to adopt an active lifestyle and engage in walking exercise and other similar activities but provided no formal prescription or strategies to change PA behavior.
Measures
Objective measurement of MVPA. The SenseWear Armband monitor (SWA; BodyMedia, Inc., Pittsburgh, PA) was used to objectively measure both the primary outcome, changes in daily bout-related and total MVPA minutes, and the secondary outcome, changes in daily steps. The SWA is a wireless multi-sensor monitor worn on the upper right triceps muscle. This device simultaneously integrates movement data from a triaxial accelerometer, several sensor-measured physiologic metrics (i.e., heat flux, galvanic skin response, skin and near-body temperatures), and entered anthropometric (i.e. height and body weight) and demographic (i.e. sex and age) information into proprietary algorithms to estimate energy expenditure and time spent engaged in activities of different levels of intensity. The SWA demonstrates acceptable levels of agreement with criterion measures of total and activity-related energy expenditure (21,22), provides comparable MVPA estimates to those from other objective monitors (23), and is increasingly used to objectively quantify PA in the bariatric surgery population (4,23-25).
Participants were asked to wear the SWA during all waking hours for 7 consecutive days at both baseline and post-intervention follow-up. The SenseWear Professional Software (Version 7.0) was used to determine the number of days and hours/day that participants wore the SWA. The number of minutes participants spent in bout-related and total MVPA was determined using metabolic equivalent (MET) values, where activities ≥3 METs were classified as MVPA (10). Time spent in bout-related MVPA was calculated based on the rule that a participant had to be active for 10 minutes at or above the 3 METs threshold for a bout to be counted. Participants’ data were considered valid if they wore the SWA for ≥6 hours/day on ≥4 days during both the baseline and post-intervention follow-up periods. These criteria have been shown to maximize sample size (26,27) and yield estimates of bout-related and total MVPA in overweight/obese individuals before and after lifestyle intervention that are comparable to those based on more stringent criteria (26).
Other Measures. Participants completed self-report questionnaires assessing demographic characteristics (i.e. age, sex, race/ethnicity, marital status, educational level, employment) at baseline. Weight was measured in light street clothing, without shoes, and on a calibrated scale at both baseline and post-intervention.
Statistical Analysis
All analyses were performed using SPSS Statistics for Windows (version 20.0; SPSS, IBM Corp, Armonk, NY). Descriptive statistics including means and standard deviations (SD) and frequency counts were calculated to describe participants’ baseline characteristics and their MVPA at baseline and post-intervention follow-up. T-test and chi-square were used to compare the baseline characteristics of participants in SC and PAI. Changes in bout-related and total MVPA minutes and daily steps from baseline to post-intervention follow-up were assessed via separate analysis of covariance (ANCOVA) adjusting for baseline MVPA minutes and SWA wear time at both baseline and post-intervention follow-up. Analyses followed the intent-to-treat (ITT) principle in which missing data at post-intervention follow-up was replaced by baseline MVPA minutes (assuming no change in MVPA minutes). Due to evidence of minor violations of the assumptions of ANCOVA (i.e., normal distribution of the outcome, homogeneity of variances), non-parametric tests (28) were conducted to confirm the pattern of results obtained from the ANCOVAs. The pattern of results for the parametric and non-parametric tests was identical (data not shown); only the results of the ANCOVAs are reported below. Chi-square was used to compare the proportion of participants in the PAI and SC groups who achieved ≥ 150 minutes per week of bout-related MVPA at baseline and post-intervention follow-up. All tests of statistical significance were two-tailed, with α=0.05. This trial was designed to have 80% power to detect significant between-group differences in bout-related MVPA of at least 11 minutes/day (a large effect equivalent to Cohen’s d ≥ 0.95) with a sample size of at least 75.
The effect of different activity monitor weartime requirements on the analysis of primary outcomes was assessed in sensitivity analyses in which a covariate representing two different weartime requirements (0 = less stringent requirement of ≥6 hours/day, 1 = more stringent requirement of ≥10 hours/day) were added to the ANCOVAs. Consistent with previous research showing that using the less stringent criterion does not adversely affect analysis of change in MVPA (26), there was no effect of weartime on the results (p > 0.50 for bout-related and total MVPA) in the sensitivity analysis. Thus, the results of the analyses using the less stringent criterion are reported below.
RESULTS
Recruitment and Retention
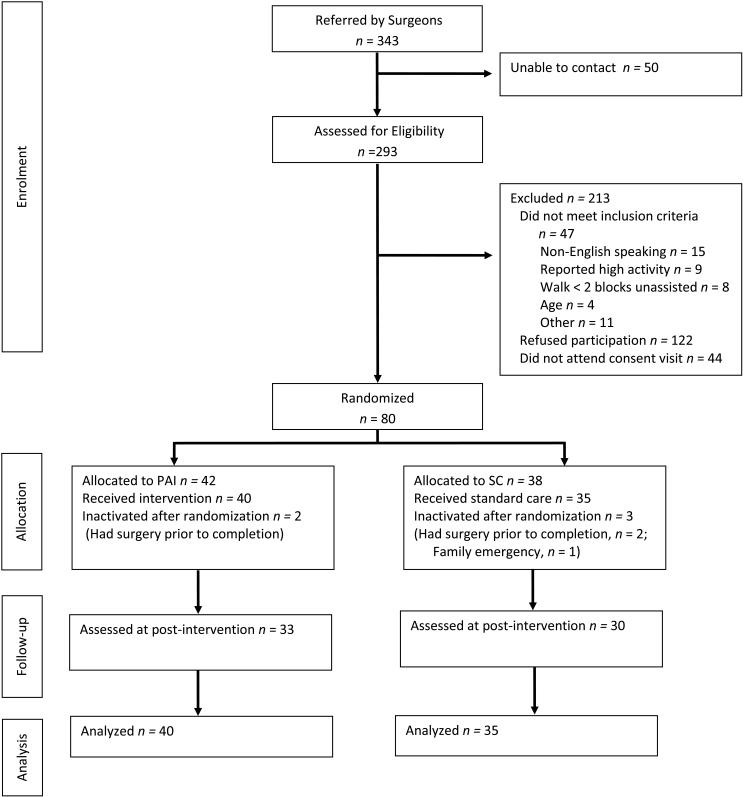
The CONSORT diagram is depicted in Figure 1. Of 343 patients referred, 293 were reached by telephone and screened for eligibility. Of those screened, 213 were excluded, resulting in a total of 80 participants who were randomized. Five participants were inactivated after randomization due to undergoing surgery prior to study completion (n=4) and family emergency prior to beginning the intervention period (n=1). Of the remaining 75 participants, 63 (84%) completed the 6-week follow-up primary end point. All attrition was due to loss of contact with participants. The rate of retention between the PAI and SC groups at post-intervention follow-up was not significantly different (82.5% in PAI vs. 85.7% in SC, p=0.76). Participants who completed the study were significantly older than participants who did not complete the study (47.1±8.4 vs. 40.3±9.4 years old, p=0.01].
Figure 1.
CONSORT flow diagram includes data on surgeon referrals, patient enrollment, allocation to intervention and control groups, assessment at post-intervention follow-up, and primary analysis.
Participant Characteristics
Participants’ baseline characteristics are reported in Table 1. Most participants were female (87%). Twenty-one percent of participants were non-White and approximately 12% were Hispanic. Approximately half of participants were married, 28% had a college degree, and 63% had full-time employment. Participants in the PAI condition weighed significantly more compared to those in the SC condition at both baseline (125.7±21.0 vs. 114.9±18.9 kg, p=0.02) and post-intervention follow-up (125.3±20.5 vs. 115.0±18.7, p=0.02), but there was not a significant difference in BMI or other characteristics. Neither group experienced significant weight loss over the course of the intervention period. Of the 63 participants who completed the preoperative intervention outcomes assessment, 41 went on to have surgery. Of these 41 participants, the average length between completion of the assessment and surgery was 90.0 ± 64.5 days.
Physical Activity Intervention Attendance
Thirty-three of the 40 (83%) participants who received PAI also completed PAI. Among these 33 completers, 100% attended all 6 weekly sessions.
Intention-to-Treat Analysis
On average, participants wore the SWA monitor for 11.4±1.8 hours per day on 6.5±1.6 days during baseline and 12.3±1.6 hours per day on 6.4±1.2 days during post-intervention follow-up. There were no statistically significant differences in SWA monitor wear time between the PAI and SC groups during the baseline and post-intervention follow-up periods (p > 0.05) (Table 2).
Table 2.
Baseline characteristics of participants assigned to the Physical Activity Intervention (PAI) and Standard Surgical Care Control (SC) Conditions
| Full Sample (N = 75) |
PAI Condition (N = 40) |
SC Condition (N = 35) |
|
|---|---|---|---|
|
|
|||
| Sex (%) | |||
| Men | 13.3 | 15.0 | 11.4 |
| Women | 86.7 | 85.0 | 88.6 |
| Age, mean (SD), years | 46.0 (8.9) | 44.2 (9.2) | 48.1 (8.1) |
| Race (%) | |||
| American Indian | 2.7 | 5.0 | 0.0 |
| Black | 5.3 | 5.0 | 5.7 |
| White | 78.7 | 77.5 | 80.0 |
| Other | 13.3 | 12.5 | 14.3 |
| Ethnicity (%) | |||
| Hispanic | 12.0 | 12.5 | 11.4 |
| Non-Hispanic | 88.0 | 87.5 | 88.6 |
| Marital status (%) | |||
| Single | 20.6 | 23.1 | 17.6 |
| Married | 49.3 | 48.7 | 50.0 |
| Separated/Divorced/Widowed | 30.1 | 28.2 | 32.4 |
| Education (%) | |||
| High school or less | 26.6 | 17.5 | 37.1 |
| Some college | 45.3 | 55.0 | 34.3 |
| College or University Degree | 21.3 | 20.0 | 22.9 |
| Graduate Degree | 6.8 | 7.5 | 5.7 |
| Employed (%) | 62.7 | 70.0 | 54.3 |
| Professional, administrator, or executive | 44.9 | 55.2 | 30.0 |
| Clerical work, administrative support, sales | 36.7 | 34.5 | 40.0 |
| Crafts, trade, factory work, service or labor | 18.4 | 10.3 | 30.0 |
| Unemployed (%) | 36.0 | 27.5 | 45.7 |
| Homemaker | 31.0 | 42.9 | 20.0 |
| Retired or disabled | 55.2 | 50.0 | 60.0 |
| Student | 13.8 | 7.1 | 20.0 |
| Weight, mean (SD), kg | 120.7 (20.6) | 125.7 (21.0)* |
114.9 (18.9) |
| Body Mass Index, mean (SD), kg/m2 | 45.0 (6.5) | 45.6 (7.0) | 44.4 (5.8) |
For comparison of PAI and SC, P < 0.05
Changes in MVPA from baseline to 6-week post-intervention follow-up are depicted in Figure 2 by condition. The PAI condition was more effective for increasing bout-related MVPA compared to the SC condition (p=0.001). Participants in the PAI condition increased bout-related MVPA by an average of 16.6±20.7 minutes/day [Median=10.6, Quartile (Q1)=0.0, Q3=26.7 minutes/day; range=−2.86 to 76.93 minutes/day) at post-intervention follow-up, from 4.4±5.6 at baseline to 21.0±21.4 minutes/day. By contrast, SC participants on average demonstrated no change in bout-related MVPA (−0.3±12.7 minutes/day; Median=0.0, Q1=−2.1, Q3=3.2 minutes/day; range=−47.6 to 34.17 minutes/day) from baseline to post-intervention follow-up (7.9±16.6 to 7.6±11.5 minutes/day).
Figure 2.
Daily Moderate-to-Vigorous Physical Activity (MVPA) in the Physical Activity Intervention (PAI) and Standard Surgical Care Control (SC) Conditions at Baseline and Post-Intervention Follow-up
Note. For comparison of PAI and SC in baseline to post-intervention follow-up changes in daily minutes of bout-related and total MVPA adjusting for baseline values and objective monitor wear time, * P = 0.001
The PAI condition was also more effective than the SC condition for increasing total MVPA (p=0.001). Participants in the PAI condition increased total MVPA by an average of 21.0±26.9 minutes/day (Median=13.2, Q1=0.4, Q3=32.9 minutes/day; range=−13.3 to 98.5 minutes/day) at post-intervention follow-up, from 30.9±21.2 to 51.9±30.0 minutes/day. Seventy-nine percent of the change in total MVPA among PAI participants was due to increases in bout-related MVPA. Conversely, participants in the SC condition on average showed no change in total MVPA (−0.1±16.3 minutes/day; Median=−0.004, Q1=−6.0, Q3=9.5 minutes/day; range=−47.8 to 41.3 minutes/day) from baseline to post-intervention follow-up (33.7±33.2 to 33.6±28.5 minutes/day).
Likewise, the PAI condition was more effective than the SC condition for increasing daily steps (p<0.001). Participants in the PAI condition increased daily steps from baseline to post-intervention follow-up by an average of 2027.6±1886.9 (4629.4±2590.4 to 6935.4±3001.6 steps/day), whereas participants in the SC condition increased daily steps by an average of 202.7±1374.3 (4629.4±2590.4 to 4832.0±2972.8 steps/day).
Adherence to Physical Activity Guidelines
We next compared the proportion of participants in the PAI and SC groups who met the national guideline of 150 weekly minutes of bout-related MVPA (10) at baseline and post-intervention follow-up. In intent-to-treat analyses with baseline value carried forward for participants with missing data, none of the PAI participants met the guideline compared to 8.6% (n=3) of SC participants at baseline (p = 0.10). At post-intervention, 30% of PAI participants (n=12) met the guideline compared to 14.3% (n=5) of SC participants (p = 0.11). Among completers only, a greater proportion of PAI participants met the guideline compared to SC participants at post-intervention (36.4% vs. 13.3%, p = 0.04).
DISCUSSION
It is recommended that bariatric surgery patients increase participation in PA preoperatively (7-8); however, interventions to help patients adopt this behavior change during this period are sparse. The current study is the first randomized controlled trial to test the efficacy of a preoperative behavioral intervention to increase objectively-measured free-living MVPA in severely obese patients seeking bariatric surgery. A particular emphasis of the intervention was increasing MVPA minutes/day accumulated in bouts ≥10 minutes, given that accumulating MVPA in episodes of this duration is a focus of national PA guidelines (10). Results showed that at baseline, participants in both the physical activity intervention (PAI) and standard surgical care control (SC) groups performed little or no bout-related MVPA, consistent with previous research that has objectively assessed preoperative PA patterns (5-6). However, at post-intervention follow-up, participants in the PAI condition on average achieved a near 5-fold increase in daily bout-related MVPA (from mean of 4.4 to 21.0 minutes/day), whereas participants in the SC condition demonstrated no change (from mean of 7.9 to 7.6 minutes/day). Overall, PAI participants on average attained a level of bout-related MVPA (147 minutes/week) at follow-up that was consistent with national PA guideline (i.e. ≥150 minutes/week) (10), and a significantly greater proportion of participants who completed PAI met this guideline compared to those in the SC group (36.4% vs. 14.3%). Moreover, the proportion of PAI completers who met the PA guideline (36.4%) was similar to that found among normal-weight controls (40%) in a previous study conducted by our group (5). Participants in the PAI condition also demonstrated a larger increase in total daily MVPA compared to SC participants, with almost all of the change (79%) due to increased performance of bout-related MVPA.
The results of this study suggest that with intervention, bariatric surgery patients can achieve significant increases in MVPA even before weight loss and other postoperative outcomes are experienced. This is particularly important given that higher PA levels and positive PA attitudes and beliefs preoperatively are associated with higher PA levels postoperatively (1,3,29). Moreover, previous studies involving use of similar objective PA measures show that postoperative patients, despite the benefits of bariatric surgery, do not reach the level of bout-related MVPA achieved by the intervention participants in the current preoperative study (4,5). Thus, the preoperative period may serve as an important “teachable moment” for targeting motivational barriers to PA that may go unresolved postoperatively (30) and facilitating the “behavioral surgery” mindset that bariatric surgery is only a tool and changes in PA and other relevant behaviors are necessary to fully harness the power of this tool (31).
This study has several important strengths including the randomized controlled study design, a structured intervention modeled after the PA component of the lifestyle intervention used in the DPP and Look AHEAD trials (11,12), objective measurement of PA study outcomes, and high retention at the post-intervention follow-up assessment. This study also has several limitations. First, the intervention only lasted 6 weeks and measurement of the primary outcome was based on 1-week follow-up; however, future reports will determine if intervention-related increases in MVPA achieved preoperatively are maintained postoperatively. Second, while the duration of the intervention was intended to be relatively short to afford sufficient time for implementation during the preoperative period and reduce participant burden, it was still intensive requiring several face-to-face visits. Thus, it will be important to determine whether lower intensity interventions can produce similar changes in activity behaviors. Third, generalizability of the results to the general bariatric surgery population may be limited given that nearly 3 out of 4 patients who underwent initial screening were excluded, the vast majority of whom refused to participate. Additionally, 44 individuals who initially agreed to participate did not attend the initial study orientation. However, baseline PA levels of current study participants were similar to those shown in previous observational studies that used objective monitors to assess PA patterns preoperatively (2,3,5,6). Regardless, more research is clearly needed to identify effective strategies for recruiting and retaining bariatric surgery patients in adjunctive PA and other behavioral interventions.
In conclusion, a 6-week behavioral intervention, involving tailored face-to-face intervention sessions focused on individualized instruction in behavioral and cognitive strategies to increase daily home-based walking exercise, was effective for increasing bout-related MVPA in severely obese patients seeking bariatric surgery. Future studies are needed to determine whether preoperative increases in bout-related MVPA are maintained postoperatively and impact surgical outcomes.
Acknowledgements
This research was supported by a grant from the National Institutes of Health/National Institute of Diabetes & Digestive & Kidney Diseases (K01 DK083438, PI: Bond). The authors would like to thanks the Bari-Active subjects for their participation.
Source of Support: National Institute of Diabetes and Digestive and Kidney Diseases (1 K01 83438)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: clinicaltrials.gov Identifier: NCT00962325
References
- 1.King WC, Bond DS. The important of preoperative and postoperative physical activity counseling in bariatric surgery. Exerc Sport Sci Rev. 2013;41:26–35. doi: 10.1097/JES.0b013e31826444e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bond DS, Jakicic JM, Unick JM, et al. Pre- to postoperative physical activity changes in bariatric surgery patients: self-report vs. objective measures. Obesity (Silver Spring) 2010;18:2395–7. doi: 10.1038/oby.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King WC, Hsu JY, Belle SH, et al. Pre- to postoperative changes in physical activity: report from the longitudinal assessment of bariatric surgery-2 (LABS-2) Surg Obes Relat Dis. 2012;8:522–32. doi: 10.1016/j.soard.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman N, Hill K, Taylor S, Hassanali M, Straker L, Hamdorf J. Patterns of physical activity and sedentary behaviour following bariatric surgery: an observational study. Surg Obes Relat Dis. 2013 Oct 25; doi: 10.1016/j.soard.2013.10.012. doi:10.1016/j.soard.2013.10.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Bond DS, Jakicic JM, Vithiananthan S, et al. Objective quantification of physical activity in bariatric surgery candidates and normal-weight controls. Surg Obes Relat Dis. 2010;6:72–8. doi: 10.1016/j.soard.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King WC, Belle SH, Eid GM, et al. Physical activity levels of patients undergoing bariatric surgery in the Longitudinal Assessment of Bariatric Surgery study. Surg Obes Relat Dis. 2008;4:721–8. doi: 10.1016/j.soard.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackburn GL, Hutter MM, Harvey AM, et al. Expert panel on weight loss surgery: executive report update. Obesity (Silver Spring) 2009;17:842–62. doi: 10.1038/oby.2008.578. [DOI] [PubMed] [Google Scholar]
- 8.Poirier P, Cornier MA, Mazzone T, et al. Bariatric surgery and cardiovascular risk factors: a scientific statement from the American Heart Association. Circulation. 2011;123:1683–701. doi: 10.1161/CIR.0b013e3182149099. [DOI] [PubMed] [Google Scholar]
- 9.Baillot A, Mampuya WM, Comeau E, Méziat-Burdin A, Langlois MF. Feasibility and impacts of supervised exercise training in subjects with obesity awaiting bariatric surgery: a pilot study. Obes Surg. 2013;23:882–91. doi: 10.1007/s11695-013-0875-5. [DOI] [PubMed] [Google Scholar]
- 10.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 11.Jakicic JM, Wing RR, Butler BA, Robertson RJ. Prescribing exercise in multiple short bouts versus one continuous bout: effects on adherence, cardiorespiratory fitness, and weight loss in overweight women. Int J Obes Relat Metab Disord. 1995;19:893–901. [PubMed] [Google Scholar]
- 12.The Diabetes Prevention Program Research Group (DPP): description of lifestyle intervention Diabetes Care. 2002;25:2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Look AHEAD Research Group. Wadden TA, West DS, Delahanty L, et al. The Look AHEAD study: A description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prochaska JO, Marcus BH. The transtheoretical model: applications to exercise. In: Dishman RK, editor. Advances in exercise adherence. Human Kinetics; Champaign IL: 1994. pp. 161–180. [Google Scholar]
- 15.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50:179–211. [Google Scholar]
- 16.Bandura A. Self-efficacy: the exercise of control. W.H. Freeman and Company; New York: 1997. pp. 1–521. [Google Scholar]
- 17.Deci E, Ryan R. Intrinsic motivation and self-determination in human behavior. Plenum Publishing Company; New York: 1985. pp. 3–367. [Google Scholar]
- 18.Dalle Grave R, Calugi S, Centis E, El Ghoch M, Marchesini G. Cognitive-behavioral strategies to increase the adherence to exercise in the management of obesity. J Obes. 2011;2011:348293. doi: 10.1155/2011/348293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olander EK, Fletcher H, Williams S, Atkinson L, Turner A, French DP. What are the most effective techniques in changing obese individuals’ physical activity self-efficacy and behaviour: a systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2013;10:29. doi: 10.1186/1479-5868-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persinger R, Foster C, Gibson M, Fater DC, Porcari JP. Consistency of the talk test for exercise prescription. Med Sci Sports Exerc. 2004;36:1632–6. [PubMed] [Google Scholar]
- 21.Johannsen DL, Calabro MA, Stewart J, et al. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Med Sci Sports Exerc. 2010;42:2134–40. doi: 10.1249/MSS.0b013e3181e0b3ff. [DOI] [PubMed] [Google Scholar]
- 22.Mackey DC, Manini TM, Schoeller DA, et al. Validation of an armband to measure daily energy expenditure in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1108–13. doi: 10.1093/gerona/glr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unick JL, Bond DS, Jakicic JM, et al. Comparison of two objective monitors for assessing physical activity and sedentary behaviors in bariatric surgery patients. Obes Surg. 2012;22:347–52. doi: 10.1007/s11695-011-0491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josbeno DA, Kalarchian M, Sparto PJ, Otto AD, Jakicic JM. Physical activity and function in individuals post-bariatric surgery. Obes Surg. 2011;21:1243–9. doi: 10.1007/s11695-010-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bond DS, Unick JL, Jakicic JM, et al. Physical activity and quality of life in severely obese individuals seeking bariatric surgery or lifestyle intervention. Health Qual Life Outcomes. 2012;10:86. doi: 10.1186/1477-7525-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerome GJ, Young DR, Laferriere D, Chen C, Wollmer WM. Reliability of RT3 accelerometers among overweight and obese adults. Med Sci Sports Exerc. 2009;41(1):110–14. doi: 10.1249/MSS.0b013e3181846cd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller GD, Jakicic JM, Rejeski WJ, et al. Effect of varying accelerometry criteria on physical activity: the look ahead study. Obesity (Silver Spring) 2013;21:32–44. doi: 10.1038/oby.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quade D. Rank analysis of covariance. J Am Stat Assoc. 1967;62:1187–1200. [Google Scholar]
- 29.Wouters EJ, Larsen JK, Zijlstra H, van Ramshorst B, Geenen R. Physical activity after surgery for severe obesity: the role of exercise cognitions. Obes Surg. 2011;21:1849–9. doi: 10.1007/s11695-010-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bond DS, Thomas JG, Ryder BA, Vithiananthan S, Pohl D, Wing RR. Ecological momentary assessment of the relationship between intention and physical activity behavior in bariatric surgery patients. Int J Behav Med. 2013;20:82–7. doi: 10.1007/s12529-011-9214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kral JG, Näslund E. Surgical treatment of obesity. Nat Clin Pract Endocrinol Metab. 2007;3:574–83. doi: 10.1038/ncpendmet0563. [DOI] [PubMed] [Google Scholar]