Abstract
Inflammatory bowel diseases (IBD) are chronic and progressive inflammatory disorders of the gastrointestinal tract. In IBD, protein serological biomarkers could be relevant tools for assessing disease activity, performing early-stage diagnosis and managing the treatment. Using the interleukin-10 knockout (IL-10−/−) mouse, a model that develops a time-dependent IBD-like disorder that predominates in the colon; we performed longitudinal studies of circulating protein biomarkers in IBD. Circulating protein profiles in serum samples collected from 30-, 93-, and 135-day-old IL-10−/− mice were investigated using two-dimensional differential gel electrophoresis and MALDI TOF/TOF tandem mass spectrometry. A total of 15 different proteins were identified and confirmed by ELISA and Western blot to be differentially accumulated in serum samples from mid- to late-stage IL-10−/− mice compared to early non-inflamed IL-10−/− mice. The use of another model of colitis and an extra-intestinal inflammation model validated this biomarker panel and demonstrated that comprised some global inflammatory markers, some intestinal inflammation-specific markers and some chronic intestinal inflammation markers. Statistical analyses using misclassification error rate charts validated the use of these identified proteins as powerful biomarkers of colitis. Unlike standard biomarker screening studies, our analyses identified a panel of proteins that allowed the definition of protein signatures that reflect colitis status.
Keywords: Serological Biomarkers, IBD, 2D-DIGE, Diagnostics, Therapeutics
Introduction
Inflammatory bowel diseases (IBDs) are chronic inflammatory conditions of the gastrointestinal (GI) tract that affect about 1.4 million people in the United States and approximately 2.2 million people in Europe [1, 2]. Two major clinical forms of IBD have been extensively studied: Crohn's disease (CD) and ulcerative colitis (UC). In CD, inflammation can occur anywhere in the GI tract, even if it primarily affects the ileum, whereas in UC, the colonic mucosa is principally involved [3]. Both diseases are thought to feature alterations in the immune response to GI microbiota in individuals genetically predisposed to IBD alterations characterized by intestinal epithelial barrier disruption and an influx of immune cells [4].
IBD is a substantial public health problem whose efficient assessment remains a difficult challenge. The clinical diagnosis of IBD is achieved through colonoscopy, possibly complemented by parallel analyses of some blood markers. Serological biomarkers are already in use to assess IBD activity, provide early stage diagnosis, evaluate prognosis, and monitor remission stage. Notable among currently available biomarkers are serum C-reactive protein (CRP) and calprotectin [5, 6]. However, CRP and calprotectin levels are increased under any inflammatory condition [7, 8], and thus are not specific for inflammation of intestinal origin, significantly limiting their usefulness in IBD. Effective diagnosis and surveillance of complex multi-factorial disorders, like IBD, could be improved through screening of easily accessible biomarkers, such as circulating serum protein, using a more defined expression pattern capable of discriminating the degree of inflammation. Such an approach could improve prediction of IBD progression, which remains a substantial challenge. Furthermore, the development of reliable non-invasive biomarkers would be highly valuable for earlier detection, which is extremely important for treatment effectiveness, and thus could prove beneficial for the treatment of IBD patients and monitoring their response to treatment.
Multiple animal models of IBD have been developed [9]. Although these models do not adequately recapitulate the full complexity of the human disease, they are nonetheless valuable and indispensable tools, providing a wide range of options for investigating the involvement of various factors in the pathogenesis of IBD. Importantly, they can be used to evaluate different therapeutic options that cannot be investigated in humans. The use of a mouse model for IBD biomarker discovery also allows easy access to large numbers of samples from a uniform genetic background, a controlled environment and uniform sample collection.
One of the most commonly used mouse models of intestinal inflammation is the interleukin-10 knockout (IL-10−/−) mouse model. IL-10 is a potent suppressor of macrophage activation in vitro. It inhibits the production of inflammatory cytokines such as IL-1, IL-6, and tumor necrosis factor α (TNF-α) by macrophages stimulated with lipopolysaccharide (LPS) or interferon-γ (IFNγ) [10]. Mice with a targeted deletion of the IL-10 gene, first generated and phenotyped in 1993, spontaneously develop chronic enterocolitis with massive infiltration of lymphocytes, activated macrophages, and neutrophils in a Th1 cell-mediated manner [11, 12]. In IL-10−/− mice, lymphocyte development and antibody responses are normal, but most animals exhibit growth retardation and anemia, and suffer from chronic enterocolitis. Alterations in the intestine include extensive mucosal hyperplasia, inflammatory reactions, and aberrant expression of major histocompatibility complex class II molecules on epithelia [12]. The predictability of the timing of colitis in IL-10−/− mice allows longitudinal assessment of blood samples at various stages of colitis progression.
Because serum is an easily accessible source material, it plays a significant role in proteomic approaches designed to identify biomarkers for the early detection of numerous diseases. Proteomics is a powerful and effective approach for rapidly evaluating protein profiles in serum. One frequently used proteomic tool is two-dimensional differential gel electrophoresis (2D-DIGE), an advanced, sensitive gel-based separation and quantification approach in which protein samples are pre-labeled with different fluorescent dyes, mixed, and run simultaneously on the same gel. One of the greatest challenges for proteome analysis is the reproducible fractionation of complex protein mixtures while still retaining the ability to qualitatively and quantitatively address the relationships between two or more samples. To date, two-dimensional gel electrophoresis is the only method that allows a suitable separation of complex protein mixtures and provides the means for the accurate and reproducible visualization of their expression profile. 2D-DIGE and the use of Cydye chemistry further allow the separation and quantitative analysis of two or more different protein samples within the same gel, thus minimizing any gel to gel variations. Two-dimensional gel electrophoresis coupled with mass spectrometry (MS) is a typical proteomic approach for the identification of new biomarker candidates [13, 14]. However, to date, no studies have used 2D-DIGE for the analysis of serum in the context of IBD biomarker discovery.
Here, using 2D-DIGE and MS, we identified a total of 15 proteins that were differentially accumulated in serum samples from mid- to late-stage colitis in IL-10−/− mice compared to early non-inflamed IL-10−/− mice. Unlike traditional biomarker screening studies, our analysis identified a panel of proteins that allowed the definition of different protein signatures that reflect colitis status.
Materials and methods
Mice
Three week-old female C57BL/6 and IL-10−/− mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were group housed under a controlled temperature (25°C) and photoperiod (12:12-h light–dark cycle) and allowed unrestricted access to standard mouse chow diet and tap water. All studies were performed in accordance with the Institutional Animal Care and Use Committee at Georgia State University (Atlanta, GA). All procedures were approved and are registered in the protocol IACUC ID: A11025 (approval date from 8/30/2011 to 8/30/2014).
Sample collection and preparation
Blood samples were collected in serum separator tubes (BD Microtainer) by retro-orbital puncture from IL-10−/− mice at 30 days, 93 days and 135 days of age. Blood samples were centrifuged to obtain clear serum.
Albumin and IgG were removed from serum samples by two consecutive applications of the SpinTrap® column (GE Healthcare Life Science, Piscataway, NJ), according to the manufacturer's protocol. Protein concentrations were then measured using the DC protein assay kit (Bio-Rad). The protein solution was either used for Western Blot or for 2D analysis after 2D-clean-up step. For 2D-clean-up, one hundred micrograms of protein were precipitated, purified and cleaned by a 2D-clean-up kit (GE Healthcare) according to manufacturer protocol. Pellets of precipitated proteins were resuspended in 60μl of rehydration buffer (7 M urea, 2 M thiourea, 4% CHAPS, 25mM Tris-HCl pH 8.8).
Sample labeling and two-dimensional gel electrophoresis
Twenty-five micrograms of Day 30 IL-10−/− (or Day-2 CAIA) serum sample were labeled with 200 pmol N-hydroxysuccinimidyl-ester of cyanine dye Cy3 and 25 micrograms of Day 93 and Day 135 IL-10−/− (or Day 12 CAIA) serum sample were labeled with 200 pmol N- hydroxysuccinimidyl-ester of cyanine dye Cy5 (GE Healthcare Life Science, Piscataway, NJ). After quenching with 10 mM lysine, the labeled proteins were mixed. Sample buffer (7 M urea, 4 M thiourea, 4% CHAPS, 2% DTT, 2% IPG buffer, pH 4-11 NL (GE Healthcare Life Science, Piscataway, NJ)) and Rehydration Solution (7 M urea, 4 M thiourea, 4% CHAPS, 1% DTT, 1% IPG) were added to a final volume of 350 μl for each gel. First-dimension IsoElectric Focusing (IEF) was performed using 24-cm IPG strips (pH 4–7, GE Healthcare Life Science, Piscataway, NJ) in Ettan IPGphor (GE Healthcare Life Science, Piscataway, NJ). After IEF, the strips were equilibrated, reduced, alkylated and stained by sequential incubation in 1.5% DTT equilibration buffer (50 mM Tris–HCl (pH 8.8), 6 M urea, 30% glycerol, and 2% SDS) and 4.5% iodoacetamide equilibration buffer slightly colored with bromophenol blue for 20min each. The second-dimension SDS-polyacrylamide gel electrophoresis was conducted on a 10% polyacrylamide gel in the Ettan DALT II system separation unit (GE Healthcare Life Science, Piscataway, NJ) until the tracking dye reached the bottom of the gel.
After completion of two-dimensional electrophoresis, gel images were acquired on a Typhoon Trio (GE Healthcare) at appropriate wavelengths for Cy3 and Cy5 dyes and analyzed using the DeCyder image analysis software (v. 7.0, GE Healthcare Life Science, Piscataway, NJ). The gels were then visualized by colloidal Coomassie staining (SimplyBlue, Invitrogen, Carlsbad, CA). The Coomassie stained gel was scanned again with Typhoon Trio scanner. The Coomassie stained gel image was matched and aligned with previous Cy3 and Cy5 fluorescence images; a pick list of proteins of interest was made and subsequent MS/MS analysis was performed based on protein abundance that increased or decreased more than 1.5-fold. 3D views of individual spots for one of three independent experiments were generated with DeCyder image analysis software.
Spot picking and mass spectrometric protein identification
The generated pick list was exported to Ettan Spot Picker (GE Healthcare), and protein spots were excised and transferred to a micro titer plate by Ettan Spot Picker. The picked gel pieces were washed first with dd-H2O and subsequently with washing solution I (50% ethanol, 10% acetic acid), and washing solution II (50% acetonitrile, 100 mM ammonium bicarbonate, pH 8.3). The washed gel pieces were finally dehydrated with 100% acetonitrile and dried under speed-vac. The dried gel pieces were either digested in trypsin or kept at −80°C until they were treated with trypsin for the mass spectrometry peptide analysis. Briefly, the gel pieces were incubated with an appropriate amount of trypsin (Modified Trypsin Gold, Promega, Madison, WI) in ProteaseMAX Surfactant (Promega, Madison, WI) at 37°C for 2-3 hours. After incubation, the digested peptides were extracted with 2.5% of trifluoroacetic acid. The extracted peptides were further purified and concentrated by ZipTip, a micro-reverse phase column (Millipore, Billerica, MA) according to the manufacturer's protocol. Extracted peptides were then analyzed by 4800 MALDI TOF/TOF tandem mass spectrometer (AB Sciex, Framingham, MA) with MS/MS tandem mode. Protein identifications were carried out by Mascot search engine (Matrix Science Inc, Boston, MA) against the Swiss-Prot or NCBI protein databases.
H&E staining of colonic tissue
Mouse colons were fixed in 10% buffered formalin for 24 hours at room temperature and then embedded in paraffin. Tissues were sectioned at 5-μm thickness and stained with hematoxylin & eosin (H&E) using standard protocols. Images were acquired using a Zeiss Axioskop 2 plus microscope (Carl Zeiss MicroImaging) equipped with an AxioCam MRc5 CCD camera (Carl Zeiss).
ELISA
Haptoglobin and hemopexin levels were quantified in serum using mouse hemopexin and mouse haptoglobin ELISA kits (GenWay Biotech. Inc, San Diego, CA) according to the manufacturer's instructions.
Western blotting
Twenty micrograms of serum proteins were resolved on polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad). Membranes were then probed with primary antibodies: anti-A1BG (dilution 1:250; Santa Cruz # sc-132613, Dallas, TX), anti-PZP (dilution 1:1000; Santa Cruz # 8516, Dallas, TX) or anti-peroxiredoxin 2 (dilution 1:250; Abcam # ab109367). After washes, membranes were incubated with appropriate horseradish peroxidaseconjugated secondary antibodies (dilution 1:5000, GE Healthcare Biosciences, Pittsburgh, PA), and blots were detected using the Enhanced Chemiluminescence Detection Kit (GE Healthcare Biosciences).
Bioinformatic analysis
Functional network analysis of the identified proteins by 2D-DIGE and their association with biological processes or diseases were performed by Coremine Medical (http://www.coremine.com/medical/#search), formerly Pubgene [15]. p values represent the significance of the association between the protein and the disease or biological function.
Statistical analysis
Statistical analysis for significance (p<0.05) was determined using Student's t-test (GraphPad Prism). Differences were noted as significant with *: p<0.05. Prediction Analysis for Microarrays (PAM) package within R software was used to analyze misclassification error rates, to generate protein abundance plots and to generate a heatmap.
Results
Characterization of the IL-10−/− mouse model
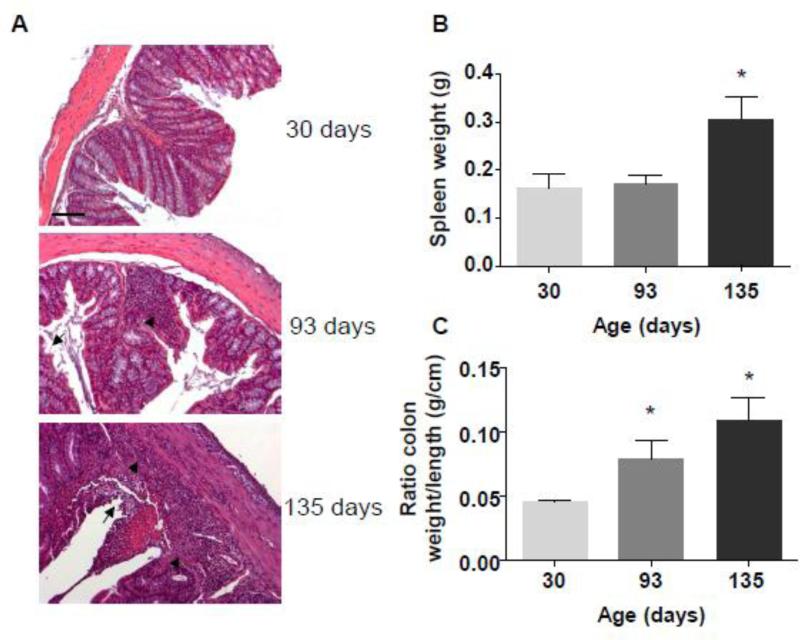
IL-10 is a well-known suppressor of TH1 cells and macrophage effector functions. In vitro studies have shown that IL-10 inhibits IL-12 production, TNF-α production and T-cell proliferation, and may also promote the formation of antigen-specific regulatory T-cells [11, 16]. IL-10−/− mice spontaneously develop chronic enterocolitis with massive infiltration of lymphocytes, activated macrophages, and neutrophils [12]. To confirm the usefulness of the IL-10−/− model as a tool for investigating protein accumulation profiles during intestinal inflammation, we first assessed the development of colitis in these mice. Female IL-10−/− mice were monitored for colitis development for 15 weeks: at weaning (day 30), 15 weeks post- weaning (day 135), and at an intermediate time point (day 93). Histological features, assessed by H&E staining, revealed that 135-day-old IL-10−/− mice exhibited signs of robust inflammation, with global immune cell infiltration (arrow head) and epithelial erosion (arrow) (Figure 1A). This histological analysis showed that mice at day 93 showed milder signs of inflammation marked by incipient erosion (arrow) and local lymphocyte infiltrations (arrow head). We also measured weights of spleens (Figure 1B) for which an increased weight positively correlate with the extent of inflammation. Colon weight and colon length were measured to make the colon weight/colon length ratio (Figure 1C), correlating with intestinal inflammation, as previously described [17]. For 135 day-old mice, spleen weights were significantly increased compared to 30 day-old mice. Likewise, the colon weights/colon length ratios of 93 and 135 day-old mice were increased compared to those of 30 day-old mice. Taken together with the histological features, these data confirm that IL-10−/− mice developed colitis in a time-dependent manner in our vivarium. In subsequent proteomic studies, protein profiles in serum samples from 93- and 135-day-old IL-10−/− mice, which developed mild and severe colitis, respectively, will be compared to those from 30-day-old mice, which did not exhibit any sign of colitis.
Figure 1. IL-10−/− mice develop spontaneous colitis.
A, Representative H&E-stained colonic sections from 30-, 93-, and 135-day-old mice. Arrow: erosion; arrowhead: lymphocyte infiltration. Scale bar: 100 μm. B, C, Spleen weight (B) colon weight/colon length ratios (C) were measured at three different mouse age: 30 days old, 93 days old, and 135 days old mice. *p<0.05 compared to 30 days old.
2D-DIGE analysis and identification of proteins associated with colitis progression by MALDI-TOF/TOF mass spectrometry
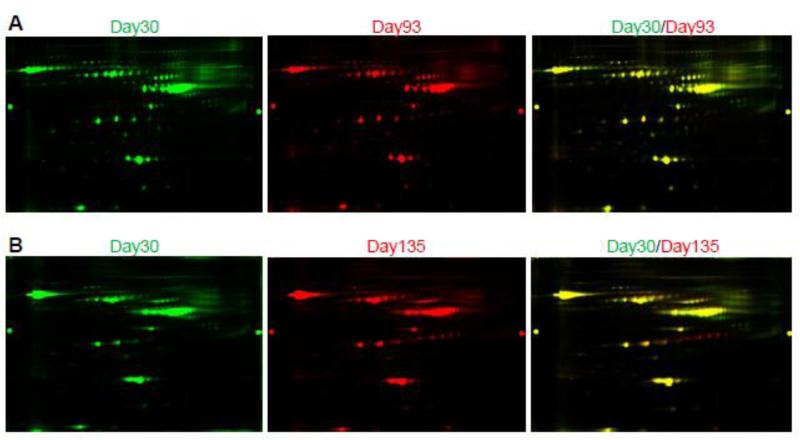
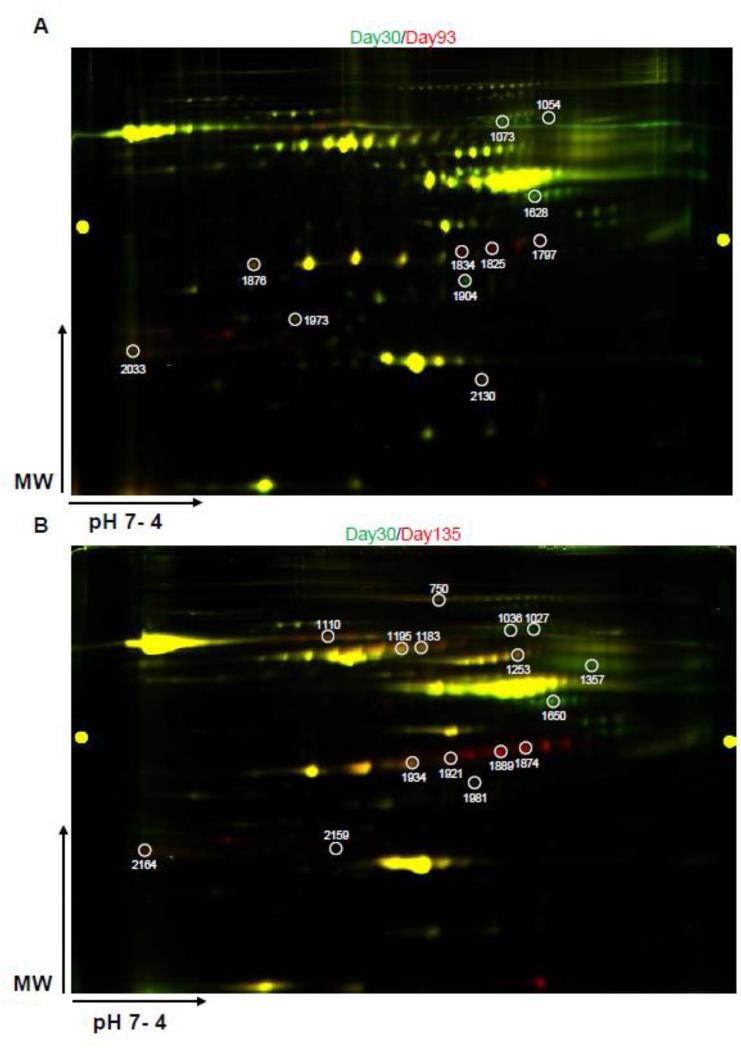
Two paired samples, one containing equal amounts of day 30 and day 93 serum proteins and one containing equal amounts of day 30 and day 135 serum proteins, were labeled with Cy3 (day 30) and Cy5 (day 93 or 135) dyes for 2D-DIGE analysis. Representative 2D-DIGE gel images are shown in Figure 2A and 2B. A quantitative analysis, performed from 3 independent experiments, identified a total of 11 spots with intensity changes (i.e., ≥1.5-fold difference in abundance of the spot volume) between day-30 and day-93 samples (Table 1) and 16 spots with volume difference between day-30 and day-135 samples (Table 2). The average of fold changes of spot abundance is represented. Those protein spots were also shown in Figure 3A and Figure 3B, with spot number identification. Matrix-assisted laser desorption/ionization time-of-flight/time-of-flight MS (MALDI-TOF/TOF-MS) analyses revealed that these 11 and 16 spots corresponded to 9 and 12 unique protein accession numbers, respectively (Table 1 and 2). Three proteins were down-regulated and six proteins were up-regulated in the serum of 93-day-old IL-10−/− mice (mild colitis) compared to 30-day-old IL-10−/− mice, which do not display any colitis symptoms. When comparing samples from day 30 and day 135, four proteins were down-regulated and eight were up-regulated in the serum of 135-day-old IL-10−/− mice (severe colitis) compared to 30-day-old IL-10−/− mice. Spot number, GI accession number, protein name, protein molecular weight, isoelectric point (PI), peptide count, percentage of total ion confidence interval (CI), average fold change, and overall trend are described in Table 1 (day 30 vs. day 93) and Table 2 (day 30 vs. day 135). The following proteins were found to be down-regulated in both day 93 and day 135 samples compared to day 30 samples: similar to alpha-1-B glycoprotein (A1BG), serpin peptidase inhibitor, clade A, member 1 (SERPINA1), and collagen, type I, alpha 1 (COL1A1). Contrapsin was down-regulated only in day 135 compared to day 30 samples. Haptoglobin (HP), pregnancy zone protein (PZP), and hemoglobin alpha 1 (HBA1) were up-regulated at both day 93 and day 135 compared to day 30. HP displayed a greater up-regulation at day 135 (+40.36 and. +86.19) than at day 93 (+20.80 and +26.09), both compared to day 30. Histocompatibility 2 Q region locus 10 (H2-Q10), complement component 3 (C3), and peroxiredoxin 2 (PRDX2) were up-regulated only in day-93 samples compared to day-30 samples, whereas inter alpha-trypsin inhibitor, heavy chain 4 (ITIH4), transferrin (TF), hemopexin (HPX), kininogen 1 (KNG1), and thrombospondin 1 (THBS1) were up-regulated only in day-135 samples compared to day 30 samples.
Figure 2. Composite image illustrating one representative 2D-DIGE gel.
A, Representative 2D-DIGE image of protein accumulation in sera of 30-day-old mice (Cy3, green fluorescence) and 93-day-old mice (Cy5, red fluorescence), and a composite overlay image generated by superimposition. B, Representative 2D-DIGE image of proteins accumulation in sera of 30-dayold mice (Cy3, green fluorescence) and 135-day-old mice (Cy5, red fluorescence), and a composite overlay image generated by superimposition. One of three independent experiments is shown.
Table 1.
Identification of serum proteins differentially expressed between 30-day-old and 93-day-old IL-10−/− mice
| Spot no.a | GI Accession no.b | Protein narre | Protein MW (kDa) | Protein PI | Peptide count | Total ion C.I. %c | Average fold changed | Overall trend* |
|---|---|---|---|---|---|---|---|---|
| 1073 | 51829721 | Similar to Alpha-1-B Glycoprotein | 56.5 | 6.33 | 3 | 100 | −2.68 ± 0.65 | down |
| 1054 | 51829721 | Similar to Alcha-1-B Glycoprotein | 56.5 | 6.33 | 3 | 100 | −5.02 ± 1.73 | down |
| 1628 | 6678079 | SERPINA1 | 45.9 | 5.3 | 5 | 100 | −2.13 ± 0.25 | down |
| 1834 | 2144486 | Haptoglobin | 38.8 | 6.08 | 4 | 100 | 20.80. ± 4.68 | up |
| 1825 | 2144486 | Haptoglobin | 38.8 | 6.08 | 2 | 100 | 26.09 ± 7.13 | up |
| 1797 | 6754132 | H2-Q10 | 37.3 | 5.13 | 4 | 100 | 3.90 ± 0.69 | up |
| 1876 | 34785996 | Pregnancy Zone Protein | 165.8 | 6.24 | 8 | 100 | 1.54 ± 0.05 | up |
| 1904 | 37589303 | Collagen, type I, alpha 1 | 117.8 | 5.58 | 2 | 100 | −4.02 ± 0.41 | down |
| 1973 | 28175786 | Complement component 3 | 186.4 | 6.29 | 1 | 100 | 2.44 ± 0.40 | up |
| 2033 | 28175802 | Hemoglobin alpha 1 | 151 | 7.96 | 2 | 100 | 1.88 ± 0.18 | up |
| 2130 | 51980699 | Peroxiredoxin 2 | 21.8 | 5.20 | 7 | 100 | 2.29 ± 0.67 | up |
Spot no. generated by DeCycler Image analysis software (v. 7.0, GE Healthcare), referencing the spots shown on the representative image in Figure 3.
Accession number from NCBI database.
C.I. % rates the confidence level of the Protein Score or Ion Score. The closer the Confidence Interval (C.I. %) is to 100%, the more likely the protein is correctly identified.
Average ratio of protein expression between day 30 and day 93, calculated from 3 independent experiments and represented as mean ± SEM.
up: up-regulated in day-93 compared to day-30 group; down: down-regulated in day-93 group compared to day-30 group.
Abbreviations: SERPINA1: Serpin peptidase inhibitor, clade A, member 1; H2-Q10: Histocompatibility 2, Q region locus 10.
Table 2.
Identification of serum proteins differentially expressed between 30-day-old and 135-day-old IL-10−/− mice
| Spot no.a | GI Accession no.b | Protein name | Protein MW (kDa) | Protein PI | Peptide count | Total ion C.I. %c | Average fold changed | Overall trend* |
|---|---|---|---|---|---|---|---|---|
| 750 | 16741341 | ITIH4 | 104.6 | 6.05 | 5 | 100 | 1.81 ± 0.01 | up |
| 1027 | 51829721 | Similar to Alpha-1-B Glycoprotein | 56.5 | 6.33 | 2 | 99.99 | −8.39 ± 1.73 | down |
| 1036 | 51829721 | Similar to Alpha-1-B Glycoprotein | 56.5 | 6.33 | 2 | 99.93 | −6.67 ± 1.47 | down |
| 1110 | 17046471 | Transferrin | 76.7 | 6.92 | 1 | 86.2 | 2.27 ± 0.52 | up |
| 1183 | 23956086 | Hemopexin | 51.3 | 7.64 | 3 | 100 | 2.21 ± 0.05 | up |
| 1195 | 23956086 | Hemopexin | 51.3 | 7.64 | 4 | 100 | 1.66 ± 0.03 | up |
| 1253 | 50082914 | Kininogen 1 | 53.2 | 4.88 | 3 | 100 | 1.84 ± 0.003 | up |
| 1357 | 54173 | Contrapsin | 46.6 | 5.04 | 7 | 100 | −2.04 ± 0.18 | down |
| 1650 | 6678079 | SERPINA1 | 45.9 | 5.44 | 3 | 100 | −2.82 ± 0.93 | down |
| 1874 | 2144486 | Haptoglobin | 38.8 | 6.08 | 3 | 100 | 40.36 ± 5.05 | up |
| 1889 | 2144486 | Haptoglobin | 38.8 | 6.08 | 4 | 100 | 86.19 ± 12.21 | up |
| 1921 | 34785996 | Pregnancy Zone Protein | 165.8 | 6.24 | 5 | 100 | 2.98 ± 0.03 | up |
| 1934 | 34785996 | Pregnancy Zone Protein | 165.8 | 6.24 | 6 | 100 | 1.92 ± 0.07 | up |
| 1981 | 37580303 | Collagn, type I, alpha 1 | 117.8 | 5.58 | 1 | 99.99 | −4.22 ± 0.27 | down |
| 2159 | 554390 | Thrombospondin 1 | 53.8 | 6.16 | 2 | 100 | 1.72 ± 0.10 | up |
| 2164 | 28175802 | Hemoglobin alpha 1 | 151l | 7.96 | 1 | 89.68 | 2.32 ± 0.29 | up |
Spot no. generated by DeCycler Image analysis software (v. 7.0, GE Healthcare), referencing the spots shown on the representative image in Figure 3.
Accession number from NCBI database.
C.I.% rates the confidence level of the Protein Score or Ion Score. The closer the Confidence Interval (C.I.%) is to 100%, the more likely the protein is correctly identified.
Average ratio of protein expression between day 30 and day 135, calculated from 3 independent experiments and represented as mean ± SEM.
up: up-regulated in day-135 compared to day-30 group; down: down-regulated in day-135 group compared to day-30 group.
Abbreviations: ITIH4: Inter alpha-trypsin inhibitor, heavy chain 4; SERPINA1: Serpin peptidase inhibitor, clade A, member 1.
Figure 3. Serum proteins differentially expressed between 93- or 135-day-old IL-10−/− mice and 30-day-old IL-10−/− mice.
A, Green spots represent proteins more up-regulated in serum from 30-day-old IL-10−/− mice, whereas red spots indicate proteins up-regulated in serum from 93-day-old IL-10−/− mice. Spots corresponding to identified proteins spots are circled and numbered (Cf. Table 1). B, Green spots represent proteins up-regulated in serum from 30-dayold IL-10−/− mice, whereas red spots indicate proteins up-regulated in serum from 135-day-old IL-10−/− mice. Identified protein spots are circled and numbered (Cf. Table 2).
Validation of the putative serum protein biomarkers identified
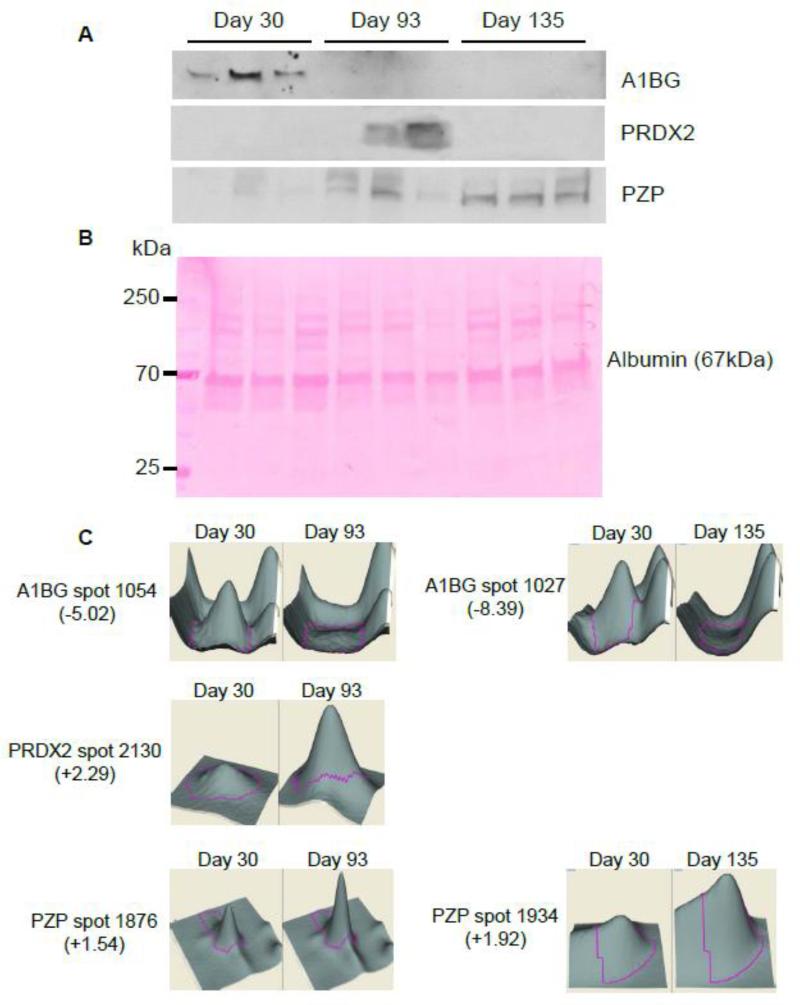
To validate these 2D-DIGE results, we determined the accumulation levels of the identified proteins previously shown to have the greatest changes in 93- and 135-day-old IL-10−/− mice serum samples compared to 30-day-old IL-10−/− mice (A1BG, PRDX2, PZP, HP, and HPX) by enzyme-linked immunosorbent assay (ELISA) or Western blotting.
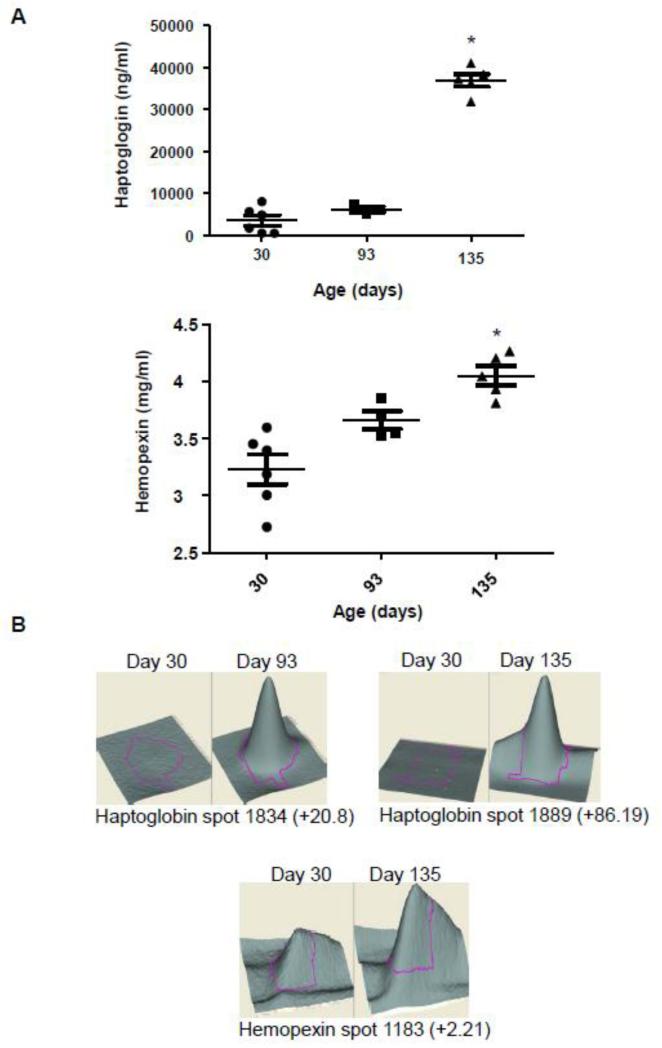
The accumulation of A1BG, PRDX2 and PZP in the serum of IL-10−/− mice was analyzed by Western blot (Figure 4A). Ponceau Red staining, considered as an alternative method for Western Blot loading control, is shown in Figure 4B instead of β-actin which is not suitable for serum protein normalization since serum does not contain any blood cells [18]. The intensity of the main band observed in Ponceau Red-stained gels (albumin, 67 kDa) was similar for all lanes, confirming equal protein loading. The accumulation of A1BG was decreased in the serum of mice with mild colitis (day 93) and severe colitis (day 135) compared to non-colitic mice (day 30) (Figure 4A). These results are well-correlated with the 2D-DIGE results, which showed a 5.02-fold decrease in spot volume between day 93 and day 30 and a 8.39-fold decrease between day 30 and day 135, as shown in a 3D view of the spots (Figure 4C). PRDX2 accumulation was shown to increase in the serum of mice with mild colitis (day 93) compared to non-colitic mice (day 30) (Figure 4A). At day 135 (severe colitis), PRDX2 levels were unchanged compared to those of non-colitic mice. Thus, PRDX2 appears to be transiently overexpressed during the establishment of intestinal inflammation (Figure 4A). This observation confirms the 2D-DIGE results, which showed a 2.29-fold increase in PRDX2 spot volume between day 93 and day 30, and no difference in spot volume between day 135 and day 30 (Figure 4C). Western blot analyses showed a progressive increase in PZP protein in serum throughout colitis development, consistent with spot volume data, which showed a 1.54-fold increase in PZP at day 93 compared to day 30 and a 1.92-fold increase at day 135 compared to day 30 (Figure 4A). An analysis of HP and HPX accumulation in the serum of IL-10−/− mice by ELISA showed an increase in HP in severe colitic IL-10−/− mice (day 135) compared to mice with mild colitis (day 93) or compare to non-colitic mice (day 30) (Figure 5A). This result is in accordance with the 2D-DIGE results, which showed approximately an 86-fold increase in HP spot volume at day 135 compared to day 30 (Figure 5B). However, 2D-DIGE also showed an increase in HP at day 93 (20.8-fold) compared to day 30. HPX was significantly increased in the serum of IL-10−/− at day 135 compared to day 30, a result that correlated with spot volume, which showed a 2.21-fold increase in HPX at day 135 compared to day 30 (Figure 5A and 5B).
Figure 4. Western blot analysis of putative IBD biomarkers A1BG, PRDX2, and PZP, and corresponding 3D view.
A, Accumulation of A1BG, PRDX2, and PZP in serum samples from mice with mild/moderate or severe colitis compared to that in serum from non-colitic mice was validated by Western blotting. B, Ponceau Red-stained Western blot membrane, used as a loading control. Albumin is visible at 67 kDa. C, 3D rendering of A1BG, PRDX2, and PZP protein spots enlarged to show the differences in their expression between non-colitic mice (30 days old) and mice with moderate (93 days old) and severe (135 days old) colitis. The 3D views of 1 out of 3 independent experiments are shown.
Figure 5. ELISA analysis of putative IBD biomarkers HP and HPX and corresponding 3D view.
A, The accumulation of HP and HPX in serum samples from mice with mild/moderate or severe colitis compared with that in non-colitic mice was validated by ELISA. B, A 3-D view of HP and HPX protein spots enlarged to show the differences in their expression between noncolitic mice (30 days old) and mice with mild/moderate (93 days old) and severe (135 days old) colitis. *p<0.05 compared to 30-day-old non-colitic mice. The 3D views of 1 out of 3 independent experiments are shown.
Taken together, these results confirm that the proteins identified here are differentially accumulated during the progression of the colitis. Some proteins are decreased (A1BG); others are increased throughout the establishment of the disease (PZP, HP, HPX); and some others are transiently increased (PRDX2) or increased later in disease progression (TF).
In order to investigate whether the biomarkers identified are specific for the subtype of intestinal inflammation, we used a model of acute colitis by treating mice with 3% DSS. Both Lcn2 quantification (a marker of inflammation [19]) and histological examination confirmed that mice developed acute colitis (Figure S1A). We next investigated the accumulation of some biomarkers previously identified by Western blot (Figure S1B) or ELISA (Figure S1C), and found that PZP and PRDX2, previously found to be increased in colitic IL10−/− mice, were not altered in mice treated with DSS. A1BG was observed to be decreased after DSS treatment and HP and HPX were drastically increased at D7, showing that these three proteins were altered in the same way as was previously observed in colitic IL10−/− mice. This suggests that these markers are non-specific to chronic colitis, and may be general markers of any form of intestinal inflammation or inflammation in general. Importantly, PZP and PRDX2 expressions were found to be unmodified during DSS treatment, suggesting that these markers are specific to a chronic inflammatory state.
We next aimed to determine whether the protein panel identified is specific to intestinal inflammation or could be associated with any kind of inflammation. As a model of extra-intestinal inflammation, we used collagen antibody-induced arthritis (CAIA). Lcn-2 was increased in the serum of arthritic mice at day 12 and returned to basal level at day 22 (terminal time point), and the clinical score was found to be increased at days 12 and 22 compared to the pre-treatment time point (day -2) (Figure S2A). 2D-DIGE analysis was performed to compare total serum protein accumulation between day -2 and day 12 (Figure S2B). Spots corresponding to the proteins previously identified in the IL10−/− model were picked, as well as one additional spot found to have a specific alteration in the arthritis model. The proteins ITIH4, HPX, HP, H2Q-10 and C3 were found to be up-regulated in the arthritis mouse model (Figure S2B and Table 3), and serpin A1 was found to be down-regulated during arthritis, as it was previously observed in the IL10−/− colitic mice. However, contrapsin, PZP and COLA1A were importantly found to be unmodified during arthritis, while those proteins were previously found either down-regulated or up-regulated in the IL10−/− colitis model. Serum amyloid P-component and Transthyretin were respectively found to be specifically up-regulated or down-regulated in the arthritis model. In addition, we analyzed PZP, A1BG and PRDX2 expression by Western blot and HPX and HP expression by ELISA. Consistent with the results obtained by 2D-DiGE, HPX and HP were found to have an expression drastically increased in the serum of day 12 arthritic mice that returns to a normal level at day 22, which is in agreement with the 2D-DiGE results comparing day -2 and day 22 (Figures S2 and S3). A1BG was decreased in the serum of day 22 mice compared to day -2, as it was previously observed using the IL10−/− model. Importantly, levels of PRDX2 and PZP were not modified in the serum of arthritis mice (Figure S2C). Altogether, these results confirmed that among the proteins of the panel previously identified, some are specific to intestinal inflammation development (PZP, COL1A1, PRDX2) with some of them specific to the IL10−/− model (PZP, PRDX2), some are specific to the development of arthritic (Serum amyloid P-component and Transthyretin), and some are found to have an altered expression in any inflammatory conditions (HP, ITIH4, HPX, C3 and A1BG). This combination of specific and non-specific marker makes the panel powerful and the signature unique.
Table 3.
Identification of serum proteins of CAIA model – comparison Day -2 versus Day 12
| Spot no.a | GI Accession no.b | Protein name | Protein MW (kDa) | Protein PI | Peptide count | Total ion C.I. %c | Avrerage fold changed | Overall trend* |
|---|---|---|---|---|---|---|---|---|
| 732 | 16741341 | ITIH4 | 104.6 | 6.05 | 4 | 100 | 2.1 ± 0.22 | up |
| 746 | 16741341 | ITIH4 | 104.6 | 6.05 | 3 | 100 | 1.86 ± 0.24 | up |
| 1379 | 23956086 | Hemopexin | 51.3 | 7.64 | 3 | 100 | 8.09 ± 2.1 | up |
| 1410 | 23956086 | Hemopexin | 51.3 | 7.64 | 3 | 100 | 6.95 ± 2.17 | up |
| 1450 | 54173 | Contrapsin | 46.6 | 5.04 | 8 | 100 | 1.1 ± 0.07 | - |
| 1798 | 40254615 | SERPINA1 | 45.9 | 5.3 | 2 | 100 | −2.23 ± 0.20 | down |
| 1991 | 2144486 | Haptoglobin | 38.8 | 6.08 | 2 | 86.7 | 9.79 ±2.5 | up |
| 2052 | 2144486 | Haptoglobin | 38.8 | 6.08 | 3 | 100 | 67.6 ± 23.7 | up |
| 2042 | 6754132 | H2-Q10 | 37.3 | 5.13 | 5 | 100 | 19.41 ± 3.9 | up |
| 2090 | 34785996 | Pregnancy Zone Protein | 165.8 | 6.24 | 3 | 100 | 1.02 ± 0.05 | - |
| 2102 | 34785996 | Pregnancy Zone Protein | 165.8 | 6.24 | 2 | 100 | 1.1 ± 0.04 | - |
| 2198 | 37589303 | Collagen, type I, alpha 1 | 117.8 | 5.58 | 2 | 100 | 1.19 ± 0.99 | - |
| 2242 | 28175786 | Complemnt component 3 | 186.4 | 6.29 | 1 | 100 | 2.32 ± 0.2 | up |
| 2288 | 38174334 | Serum amyloid P-component | 26.2 | 5.98 | 2 | 100 | 3.04 ± 0.19 | up |
| 2553 | 56541070 | Transthyretin | 15.8 | 5.77 | 3 | 100 | −1.99 ± 0.098 | down |
Spot no. generatd by DeCycler Image analysis software (v. 7.0, GE Healthcare), referencing the spots shown on the representative image in Figure 3.
Accession number from NCBI database.
C.I.% rates the confidence level of the Protein Score or Ion Score. The closer the Confidence Interval (C.I.%) is to 100%, the more likely the protein is correctly identified.
Average ratio of protein expression between day 12 and day-2, calculated from 3 independent experiments and represented as mean ± SEM.
up: up-regulated in day 12 compared to day-2 group; down: down-regulated in day 12 group comapred to day 2 group; -: no alteration in day 12 compared to day -2.
Abbreviations: SERPINA1: Serpin peptidase inhibitor, clade A, member 1; H2-Q10: Histocompatibility 2, Q region locus 10.
Serum protein signature of colitis
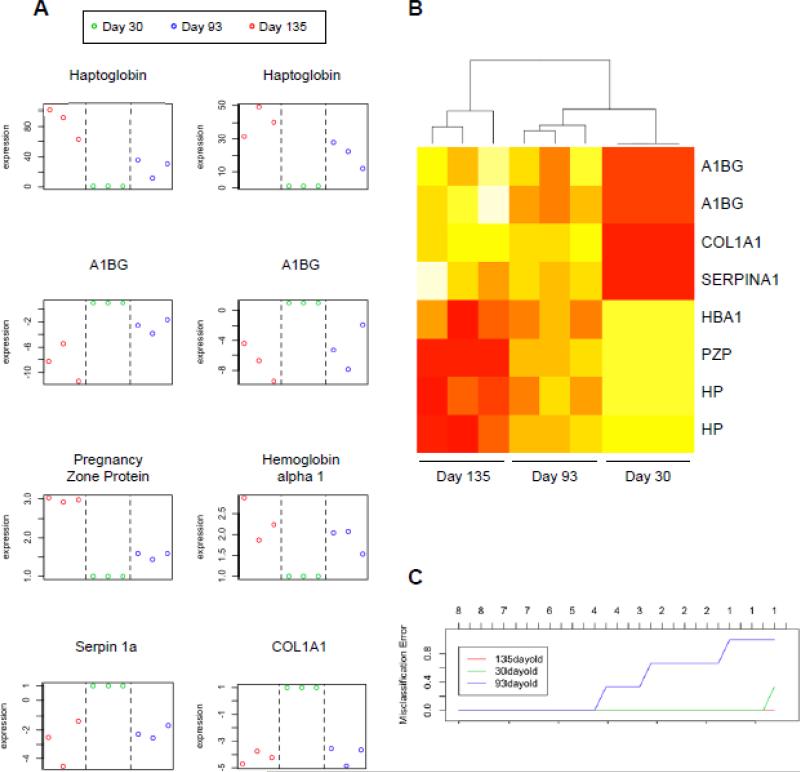
In an effort to statistically define a protein signature that could be used to distinguish non-colitis mice from mild and severe colitis mice, we statistically analyzed the differences in protein accumulation profiles between the sera of severe and mild colitis mice versus non-colitis mice. For this, we analyzed the six proteins that were deregulated at both day 135 and day 93, namely HP, A1BG, PZP, HBA1, SERPINA1 and COL1A1. A schematic plot representation of relative abundance of the spot volume of these six proteins (for HP and A1BG, the plots for the two spots with the same accession number were shown) is shown in Figure 6A, and a heat map representation of relative spot volume abundance is shown in Figure 6B. These data demonstrated clear clustering of mild colitis (day 93), severe colitis (day 135), and non-colitis mice, and mild colitis profile is intermediate between non-colitis and severe colitis groups. To statistically validate those proteins signature, we estimated the misclassification error rate when the identified proteins were used as biomarkers to assess the colitis status of mice (Figure 6C). For this analysis, we used the Prediction Analysis for Microarrays tool, a package written in R statistical software and designed to carry out sample classifications from gene expression data via the nearest shrunken centroid method. We demonstrated that using four of the six different proteins yielded a misclassification error rate of 0 (i.e., 0%) for all the experimental group, allowing a perfect discrimination of 30-day-old mice, 93-day-old mice and 135-day-old mice, confirming that the protein set identified by 2D-DIGE could be used as a signature of mild and severe colitis. In addition, 135-day-old mice could be discriminated from 93- and 30-day-old mice with a misclassification rate of 0 using only a single protein (Figure 6C). Heat maps depicting the expression profiles of all 11 differentially accumulated proteins in day 93 versus day 30 comparisons and all 16 differentially accumulated proteins in day 135 versus day 30 comparisons are shown in Figure S4A and S4B.
Figure 6. Statistical analysis of identified proteins as biomarkers of intestinal inflammation.
A, Schematic plot representation of the relative abundance of the six identified protein spots commonly deregulated in 93- and 135-day-old samples compared to the 30-dayold mouse serum samples. The y-axis represents relative values (day 30 = 1). B, Heat map representation of proteins identified by 2D-DIGE. The heat map indicates the relative abundance of the six protein spots commonly deregulated in 93 and 135 days old samples compared to 30 days old samples. For each sample, columns show the relative abundance of the proteins listed in the right (white, low expression; red, high expression). The accumulation of proteins was clustered using unsupervised hierarchical clustering. C, Overall misclassification error rate obtained using the six identified proteins and their application as biomarkers for colitis. A1BG: Alpha-1-B Glycoprotein; COL1A1: Collagen, type I, alpha 1; HBA1: Hemoglobin Alpha 1; PZP: Pregnancy Zone Protein; HP: Haptoglobin; SERPINA1: Serpin peptidase Inhibitor, clade A, member 1.
Biological processes and diseases associated with the identified proteins
The previously identified proteins were analyzed using the Coremine Program to investigate functional aspects of the identified proteins and their association with biological processes and diseases. The 12 identified proteins (HP, HPX, KNG1, SERPINA1, TF, THBS1, COL1A1, ITIH4, PZP, PRDX2, A1BG and HBA1) were found to be associated with 15 biological functions: gene expression, glycosylation, pathogenesis, growth, digestion, signal transduction, secretion, metabolism, cell differentiation, translation, phosphorylation, immune response, protein denaturation, aging, and menopause (Table 4, Figure S5). The p-values shown represent the significance of the association between the protein and the disease. Interestingly, an analysis of the associations of these 12 proteins with diseases using Coremine showed that the top two diseases associated with the 12 proteins were inflammation and acute-phase reaction (Table 5, Figure S6), with p-values of 0.0082 and 0.0172, respectively. The acute-phase reaction is part of the early defense or innate immune system that is triggered by various stimuli, including trauma, infection, stress, neoplasia, and inflammation. The acute-phase reaction results in a complex systemic response that serves the goal of reestablishing homeostasis and promoting healing [20]. This demonstration of a strong association between our protein panel and inflammation-related processes supports the identified serum proteins as a marker signature of IBD.
Table 4.
15 biological processes associated with twelve identified proteins
| Biological process | Significance (p value) a |
|---|---|
| Gene expression | 0.00750 |
| Glycosylation | 0.0102 |
| Pathogenesis | 0.0137 |
| Growth | 0.0139 |
| Digestion | 0.0202 |
| Signal transduction | 0.0218 |
| Secretion | 0.0236 |
| Metabolic process | 0.0282 |
| Cell differentiation | 0.0301 |
| Translation | 0.0543 |
| Phosphorylation | 0.0559 |
| Immune response | 0.0584 |
| Protein denaturation | 0.202 |
| Aging | 0.261 |
| Menopause | 0.277 |
p values represent the significance of the as sociation between the protein and the biological function
Table 5.
Top 15 diseases associated with twelbe identified proteins
| Diseases | Signficance (p value)a |
|---|---|
| Inflammation | 0.00842 |
| Acute-Phase reaction | 0.0172 |
| Neoplasms | 0.0182 |
| Liver carcinoma | 0.0216 |
| Exanthema | 0.0256 |
| Liver neoplasms | 0.0258 |
| Carcinoma | 0.0373 |
| Sprains and strains | 0.0695 |
| Malignant neoplasms of ovary | 0.116 |
| Melanoma | 0.124 |
| Malignant neoplasms of breast | 0.126 |
| Hypertensive disease | 0.148 |
| Chronic disease | 0.149 |
| Prostatic Neoplasms | 0.173 |
| Degenerative polyarthritis | 0.178 |
p values represent the significance of the association between the protein and the disease
Discussion
Proteomics is a powerful approach for biomarker identification, and its application to body fluids, like serum, presents new opportunities for the identification of novel, highly sensitive, and specific markers for the early detection of inflammatory bowel disease. In a longitudinal study, we used the well-established IL-10−/− mouse model of IBD in conjunction with a proteomic approach to identify mouse serum protein biomarkers that could be used to predict and follow the degree of intestinal inflammation during the progression of IBD. The concept driving our study was that deregulated proteins identified in serum could be used as part of a panel, yielding to a biomarker signature capable of discriminating between colitis and noncolitis, as well as between different stages of colitis.
Using serum samples from IL-10−/− colitis mice and 2D-DIGE technology, we identified 15 proteins (HP, HPX, KNG1, SERPINA1, TF, THBS1, COL1A1, ITIH4, PZP, PRDX2, A1BG, HBA1, Contrapsin, H2-Q10 and C3) whose accumulation was altered under intestinal inflammatory conditions. All these proteins have been previously linked to various inflammatory conditions, confirming that these markers are associated with the inflammatory state. For example, chromatography has demonstrated an association between sepsis, a whole-body, severe inflammation, and a decrease in A1BG protein [21]. It has been reported that COL1A1 is increased in mouse models of colitis, including dextran sodium sulfate (DSS) and 2,4,6-trinitrobenzenesulfonic acid (TNBS) models [22, 23]. C3 has been identified as an autoantigen in IBD [24], and it has been shown that HP is increased in the sera of patients with colon cancer and chronic inflammatory diseases as well as in the mouse model of DSS-induced colitis [25, 26]. IBD is associated with anemia, which varies considerably in severity among IBD patients [27]. Depending on anemia status, TF has been found to either increase or decrease in the sera of IBD patients [28]. In patients suffering from anemia of chronic disease, TF levels are reduced compared to those in control individuals [28]. Iron-deficiency anemia (IDA), which can occur with chronic bleeding, is associated with higher serum concentrations of TF [28]. In our study, TF levels were not modified at day 93 (mild colitis) but were increased at day 135 (severe colitis), attesting to the differences in degree of bleeding according to the severity of colitis. HPX has been found to be increased in the sera of UC and CD patients compared to control patients [29], and is also increased in injured rat intestinal mucosa [30]. ITIH4 levels have been shown to increase during experimental aspergillosis [31]; however, ITIH4 is considered as an anti-inflammatory protein whose down-regulation is a marker of acute ischemic stroke [32]. In the present study, this protein was upregulated at the later 135-day time point (severe colitis) but was normally expressed at the intermediate time point (moderate colitis). It has been shown that KNG1 is involved in the pathogenesis of colitis in an animal model, as evidenced by attenuated DSS-induced colitis in KNG1-deficient rats [33], and that PRDX2 is involved in the persistence of pro-inflammatory cells in chronic inflammation (lymphocytes from rheumatoid arthritis patients) [34]. THBS1 has been found to play a role in intestinal inflammation and carcinogenesis [35]. Finally, PZP has not been linked to an inflammatory status. In addition, a recent study, which analyzed the serum protein profile of early and advanced Crohn's disease, notably reports haptoglobin, complement C3 or α1-anti-trypsin (serpin family) with modified serum accumulations [36]. Interestingly, this study showed that C3 was only deregulated in early CD, correlating with our results that showed C3 over-representation at day 93 only [36].
This biomarker panel was discovered using IL10−/− mice that develop colitis in a time-dependent manner. While the appearance of the inflammation was described to be only colonic in several studies [11, 37], in other studies small intestinal inflammation was also observed [12, 38]. Therefore, we cannot exclude that some proteins of our panel could not discriminate between colitis and ileitis. The use of the DSS model that exclusively develops colonic inflammation can validate some biomarkers of the panel. Proteins found to have an altered expression in the IL10−/− model but not in the DSS model (that exclusively develops colonic inflammation) could therefore be either specific to chronic colitis versus acute colitis or specific to ileitis versus colitis.
Our bioinformatics analysis allowed us to establish a network of associations between the identified proteins (HP, HPX, KNG1, SERPINA1, TF, THBS1, COL1A1, ITIH4, PZP, PRDX2, A1BG and HBA1) and biological processes (Table 4 and Figure S5) and diseases (Table 5 and Figure S6). This analysis showed that the proteins in the panel identified by 2D-DIGE are associated with biological processes relevant to the induction and progression of IBD. For example, cell differentiation and immune response, which are key processes in the induction and progression of inflammation, were among the biological mechanisms impacted by altered accumulation of identified proteins. A central component of chronic intestinal inflammation in IBD is a perpetuated and deregulated immune response against commensal bacteria of the gut [39]. Moreover, the immunopathologic events underlying these two major forms of IBD are indeed marked by very different T-cell differentiation pathways, but both share an increase in T-cell differentiation and activation [40]. In addition, our bioinformatics study associated our panel of protein with 15 diseases. The top two diseases are inflammation and acute-phase response, further validating the panel of identified serum protein as colitis biomarkers.
The power and innovation of our study lies in the use of a combination of several biomarkers to define a signature of IBD. The use of two other models of inflammation (DSS and CAIA) showed that the biomarker panel identified for IBD comprises some global inflammatory markers, some intestinal inflammation specific markers and some chronic intestinal inflammation markers. This combination of specific and non-specific markers makes the panel powerful and the signature unique. Our study revealed that six proteins (A1BG, COL1A1, SERPINA1, HBA1, PZP and HP) were deregulated in common in the serum of 93- and 135-dayold mice compared to serum samples from 30-day-old mice. The use of a single protein was found to be sufficient to discriminate severe colitis (135-day-old mice) from both the absence of colitis (30-day-old mice) and mild colitis (93-day-old mice). More importantly, four proteins were found to be sufficient to classify a mouse in one of the three experimental groups. Thus, instead of using only one biomarker that is specific for a single stage of the disease, we used a panel of serum proteins as biomarker signature, exploiting their specific patterns of expression to discriminate between disease subtype and progression status, and/or predict therapeutic responses. Collectively, our results highlight the power of multiple biomarkers with different patterns of expression in predicting, following and diagnosing colitis, demonstrating the predictive potential of our protein signature panel.
Our study suggests that the deregulated biomarkers identified in mice could serve as a signature panel for the early diagnosis and effective surveillance of IBD in humans. However, because of its novelty, this concept will require additional studies to determine whether the protein biomarker signatures identified in our animal model can be translated to humans. We also anticipate that this approach might aid in identifying patients who are likely to experience disease recurrence after treatment, to predict response to therapy, and to discriminate between different subtypes of IBD in humans. Moreover, microchip ELISA could be integrated with cell phone/CCD-based colorimetric measurement technology to detect specific biomarker signatures at the point-of-care (POC), facilitating the creation of bedside technologies for diagnostics and treatment monitoring. Our study provides proof of principle of the concept of a serum protein signature associated with IBD. A similar longitudinal study in human would help pave the way to personalized medicine in which patient-to-patient differences in molecular signatures would allow the assessment of disease status and personalized drug management.
Supplementary Material
Significance.
Crohn's disease (CD) and ulcerative colitis (UC) are the most common inflammatory bowel diseases (IBD) occurring in humans. The major current diagnosis tool is colonoscopy, which is invasive and could lead to false diagnosis. The emergence of serological biomarkers enables the use of new diagnosis tools such as protein signatures for IBD diagnosis/management. Using 2D-DiGE coupled to mass spectrometry, our longitudinal study in a mouse model of colitis identified a signature of protein biomarkers for specific stages of disease.
Highlights.
- A longitudinal study was performed in the IL10−/− mouse model of IBD using 2D-DiGE
- A panel of proteins was found to be differentially expressed in serum of colitic mice compare to non colitic mice
- We provide proof of principle of the concept of serum protein signature of IBD
- This specific signature could serve for diagnosis, surveillance and drug management of IBD in human
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs (BX002526) and the National Institutes of Health of Diabetes and Digestive and Kidney (RO1-DK-071594 to D.M), the American Heart Association Postdoctoral Fellowship Grant 13POST16400004 (to B.X.). D. Merlin is a recipient of a Research Career Scientist Award from the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Saleh M, Elson CO. Experimental inflammatory bowel disease: insights into the host-microbiota dialog. Immunity. 2011;34:293–302. doi: 10.1016/j.immuni.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakata K, Yamashita T, Maeda M, Moriyama Y, Shimada S, Tohyama M. Cloning of a lymphatic peptide/histidine transporter. Biochem J. 2001;356:53–60. doi: 10.1042/0264-6021:3560053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res. 2012;159:313–25. doi: 10.1016/j.trsl.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meuwis MA, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Piver E, et al. Serum calprotectin as a biomarker for Crohn's disease. J Crohns Colitis. 2013 doi: 10.1016/j.crohns.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Cury DB, Mizsputen SJ, Versolato C, Miiji LO, Pereira E, Delboni MA, et al. Serum calprotectin levels correlate with biochemical and histological markers of disease activity in TNBS colitis. Cell Immunol. 2013;282:66–70. doi: 10.1016/j.cellimm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshu CE, Tsilidis KK, Peskoe SB, Giardiello FM, Dluzniewski PJ, Nelson WG, et al. The association between circulating high-sensitivity C-reactive protein concentration and pathologic measures of colonic inflammation. Cancer Causes Control. 2014 doi: 10.1007/s10552-014-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev. 2007;59:1073–83. doi: 10.1016/j.addr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 11.Davidson NJ, Leach MW, Fort MM, Thompson-Snipes L, Kuhn R, Muller W, et al. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996;184:241–51. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 13.Clement CC, Aphkhazava D, Nieves E, Callaway M, Olszewski W, Rotzschke O, et al. Protein expression profiles of human lymph and plasma mapped by 2D-DIGE and 1D SDS-PAGE coupled with nanoLC-ESI-MS/MS bottom-up proteomics. J Proteomics. 2013;78:172–87. doi: 10.1016/j.jprot.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F, Chen DN, He CW, Zhou Y, Olkkonen VM, He N, et al. Identification of urinary Gc-globulin as a novel biomarker for bladder cancer by two-dimensional fluorescent differential gel electrophoresis (2D DIGE). J Proteomics. 2012;77:225–36. doi: 10.1016/j.jprot.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Jenssen TK, Laegreid A, Komorowski J, Hovig E. A literature network of human genes for high-throughput analysis of gene expression. Nat Genet. 2001;28:21–8. doi: 10.1038/ng0501-21. [DOI] [PubMed] [Google Scholar]
- 16.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 17.Morteau O, Morham SG, Sellon R, Dieleman LA, Langenbach R, Smithies O, et al. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J Clin Invest. 2000;105:469–78. doi: 10.1172/JCI6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, Martinez-Augustin O, et al. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem. 2010;401:318–20. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 19.Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 2012;7:e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cray C, Zaias J, Altman NH. Acute phase response in animals: a review. Comp Med. 2009;59:517–26. [PMC free article] [PubMed] [Google Scholar]
- 21.Baum A, Pohl M, Kreusch S, Cumme GA, Ditze G, Misselwitz J, et al. Searching biomarker candidates in serum using multidimensional native chromatography. II Method evaluation with Alport syndrome and severe inflammation. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;876:31–40. doi: 10.1016/j.jchromb.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Wu F, Chakravarti S. Differential expression of inflammatory and fibrogenic genes and their regulation by NF-kappaB inhibition in a mouse model of chronic colitis. J Immunol. 2007;179:6988–7000. doi: 10.4049/jimmunol.179.10.6988. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi H, Suzuki K, Nagata M, Kawase T, Sukumaran V, Thandavarayan RA, et al. Irsogladine maleate ameliorates inflammation and fibrosis in mice with chronic colitis induced by dextran sulfate sodium. Med Mol Morphol. 2012;45:140–51. doi: 10.1007/s00795-011-0550-7. [DOI] [PubMed] [Google Scholar]
- 24.Lundgren BA, Rorsman F, Portela-Gomes GM, Grimelius L, Ekdahl KN, Nilsson B, et al. Identification of complement C3 as an autoantigen in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2010;22:429–36. doi: 10.1097/MEG.0b013e32833283b1. [DOI] [PubMed] [Google Scholar]
- 25.Hou YC, Chu CC, Ko TL, Yeh CL, Yeh SL. Effects of alanyl-glutamine dipeptide on the expression of colon-inflammatory mediators during the recovery phase of colitis induced by dextran sulfate sodium. Eur J Nutr. 2013;52:1089–98. doi: 10.1007/s00394-012-0416-3. [DOI] [PubMed] [Google Scholar]
- 26.Park SY, Yoon SJ, Jeong YT, Kim JM, Kim JY, Bernert B, et al. N-glycosylation status of beta-haptoglobin in sera of patients with colon cancer, chronic inflammatory diseases and normal subjects. Int J Cancer. 2010;126:142–55. doi: 10.1002/ijc.24685. [DOI] [PubMed] [Google Scholar]
- 27.Weiss G, Gasche C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica. 2010;95:175–8. doi: 10.3324/haematol.2009.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theurl I, Mattle V, Seifert M, Mariani M, Marth C, Weiss G. Dysregulated monocyte iron homeostasis and erythropoietin formation in patients with anemia of chronic disease. Blood. 2006;107:4142–8. doi: 10.1182/blood-2005-08-3364. [DOI] [PubMed] [Google Scholar]
- 29.Weeke B, Jarnum S. Serum concentration of 19 serum proteins in Crohn's disease and ulcerative colitis. Gut. 1971;12:297–302. doi: 10.1136/gut.12.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takagi T, Naito Y, Okada H, Takaoka M, Oya-Ito T, Yamada S, et al. Hemopexin is upregulated in rat intestinal mucosa injured by indomethacin. J Gastroenterol Hepatol. 2012;27(Suppl 3):70–5. doi: 10.1111/j.1440-1746.2012.07076.x. [DOI] [PubMed] [Google Scholar]
- 31.Desoubeaux G, Jourdan ML, Valera L, Jardin B, Hem S, Caille A, et al. Proteomic demonstration of the recurrent presence of inter-alpha-inhibitor H4 heavy-chain during aspergillosis induced in an animal model. Int J Med Microbiol. 2013 doi: 10.1016/j.ijmm.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Kashyap RS, Nayak AR, Deshpande PS, Kabra D, Purohit HJ, Taori GM, et al. Inter-alpha-trypsin inhibitor heavy chain 4 is a novel marker of acute ischemic stroke. Clin Chim Acta. 2009;402:160–3. doi: 10.1016/j.cca.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Khan MM, Liu Y, Khan ME, Gilman ML, Khan ST, Bromberg M, et al. Upregulation of tissue factor in monocytes by cleaved high molecular weight kininogen is dependent on TNF-alpha and IL-1beta. Am J Physiol Heart Circ Physiol. 2010;298:H652–8. doi: 10.1152/ajpheart.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabo-Taylor KE, Eggleton P, Turner CA, Faro ML, Tarr JM, Toth S, et al. Lymphocytes from rheumatoid arthritis patients have elevated levels of intracellular peroxiredoxin 2, and a greater frequency of cells with exofacial peroxiredoxin 2, compared with healthy human lymphocytes. Int J Biochem Cell Biol. 2012;44:1223–31. doi: 10.1016/j.biocel.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutierrez LS. The role of thrombospondin 1 on intestinal inflammation and carcinogenesis. Biomark Insights. 2008;3:171–8. doi: 10.4137/bmi.s630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piras CS,A, Greco V, Cassinotti A, Maconi G, Ardizzone S, Amoresano A, Bianchi Porro G, Bonizzi L, Roncada P. Serum protein profiling of early and advanced Crohn's disease. EuPA Open Proteomics. 2014;3:48–59. [Google Scholar]
- 37.Sydora BC, Tavernini MM, Wessler A, Jewell LD, Fedorak RN. Lack of interleukin-10 leads to intestinal inflammation, independent of the time at which luminal microbial colonization occurs. Inflamm Bowel Dis. 2003;9:87–97. doi: 10.1097/00054725-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Fu S, Sun S, Li Z, Guo B. Inflammasome activation has an important role in the development of spontaneous colitis. Mucosal Immunol. 2014 doi: 10.1038/mi.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 40.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–70. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.