Abstract
Midbrain dopamine neurons fire irregularly, with interspersed clusters of high-frequency spikes, commonly called ‘bursts’. In this review we examine such heterogeneity in activity, and provide insight into how it can participate in psychiatric conditions such as drug addiction. We first describe several techniques used to evaluate dopamine neuron activity, and comment on the different measures that each provides. We next describe the activity of dopamine neurons in ‘basal’ conditions. Specifically, we discuss how the use of anesthesia and reduced preparations may alter aspects of dopamine cell activity, and how there is heterogeneity across species and regions. We also describe how dopamine cell firing changes throughout the peri-adolescent period and how dopamine neuron activity differs across the population. In the final section, we discuss how dopamine neuron activity changes in response to life events. First, we focus attention on drugs of abuse. Drugs themselves change firing activity through a variety of mechanisms, with effects on firing while drug is present differing from those seen after drug discontinuation. We then review how stimuli that are rewarding, aversive, or salient can evoke changes in firing rate and discharge pattern of dopamine neurons, and provide behavioral relevance of dopamine signaling. Finally, we discuss how stress can modulate dopamine neuron firing and how this may contribute to the role that stressful experiences play in psychiatric disorders such as addiction and depression.
Keywords: Dopamine, firing, bursting, VTA, SNc, drug addiction, tonic, phasic, adolescence, psychostimulants, anesthesia
1. INTRODUCTION
Midbrain dopamine neurons are essential mediators of a number of normal behaviors including voluntary movement, feeding, associative learning, and motivation. In addition, several pathologies are related to dysfunction of these neurons including Parkinson's disease and drug addiction. Given this varied set of behaviors, it is not surprising that the search for a single function for dopamine has been elusive. Concurrent with the search for one role has been a supposition that dopamine neurons themselves form a homogeneous group with similar properties and responses to stimuli. This picture has gradually shifted during the last decade as a new appreciation for heterogeneity of dopamine neurons both within and between individuals has become accepted. In this review, we will examine this heterogeneity as it relates to several features including firing rates and patterns, neuroanatomy, anesthesia, species, development, and life events (e.g. exposure to abused drugs and stress).
2. ANATOMY OF THE DOPAMINE SYSTEM
The anatomy of the dopamine system is described in detail in other contributions to this special issue (Walsh and Han, 2014; Yetnikoff et al., 2014); here we only present a very brief summary. The majority of dopamine neurons of the midbrain originate in the ventral tegmental area (VTA, also known as the A10 cell area) and the substantia nigra pars compacta (SNc, A9 cell area), with VTA placed ventromedial to SNc. These dopamine neurons send projections to several forebrain regions – most prominently the striatal complex, which encompasses nucleus accumbens (NAc; ventral striatum) and caudate putamen (CPu; dorsal striatum). Dopamine neurons also project to the amygdala, cortex, and hippocampus. Projections from the SNc are more localized to the CPu, whereas those from the VTA include the aforementioned structures.
Inputs to the dopamine neurons originate from wide variety of areas, with excitatory projections from cortex, lateral dorsal tegmental nucleus (LDTg), and pedunculopontine tegmental nucleus (PPTg) thought to be of particular importance for driving activity. Inhibitory GABAergic inputs are largely from areas such as ventral pallidum (VP) and rostromedial tegmental nucleus (RMTg). Furthermore, intermingled with dopamine neurons are a sizable population of non-dopamine neurons which include GABAergic neurons and glutamate neurons. These can also modulate the activity of dopamine neurons (Dobi et al., 2010; Tan et al., 2012). In addition, in some cases dopamine neurons co-release other neurotransmitters including glutamate, GABA, neurotensin, and cholecystokinin (German and Liang, 1993; Stuber et al., 2010; Tritsch et al., 2012), adding more heterogeneity to the midbrain.
3. EVALUATING DOPAMINE NEURON ACTIVITY
Dopamine neuron activity, and more broadly activity in the mesolimbic dopamine projection, can be assayed in a number of ways. These include in vivo methods in both awake and anesthetized subjects, as well as ex vivo methods in tissue slices.
3.1 Direct measures of dopamine neuron activity
Historically, the majority of work on dopamine neuron activity has been conducted in vivo in anesthetized rats and has used electrophysiology to record extracellular action potentials. Using this technique many of the characteristics that are associated with dopamine neurons were derived, including their electrophysiological properties and response to pharmacological agents (Grace and Bunney, 1983). These characteristics are detailed below, in section 4 on basal activity. A few studies have used sharp electrodes to record intracellularly but this is difficult due to challenges of recording in a deep-lying structure such as the midbrain (Grace, 1988).
Using extracellular recordings, activity of dopamine neurons has also been analyzed in awake animals. Of these studies, most have been performed in head-fixed non-human primates. With this technique, the mobility of the electrode and the stability of the subject mean that dopamine neurons can be easily found and recorded for long periods of time. Recordings in awake freely-moving rodents using multi-wire electrodes tend to be more difficult and typically result in a low yield of cells. However, recent technological advances, such as improvements in electrode construction, have made these recordings more manageable to perform in rodents (Cohen et al., 2012; Kim et al., 2012). Finally, in rare cases, it has been possible to achieve recordings of dopamine cells from human subjects undergoing surgery to ameliorate Parkinson's Disease (Zaghloul et al., 2009).
In vitro, firing of dopamine neurons has been measured in various preparations including ex vivo tissue slices, organotypic slice cultures, and primary dissociated cell cultures (Shepard and Bunney, 1988; Chiodo and Kapatos, 1992; Masuko et al., 1992; Steensen et al., 1995). Here, whole-cell, cell-attached, or extracellular methods can be used to record spontaneous action potentials (Sanghera et al., 1984; Kita et al., 1986; Brodie and Dunwiddie, 1987; Shepard and Bunney, 1988; Mueller and Brodie, 1989; Paladini et al., 2007). The particular method used can influence firing rate. For example, whole cell recordings in current-clamp mode often exhibit run-down of firing such that spontaneous action potentials cease within several minutes of membrane rupture. For this reason cell-attached, extracellular, or perforated patch recordings are preferred.
In recent years there has been lively debate regarding the ability to classify neurons as dopaminergic or not using electrophysiological criteria. Accurate identification of dopaminergic phenotype is crucial when considering heterogeneity across the population, as it is likely to be a key source of variability (Margolis et al., 2006, 2010; Zhang et al., 2010; Chieng et al., 2011; Ungless and Grace, 2012). Currently it is thought that, in the SNc, electrophysiological criteria reliably identify dopamine neurons both in vivo and ex vivo. In the VTA, electrophysiological and pharmacological criteria fairly robustly identify dopamine neurons in vivo (Ungless and Grace, 2012). However, identification ex vivo requires post hoc immunohistochemistry to confirm dopaminergic identity. Recently, the advent of transgenic technologies has meant that researchers working in mice, and more recently rats, can utilize expression of a fluorescent reporter to confirm dopaminergic identity ex vivo (Labouèbe et al., 2007; Witten et al., 2011). In vivo, optogenetics has also been used to confirm either a dopaminergic or GABAergic phenotype (Cohen et al., 2012). For example, neurons of a specific phenotype (e.g. dopamine or GABA) can be genetically engineered to express light-activated channels such as channelrhodopsin. If light evokes excitation of the cell being recorded with short latency (e.g. < 5 ms) then there is more certainty that that particular cell is from the transfected population. However, in these circumstances, if a particular genetic manipulation (e.g. overexpression of a channel) can itself alter neuronal properties should be considered (see section 4.4 on heterogeneity across species).
3.2 Indirect measures of dopamine neuron activity
It is also possible to assess activity of dopamine neurons in an indirect fashion without recording action potentials. For example, at the level of the dopamine soma, whole cell recordings made in ex vivo slices can assess a number of parameters related to expression of channels and post-synaptic receptors, which may provide an indirect measure of the cell's excitability or responsiveness to synaptic input, even made in a reduced preparation (i.e. ex vivo). Of these, perhaps the most noteworthy, is the use of AMPA/NMDA ratio, which is thought to provide a read out of the strength of glutamatergic input to the neuron and is often used as a measure of long-term potentiation that has occurred in vivo. This measure has been shown to increase in response to a number of manipulations including cocaine exposure (Ungless et al., 2001; Chen et al., 2008), stress exposure (Saal et al., 2003), and Pavlovian learning (Stuber et al., 2008). However, it is important to note that the precise effect that this alteration in synaptic strength has on firing activity in dopamine neurons in vivo is still unclear. In fact, comparison across different studies using a well-defined protocol such as self-administration of cocaine shows that the temporal dynamics of AMPA/NMDA ratio vs. firing rate do not match up, indicating that these two phenomena are not necessarily interchangeable (Marinelli et al., 2003; Borgland et al., 2004; Chen et al., 2008; McCutcheon et al., 2009).
Another method of assaying dopamine neuronal activity at the soma is calcium imaging. Using calcium indicators, which may be genetically-encoded or not, activity of dopamine neurons may be optically monitored. The recent adoption of fiber optic bundles has permitted such measurements to be made in awake, behaving animals (Vincent et al., 2006; Soden et al., 2013). However, the time resolution of this in vivo technique is lower than traditional electrophysiology and, at least at the moment, it is not possible to distinguish individual action potentials with confidence.
Finally, it is also possible to assess the output of dopamine neurons by measuring the amount of neurotransmitter with either microdialysis/HPLC (Church et al., 1987; Hernandez et al., 1987; Sharp et al., 1987) or electrochemistry (voltammetry/amperometry) (Stamford et al., 1984; Garris et al., 1997; Phillips et al., 2003). This can be done in projection targets of dopamine neurons, as well as within the midbrain itself. This is because dopamine is not only released at the terminals, but it is also released somato-dendritically, at the level of the dopamine soma. Overall, these methods are generally used in vivo and provide important information on the actual consequences of changes in dopamine neuron activity. With this in mind, however, it is important to note that somatic action potentials do not necessarily translate into neurotransmitter release; several processes may modulate the effect of each action potential including the expression of monoamine transport molecules, catecholamine metabolizing enzyme, and technical factors, e.g. probe size, geometry, and location (Floresco et al., 2003; Phillips and Wightman, 2004). They can also be influenced by proximity to other action potentials (summation) and pre-synaptic modulation of neurotransmitter release (Gonon, 1988; Grace, 1995; Schmitz et al., 2003; Exley and Cragg, 2008). Moreover, in the striatum, action-potential independent dopamine release can be evoked via activity in cholinergic interneurons, highlighting another mechanism by which firing of dopamine cells can be uncoupled from dopamine release (Cachope et al., 2012; Threlfell et al., 2012).
4. ‘BASAL’ ACTIVITY
Dopamine neurons fire with characteristic patterns of activity in basal conditions (i.e. without stimulation), and these differ across in vivo and ex vivo preparations. Most of this section will review studies made in vivo, in anesthetized rats, as these are the most abundant.
4.1 Heterogeneity in firing patterns
In vivo in anesthetized adult rats, dopamine neurons of the VTA fire action potentials from ~0.5 to ~10 Hz (average ~4.5 Hz), and this activity is normally-distributed, with the majority of cells firing around 4-5 Hz (see Grace and Bunney, 1984b). The firing pattern is irregular and is interspersed with clusters of action potentials emitted at high frequency (~2-5 spikes at approximately 10-20 Hz). These clusters of action potentials are commonly known as ‘bursts’ (Grace and Bunney, 1984b) and are thought to be critical for many, if not all, of dopamine's functions in the mesolimbic system. The phenomenon of bursting, including criteria used to identify burst events, is covered in detail later-on in this section and in Figure 1. A primary reason underlying the importance of bursting for dopamine's physiological function is the capacity for bursts to transiently increase dopamine concentration to the high levels required to activate certain populations of post-synaptic dopamine receptors. In addition, it is thought that these high concentrations may enable dopamine to overwhelm re-uptake mechanisms and ‘spillover’ to nearby sites (Dreyer et al., 2010).
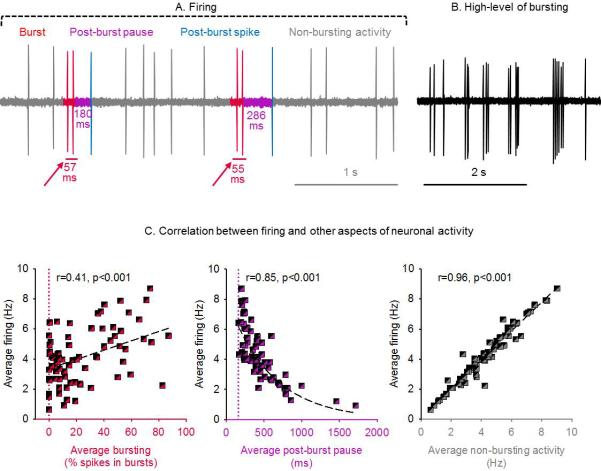
Figure 1.
Dopamine neuron firing patterns in vivo. (A) In vivo extracellular recording of a VTA dopamine neuron from a chloral hydrate anesthetized rat. Firing can be divided into bursting (red) and non-bursting (grey) activity. An inter-spike interval of <80 ms defines the beginning of a burst. A burst is terminated by an interspike interval of >160 ms, which is the post-burst pause (purple); the first action potential following it is the post-burst spike (blue). (B) Recording under similar conditions as (A) of a neuron exhibiting a high level of bursting. (C) Correlation between average firing rate and bursting (percent of spikes emitted in bursts; left panel) is poor (r=0.41, p<0.001) due to inclusion of many post-burst pauses (middle panel, r=0.85, p<0.001) that lower firing rate. Non-bursting activity correlates very well with average firing rate (right panel, r=0.96, p<0.001). The red vertical hashed line indicates 0% bursting; the purple vertical hashed line indicates 160 ms (i.e. the minimum interspike interval terminating a burst event). Correlations are performed by fitting data to a straight line for (A) and (B) and to an exponential decay function for (C). Data collected from male adult (2-4 months) singly-housed rats across studies in our lab, n=85 cells from 34 rats.
Figure 1 shows a representative firing trace. The overall firing rate is the sum of bursting and ‘non-bursting’ spikes, together with the pauses between spikes (inter-spike intervals, ISIs). Bursts are often followed by an ISI with long duration (a post-burst ‘pause’); these pauses can lower the overall firing rate; therefore, as described by Grace (Grace and Bunney, 1984b), the amount of bursting does not correlate very closely with the overall firing rate (correlation: r=0.38). We reproduced this finding in our lab (correlation: r=0.41) and show it in Figure 1. Instead, the overall firing rate correlates best with non-bursting activity as is shown in Figure 1 (correlation: 0.96). Non-bursting activity is seldom reported; it represents the firing rate of all spikes that are not part of a burst or a post-burst spike.
Bursting can be analyzed in terms of ‘amount of bursting’. In this case one can examine the proportion of spikes emitted in bursts, the frequency of burst events, or the time spent bursting. Bursting can also be analyzed in terms of ‘burst characteristics’. In this case one can examine the number of spikes within each burst, the duration of the burst events, and the frequency of the spikes within each burst (Figure 1). All of these parameters vary greatly across cells, even under resting conditions. Thus, the amount of bursting is observed in a continuum from no bursting at all (rare, only in a small proportion of neurons) to high levels of bursting, such as. 80-90% of spikes emitted in bursts (Figures 1 and 2); the average is approximately 30% (Grace and Bunney, 1984b); but, as explained later, this can vary across study and conditions. Similarly, the frequency of burst events also shows a continuum. Burst event frequency can range from rare (e.g. never or only a few times per minute) to frequent (e.g. 1-2 Hz, i.e. a burst event every 0.5-1 s), with an average event frequency of 0.3-0.5 Hz, i.e. a burst every 2-3 s (Figure 2). Some studies classify neurons as ‘bursting’ and ‘non-bursting’, based on different criteria that vary across papers and labs. For example, neurons are classified as ‘bursting’ if they have more than two ‘triplets’ (a three-spike burst) over 500 consecutive spikes (Grace and Bunney, 1984b), or if they show more than a certain percentage of bursting. However, as mentioned above, there is a continuum from non-bursting to bursting across cells, and this continuum is very gradual. As Figures 1 and 2 show, bursting is it is not an ‘all or nothing’ phenomenon, nor does it have a bimodal distribution across the neuronal population. Similarly, Mameli-Engvall et al. (2006) recorded from VTA neurons in mice and found that cells existed on a continuum with respect both to average firing rate and number of spikes within a burst. In addition, dopamine cells fire stably with respect to these parameters over time. Because of this, separating neurons into two categories, or the idea that is commonly presented in the literature, whereby neurons belong to either one category or another, or have two distinct firing patterns, can be misleading. The frequency of burst events and their characteristics may be altered by stimuli or environmental circumstances (Marinelli et al., 2003; Dahan et al., 2007; Anstrom et al., 2009; Wanat et al., 2009), but this is quite different to the situation that is frequently described, where cells in ‘non-bursting mode’ (emitting no spikes in clusters), suddenly shift their firing pattern to ‘bursting mode’ (for example see Bioulac et al., 1997; Drion et al., 2010; Lalive et al., 2014).
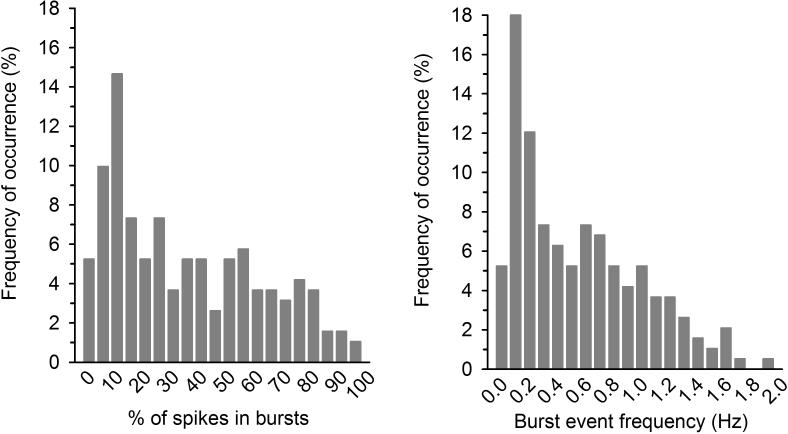
Figure 2.
Dopamine neurons exist on a continuum with respect to bursting activity. Histograms show the frequency of occurrence (in %) for the proportion of spikes emitted in bursts (left panel) and burst event frequency (right panel). VTA dopamine neurons were recorded in vivo in chloral hydrate anesthetized rats. Distribution is smooth and monophasic demonstrating that there is no clear distinction between bursting and non-bursting neurons. For these data, mean ± SEM for % of spikes emitted in bursts is 32.1 ± 1.9 and mean ± SEM for burst event frequency is 0.5 ± 0.03 Hz. Note that the majority of bursts occur at <0.2 Hz (i.e. once every 5 s) and only very few occur above 1 Hz (i.e. once per second). Data collected from male adult (2-4 months) singly-housed rats across studies in our lab, n=191 cells from 73 rats.
Although bursting is commonly referred to and is an accepted phenomenon, defining bursts in practice is not straight-forward. Classically, bursts have been defined as clusters of spikes in which the first ISI is <80 ms and the last ISI of the cluster is <160 ms (Figure 1) (Grace and Bunney, 1984b). These parameters are based on the fact that the frequency of spikes within a burst tends to undergo adaptation, i.e. the ISI often increases within the burst. The average duration of a burst varies from <80 ms, when the burst is composed of only two spikes (a ‘doublet’) to longer (80 ms to several hundreds of ms), when the burst is composed of several spikes (a ‘triplet’ or longer). On average, however, bursts last 80-200 ms and are of 2-5 spikes in basal conditions, although longer bursts are possible [(see Grace and Bunney, 1984a) and Figure 1], especially after behavioral or pharmacological manipulations (see section 5.1 on heterogeneity in response to addictive drugs).
Several other types of burst detection methods have been proposed with some offering purely descriptive measures and others providing a test of statistical significance. The latter include analyses such as the Poisson Surprise method, Rank Surprise, and Robust Gaussian Surprise, which are able to statistically identify bursts and pauses in spike trains (Legéndy and Salcman, 1985; Gourévitch and Eggermont, 2007; Ko et al., 2012). Alternatively, slow oscillations in activity can be studied; when slow oscillations are present, they correlate with the amount of bursting (Zhang et al., 2008).
Finally, not all dopamine neurons are spontaneously active, both in vivo or ex vivo. This is an important point because manipulations that activate silent cells are very difficult to study, especially with extracellular in vivo methods that involve moving an electrode slowly through the midbrain until a cell is detected. Grace and colleagues developed one method to assay the number of active vs. silent cells, that is, the “population activity” (Bunney and Grace, 1978). This involves counting the number of cells detected each time the electrode is lowered from the dorsal to ventral aspect of the midbrain (i.e. counting the cells/track). This is a powerful technique, and requires recording expertise (i.e. the ability to consistently find cells during the recording procedure). Using this approach, a number of manipulations have been shown to alter the number of spontaneously active cells, including acute and chronic treatment with neuroleptics (Bunney and Grace, 1978; Chiodo and Bunney, 1983; White and Wang, 1983) and activation or disruption of input from various brain regions such as striatum and cortex (Shim et al., 1996; West and Grace, 2000). Although difficult to assess the exact proportion of silent cells, certain manipulations (e.g. acute neuroleptic administration) increase the number spontaneously active dopamine cells in the VTA by 2-3 fold suggesting that, at baseline, less than half of the dopamine cells may be active. In another study, however, very few, if any, silent dopamine cells were found in SNc (Dai and Tepper, 1998) although it was suggested that this may have resulted from the antidromic stimulation procedure being used (West and Grace, 2000). The study of population activity can also distinguish between manipulations that increase firing activity in already active cells and manipulations that change the number of spontaneously active cells (Floresco et al., 2001, 2003). Manipulations that increase the number of spontaneously active cells also tend to alter the distribution of firing rates, from a normal distribution to a biphasic distribution. This is due to activation of silent cells, which can fire at a lower rate than spontaneously active cells; therefore, when more cells shift from silent to active, overall firing rate does not change or decreases slightly (Floresco et al., 2001, 2003).
4.2 Heterogeneity in the meaning of “bursting” and “phasic”
It is important to note that the term “burst” when referring to spike data from dopamine neurons has a stricter definition than simply a “burst of activity”, which is often used to refer to any transient increase in spike frequency. One example is in the seminal work of Schultz and colleagues (reviewed in more detail in section 5.2 on heterogeneity in response to brief environmental stimuli), in which rewards (and conditioned predictors of reward) are said to evoke “a short burst of impulses” or a “burst of activity” (Schultz et al., 1997). Here, a burst is used to mean that presentation of the reward increases the probability of spiking (firing), not necessarily of bursting. In fact, these data show that neuronal activity does not always reach firing rates or ISIs that would be consistent with bursting (i.e. <80 ms; 12.5 Hz). Therefore, the term “bursting” denotes different things across the literature (Anstrom et al., 2007).
When describing dopamine signaling, the terms tonic and phasic are also used in a variety of ways. For example, both are used to refer to patterns of neuronal activity as well as patterns of dopamine release in terminal regions. With respect to neuronal activity, in many electrophysiology studies, phasic is used as a strict synonym for ‘bursting’ (Floresco et al., 2003; Tsai et al., 2009; Zweifel et al., 2009). As such, neuronal activity is referred to as either ‘tonic’ or ‘phasic’, to distinguish between single-spike and burst firing patterns. In other studies, however, ‘tonic’ and ‘phasic’ are unrelated to firing patterns; instead, they refer to temporal changes in firing activity (similar to the Schultz studies mentioned above). With respect to dopamine release, these terms are used to differentiate between the rapid fluctuations in dopamine concentration that occur on a second-to-second timescale (phasic) vs. the level of extracellular dopamine, which is more stable or changes more slowly, in minutes to hours (tonic) (Grace, 1995). These different measures of dopamine neuron output are usually measured with voltammetry or microdialysis, respectively.
Phasic dopamine release events measured with voltammetry are often called transients. They occur spontaneously, as well as in response to electrical stimulation of dopamine cell bodies, administration of drugs, and behaviorally salient events such as presentation of an unpredicted reward (Garris et al., 1997; Roitman et al., 2004; Aragona et al., 2008). These transients almost certainly result from bursting of dopamine neurons as burst firing has been shown to be both necessary and sufficient for the production of dopamine transients (Tsai et al., 2009; Zweifel et al., 2009). However, frequency of burst events in dopamine neurons at baseline (~1 every 2-3 s) is generally higher than the frequency of spontaneous dopamine transients (1 every 1-5 minutes) therefore it is unlikely that each observed transient is the result of a burst event in a single neuron. This discrepancy may be resolved by considering that most release events resulting from burst events of single neurons occur under the detection threshold of voltammetry. Instead, observed dopamine transients likely result from synchronous or overlapping burst events in several neurons. This concomitant activation occurs in situations that robustly evoke dopamine transients (e.g. reward presentation or electrical/optogenetic stimulation). Finally, the number of observed transients is also limited by tightly controlled re-uptake mechanisms that prevent dopamine diffusing to the voltammetry electrode; psychostimulants increase transient frequency due, at least in part, to the blockade of this re-uptake (Venton and Wightman, 2007; Aragona et al., 2008; Park et al., 2011).
Another area of debate concerns the origin of tonic extracellular dopamine. Although difficult to measure precisely, the tonic level of extracellular dopamine is thought to be in the low nanomolar range. Recent studies indicate that this concentration is mostly made up of phasic dopamine release events that are averaged across time, rather than resulting from neurotransmitter release from single spikes (Owesson-White et al., 2012). There are several reasons why single spike activity is unlikely to lead to significant dopamine release including low pre-synaptic probability of release and tight control of dopamine diffusion by dopamine re-uptake mechanisms
4.3 Heterogeneity across recording conditions and state of wakefulness
The state of arousal/wakefulness of the subject, including depth of anesthesia if any, is a potential contributor to heterogeneity of dopamine cell activity. Activity is driven, for the most part, by afferents arising from a wide range of structures; therefore, any alteration in the input from these structures caused by anesthesia is likely to influence firing rate and pattern. In addition, as described below, the proportion of studies conducted in anesthetized vs. non-anesthetized animals varies greatly across species, which may often confound attempts to compare similar studies or even lead to ‘species differences’ being invoked as a source of variance across studies. Due to this imbalance, the section below will concentrate on studies performed in rats across differing states of wakefulness.
In vivo, anesthetized
The predominant preparation used to assay dopamine neuron activity is in vivo under anesthesia. A number of anesthetics have been used to conduct these studies, although chloral hydrate is by far the most popular, due to its preservation of busting activity, which is assumed to reflect intact synaptic input. This is in contrast to an anesthetic such as ketamine, which although routinely used for surgical procedures, is rarely used in studies of the dopamine system due to its blockade of NMDA receptors; NMDA receptors are necessary for burst firing of dopamine neurons (Overton and Clark, 1997). One consideration with the prominent use of chloral hydrate is that one of its primary metabolite, trichloroethanol, has been shown to have an excitatory effect on dopamine cell firing (Appel et al., 2006).
Under chloral hydrate anesthesia, the average firing rate in the VTA of adult male rats is, as reported previously, ~4.5 Hz and ~30% of the spikes are emitted in bursts (Grace and Bunney, 1984b; Marinelli et al., 2006), although this can vary across studies. Even under chloral hydrate, however, depth of anesthesia (light vs. deep) has a profound effect on firing rate and the propensity to burst (Fà et al., 2003). This might be of particular concern in situations in which experimental groups might differ in their response to the same anesthetic (e.g. across ages or following exposure to drugs or stress). For this reason, wherever possible depth of anesthesia should be closely monitored and regulated to ensure consistent depth of anesthesia (for example by monitoring body temperature, breathing rates, or EEG activity).
Urethane has been another popular choice when studying dopamine neuron activity. Under urethane, neurons fire more slowly and exhibit less bursting than under chloral hydrate (Tepper et al., 1995; Paladini and Tepper, 1999; Pistis et al., 2004). For example, in adult male rats, firing rate under urethane is approximately 2.7 Hz, and bursting less than 16% (Pistis et al., 2004). However, once anesthetized, depth of anesthesia fluctuates very little under urethane so recordings may be stable for long periods and the need for close monitoring is reduced (Flecknell, 1987).
Isoflurane has been used more recently and under this anesthetic, based on the limited amount of available data, firing rates are perhaps slightly higher than those seen under chloral hydrate anesthesia (i.e. 5.0 Hz vs. 4.5Hz), and bursting is slightly more (i.e. 45% of spikes emitted in bursts, vs. about 30%) (Panin et al., 2012).
In vivo, awake and immobilized
Several studies have made in vivo recordings from animals that are un-anesthetized but immobilized by some other means. Of these, paralysis with an agent such as d-tubocurarine has been commonly performed. Using this technique, differences have been observed in responses of dopamine cells when compared with anesthetized preparations. An example is the finding by one group that depolarization block induced by neuroleptics, which is seen in chloral hydrate anesthetized animals, is not seen when rats are anesthetized with a variety of paralyzing agents (Mereu et al., 1995; Melis et al., 1998). Results such as this question if recordings made in anesthetized subjects are representative of the un-anesthetized subject. However, criticisms have also been made of experiments in paralyzed animals, as stress which can easily be induced in paralyzed rats is also a potent modulator of dopamine cell firing and may confound results (Lodge and Grace, 2011).
More recently, to circumvent the stress-related effects associated with paralysis, a few studies in rodents have immobilized subjects after repeated handling and habituation (Fà et al., 2003; Dahan et al., 2007). It is worth noting that this technique, although rare in rodents, is the method of choice in non-human primate studies. Using this technique in rats, Dahan and colleagues (2007) examined dopamine neuron firing across sleep/wake cycles. They reported firing properties consistent with those reported in freely-moving and choral hydrate anesthetized rats. Thus, during wakefulness, firing rate of dopamine neurons was approximately 4 Hz and the percentage of spikes in burst was approximately 30%. Results were similar during slow-wave sleep. However, during REM sleep, bursting increased dramatically. In a different study, electrophysiological and pharmacological properties of dopamine neurons were compared explicitly across different in vivo conditions: conscious, head-restrained vs. lightly and deeply chloral hydrate anesthetized (Fà et al., 2003). Although similar average firing rate was found across preparations, the number of spontaneously active cells and the percentage of bursting neurons was higher in un-anesthetized vs. anesthetized conditions. The authors concluded that chloral hydrate anesthesia suppresses excitatory input to dopamine neurons that normally determines the number of spontaneously active cells and their bursting activity.
In vivo, awake and freely moving
The studies with the most relevance to the physiological role of dopamine cells are likely to be those conducted in awake, behaving subjects. Several groups have recorded from mice and rats although technical challenges (targeting ventral structures, relatively low density of dopamine cells) mean there are fewer studies than in other regions such as the cortex, hippocampus, or NAc. When comparing across preparations, another consideration is that analysis of baseline or ‘at rest’ firing rates are often absent from these reports. In general, analysis focuses on modulation of spike rate from baseline. Whether such a ‘baseline' exists in these studies is debatable, as in most tasks there might be effects on firing caused by the preceding or succeeding trials.
In a seminal study by Hyland and colleagues, the authors explicitly compared firing patterns obtained in their recordings of freely-moving rats with those from chloral hydrate anesthetized subjects (Hyland et al., 2002). They concluded that, in general, neurons responded similarly in awake rats as they did in anesthetized preparations: cells showed similar firing patterns. Moreover, baseline firing rates were similar to what is observed in most anesthetized preparations: 3.7 Hz in one study, Hyland et al. (2002), and 4.6 Hz in another (Pan and Hyland (2005); the percentage of spikes emitted in bursts was also similar across conditions. It was noted that the proportion of doublets (a burst of two spikes) to bursts containing three or more spikes was higher in awake rats than in anesthetized rats; consequently the intraburst frequency was also elevated in awake vs. anesthetized conditions (Hyland et al., 2002);this is because the first two spikes in a burst usually have a shorter ISI compared with that of subsequent spikes in the burst; therefore, bursts of two spikes have higher intraburst frequency than bursts of more than two spikes. Other studies that have conducted a full analysis of baseline properties of cells (Anstrom and Woodward, 2005; Puryear et al., 2010; Martig and Mizumori, 2011) report similar results. However, the majority of studies recording dopamine neurons in awake rodents have focused on modulations by significant events, such as reward presentation or operant response; therefore determining how similar baseline firing properties are to the anesthetized preparation is challenging (Roesch et al., 2007; Jin and Costa, 2010; Kim et al., 2010, 2012; Cohen et al., 2012; Jo et al., 2013).
Ex vivo
In tissue slices, dopamine neurons fire very differently compared with the intact animal. In general, this means that they fire with lower frequency (e.g. 1-3 Hz) and do not exhibit bursts. This is believed to be due to the inputs to dopamine neurons being severed, in particular excitatory glutamatergic inputs from regions that drive firing in vivo (Kitai et al., 1999).
Several groups have tried to reproduce bursting in ex vivo preparations, so as to better understand the mechanisms underlying this phenomenon. This is described in more detail in another contribution to this special issue (Roeper, 2014).
Regardless of the method, inducing burst events in the tissue slice does not faithfully reproduce the firing patterns seen in vivo. In the tissue slice, burst events tend to be long, with numerous spikes/burst. Furthermore, bursts are isolated events among pacemaker (regular) firing activity; in other words, the cell clearly ‘switches’ from one mode of firing to the other. Instead in vivo, irregular firing is interspersed with short burst events, without marked ‘switching’ (Figure 3).
Figure 3.
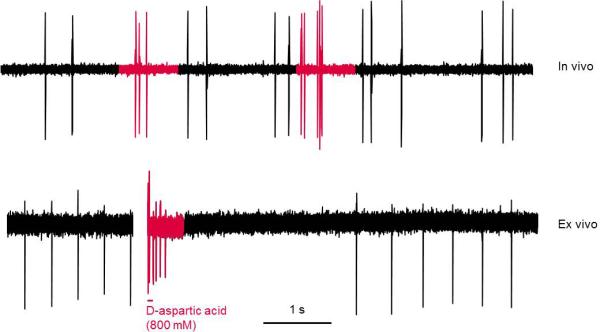
Dopamine neurons burst differently between in vivo and ex vivo preparations. Top panel: representative trace showing spontaneous bursting of a dopamine neuron in vivo in a chloral hydrate anesthetized rat. Bottom panel: representative trace showing bursting of a dopamine neuron recorded in cell-attached configuration in the ex vivo tissue slice preparation. Bursting was induced by iontophoretic application of D-aspartic acid in the presence of glycine in the perfusion bath. In vivo, firing activity consists of irregular single spikes interspersed with short (2-5) spike bursts. In contrast, in ex vivo slices dopamine neurons fire in an extremely regular pacemaker-like fashion with induced bursts consisting of many spikes and being followed by a long post-burst pause. In vivo trace collected in our lab; ex vivo trace provided by Dr. Michael Beckstead.
4.4 Heterogeneity across species
Inter-species comparisons are difficult because different species are usually recorded differently by different labs. In addition, different structures are often recorded, or different analyses are performed. For example, monkeys are usually awake, habituated, head-restrained, whereas rodents are typically anesthetized. Most studies in monkeys report data from both SNc and VTA and they do not always analyze the two structures separately. In contrast, studies in rodents usually separate the two areas (see below). With respect to firing patterns in monkeys, bursting and non-bursting activity is not typically analyzed in a formal way as it has been in the rat. Thus, it is not known whether the amount of bursting and characteristics of each burst are similar across non-human primates and rodents.
Comparisons across rodents (i.e. mice vs. rats) are also difficult. Not only are studies performed in different labs, but they are also performed in different conditions and with different anesthetics. Studies in rats tend to be performed in young-adult males. Furthermore, rats are sometimes housed in isolation (i.e. singly-housed). Instead, studies in mice are sometimes performed in both males and females, and over a large age-span (usually from adolescence until late adulthood); also, mice are usually housed in groups (i.e. group-housed).
In fact, firing rates of dopamine neurons in mice vary dramatically across the literature. Some studies show firing rates of 0 Hz (i.e. inactive neurons, which only became active upon stimulation; Tsai et al., 2009), whereas others show very high firing rates of up to 10 Hz (Stamatakis and Stuber, 2012). These differences could be due to differences in anesthetic or recording conditions, as well as the region that is being recorded. Furthermore, studies are often performed in genetically-modified mice, or mice that have received optogenetic manipulations, which could all influence basal activity of dopamine neurons.
We compiled recordings made in our lab over several years, to examine the average firing rates of adult rats and adult mice under similar recording conditions (chloral hydrate anesthesia). We found that, on average, the firing rate in group-housed mice (3.6 Hz) is slightly lower than that of group-housed rats (4.2 Hz). This could be due to greater sensitivity of mice to D2-mediated autoinhibition of neuronal firing (Courtney et al., 2012). We also found that the firing rate of singly-housed rats is slightly higher still (4.6 Hz). In fact the Gaussian distribution of firing rates that is classically described in the literature (Grace and Bunney, 1984a) is shifted across species and recording conditions (Figure 4). We also found that mice exhibit less bursting activity than rats (approximately 8-10% of spikes emitted in bursts, vs. approximately 30%). In another study, mice also showed lower bursting than rats; however they showed higher firing (Panin et al., 2012).
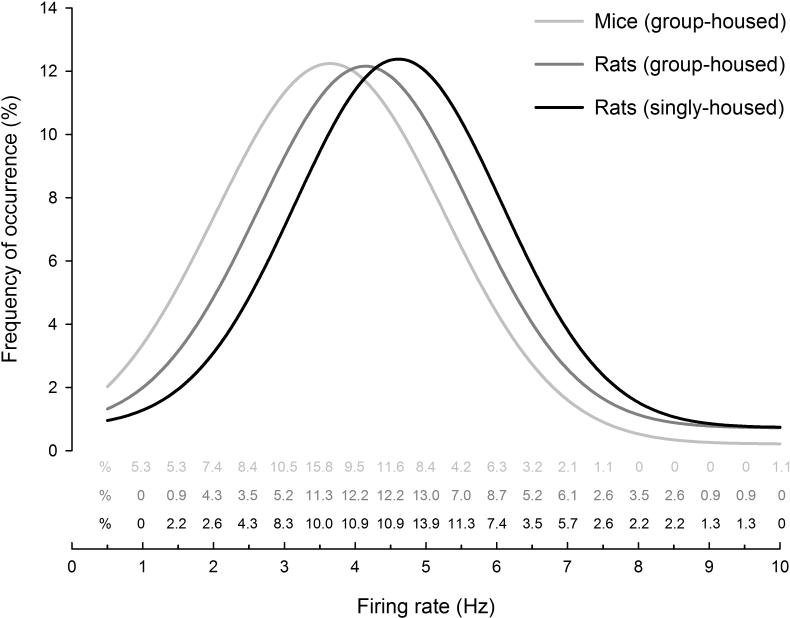
Figure 4.
Firing rate comparison between mice, rats, and housing conditions. Firing rate distributions of VTA dopamine neurons from mice (group-housed) and rats (group-housed or singly-housed) recorded in vivo under chloral hydrate anesthesia. Although there is significant overlap, mice (group-housed) show lower average firing rate than rats (group-housed or singly-housed) [ANOVA between-subjects effect for Condition, comparing mice (group-housed), rats (group-housed), and rats (singly-housed), F2,437=12.40, p<0.001, Newman Keul's post-hoc test: mice (group-housed) vs. rats (group-housed), p=0.01; mice (group-housed) vs. rats (singly-housed), p<0.001; rats (group-housed) vs. rats (singly-housed), p=0.06)]. Data are binned in 0.5 Hz intervals and values are shown as text above the x-axis. Curves are fitted to a peak Gaussian distribution: r=0.95, F=49.2, p<0.001 for mice (group-housed), r=0.95, F=44.2, p<0.001; r=0.97 for rats (group-housed), and F=81.6, p<0.001for rats (singly-housed). Data collected across studies in our lab; group-housed mice: male and female adults (2-4 months), n=95 cells (male, n=67, mean=3.7 ± 0.2 Hz; female, n=28, mean=3.2 ± 0.3Hz, ); group-housed rats: male adults (3-4 months), n=115 cells (mean=4.2 ± 0.2 Hz); singly housed rats: male adult (2-4 months), n=230 cells (mean=4.6 ± 0.08 Hz). On average 2-4 cells, and no more than 6, were recorded from each animal.
4.5 Heterogeneity across regions
Firing rates are similar across the VTA and SNc (Clark and Chiodo, 1988; Grenhoff et al., 1988), although our lab noted slightly, yet not always significantly, higher firing rates in the VTA vs. SNc from young adult singly-housed rats (Table 1, Figure 5). This was also noted in another study (Zhang et al., 2008). The amount of bursting, on the other hand, is higher in the VTA vs. SNc (Clark and Chiodo, 1988; Grenhoff et al., 1988; Zhang et al., 2008; Panin et al., 2012) (Table 1, Figure 5). Similarly, burst size is slightly larger in the VTA vs. SNc (Table 1). Mechanisms for this difference are described in another contribution to this special issue (Roeper, 2014) but likely involve differential expression of a number of proteins including D2 autoreceptors, the calcium binding protein, calbindin D-28, and hyperpolarization-gated cation channels (HCN) (Neuhoff et al., 2002; Lammel et al., 2008). Within the SNc, one study reported higher firing rates in the rostral SNc vs. the caudal SNc (Shepard and German, 1984). Within the VTA, the firing of dopamine neurons that project to the PFC is higher than that projecting to the striatal complex, probably because neurons projecting to the PFC lack D2-class autoreceptors, which are responsible for auto-inhibiting the activity of dopamine neurons (Bannon and Roth, 1983; Chiodo et al., 1984; White and Wang, 1984; Lammel et al., 2008; though see Gariano et al., 1989). Neurons projecting to the PFC only represent a small percentage of midbrain dopamine cells (see White and Wang, 1984).
Table 1.
Regional differences in dopamine neuron firing. Firing rates and patterns for dopamine neurons in VTA and SNc recorded in vivo, in chloral hydrate anesthetized rats. Rats were young-adults (2-3 months) and singly-housed. Relative to SNc neurons, VTA neurons show a trend to firing faster, emit more spikes in bursts (% of spikes emitted in bursts), exhibit a greater proportion of burst events (burst event frequency), and exhibit more spikes per burst. Data from Marinelli and White (2000).
| VTA (n=106) | SNc (n=113) | T test value | p-value | |
|---|---|---|---|---|
| Firing (Hz) | 4.6 ± 0.2 | 4.2 ± 0.17 | 1.52 | = 0.13 |
| % of spikes emitted in bursts | 39.5 ± 2.5 | 25.6 ± 1.9 | 4.44 | < 0.001 |
| Burst event frequency (Hz) | 0.63 ± 0.04 | 0.45 ± 0.04 | 3.17 | < 0.01 |
| Spikes per burst | 2.96 ± 0.09 | 2.48 ± 0.06 | 4.52 | < 0.001 |
Figure 5.
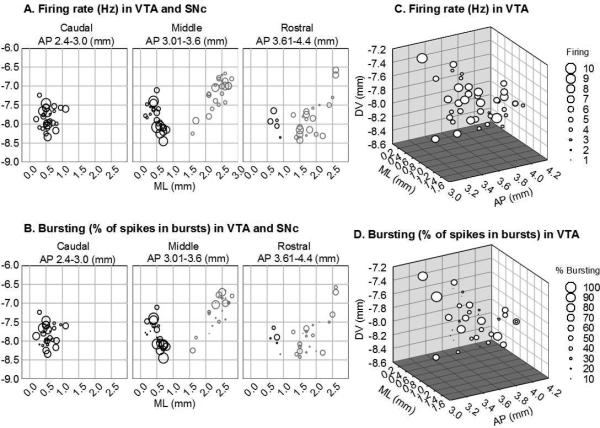
Activity of dopamine neurons within the VTA and SNc. (A,B) Scatter plots showing (A) firing and (B) bursting of dopamine neurons with respect to location in midbrain (VTA and SNc); activity is coded by marker size. VTA neurons are black; SNc neurons are grey. Left panels show caudal sections, middle panels show middle sections, and right panels show rostral sections. VTA neurons burst more than SNc neurons (T98=3.52, p<0.001), and they have slightly higher firing rates (T98=2.17, p<0.05). Within each region (VTA or SNc), there is no regional distribution in firing rate (multiple regression between firing rate and AP, ML, and DV for VTA: r=0.18, F3,51=0.58, n.s; for SNc: r=0.14, F3,41=0.26, n.s.) or bursting activity (multiple regression between bursting activity and AP, ML, and DV for VTA: r=0.16, F3,51=0.47, n.s; for SNc: r=0.21, F3,41=0.66, n.s.) (C,D) Three dimensional scatter plot showing (C) firing and (D) bursting of dopamine neurons with respect to location in the VTA; activity is coded by marker size. Within the VTA, there is no regional distribution in firing rate (multiple regression between firing rate and AP, ML, and DV: r=0.30, F3,39=1.33, n.s.) or bursting activity (multiple regression between bursting activity and AP, ML, and DV: r=0.27, F3,39=1.02, n.s.). Measurements are in mm; AP and ML are from interaural line; DV is from surface of cortex. Neurons were recorded in vivo under chloral hydrate anesthesia from two separate experiments. (A & B): singly-housed young-adult (2-3 months) male rats, extracted from Marinelli and White (2000); (C & D): group-housed adolescent (42-46 days) and adult (3-4 months) male rats, extracted from Wong et al (2013).
Recently, the idea of segregated pathways within the VTA has gained prominence (Roeper, 2013). Lammel and colleagues (Lammel et al., 2008) showed that dopamine neurons projecting to the medial PFC, NAc core and medial shell cluster in the medial posterior VTA, and are predominantly located in the medial paranigral nucleus of the VTA and the medioventral parabrachial nucleus of the VTA. In contrast, neurons projecting to the NAc lateral shell are more scattered throughout the parabrachial nucleus of the VTA and they are mostly absent from the paranigral nucleus.
Inputs to VTA neurons are also segregated (Lammel et al., 2012). Thus, the LDTg predominantly projects to VTA neurons that send axons to NAc shell; on the other hand, the lateral habenula predominantly projects to VTA neurons that project to medial PFC. Interestingly, these different pathways exhibited stimulus-specific plasticity with VTA-NAc shell connection being strengthened by exposure to cocaine and VTA-mPFC pathway being strengthened by an aversive stimulus, cold water swim (Lammel et al., 2011).
We analyzed our in vivo anesthetized data to test if there is regional diversity in firing rate or pattern within the midbrain. We did not note any apparent differences in firing rates or bursting activity within different regions of the VTA (Figure 5). This suggests that, despite the abovementioned segregation, each VTA region contains neurons that do not exclusively project to, or receive input from, a single/specific brain region. Thus, although there may be a tendency for neurons projecting to a particular area to fire in a certain manner and be localized to one sub-region of the VTA, these tendencies are not absolute and, consequently, there is no clear demarcation between one population and another.
It is worth noting that the extent to which sampling regions overlap across techniques is uncertain and difficult to fully assess. In vivo studies usually report recording coordinates, whereas ex vivo studies predominantly illustrate location with a diagram or photograph, in which it is often difficult to evaluate the precise coordinates. With these caveats in mind, it appears that recordings in vivo are usually close to the midline (~0.4-1.0 mm from the midline), whereas recordings ex vivo tend to be more lateral (~0.8-1.5 mm from the midline), just medial to the medial terminal nucleus of the accessory optical tract (MT). These differences are illustrated in Figure 6.
Figure 6.
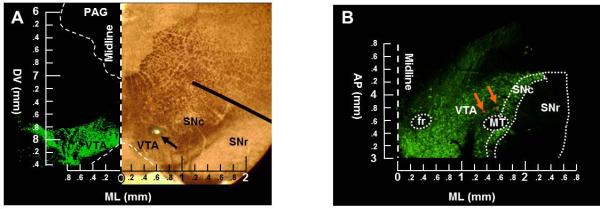
Differences in VTA sampling location between in vivo and ex vivo studies. (A) Example of a coronal tissue slice examined under a bright-field microscope after in vivo extracellular recording from our lab (right image). Arrow denotes the recording location. Left image shows a section processed for tyrosine hydroxylase immunoreactivity (green), as a marker for dopamine neurons, and photographed with a fluorescent microscope. Coordinates of the recorded neuron are AP3.4, ML0.6, DV7.9. Measurements are in mm; AP and ML are from interaural line; DV is from surface of cortex. (B) Example of a horizontal tissue slice processed for TH immunoreactivity (green) after ex vivo whole-cell patch clamp recording and photographed with a florescent microscope. Recorded cells were filled with neurobiotin (red). Orange/yellow represents overlay, indicating that the recorded neurons were dopaminergic. Coordinates for these neurons are AP3.7, ML1.3, DV8.2 for one neuron and AP3.8, ML1.5, DV8.2 for the other neuron. Measurements are in mm; AP is from interaural line and DV is from surface of cortex. PAG, periaqueductal gray; SNr, substantia nigra pars reticulata; fr, habenulo-interpeduncular tract; MT, medial terminal nucleus of accessory optic tract.
4.6 Heterogeneity across ages
A few studies have examined how dopamine neuron firing changes during development. Firing of dopamine neurons increases from birth to postnatal day 28-35 (Pitts et al., 1990; Tepper et al., 1990; Choong and Shen, 2004), when autoreceptors become functionally mature (Wang and Pitts, 1995). Separate studies show that dopamine neuron activity declines progressively during adulthood (Freeman et al., 1989; Lavín and Drucker-Colín, 1991). Indeed, we recently performed a comprehensive analysis of dopamine cell firing from weaning to adulthood and showed that dopamine neuron activity displays an inverted-U-shaped curve, with a peak around puberty (postnatal day 45) (McCutcheon and Marinelli, 2009; McCutcheon et al., 2012a), see Figure 7. Bursting amount and characteristics, on the other hand, do not vary consistently across ages. Age-differences in neuronal activity are important to recognize because in vivo studies use young-adult and adult animals whereas ex vivo studies frequently use pre-pubertal rodents, which are often mislabeled as ‘adults’ (McCutcheon and Marinelli, 2009). Such age differences are also of relevance due to their potential role in psychiatric disorders; several disorders either peak or exhibit their onset during adolescence/puberty (Paus et al., 2008). For example, in rats, we found that the elevated firing rate observed during adolescence is associated with an increased propensity to acquire self-administration of cocaine in these animals. Administering quinpirole, which is known to reduce firing rates, reduces drug intake in adolescents to levels seen in adults; instead, administering eticlopride, which is known to enhance firing rates, increases intake in adults to levels seen in adolescents (Wong et al., 2013).
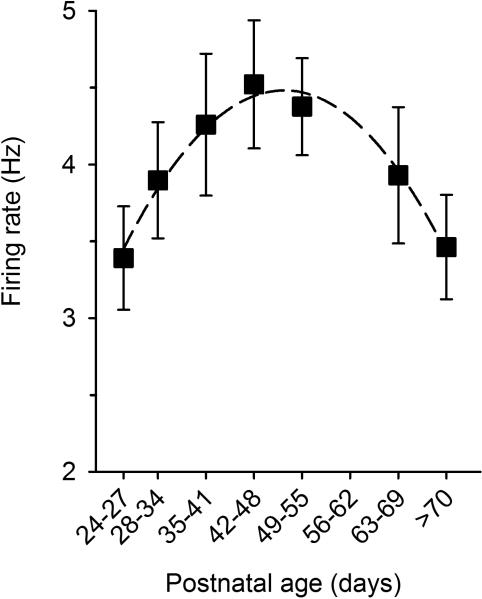
Figure 7.
Firing rates of VTA dopamine neurons across ages. Dopamine neurons were recorded in vivo in group-housed male rats under chloral hydrate anesthesia. Firing rate is low soon after weaning, it peaks around puberty (postnatal day 45), and decreases thereafter. Values are mean ± SEM. Data from Marinelli and McCutcheon (2009).
4.7 Heterogeneity across individuals
Dopamine neuron activity also varies across individuals and this variability is correlated with behavioral differences. This was shown most prominently in studies of ‘high-responder’ vs. ‘low-responder’ rats. Outbred rats can be classified as high-responders (HRs) or low-responders (LRs) based on their locomotor response to a novel environment: HRs show greater locomotion, relative to LRs. This classification is associated with several other phenotypes, including propensity to acquire self-administration of psychostimulants (Piazza et al., 1990; Cain et al., 2004), opiates (Ambrosio et al., 1995), and ethanol (Nadal et al., 2002). Dopamine neuron activity and bursting is also associated with this designation, with HRs showing elevated firing and bursting relative to LRs (Marinelli and White, 2000). This elevated activity results in elevated dopamine efflux in forebrain regions (Hooks et al., 1992). Analysis of firing rate distribution in the study by Marinelli and White (2000) showed that both HRs and LRs exhibit a Gaussian distribution of firing rates; however, the curve is rightward-shifted in HRs, relative to LRs. This suggests that the elevation in firing rate observed in HRs results from a generalized increase in activity across the entire neuronal population rather than being due to a specific effect on one sub-population of cells (e.g. PFC-projecting neurons).
A general increase in dopamine neuron activity is also observed in alcohol-preferring rats (Morzorati, 1998; Morzorati and Marunde, 2006). Together, these studies suggest that elevated dopaminergic activity is associated with propensity to self-administer drugs of abuse.
5. DOPAMINE NEURON ACTIVITY IN RESPONSE TO ‘LIFE EVENTS’
The activity of dopamine neurons is modulated by different types of life event including exposure to addictive drugs, environmental stimuli, and stress. In studies that have analyzed firing patterns, these changes can involve either non-bursting, bursting, or both.
5.1 Heterogeneity in response to addictive drugs
The dopamine system has long been implicated in the neural and behavioral response to addictive drugs. For several decades it has been known that all addictive drugs increase dopamine tone in the forebrain (Di Chiara et al., 1992). However, the exact processes that contribute to this increase vary depending on drug and are still under debate. As highlighted below, the increase in dopamine concentration does not necessarily reflect an elevation in dopamine neuron activity. While considering these studies, it is also important to note that the effects of acute drugs are often very different to effects of repeated drugs or to the effects at different time points after discontinuation from the drug treatment (i.e. without drugs ‘on board’). As such, it is essential to use terminology about ‘effects of drugs’ in a precise manner so that the effects of drug on board are discriminated from the consequences of discontinuation.
5.1.1 Psychostimulants
The main target of psychostimulants is the dopamine transporter (DAT). According to traditional models, blockade of DATs results in increases in extracellular dopamine, which in turn activates somato-dendritic dopamine D2-class autoreceptors and hyperpolarizes the membrane (Wang, 1981). Thus, acute administration of psychostimulants decreases cell firing as has been shown with cocaine (Einhorn et al., 1988; Brodie and Dunwiddie, 1990; Lacey et al., 1990; Bunney et al., 2001), amphetamine (Rebec and Segal, 1978), methylphenidate (Rebec and Segal, 1978; Federici et al., 2005), and methylenedioxymethamphetamine (MDMA) (Kelland et al., 1989; Matthews et al., 1989; White et al., 1996).
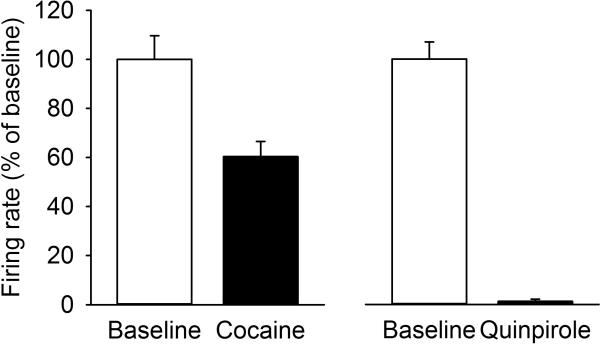
However, the suppression of firing rate following psychostimulants is only partial and not as complete as is seen after agonist activation of D2-class autoreceptors (Figure 8). This suggests psychostimulants might also excite dopamine neurons via other mechanisms. In fact, psychostimulants have effects on activity of dopamine cells that are independent of their effects on dopamine re-uptake. For example, if the dopamine-mediated suppression of firing is blocked, amphetamine excites dopamine neurons via an adrenergic mechanism (Shi et al., 2000). At high doses, cocaine has been shown to disinhibit dopamine neurons by blocking voltage-gated sodium channels in VTA GABA neurons (Steffensen et al., 2008) and cocaine may also suppress GABAergic input to dopamine cells from the RMTg (Lecca et al., 2011). Methamphetamine also has dose-dependent effects on firing rate whereby a low dose of drug increases firing rate via a transporter-mediated depolarizing current (Branch and Beckstead, 2012). Finally, amphetamine may modulate dopamine cell activity indirectly via its effects on dopamine cell inputs arising from other brain regions (Bunney and Aghajanian, 1977; Paladini et al., 2001; Paladini and Williams, 2004; Shi et al., 2007).
Figure 8.
Cocaine and D2-class autoreceptor agonist (quinpirole) differentially decreases the firing rate of dopamine neurons. Cocaine decreases firing rate to approximately 60 % of initial firing, whereas quinpirole silences firing completely [ANOVA between-subjects effect for Drug, comparing cocaine and quinpirole: F1,48=18.91, p<0.001; within-subjects effect for time, comparing pre-drug (i.e. baseline) and post-drug F1,48=140.08, p<0.001; interaction Drug × Time F1,48=25.51, p<0.001). Drugs were administered intravenously through the tail vein (Cocaine as a bolus dose of 1 mg/kg and quinpirole as a cumulative dose of 1 mg/kg). Baseline is the average firing rate over 3 minutes of recording; drug response is the average firing rate over one minute at the time of maximal suppression (1 min post cocaine, 11 min post quinpirole). Dopamine neurons were recorded in vivo, in singly-housed adult (2-3 months) male rats under chloral hydrate anesthesia. n=20 cells from 20 rats for cocaine; n=30 cells from 30 rats for quinpirole. Values are mean ± SEM.
In awake behaving animals the effects of psychostimulants may also differ. A recent report showed that acute cocaine does not decrease cell firing in awake subjects, but it does in anesthetized subjects (Koulchitsky et al., 2012). Furthermore, using voltammetry, cocaine has been shown to increase phasic dopamine release events, likely by increasing bursting activity. Interestingly, this effect is only observed in NAc shell and not NAc core, suggesting regional heterogeneity in some effects of drugs (Aragona et al., 2008).
In contrast to the effect of acute administration, discontinuation from psychostimulant exposure increases dopamine neuron activity. Thus, a transient increase in firing frequency is observed after discontinuing psychostimulant exposure in models of self-administration and experimenter-administered drug (White and Kalivas, 1998; Marinelli et al., 2003). This ‘rebound increase’ in activity is most apparent for non-bursting activity, which is elevated on days 1-3 after discontinuing cocaine exposure and, to a lesser extent, for amount of bursting activity, which is elevated only on day 1 after discontinuing cocaine exposure. In situations with elevated bursting, both the frequency of the burst events and the characteristics of the bursts are affected, reaching up 5-7 spikes on average, and emitted as frequently as every 0.4-0.5 s (every 1.1 s on average) (Marinelli et al., 2003).
Behaviorally, drug-taking persists long after firing rate and bursting return to baseline so the changes seen during withdrawal are not thought to be a direct correlate of addiction. Instead, the elevation in activity is believed important for driving plasticity in the forebrain, particularly NAc, that is essential for long-lasting drug-associated memories (Wolf, 1998; Mameli et al., 2009). In support of this view, the persistence of enhanced dopamine neuron activity is greater in individuals with increased propensity to acquire cocaine self-administration (high-responder rats) (McCutcheon et al., 2009).
5.1.2. Other drugs (opiates, alcohol, nicotine)
Oppositely to what is seen with psychostimulants, most other drugs increase dopamine neuron activity when administered acutely and decrease dopamine cell activity when they are discontinued. This applies to opiates (Gysling and Wang, 1983), alcohol (Mereu et al., 1984; Brodie et al., 1999), nicotine (Lichtensteiger et al., 1982; Mereu et al., 1987), and cannabinoids (Gessa et al., 1998). The mechanisms underlying these effects are varied. With opiates, conventionally, the increase is thought to be due to activation of µ opioid receptors on GABAergic neurons within the VTA, or possibly the RMTg, which provide constitutive inhibitory input to dopamine cells; their inhibition through µ opioid receptor activation, results in a disinhibition of dopamine cells (Johnson and North, 1992; Melis et al., 2000; Lecca et al., 2011, 2012). However, recent studies suggest that opiate action on dopamine cells is more complex, and that there is heterogeneity across the population of dopamine neurons, which is related to projection targets (Ford et al., 2006; Margolis et al., 2008).
5.1.3. Role of dopamine in drug-seeking and craving
As drugs can have a variety of effects on dopamine neurons (excitatory and inhibitory) depending on the type of drug, the dose, and whether it is ‘on-board’ or has been discontinued, it is worth considering which aspect of dopaminergic transmission could promote drug craving. A suggestion has been that drug craving and relapse in the addicted individual could be the result of two separate phenomena: ‘chronic craving’ and ‘instant craving’. These could be caused by a reduced and elevated dopaminergic tone, respectively (Pilla et al., 1999; Childress and O'Brien, 2000; Franken et al., 2005). In the first case, a hypodopaminergic state would promote the search for drug, so as to counteract the decrease in dopaminergic tone. At the same time, this hypodopaminergic state could decrease an individual's interest in stimuli that are not related to drugs, thus making the drive for drugs even stronger (Volkow et al., 2002; Melis et al., 2005). It is therefore possible that that discontinued exposure from opiates and alcohol could induce this state, as could certain aversive stimuli. On the other hand, ‘instant craving’ could be caused by an elevation in dopaminergic transmission, which acts like a trigger to precipitate relapse or enhance the drive to the drug (Grace, 2000; Leyton et al., 2002; Oswald et al., 2005). In this case, ‘at risk’ individuals, and subjects exposed to stress, cues, or drug re-exposure, could be in this category.
5.2 Heterogeneity in response to brief environmental stimuli
Many dopamine neurons respond to environmental stimuli. Early observations were that dopamine neurons showed short-latency responses to many types of events including presentation of rewarding, aversive, or salient stimuli. In addition, the discovery that responses of dopamine neurons were dynamic and changed as animals learned predictive associations between stimuli generated a great deal of interest in dopamine as ‘teaching signal’ (Schultz et al., 1997). Other interpretations were that dopamine encoded ‘sensory salience’ (Redgrave et al., 1999), or arousal, so as to facilitate goal-directed behaviors (Horvitz, 2000) and allow orienting attention and behavior towards stimuli that would otherwise go unnoticed (Joseph et al., 2003; Redgrave and Gurney, 2006). Recent work has shown a more nuanced participation of dopamine in signaling different types of stimuli as is outlined below.
5.2.1 Rewarding stimuli
Responses of dopamine neurons to natural rewards are fairly homogenous. That is, if cells respond to a rewarding stimulus they almost always do so by increasing their firing rate (Schultz, 1998; Joshua et al., 2008; Matsumoto and Hikosaka, 2009; Cohen et al., 2012). However, this simple description excludes some important considerations and ways in which reward-related responses exhibit heterogeneity. First, although reward-evoked decreases are very rarely seen, not all cells increase firing to reward. In fact, an estimate taken from several key papers recording midbrain dopamine neurons in monkeys is that ~60-80% of neurons are reward-responsive; thus, a sizeable proportion do not respond to reward (Mirenowicz and Schultz, 1996; Tobler et al., 2003; Joshua et al., 2008; Matsumoto and Hikosaka, 2009). Second, as rewards become associated with cues, dopamine neurons start responding to the predictive cues and stop responding to the rewards themselves (Schultz et al., 1997). Thus, the response to the reward is heterogeneous across time, although the response to prediction error is stable. Lastly, the magnitude of dopamine neuron responses to rewards is strongly modulated by multiple factors including reward magnitude, probability, delay, and ambiguity (Fiorillo et al., 2003; Tobler et al., 2005; Roesch et al., 2007; Bromberg-Martin and Hikosaka, 2009).
As explained previously, a reward-associated increase in dopamine neuron activity is often referred to as a ‘burst in activity’; however this typically refers to a short-lasting increase in neuronal firing, not bursting (Schultz et al., 1997). In fact, bursting activity ‘proper’ is seldom examined in these studies; rewarding stimuli are usually of short duration, and changes in bursting activity can be difficult to measure or interpret over brief periods.
Experiments using microdialysis and voltammetry to measure dopamine neuron output have widely supported these key electrophysiological findings by showing that rewarding stimuli, whether primary or conditioned, elevate dopamine levels in terminal regions (Bassareo and Di Chiara, 1999; Roitman et al., 2004; Day et al., 2007).
5.2.2. Aversive stimuli
Relative to rewarding stimuli, there is greater heterogeneity in the responses of dopamine neurons to aversive stimuli. Furthermore, the types of aversive stimuli studied vary a great deal in their magnitude and range from mild air puffs to electric shocks. Here, we describe the effects of brief (on the order of seconds), acute aversive stimuli; long-term exposure to these stimuli may be considered stressful and will be dealt with in section 5.3 in more detail.
Many different aversive stimuli have been used, which range from mild to noxious. The most commonly used stimuli are mechanical in nature and include pinches or pricks to the tail or the foot. Using these stimuli, increases in firing rate have been evoked in between 0-67% of neurons and decreases in firing rate have been evoked in between 11-83% of neurons, with all but one of the studies (Ungless et al., 2004) finding both types of response (reviewed in McCutcheon et al., 2012b). Interestingly, in Ungless et al. (2004), the only cells that were excited by the aversive stimulus were non-dopaminergic, suggesting that neurochemical phenotype might be an important factor. However, a later study by the same group showed that an aversive stimulus excites dopamine cells that are located ventrally within VTA, suggesting anatomical heterogeneity (see below). Another commonly used type of stimulus is air puffs to either face or limb. Again, a range of responses have been observed with increases noted in 11-49% of neurons and decreases in 0-51% of neurons. The slightly lower numbers of responsive cells, relative to mechanical stimuli, may reflect the milder nature of this type of stimulus or its reduced duration. A number of studies have used cues predicting an aversive stimulus and these too find a range of responses with increases seen in 3-50% of cells and decreases seen in 14-60% of cells. In addition to being classified as simply increases or decreases some researchers have described more complex responses such as biphasic responses (e.g. decrease followed by increase or increase followed by decrease) (e.g. Mileykovskiy and Morales, 2011; Wang and Tsien, 2011).
Heterogeneous responses to aversive stimuli may segregate based on location of dopamine cells in the midbrain and a mediolateral gradient has been reported in some studies. As such, in the monkey, neurons in SNc are more likely to be excited and neurons in VTA are more likely to be inhibited by a variety of aversive stimuli (Mirenowicz and Schultz, 1996; Matsumoto and Hikosaka, 2009) and in the rat, within VTA, lateral VTA neurons are more likely to be excited and medial VTA neurons more likely to be inhibited (Valenti et al., 2011). Furthermore, a dorsal-ventral gradient within rat VTA has also been reported with dorsal cells more likely to be inhibited and ventral cells more likely to be excited (Brischoux et al., 2009).
Inputs to dopamine cells are likely to be an important determinant as to how cells respond to aversive stimuli. As such, one study found that all SNc neurons were inhibited by noxious footshock and that this response was relayed via nociceptive neurons in the parabrachial nucleus (Coizet et al., 2010). In another report it was shown that a small proportion of SNc neurons were inhibited by aversive stimuli and this was tightly linked to the amount of GABAergic input from neighboring substantia nigra reticulata (Henny et al., 2012).
With respect to dopamine measurements in forebrain areas, microdialysis measurements are difficult to interpret as the low temporal resolution means that changes in extracellular dopamine may result from termination of the stimulus, rather than the stimulus itself (Puglisi-Allegra et al., 1991). In addition, in order to generate measureable changes in dopamine levels, repeated stimuli have most often been used, which may be classed as a stressful experience as is discussed below. Using voltammetry, which has a higher time resolution than microdialysis, these phenomena can be studied with more accuracy. As such, it has been shown that cues that predict an aversive footshock decrease in dopamine within NAc core but increase it in NAc shell (Badrinarayan et al., 2012) although aversive taste stimuli – whether primary or conditioned – lead to a decrease in NAc shell dopamine (Roitman et al., 2008; Wheeler et al., 2011; McCutcheon et al., 2012b). This provides some support for heterogeneity based on projection target but also the potential for heterogeneity based on sensory modality and specifics of experiment. Interestingly, a recent study showed that if rats learn to perform a cued response that prevents the occurrence of an aversive stimulus then, the predictive cue evokes an increase in NAc core dopamine, rather than the decrease that is seen when rats fail to perform the action (Oleson et al., 2012). This is of interest because in some studies of conditioned aversive stimuli subjects were able to escape experience of the aversive stimulus by responding to the cue (Mirenowicz and Schultz, 1996), which may underlie some of the heterogeneity in data sets.
It is worth noting that, in contrast to studies on rewarding stimuli, studies on aversive stimuli are performed in both anesthetized and awake preparations. This may be important as it is debatable if a truly aversive experience is possible under anesthesia. Instead, responses to these stimuli may reflect a more basic property of the stimulus such as its sensory components.
5.2.3. Neutral stimuli
Dopamine neurons respond to ‘neutral’ stimuli (i.e. those without an existing positive or negative valence), especially if they are novel or otherwise behaviorally salient (Ljungberg et al., 1992; Redgrave et al., 1999; Horvitz, 2000). These responses, which are believed to be driven from the cortex via superior colliculus (Bertram et al., 2014), may have an alerting role and either enable learning about new stimuli or be important for behavioral switching (Overton et al., 2014).
In addition, as mentioned earlier, if neutral stimuli reliably predict either a rewarding or aversive stimuli, they become conditioned predictors and evoke a similar firing response as the stimulus that they predict (Schultz, 2007).
In summary, dopamine responses show great heterogeneity to a number of different stimuli. To rewarding stimuli, while responses are homogenous in their valence they exhibit differences in occurrence and magnitude. For aversive stimuli, responses are far more heterogeneous and generally include a mixture of responses. This may reflect the existence of different pools of dopamine neurons with different functional roles (Bromberg-Martin et al., 2010).
5.3 Heterogeneity in response to stress
Stress is commonly defined as a physiological challenge with negative connotations that results in increased circulating levels of stress hormones (e.g. corticosterone/cortisol) and behavioral avoidance. However, it is important to realize that not all stress is ‘bad’ stress and, in fact, a moderate level of stress (and associated hormones) may be required for normal physiological function. Thus, the relationship between stress and behavior can be conceptualized as an inverted-U shape with too little or too much stress having a detrimental effect on a number of behaviors, such as learning and memory. Similarly, microdialysis studies have shown that an inverted-U-shaped curve exists when comparing extracellular dopamine levels in NAc in response to stress: low/moderate levels of stress increase dopamine in NAc (Imperato et al., 1989; Kalivas and Duffy, 1995; Tidey and Miczek, 1996; Barrot et al., 2000) whereas high levels of stress (intense, chronic, or unpredictable) lead to decreases in dopamine (Di Chiara et al., 1999; Mangiavacchi et al., 2001). Furthermore, increases in dopamine levels are larger in the PFC than in the striatal complex; within the striatal complex they are greatest in the NAc shell (for review see Marinelli, 2007).
With respect to dopamine neuron activity, a variety of stressors have been shown to increase this activity, consistent with the increases in dopamine efflux observed in microdialysis studies. These stressors include food restriction/deprivation (Marinelli et al., 2006; Branch et al., 2013), restraint (Anstrom and Woodward, 2005; Valenti et al., 2011), social defeat (Anstrom et al., 2009; Cao et al., 2010; Razzoli et al., 2011), and cold swim (Marinelli et al., 2006). Increases in neuronal activity are seen for overall firing rates and/or bursting amount and characteristics, according to the study. To our knowledge, decreases in neuronal activity corresponding to the descending part of the inverted-U have not been studied extensively. Chronic cold exposure and chronic mild stress were shown to cause a decrease in population activity; that is, the number of active neurons (Moore et al., 2001; Chang and Grace, 2013). This effect was selective to the medial and central part of the VTA, but not the lateral portion (Valenti et al., 2011). However, effects on firing frequency have not been observed. In fact, in response to chronic cold exposure a slight increase in proportion of bursting cells was seen (Moore et al., 2001). Brief, aversive stimuli (as described in section 5.2.2.) often lead to decreases in firing rate but these tend to be very transient in nature and thus are not likely to underlie the sustained decreases seen with microdialysis; furthermore, such brief aversive stimuli do not correspond to the descending part of the inverted-U shaped curve mentioned above.
5.3.1. Role of the stress-dopamine interaction in drug addiction
Midbrain dopamine neurons have been implicated in some psychiatric disorders that interact with stress. The stressors listed above, which increase dopamine transmission, are all associated with greater susceptibility to addiction and relapse, suggesting that stress-induced increase in dopaminergic activity could facilitate addiction and relapse (Carr, 2002; Marinelli et al., 2006; Ungless et al., 2010). In fact, blocking dopamine receptors prevents the increase in cocaine self-administration produced by food restriction stress (De Vry et al., 1989). These findings are consistent with the well-described role of stress in precipitating relapse in humans (Sinha, 2008).
5.3.2. Role of the stress-dopamine interaction in depression
With respect to other psychiatric disorders, depression seems to be linked to dopamine neuron firing. Mice that are susceptible to development of depressive-like symptoms after social defeat stress show hyperactivity of dopamine neurons (Krishnan et al., 2007; Cao et al., 2010), and this susceptibility can be mimicked by stimulating dopamine neurons in a burst-like manner (Chaudhury et al., 2013). In addition, normalizing this hyperactivity reverses depressive-like symptoms and, conversely, treatment with an anti-depressant normalizes neuronal hyperactivity (Cao et al., 2010). Interestingly, mice that are resistant to the development of depressive-like symptoms show enhanced homeostatic plasticity of intrinsic conductances within VTA neurons that project to NAc (Friedman et al., 2014). However, in another study, Tye et al. (2013) showed instead that suppression of dopamine neuron activity induced a depression-like phenotype in forced swim and the sucrose preference test. Thus, the precise role of dopamine neuron activity in mediating depression is uncertain but may involve complex interactions between inherent susceptibility or resilience and exposure to life events (e.g. stress).
6. SUMMARY
There are multiple ways in which midbrain dopamine neurons exhibit heterogeneity. Dopamine neurons fire irregularly, with interspersed burst events. The differing patterns of activity can lead to differences in neurotransmitter output and can be altered by multiple factors. Some of these factors vary on a cell by cell basis within an individual (e.g. dependent on projection site, location within midbrain); some vary on a subject to subject basis (e.g. at different ages, or in different individuals such as high and low responders); and, finally, some variation is induced by life events, (e.g. exposure to drugs or stress, learning, or conditioning). Therefore, firing rates and patterns can be altered by many stimuli, experiences, or individual parameters. Interestingly, some changes (e.g. discontinuation of drug use and adolescence) have stronger effects on non-bursting (single spike firing) than on bursting. This is important as bursting receives a great deal more attention in the literature but might be a worse predictor of various behavioral correlates or psychopathological phenomena.
Variability in wakefulness/arousal across preparations is another significant source of heterogeneity in firing rates and patterns. This seems to be tightly related to the level of afferent input onto dopamine neurons. As such, a continuum exists whereby in situations with very low levels of synaptic input (deeply anesthetized, ex vivo) the number of spontaneously active cells is low as are their firing rates and propensity to burst. In contrast, in situations when synaptic input is elevated (acute stress, reward presentation) spontaneously active cells fire faster and are more likely to exhibit bursting.
Alterations in firing activity may influence multiple elements of an individual's behavior, such as their vulnerability to drug addiction. With respect to drugs, there are important differences between the effects of drugs on dopamine neurons acutely, while drug is on board, and effects that occur after discontinuation of drug. These differences may underlie different forms of drug-seeking behaviors with some resulting from a hyperdopaminergic system and others from a hypodopaminergic system.
Responses to aversive stimuli show a great deal of variability, the meaning of which is still being interrogated; however, the existence of segregated populations with distinct input and output circuitry may help to explain this diversity. As such, the increases and decreases in dopamine neuron activity observed to similar stimuli may be subserving different downstream functions. The fact that the basic stress response overlaps so much with responses to rewarding stimuli whether drugs or food (i.e. increased levels) implies that a similar behavioral outcome might be expected to each type of stimulus. Thus, dopamine neuron activity may be more important for activating or invigorating animals vs. encoding a hedonic value of a stimulus. However, other studies show that phasic dopamine, especially in NAc shell, tracks the hedonic value of taste stimuli (Roitman et al., 2008; Wheeler et al., 2011; McCutcheon et al., 2012b). These findings may be reconciled by considering regional heterogeneity in dopamine signaling.
Finally, as well as the modes of heterogeneity which have been discussed here, emerging fields that will add to this nuanced picture involve the extent of synchrony between dopamine neurons and how this can be altered during behavior (Kim et al., 2012) and the participation of other neurotransmitters in dopamine neuron firing (Hnasko et al., 2010; Stuber et al., 2010; Tritsch et al., 2012). As these novel areas are developed, they will add further complexity to our understanding of dopamine signaling and its role in behavior.
Highlights.
Midbrain dopamine neuron activity varies with respect to firing rates and patterns across individuals and across states.
Heterogeneity in dopamine cell activity is seen over development, stressful events, drug exposure, and behavioral stimuli.
We review heterogeneity in firing and provide insight into how it may facilitate development of psychiatric conditions
ACKNOWLEDGEMENTS
This work was supported by NIH Grants DA033380 (JEM) and DA031577 (MM). We thank Dr. Michael Beckstead for providing the electrophysiology trace reported in Figure 3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ambrosio E, Goldberg SR, Elmer GI. Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav Pharmacol. 1995;6:229–237. [PubMed] [Google Scholar]
- Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstrom KK, West AR, Phillips PEM, Marinelli M. Dopaminergic burst firing and behavior: are you and I talking about the same thing?. Winter Conference on Brain Research) 40th Annual Meeting; Snowmass, CO. 2007. [Google Scholar]
- Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30:1832–1840. doi: 10.1038/sj.npp.1300730. [DOI] [PubMed] [Google Scholar]
- Appel SB, Wise L, McDaid J, Koyama S, McElvain MAMA, Brodie MS. The effects of long chain-length n-alcohols on the firing frequency of dopaminergic neurons of the ventral tegmental area. J Pharmacol Exp Ther. 2006;318:1137. doi: 10.1124/jpet.106.105148. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, Aragona BJ. Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. J Neurosci. 2012;32:15779–15790. doi: 10.1523/JNEUROSCI.3557-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon MJ, Roth RH. Pharmacology of mesocortical dopamine neurons. Pharmacol Rev. 1983;35:53–68. [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rougé-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Bertram C, Dahan L, Boorman LW, Harris S, Vautrelle N, Leriche M, Redgrave P, Overton PG. Cortical regulation of dopaminergic neurons: role of the midbrain superior colliculus. J Neurophysiol. 2014;111:755–767. doi: 10.1152/jn.00329.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bioulac B, Benazzouz A, Burbaud P, Gross C. Chronic administration of DL-allyl-glycine into the neostriatum, disorganises the firing modes of the nigral dopaminergic neurons in the rat. Neurosci Lett. 1997;226:21–24. doi: 10.1016/s0304-3940(97)00236-x. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch SY, Beckstead MJ. Methamphetamine produces bidirectional, concentration-dependent effects on dopamine neuron excitability and dopamine-mediated synaptic currents. J Neurophysiol. 2012;108:802–809. doi: 10.1152/jn.00094.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch SY, Goertz RB, Sharpe AL, Pierce J, Roy S, Ko D, Paladini CA, Beckstead MJ. Food restriction increases glutamate receptor-mediated burst firing of dopamine neurons. J Neurosci. 2013;33:13861–13872. doi: 10.1523/JNEUROSCI.5099-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Dunwiddie TV. Cholecystokinin potentiates dopamine inhibition of mesencephalic dopamine neurons in vitro. Brain Res. 1987;425:106–113. doi: 10.1016/0006-8993(87)90488-4. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Dunwiddie TV. Cocaine effects in the ventral tegmental area: evidence for an indirect dopaminergic mechanism of action. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:660–665. doi: 10.1007/BF00175709. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O. Midbrain dopamine neurons signal preference for advance information about upcoming rewards. Neuron. 2009;63:119–126. doi: 10.1016/j.neuron.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney BS, Aghajanian GK. D-Amphetamine-induced inhibition of central dopaminergic neurons: direct effect or mediated by a striatonigral feedback pathway? Adv Biochem Psychopharmacol. 1977;16:577–582. [PubMed] [Google Scholar]
- Bunney BS, Grace AA. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sci. 1978;23:1715–1727. doi: 10.1016/0024-3205(78)90471-x. [DOI] [PubMed] [Google Scholar]
- Bunney EB, Appel SB, Brodie MS. Electrophysiological effects of cocaethylene, cocaine, and ethanol on dopaminergic neurons of the ventral tegmental area. J Pharmacol Exp Ther. 2001;297:696–703. [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang H-L, Morales M, Lovinger DM, Cheer JF. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain ME, Smith CM, Bardo MT. The effect of novelty on amphetamine self-administration in rats classified as high and low responders. Psychopharmacology (Berl) 2004;176:129–138. doi: 10.1007/s00213-004-1870-2. [DOI] [PubMed] [Google Scholar]
- Cao J-L, Covington HE, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, Han M-H. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 2010;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Chang C-H, Grace AA. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry. 2013:1–8. doi: 10.1016/j.biopsych.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B, Azriel Y, Mohammadi S, Christie MJ. Distinct cellular properties of identified dopaminergic and GABAergic neurons in the mouse ventral tegmental area. J Physiol. 2011;589:3775–3787. doi: 10.1113/jphysiol.2011.210807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, O'Brien CP. Dopamine receptor partial agonists could address the duality of cocaine craving. Trends Pharmacol Sci. 2000;21:6–9. doi: 10.1016/s0165-6147(99)01422-4. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Bannon MJ, Grace AA, Roth RH, Bunney BS. Evidence for the absence of impulse-regulating somatodendritic and synthesis-modulating nerve terminal autoreceptors on subpopulations of mesocortical dopamine neurons. Neuroscience. 1984;12:1–16. doi: 10.1016/0306-4522(84)90133-7. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Bunney BS. Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci. 1983;3:1607–1619. doi: 10.1523/JNEUROSCI.03-08-01607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo LA, Kapatos G. Membrane properties of identified mesencephalic dopamine neurons in primary dissociated cell culture. Synapse. 1992;11:294–309. doi: 10.1002/syn.890110405. [DOI] [PubMed] [Google Scholar]
- Choong K, Shen R. Prenatal ethanol exposure alters the postnatal development of the spontaneous electrical activity of dopamine neurons in the ventral tegmental area. Neuroscience. 2004;126:1083–1091. doi: 10.1016/j.neuroscience.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Church WH, Justice JB, Neill DB. Detecting behaviorally relevant changes in extracellular dopamine with microdialysis. Brain Res. 1987;412:397–399. doi: 10.1016/0006-8993(87)91150-4. [DOI] [PubMed] [Google Scholar]
- Clark D, Chiodo LA. Electrophysiological and pharmacological characterization of identified nigrostriatal and mesoaccumbens dopamine neurons in the rat. Synapse. 1988;2:474–485. doi: 10.1002/syn.890020503. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coizet V, Dommett EJ, Klop EM, Redgrave P, Overton PG. The parabrachial nucleus is a critical link in the transmission of short latency nociceptive information to midbrain dopaminergic neurons. Neuroscience. 2010;168:263–272. doi: 10.1016/j.neuroscience.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney NA, Mamaligas AA, Ford CP. Species differences in somatodendritic dopamine transmission determine D2-autoreceptor-mediated inhibition of ventral tegmental area neuron firing. J Neurosci. 2012;32:13520–13528. doi: 10.1523/JNEUROSCI.2745-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- Dai M, Tepper JM. Do silent dopaminergic neurons exist in rat substantia nigra in vivo? Neuroscience. 1998;85:1089–1099. doi: 10.1016/s0306-4522(97)00615-5. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- De Vry J, Donselaar I, Van Ree JM. Food deprivation and acquisition of intravenous cocaine self-administration in rats: effect of naltrexone and haloperidol. J Pharmacol Exp Ther. 1989;251:735–740. [PubMed] [Google Scholar]
- Di Chiara G, Acquas E, Carboni E. Drug motivation and abuse: a neurobiological perspective. Ann N Y Acad Sci. 1992;654:207–219. doi: 10.1111/j.1749-6632.1992.tb25969.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry. 1999;46:1624–1633. doi: 10.1016/s0006-3223(99)00236-x. [DOI] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang H-L, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. J Neurosci. 2010;30:14273–14283. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drion G, Bonjean M, Waroux O, Scuvée-Moreau J, Liégeois J-F, Sejnowski TJ, Sepulchre R, Seutin V. M-type channels selectively control bursting in rat dopaminergic neurons. Eur J Neurosci. 2010;31:827–835. doi: 10.1111/j.1460-9568.2010.07107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn LC, Johansen PA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J Neurosci. 1988;8:100–112. doi: 10.1523/JNEUROSCI.08-01-00100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153(Suppl):S283–97. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fà M, Mereu G, Ghiglieri V, Meloni A, Salis P, Gessa GL. Electrophysiological and pharmacological characteristics of nigral dopaminergic neurons in the conscious, head-restrained rat. Synapse. 2003;48:1–9. doi: 10.1002/syn.10177. [DOI] [PubMed] [Google Scholar]
- Federici M, Geracitano R, Bernardi G, Mercuri NB. Actions of methylphenidate on dopaminergic neurons of the ventral midbrain. Biol Psychiatry. 2005;57:361–365. doi: 10.1016/j.biopsych.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Flecknell PA. Laboratory animal anaesthesia. An introduction for research workers and technicians. Academic Press; London: 1987. [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IHA, Booij J, van den Brink W. The role of dopamine in human addiction: from reward to motivated attention. Eur J Pharmacol. 2005;526:199–206. doi: 10.1016/j.ejphar.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Freeman AS, Kelland MD, Rouillard C, Chiodo LA. Electrophysiological characteristics and pharmacological responsiveness of midbrain dopaminergic neurons of the aged rat. J Pharmacol Exp Ther. 1989;249:790–797. [PubMed] [Google Scholar]
- Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Li X, Dietz DM, Pan N, Vialou VF, Neve RL, Yue Z, Han M-H. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–319. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariano RF, Tepper JM, Sawyer SF, Young SJ, Groves PM. Mesocortical dopaminergic neurons. 1. Electrophysiological properties and evidence for soma-dendritic autoreceptors. Brain Res Bull. 1989;22:511–516. doi: 10.1016/0361-9230(89)90103-2. [DOI] [PubMed] [Google Scholar]
- Garris PA, Christensen JR, Rebec GV, Wightman RM. Real-time measurement of electrically evoked extracellular dopamine in the striatum of freely moving rats. J Neurochem. 1997;68:152–161. doi: 10.1046/j.1471-4159.1997.68010152.x. [DOI] [PubMed] [Google Scholar]
- German DC, Liang CL. Neuroactive peptides exist in the midbrain dopaminergic neurons that contain calbindin-D28k. Neuroreport. 1993;4:491–494. doi: 10.1097/00001756-199305000-00007. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- Gonon F. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- Gourévitch B, Eggermont JJ. A simple indicator of nonstationarity of firing rate in spike trains. J Neurosci Methods. 2007;163:181–187. doi: 10.1016/j.jneumeth.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Grace AA. In vivo and in vitro intracellular recordings from rat midbrain dopamine neurons. Ann N Y Acad Sci. 1988;537:51–76. doi: 10.1111/j.1749-6632.1988.tb42096.x. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend. 1995;37:111–129. doi: 10.1016/0376-8716(94)01066-t. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl 2):S119–28. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984a;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984b;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenhoff J, Ugedo L, Svensson TH. Firing patterns of midbrain dopamine neurons: differences between A9 and A10 cells. Acta Physiol Scand. 1988;134:127–132. doi: 10.1111/j.1748-1716.1988.tb08468.x. [DOI] [PubMed] [Google Scholar]
- Gysling K, Wang RY. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res. 1983;277:119–127. doi: 10.1016/0006-8993(83)90913-7. [DOI] [PubMed] [Google Scholar]
- Henny P, Brown MTC, Northrop A, Faunes M, Ungless MA, Magill PJ, Bolam JP. Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons. Nat Neurosci. 2012;15:613–619. doi: 10.1038/nn.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L, Lee F, Hoebel BG. Simultaneous microdialysis and amphetamine infusion in the nucleus accumbens and striatum of freely moving rats: increase in extracellular dopamine and serotonin. Brain Res Bull. 1987;19:623–628. doi: 10.1016/0361-9230(87)90047-5. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Colvin AC, Juncos JL, Justice JB. Individual differences in basal and cocaine-stimulated extracellular dopamine in the nucleus accumbens using quantitative microdialysis. Brain Res. 1992;587:306–312. doi: 10.1016/0006-8993(92)91012-4. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Imperato A, Puglisi-Allegra S, Casolini P, Zocchi A, Angelucci L. Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. Eur J Pharmacol. 1989;165:337–338. doi: 10.1016/0014-2999(89)90735-8. [DOI] [PubMed] [Google Scholar]
- Jin X, Costa RM. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature. 2010;466:457–462. doi: 10.1038/nature09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YS, Lee J, Mizumori SJY. Effects of prefrontal cortical inactivation on neural activity in the ventral tegmental area. J Neurosci. 2013;33:8159–8171. doi: 10.1523/JNEUROSCI.0118-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph MH, Datla K, Young AMJ. The interpretation of the measurement of nucleus accumbens dopamine by in vivo dialysis: the kick, the craving or the cognition? Neurosci Biobehav Rev. 2003;27:527–541. doi: 10.1016/j.neubiorev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Joshua M, Adler A, Mitelman R, Vaadia E, Bergman H. Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J Neurosci. 2008;28:11673–11684. doi: 10.1523/JNEUROSCI.3839-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Kelland MD, Freeman AS, Chiodo LA. (+/−)-3,4-Methylenedioxymethamphetamine-induced changes in the basal activity and pharmacological responsiveness of nigrostriatal dopamine neurons. Eur J Pharmacol. 1989;169:11–21. doi: 10.1016/0014-2999(89)90812-1. [DOI] [PubMed] [Google Scholar]
- Kim Y, Matthews M, Moghaddam B. Putative -aminobutyric acid neurons in the ventral tegmental area have a similar pattern of plasticity as dopamine neurons during appetitive and aversive learning. Eur J Neurosci. 2010;32:1564–1572. doi: 10.1111/j.1460-9568.2010.07371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Wood J, Moghaddam B. Chapouthier G, editor. Coordinated activity of ventral tegmental neurons adapts to appetitive and aversive learning. PLoS One. 2012;7:e29766. doi: 10.1371/journal.pone.0029766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Kita H, Kitai ST. Electrical membrane properties of rat substantia nigra compacta neurons in an in vitro slice preparation. Brain Res. 1986;372:21–30. doi: 10.1016/0006-8993(86)91454-x. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Shepard PD, Callaway JC, Scroggs R. Afferent modulation of dopamine neuron firing patterns. Curr Opin Neurobiol. 1999;9:690–697. doi: 10.1016/s0959-4388(99)00040-9. [DOI] [PubMed] [Google Scholar]
- Ko D, Wilson CJ, Lobb CJ, Paladini CA. Detection of bursts and pauses in spike trains. J Neurosci Methods. 2012;211:145–158. doi: 10.1016/j.jneumeth.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulchitsky S, De Backer B, Quertemont E, Charlier C, Seutin V. Differential effects of cocaine on dopamine neuron firing in awake and anesthetized rats. Neuropsychopharmacology. 2012;37:1559–1571. doi: 10.1038/npp.2011.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Labouèbe G, Lomazzi M, Cruz HG, Creton C, Luján R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, Lüscher C. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;10:1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Actions of cocaine on rat dopaminergic neurones in vitro. Br J Pharmacol. 1990;99:731–735. doi: 10.1111/j.1476-5381.1990.tb12998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalive AL, Munoz MB, Bellone C, Slesinger PA, Lüscher C, Tan KR. Firing modes of dopamine neurons drive bidirectional GIRK channel plasticity. J Neurosci. 2014;34:5107–5114. doi: 10.1523/JNEUROSCI.5203-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavín MA, Drucker-Colín R. Ontogeny of the electrophysiological activity of dopaminergic cells with special reference to the influence of adrenal medullary grafts on aging. Brain Res. 1991;545:164–170. doi: 10.1016/0006-8993(91)91282-6. [DOI] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Ennas MG, Castelli MP, Muntoni AL, Pistis M. Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacology. 2011;36:589–602. doi: 10.1038/npp.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M. Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology. 2012;37:1164–1176. doi: 10.1038/npp.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legéndy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol. 1985;53:926–939. doi: 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W, Hefti F, Felix D, Huwyler T, Melamed E, Schlumpf M. Stimulation of nigrostriatal dopamine neurones by nicotine. Neuropharmacology. 1982;21:963–968. doi: 10.1016/0028-3908(82)90107-1. [DOI] [PubMed] [Google Scholar]
- Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32:507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Lüscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc: Supplementary Material. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux J-P, Faure P. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Mangiavacchi S, Masi F, Scheggi S, Leggio B, De Montis MG, Gambarana C. Long-term behavioral and neurochemical effects of chronic stress exposure in rats. J Neurochem. 2001;79:1113–1121. doi: 10.1046/j.1471-4159.2001.00665.x. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Coker AR, Driscoll JR, Lemaître A-I, Fields HL. Reliability in the identification of midbrain dopamine neurons. PLoS One. 2010;5:e15222. doi: 10.1371/journal.pone.0015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M. Dopaminergic reward pathways and the effects of stress. In: Al'Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. Academic Press; Burlington: 2007. pp. 41–84. [Google Scholar]
- Marinelli M, Cooper DC, Baker LK, White FJ. Impulse activity of midbrain dopamine neurons modulates drug-seeking behavior. Psychopharmacol. 2003;168:84–98. doi: 10.1007/s00213-003-1491-1. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Rudick CN, Hu X-T, White FJ. Excitability of dopamine neurons: modulation and physiological consequences. CNS Neurol Disord Drug Targets. 2006;5:79–97. doi: 10.2174/187152706784111542. [DOI] [PubMed] [Google Scholar]
- Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martig AK, Mizumori SJY. Ventral tegmental area and substantia nigra neural correlates of spatial learning. Learn Mem. 2011;18:260–271. doi: 10.1101/lm.1895211. [DOI] [PubMed] [Google Scholar]
- Masuko S, Nakajima S, Nakajima Y. Dissociated high-purity dopaminergic neuron cultures from the substantia nigra and the ventral tegmental area of the postnatal rat. Neuroscience. 1992;49:347–364. doi: 10.1016/0306-4522(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Champney TH, Frye GD. Effects of (+−)3,4-methylenedioxymethamphetamine (MDMA) on brain dopaminergic activity in rats. Pharmacol Biochem Behav. 1989;33:741–747. doi: 10.1016/0091-3057(89)90464-4. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J Neurophysiol. 2012a;108:1620–1630. doi: 10.1152/jn.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF. Encoding of aversion by dopamine and the nucleus accumbens. Front Neurosci. 2012b;6:137. doi: 10.3389/fnins.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age matters. Eur J Neurosci. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, White FJ, Marinelli M. Individual differences in dopamine cell neuroadaptations following cocaine self-administration. Biol Psychiatry. 2009;66:801–803. doi: 10.1016/j.biopsych.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Gessa GL, Diana M. Clozapine does activate nigrostriatal dopamine neurons in unanesthetized rats. Eur J Pharmacol. 1998;363:135–138. doi: 10.1016/s0014-2999(98)00822-x. [DOI] [PubMed] [Google Scholar]
- Melis M, Gessa GL, Diana M. Different mechanisms for dopaminergic excitation induced by opiates and cannabinoids in the rat midbrain. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:993–1006. doi: 10.1016/s0278-5846(00)00119-6. [DOI] [PubMed] [Google Scholar]
- Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol. 2005;63:101–154. doi: 10.1016/S0074-7742(05)63005-X. [DOI] [PubMed] [Google Scholar]
- Mereu G, Fadda F, Gessa GL. Ethanol stimulates the firing rate of nigral dopaminergic neurons in unanesthetized rats. Brain Res. 1984;292:63–69. doi: 10.1016/0006-8993(84)90890-4. [DOI] [PubMed] [Google Scholar]
- Mereu G, Lilliu V, Vargiu P, Muntoni AL, Diana M, Gessa GL. Depolarization inactivation of dopamine neurons: an artifact? J Neurosci. 1995;15:1144–1149. doi: 10.1523/JNEUROSCI.15-02-01144.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu G, Yoon KW, Boi V, Gessa GL, Naes L, Westfall TC. Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. Eur J Pharmacol. 1987;141:395–399. doi: 10.1016/0014-2999(87)90556-5. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy B, Morales M. Duration of inhibition of ventral tegmental area dopamine neurons encodes a level of conditioned fear. J Neurosci. 2011;31:7471–7476. doi: 10.1523/JNEUROSCI.5731-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Moore H, Rose HJ, Grace AA. Chronic cold stress reduces the spontaneous activity of ventral tegmental dopamine neurons. Neuropsychopharmacology. 2001;24:410–419. doi: 10.1016/S0893-133X(00)00188-3. [DOI] [PubMed] [Google Scholar]
- Morzorati SL. VTA dopamine neuron activity distinguishes alcohol-preferring (P) rats from Wistar rats. Alcohol Clin Exp Res. 1998;22:854–857. [PubMed] [Google Scholar]
- Morzorati SL, Marunde RL. Comparison of VTA dopamine neuron activity in lines of rats selectively bred to prefer or avoid alcohol. Alcohol Clin Exp Res. 2006;30:991–997. doi: 10.1111/j.1530-0277.2006.00113.x. [DOI] [PubMed] [Google Scholar]
- Mueller AL, Brodie MS. Intracellular recording from putative dopamine-containing neurons in the ventral tegmental area of Tsai in a brain slice preparation. J Neurosci Methods. 1989;28:15–22. doi: 10.1016/0165-0270(89)90005-8. [DOI] [PubMed] [Google Scholar]
- Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162:333–338. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, Roeper J. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci. 2002;22:1290–1302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Gentry RN, Chioma VC, Cheer JF. Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. J Neurosci. 2012;32:14804–14808. doi: 10.1523/JNEUROSCI.3087-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Brasic J, Wand GS. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30:821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Overton PG, Vautrelle N, Redgrave P. Sensory regulation of dopaminergic cell activity: Phenomenology, circuitry and function. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Owesson-White CA, Roitman MF, Sombers LA, Belle AM, Keithley RB, Peele JL, Carelli RM, Wightman RM. Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J Neurochem. 2012;121:252–262. doi: 10.1111/j.1471-4159.2012.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini CA, Beckstead MJ, Weinshenker D. Electrophysiological properties of catecholaminergic neurons in the norepinephrine-deficient mouse. Neuroscience. 2007;144:1067–1074. doi: 10.1016/j.neuroscience.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini CA, Fiorillo CD, Morikawa H, Williams JT. Amphetamine selectively blocks inhibitory glutamate transmission in dopamine neurons. Nat Neurosci. 2001;4:275–281. doi: 10.1038/85124. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Tepper JM. GABA(A) and GABA(B) antagonists differentially affect the firing pattern of substantia nigra dopaminergic neurons in vivo. Synapse. 1999;32:165–176. doi: 10.1002/(SICI)1098-2396(19990601)32:3<165::AID-SYN3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Williams JT. Noradrenergic inhibition of midbrain dopamine neurons. J Neurosci. 2004;24:4568–4575. doi: 10.1523/JNEUROSCI.5735-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci. 2005;25:4725–4732. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panin F, Cathala A, Piazza PV, Spampinato U. Coupled intracerebral microdialysis and electrophysiology for the assessment of dopamine neuron function in vivo. J Pharmacol Toxicol Methods. 2012;65:83–92. doi: 10.1016/j.vascn.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Park J, Takmakov P, Wightman RM. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. J Neurochem. 2011;119:932–944. doi: 10.1111/j.1471-4159.2011.07494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PEM, Stuber GD, Heien MLAV, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Phillips PEM, Wightman RM. Extrasynaptic dopamine and phasic neuronal activity. Nat Neurosci. 2004;7:199. doi: 10.1038/nn0304-199a. author reply 199. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminière J-M, Maccari S, Mormède P, Le Moal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol. 1990;1:339–345. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol Psychiatry. 2004;56:86–94. doi: 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Pitts DK, Freeman AS, Chiodo LA. Dopamine neuron ontogeny: electrophysiological studies. Synapse. 1990;6:309–320. doi: 10.1002/syn.890060402. [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Imperato A, Angelucci L, Cabib S. Acute stress induces time-dependent responses in dopamine mesolimbic system. Brain Res. 1991;554:217–222. doi: 10.1016/0006-8993(91)90192-x. [DOI] [PubMed] [Google Scholar]
- Puryear CB, Kim MJ, Mizumori SJY. Conjunctive encoding of movement and reward by ventral tegmental area neurons in the freely navigating rodent. Behav Neurosci. 2010;124:234–247. doi: 10.1037/a0018865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M, Andreoli M, Michielin F, Quarta D, Sokal DM. Increased phasic activity of VTA dopamine neurons in mice 3 weeks after repeated social defeat. Behav Brain Res. 2011;218:253–257. doi: 10.1016/j.bbr.2010.11.050. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Segal DS. Dose-dependent biphasic alterations in the spontaneous activity of neurons in the rat neostriatum produced by d-amphetamine and methylphenidate. Brain Res. 1978;150:353–366. doi: 10.1016/0006-8993(78)90286-x. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci. 1999;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Roeper J. Dissecting the diversity of midbrain dopamine neurons. Trends Neurosci. 2013;36:336–342. doi: 10.1016/j.tins.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Roeper J. The diversity of burst mechanisms in VTA and SN dopamine neurons. Neuroscience. 2014 [Google Scholar]
- Roesch MR, Calu DJ, Schoenbaum G. Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nat Neurosci. 2007;10:1615–1624. doi: 10.1038/nn2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PEM, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sanghera MK, Trulson ME, German DC. Electrophysiological properties of mouse dopamine neurons: in vivo and in vitro studies. Neuroscience. 1984;12:793–801. doi: 10.1016/0306-4522(84)90171-4. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Benoit-Marand M, Gonon F, Sulzer D. Presynaptic regulation of dopaminergic neurotransmission. J Neurochem. 2003;87:273–289. doi: 10.1046/j.1471-4159.2003.02050.x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(80-):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sharp T, Zetterström T, Ljungberg T, Ungerstedt U. A direct comparison of amphetamine-induced behaviours and regional brain dopamine release in the rat using intracerebral dialysis. Brain Res. 1987;401:322–330. doi: 10.1016/0006-8993(87)91416-8. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Bunney BS. Effects of apamin on the discharge properties of putative dopamine-containing neurons in vitro. Brain Res. 1988;463:380–384. doi: 10.1016/0006-8993(88)90414-3. [DOI] [PubMed] [Google Scholar]
- Shepard PD, German DC. A subpopulation of mesocortical dopamine neurons possesses autoreceptors. Eur J Pharmacol. 1984;98:455–456. doi: 10.1016/0014-2999(84)90300-5. [DOI] [PubMed] [Google Scholar]
- Shi W-X, Zhang X-Y, Pun C-L, Bunney BS. Clozapine blocks D-amphetamine-induced excitation of dopamine neurons in the ventral tegmental area. Neuropsychopharmacology. 2007;32:1922–1928. doi: 10.1038/sj.npp.1301334. [DOI] [PubMed] [Google Scholar]
- Shi W-XX, Pun CL, Zhang XX, Jones MD, Bunney BS. Dual effects of D-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J Neurosci. 2000;20:3504–3511. doi: 10.1523/JNEUROSCI.20-09-03504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim SS, Bunney BS, Shi WX. Effects of lesions in the medial prefrontal cortex on the activity of midbrain dopamine neurons. Neuropsychopharmacology. 1996;15:437–441. doi: 10.1016/S0893-133X(96)00052-8. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soden ME, Jones GL, Sanford CA, Chung AS, Güler AD, Chavkin C, Luján R, Zweifel LS. Disruption of dopamine neuron activity pattern regulation through selective expression of a human KCNN3 mutation. Neuron. 2013;80:997–1009. doi: 10.1016/j.neuron.2013.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA, Kruk ZL, Millar J, Wightman RM. Striatal dopamine uptake in the rat: in vivo analysis by fast cyclic voltammetry. Neurosci Lett. 1984;51:133–138. doi: 10.1016/0304-3940(84)90274-x. [DOI] [PubMed] [Google Scholar]
- Steensen BH, Nedergaard S, Ostergaard K, Lambert JD. Electrophysiological characterization of dopaminergic and non-dopaminergic neurones in organotypic slice cultures of the rat ventral mesencephalon. Exp brain Res. 1995;106:205–214. doi: 10.1007/BF00241116. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Taylor SR, Horton ML, Barber EN, Lyle LT, Stobbs SH, Allison DW. Cocaine disinhibits dopamine neurons in the ventral tegmental area via use-dependent blockade of GABA neuron voltage-sensitive sodium channels. Eur J Neurosci. 2008;28:2028–2040. doi: 10.1111/j.1460-9568.2008.06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, Ridder B De, Bowers MS, Joosten RN, Feenstra MG, Bonci A, de Ridder B. Reward-predictive cues enhance midbrain dopamine neurons. Science. 2008;321(80-):1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, Deisseroth K, Tye KM, Lüscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Martin LP, Anderson DR. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci. 1995;15:3092–3103. doi: 10.1523/JNEUROSCI.15-04-03092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Trent F, Nakamura S. Postnatal development of the electrical activity of rat nigrostriatal dopaminergic neurons. Brain Res Dev Brain Res. 1990;54:21–33. doi: 10.1016/0165-3806(90)90061-3. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Dickinson A, Schultz W. Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm. J Neurosci. 2003;23:10402–10410. doi: 10.1523/JNEUROSCI.23-32-10402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H-C, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324(80-):1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai H-C, Finkelstein J, Kim S-Y, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Argilli E, Bonci A. Effects of stress and aversion on dopamine neurons: implications for addiction. Neurosci Biobehav Rev. 2010;35:151–156. doi: 10.1016/j.neubiorev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Grace AA. Are you or aren't you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35:422–430. doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303(80-):2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Valenti O, Lodge DJ, Grace AA. Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci. 2011;31:4280–4289. doi: 10.1523/JNEUROSCI.5310-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Wightman RM. Pharmacologically induced, subsecond dopamine transients in the caudate-putamen of the anesthetized rat. Synapse. 2007;61:37–39. doi: 10.1002/syn.20343. [DOI] [PubMed] [Google Scholar]
- Vincent P, Maskos U, Charvet I, Bourgeais L, Stoppini L, Leresche N, Changeux J-P, Lambert R, Meda P, Paupardin-Tritsch D. Live imaging of neural structure and function by fibred fluorescence microscopy. EMBO Rep. 2006;7:1154–1161. doi: 10.1038/sj.embor.7400801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Walsh JJ, Han M-H. The heterogeneity of ventral tegmental area neurones: projection functions. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, Phillips PEM. Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev. 2009;2:195–213. doi: 10.2174/1874473710902020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Pitts DK. Ontogeny of nigrostriatal dopamine neuron autoreceptors: iontophoretic studies. J Pharmacol Exp Ther. 1995;272:164–176. [PubMed] [Google Scholar]
- Wang RY. Dopaminergic neurons in the rat ventral tegmental area. II. Evidence for autoregulation. Brain Res Rev. 1981;3:141–151. [Google Scholar]
- Wang DV, Tsien JZ. Convergent processing of both positive and negative motivational signals by the VTA dopamine neuronal populations. PLoS One. 2011;6:e17047. doi: 10.1371/journal.pone.0017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Grace AA. Striatal nitric oxide signaling regulates the neuronal activity of midbrain dopamine neurons in vivo. J Neurophysiol. 2000;83:1796–1808. doi: 10.1152/jn.2000.83.4.1796. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biol Psychiatry. 2011;69:1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Differential effects of classical and atypical antipsychotic drugs on A9 and A10 dopamine neurons. Science. 1983;221:1054–1057. doi: 10.1126/science.6136093. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Pharmacological characterization of dopamine autoreceptors in the rat ventral tegmental area: microiontophoretic studies. J Pharmacol Exp Ther. 1984;231:275–280. [PubMed] [Google Scholar]
- White SR, Obradovic T, Imel KM, Wheaton MJ. The effects of methylenedioxymethamphetamine (MDMA, “Ecstasy”) on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol. 1996;49:455–479. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, Stuber GD, Tye KM, Janak PH, Deisseroth K. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wong WC, Ford KA, Pagels NE, McCutcheon JE, Marinelli M. Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. J Neurosci. 2013;33:4913–4922. doi: 10.1523/JNEUROSCI.1371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetnikoff L, Lavezzi HN, Reichard RA, Zahm DS. An update on the connections of the ventral mesencephalic dopaminergic complex. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul KA, Blanco JA, Weidemann CT, McGill K, Jaggi JL, Baltuch GH, Kahana MJ. Human substantia nigra neurons encode unexpected financial rewards. Science. 2009;323:1496–1499. doi: 10.1126/science.1167342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Yang S, Jin G-Z, Bunney BS, Shi W-X. Oscillatory firing of dopamine neurons: differences between cells in the substantia nigra and ventral tegmental area. Synapse. 2008;62:169–175. doi: 10.1002/syn.20479. [DOI] [PubMed] [Google Scholar]
- Zhang TA, Placzek AN, Dani JA. In vitro identification and electrophysiological characterization of dopamine neurons in the ventral tegmental area. Neuropharmacology. 2010;59:431–436. doi: 10.1016/j.neuropharm.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJY, Paladini CA, Phillips PEM, Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]