Abstract
Lay Abstract
Temporal processing refers to our ability to “sense” or register the passage of time and to use that information to guide behavior. There is evidence that children with autism spectrum disorders (ASD) differ from children without ASD in their ability to process temporal information. Prior research has shown that age and working memory (the ability to hold and manipulate information in short-term memory storage) impact performance on temporal processing tasks in typically developing children, but it is not known whether there are similar associations in youth with ASD. It is also known that children with high levels of inattention and hyperactivity/impulsivity, who do not have ASD, tend to perform more poorly on measures of temporal processing. Our study examined the effects of working memory, age, and inattention/hyperactivity on the accuracy and consistency of temporal processing in 27 high-functioning youth with ASD and 25 youth without ASD. Our results show that youth with ASD are less accurate and less consistent in their ability to estimate time intervals, relative to typically developing youth. The difference in accuracy between the groups is more pronounced at younger ages, while working memory has a differential effect on consistency. Within the ASD group, inattention/hyperactivity was not associated with either accuracy or consistency. This study shows for the first time that both age and working memory affect how youth with and without ASD perceive and represent the passage of time.
Scientific Abstract
Impaired temporal processing has historically been viewed as a hallmark feature of attention-deficit/hyperactivity disorder (ADHD). Recent evidence suggests temporal processing deficits may also be characteristic of autism spectrum disorder (ASD). However, little is known about the factors that impact temporal processing in children with ASD. The purpose of this study was to assess the effects of co-morbid attention problems, working memory (WM), age, and their interactions, on time reproduction in youth with and without ASD.
Twenty-seven high functioning individuals with ASD and 25 demographically comparable typically developing individuals (ages 9–17; 85% male) were assessed on measures of time reproduction, auditory WM, and inattention/hyperactivity. The time reproduction task required depression of a computer key to mimic interval durations of 4, 8, 12, 16, or 20 seconds. Mixed effects regression analyses were used to model accuracy and variability of time reproduction as functions of diagnostic group, interval duration, age, WM, and inattention/hyperactivity.
A significant group by age interaction was detected for accuracy, with the deficit in the ASD group being greater in younger children. There was a significant group by WM interaction for consistency, with the effects of poor WM on performance consistency being more pronounced in youth with ASD. All participants tended to underestimate longer interval durations and to be less consistent for shorter interval durations; these effects appeared more pronounced in those who were younger or who had poorer working memory performance. Inattention/hyperactivity symptoms in the ASD group were not related to either accuracy or consistency.
This study highlights the potential value of temporal processing as an intermediate trait of relevance to multiple neurodevelopmental disorders.
Autism is a neurodevelopmental disorder, or group of related disorders, characterized by impairment in social interaction, social communication, and behavioral flexibility (DSM-V, American Psychiatric Association, 2013). The term “autism spectrum disorder” (ASD) has been adopted to reflect the dimensional nature of autism phenomenology and etiology. Conceptualizing autism as a spectrum disorder (the “autisms”) also draws attention to its phenotypic heterogeneity (Geschwind & Levitt, 2007) and the need to identify intermediate traits more closely related to specific genetic etiologies (Levitt & Campbell, 2009) and brain function (Levy & Ebstein, 2009). These intermediate traits can elucidate common biological pathways across disorders by characterizing dimensions at the behavioral level that are indicators of underlying neurofunctional integrity (Levy & Ebstein, 2009). In other words, moving away from highly heterogeneous symptom clusters (e.g., social function, communication), it will be important to identify more objectively measurable traits that can be quantified dimensionally, have a plausible neurobiological substrate, and could theoretically serve as clinical correlates of aberrant brain function.
Temporal processing, is one such intermediate trait that has a rich history in the neurosciences. It refers to the basic human ability to register the passage of time, connect that information to current behavior, and file it away for future use. Fueled by anecdotal and early empirical evidence that this basic ability may operate differently in ASD (Boucher, Pons, Lind, & Williams, 2007; Szelag, Kowalska, Galkowski, & Poppel, 2004; Wimpory, Nicholas, & Nash, 2002), the study of temporal processing and autism has gained momentum in recent years. Although temporal processing is a relatively novel area of ASD research, it is bolstered by a substantial literature on the measurement of time-related processes (e.g., time estimation, perception, production, and reproduction) and their associated neurobiological substrates in typically developing individuals (e.g., see Mauk & Buonomano, 2004; Meck & Benson, 2002). The circuit believed to support millisecond- and second- range timing functions includes projections to and from the basal ganglia and frontal lobe as well as connections with the cerebellum (Mauk & Buonomano, 2004). It is therefore noteworthy that a thorough review and meta-analysis of published neuroanatomical studies of ASD reported increased total volume of the cerebral hemispheres, cerebellum, and caudate nucleus, accompanied by a reduction in the size of the corpus callosum, as the most consistent findings to date (Stanfield, McIntosh, Spencer, Philip, Gaur, & Lawrie, 2008). There is also evidence from the literature to suggest that the precision of temporal estimates improves with age (Chelonis, Flake, Baldwin, Blake, & Paule, 2004). Thus, from a neurodevelopmental perspective, temporal processing shows promise as a possible clinical correlate of cerebellar-striatal-frontal circuitry.
The developing literature on temporal processing in ASD has yielded mixed results thus far (e.g., see Gowen & Miall, 2005; Radonovich & Mostofsky, 2004; Wallace & Happe, 2008), although the preponderance of evidence supports aberrant second-range temporal processing (Allman, DeLeon, & Wearden, 2011; Maister & Plaisted-Grant, 2011; Martin, Poirier, & Bowler, 2011; Szelag et al., 2004). Cross-study comparisons are complicated by differences in modality, interval duration, age range, and task structure. Several studies have reported results in relatively small samples (Allman et al., 2011; Szelag et al., 2004), which may be problematic in light of phenotypic heterogeneity in the ASDs (Matson & Nebel-Schwalm, 2007; Matson & Shoemaker, 2009; Starr, Szatmari, Bryson, & Zwaigenbaum, 2003). The most commonly used paradigm has been temporal reproduction (Maister & Plaisted-Grant, 2011; Martin et al., 2011; Wallace & Happe, 2008) which requires participants to first attend to an auditory or visual stimulus that persists for a pre-specified duration and to then reproduce the perceived duration. In the auditory domain, there is evidence that adults with ASD are more variable and less accurate than adults without an ASD diagnosis when asked to reproduce durations ranging from 1–4 seconds (Martin et al., 2011). In contrast, Wallace & Happe (2008) found that younger participants (9–18 years) with ASD did not differ from typically developing individuals when asked to reproduce auditory durations ranging from 2 to 45 seconds (Wallace & Happe, 2008). In the visual domain, one study showed children with ASD (8–13 years) had reduced accuracy for short durations (<2 seconds) and long durations (45 seconds) but not for durations in the 4–30 second range (Maister & Plaisted-Grant, 2011). The children with ASD were also more variable when reproducing the shorter durations; however, it is difficult to draw conclusions about variability given that the estimate was based on two data points per duration (Maister & Plaisted-Grant, 2011). In spite of these limitations, the existing literature suggests that variability in temporal reproductions may be an important characteristic of the performance of individuals with ASD (Maister & Plaisted-Grant, 2011; Martin et al., 2011; Szelag et al., 2004). Adequate assessment of variability requires larger sample sizes as well as multiple repetitions of the same duration in order to calculate reliable estimates of error variance. There are therefore unanswered questions regarding the precision of temporal estimations in children with ASD and whether precision improves with age, as has been shown in prior studies of typically developing children (Chelonis et al., 2004).
Temporal processing deficits have also been repeatedly demonstrated in Attention Deficit/Hyperactivity Disorder (ADHD; Toplak, Dockstader, & Tannock, 2006) and have been linked to variability in executive functions such as working memory (Bauermeister, Barkley, Martinez, Cumba, Ramirez, Reina, Matos, & Salas, 2005). There is preliminary evidence that working memory may play a role in ASD as well; for example, temporal processing deficits were found to be associated with short-term visual-spatial memory (Maister & Plaisted-Grant, 2011) and auditory working memory in children with ASD (Allman et al., 2011); however, as with prior studies, the authors did not report on the presence or level of co-morbid attention problems. Under the prior diagnostic classification system (DSM-IV, American Psychiatric Association, 2000), attention-related problems were subsumed by the ASD diagnosis even when children displayed clinically significant symptoms of ADHD. In other words, children who were diagnosed with ASD prior to 2013 would not have been diagnosed with ADHD, even if they exhibited symptoms consistent with that diagnosis. Estimates of ADHD symptoms in ASD are quite high (Leyfer, Folstein, Bacalman, Davis, Dinh, Morgan, Tager-Flusberg, & Lainhart, 2006), and have been shown to exacerbate deficits in verbal working memory (Yerys, Wallace, Sokoloff, Shook, James, & Kenworthy, 2009), raising the possibility that disrupted temporal processing is symptomatic of attention-related deficits rather than autism per se. Thus, in order to establish that temporal processing deficits are also characteristic of ASDs, quantification of co-morbid attention-related problems is imperative.
The primary aims of this study were: (1) to compare ASD and typically developing youth on accuracy and consistency of time reproduction across different interval durations; (2) to characterize the contribution of working memory to accuracy and consistency (variability) of time reproduction in youth with and without ASD; (3) to examine the relationship between age and time reproduction in both groups; and (4) to address limitations in the literature to date by assessing the impact of concomitant inattention/hyperactivity on time reproduction in youth with ASD.
We hypothesized that: (1) individuals with ASD would be less accurate and less consistent than individuals without ASD; (2) auditory working memory and age would be positively associated with time-reproduction performance; and (3) within the ASD group, inattention/hyperactivity would be inversely related to accuracy and consistency. Finally, we explored the joint effects of these various features on time-reproduction performance, with a particular eye toward examining whether age and working memory would have differential effects on time reproduction performance in youth with ASD.
Methods
Participants
Twenty-seven high functioning children and adolescents with a diagnosis of ASD and 25 typically developing (TD) individuals who were demographically comparable in terms of age and gender, and ranged in age from 9 to 17, took part in the experiment (see Table 1). Participants were recruited through a combination of referrals from pediatric offices, local schools, other autism studies at UCLA, and word of mouth (e.g., participating families recommended the study to friends or acquaintances). Additional recruitment occurred through flyers posted on autism-related websites and distributed at local events. To determine eligibility, all participants in the ASD group were seen at the University of California, Los Angeles (UCLA) Autism Evaluation Clinic for assessment of intellectual functioning and a diagnostic evaluation. The clinical diagnosis of ASD was confirmed by the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 1999) and the Autism Diagnostic Interview Schedule – Revised (ADI-R; Lord, Rutter, & LeCouteur, 1994). All participants were determined to have developed language and a verbal IQ greater than 75. Exclusion criteria included a history of head injuries, seizures, other neurological disorders, and psychiatric disorders other than autism. Eligibility criteria for inclusion in the control group consisted of no prior history of neurological, psychological or psychiatric diagnoses or treatment.
Table 1.
Participant characteristics and p-values for between-group comparisons
| ASD Group | TD Group | P-value | |
|---|---|---|---|
| N | 27 | 25 | --- |
| Age (years) | 12.68 (2.85) | 13.41 (2.32) | 0.3151 |
| Sex | 85% male | 88% male | 0.7764 |
| IQ | 101.31 (11.24) | 106.96 (11.46) | 0.0817 |
| Working memory | 9.54 (2.80) | 10.82 (2.91) | 0.1280 |
| Inattention/hyperactivity | 64.38 (9.25) | 51.53 (2.52) | <0.0001 |
Study Procedures
All participants and their caregivers underwent the informed consent process as part of a large, ongoing study of children with ASD. The time reproduction task was an addition to an extensive battery of questionnaires, computerized measures, and brain imaging that participants completed over multiple days. All study procedures were approved by the UCLA Institutional Review Board.
The parent-report version of the Achenbach Child Behavior Checklist (CBCL; Achenbach, 1991), which consists of 118 questions that cover eight different behavioral domains, was completed for individuals in both groups. The Attention Problems subscale includes items related to inattention, distractibility, sustained attention, restlessness, and impulsivity. For the purpose of this study, this subscale was used as a global measure of inattention/hyperactivity. All raw scores for the Attention Problems subscale were converted to standard scores (T-scores; M=50, SD=10).
The Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV; Wechsler, 2003) was used to assess general intellectual ability. In addition to the Full Scale Intelligence Quotient (FSIQ), the WISC-IV yields subtest scores in several domains. We used the Letter-Number Sequencing subtest as a measure of auditory attention and working memory. All raw scores were converted to standard scores (WISC-IV Scaled Score; M=10, SD=3).
Time Reproduction (TR) measures an individual’s ability to estimate a temporal duration and to then utilize that estimate to execute a motor response, the “reproduction” (Barkley, Edwards, Laneri, Fletcher, & Metevia, 2001). We used a previously-validated computerized time reproduction paradigm (Barkley, 1998b) that displays two light-bulbs simultaneously on the computer screen. The light-bulb on the left was turned on for an interval of 4, 8, 12, 16 or 20 seconds and when it went off the participant held the space bar down in order to “light up” the bulb on the right for the same amount of time (i.e., reproduce the interval). Prior to beginning the task, participants were given an opportunity to practice to ensure comprehension of the task instructions. Each of the 5 temporal durations was repeated 4 times in a random order, resulting in a total of 20 test trials. No performance-based feedback was provided; however, verbal praise and encouragement for effort were given to keep participants oriented and to maximize motivation to do well.
Data Analysis
To measure time-reproduction performance, we used the coefficient of accuracy (CoA), calculated by dividing the subject’s estimate of the temporal interval by the actual length of the interval presented, yielding a percentage measure of error. A score of 1.0 represents perfect accuracy whereas scores lower and higher than 1.0 represent under- and over-estimates, respectively. The CoA, which is also sometimes referred to as the duration judgment ratio, is a common outcome measure in studies of time reproduction (e.g., Hurks & Hendriksen, 2011; Kerns et al., 2001; Mullins et al., 2005; Plummer & Humphrey, 2008). We averaged the CoAs from the four repetitions of each trial type (4, 8, 12, 16, and 20 second durations) to create response accuracy scores. Response consistency was defined as the standard deviation of the four repetitions for each of the time durations. Consistency has been less frequently examined than accuracy, with most studies using the average of either the coefficient of accuracy or the absolute discrepancy as the primary outcome; however, there is evidence to suggest that the standard deviation of the coefficient of accuracy, as a measure of intra-individual variability, is an important consideration in time reproduction studies (Plummer & Humphrey, 2008). Prior to the calculation of the average accuracy and consistency scores, we eliminated trials that were believed to be the result of participant error (e.g., participant’s finger slipped off the space bar) and were therefore invalid. These were defined as subject duration estimates of less than 0.15 sec and amounted to 1.23% of the total number of observations at the trial level. It should be noted that the coefficient of accuracy is also prone to outliers, which can have a substantial impact on analyses. However, extreme values and high variability are also a potentially important aspect of the clinical phenotype; in particular, such performance patterns could be attributable to attentional difficulties, which are a major focus of our analyses. We therefore retained outlying values of accuracy and consistency in our primary models. However, since it is undesirable to have results which are driven by a small number of unusual data points, we performed sensitivity analyses in which the outlying observations were truncated to assess the stability of our findings. Specifically, we considered a point to be an outlier if it was more than 3 standard deviations away from the mean, as that is the approximate cutoff beyond which no such values would be expected in a sample with n=125–135 observations (the numbers of response accuracy and consistency values available in the TD and ASD groups, respectively). We determined outlier status separately for the two groups since their distributions were expected to differ. The primary models were rerun with the outlying observations thresholded to the value 3 standard deviations above (or below) the mean.
We used mixed effects repeated measures models with maximum likelihood estimation to analyze the impact of our predictor variables on (1) response accuracy (average CoA) and (2) response consistency (standard deviation of CoA). Specifically, the models comparing the diagnostic groups included group (ASD versus typically developing) as the between subjects factor, interval duration (4,8,12,16 or 20 seconds) as the repeated (within subjects) factor and working memory, age (range 9–17), IQ, and sex as covariates, along with the corresponding two-way interactions (group by interval, group by memory, group by age, interval by age, interval by memory, age by memory) to look at differential patterns of time reproduction. Parental ratings of inattention/hyperactivity were added in follow-up models within the ASD group only to determine whether co-morbid inattention/hyperactivity in these subjects is associated with reduced accuracy and consistency. (These variables were not included in the primary models as they showed little variability in the control group; indeed clinically meaningful levels of such symptomatology were exclusionary for these subjects). Unstructured covariance matrices were used to model the repeated measures since there were no a priori hypotheses about the patterns of relationships among the measurements. Subject-level random intercepts were also included to account for the fact that individuals may have their own baseline time reproduction performance level (consistently high or low) across trials. A maximum likelihood procedure was used for parameter estimation rather than (the typically lower bias) restricted maximum likelihood to allow likelihood ratio tests for nested models based on groups of fixed effects. However, estimates were comparable when using restricted maximum likelihood (results not shown). All statistical tests used a two-sided significance level of α=0.05.
Results
Mean age, IQ, and gender distribution by group are shown in Table 1. There were no significant group differences on any of these variables or on the WISC-IV Letter-Number Sequencing task (auditory working memory). There was a statistically significant between-group difference for the CBCL Attention Problems subscale, t(43)= 5.89, p<0.0001, with the ASD group evidencing more attention problems than the control group. As expected, the distribution of inattention/hyperactivity ratings within the control group centered on the normative mean with very little variability (M=51.53, SD=2.52). This was in contrast to the ASD group, for which parent ratings of inattention/hyperactivity were significantly higher than the normative mean and more widely distributed than in the control group (M=64.38, SD=9.25).
To assess strategy utilization, we surveyed 38 of the participants after they had completed the task. We first asked them if they had used a strategy and, if so, to describe it. We then followed up by asking them directly if they had counted. Seventeen out of 19 in the ASD group and 19 out of 19 in the TD group reported that they had counted to keep track of time passing. In other words, almost all participants attempted to use a counting strategy to keep track of time passing. Assessing the effectiveness of this strategy (e.g., consistent pacing and correct sequencing) was beyond the scope of this project.
Full sample analysis: Accuracy
In the initial mixed models for accuracy, terms involving IQ and sex along with several of the two-way interactions were not significant, and were thus removed in a stepwise fashion to reduce the number of degrees of freedom used and to improve the precision of the remaining parameter estimates. The final model included main effects of group (p = .0369), interval duration (p < .0001), age (p = .0009), and auditory working memory (p = .0005), along with interactions between group and age (p = .0371), auditory working memory and interval duration (p = .0043), age and interval duration (p < .0001), age and auditory working memory (p = .0021) and group by interval duration (p = .5491). (See Table 2A for details.) This latter term was retained in the model despite being non-significant because it represented a core experimental question. The group by age interaction indicated that individuals with ASD were less accurate than typically developing individuals, with that difference being more pronounced in younger subjects. There were also significant interactions between interval duration and age, and between interval duration and working memory; overall, participants tended to more severely underestimate longer interval durations but this effect was more pronounced in younger subjects and subjects with poorer auditory working memory. We also found that the effects of age and working memory on accuracy were interdependent, as the negative impact of poor auditory working memory was exacerbated in younger children.
Table 2.
F-statistics and p-values for analyses run with both groups (A) and with the ASD-only group (B).
| (A) | ||||
|---|---|---|---|---|
| FULL SAMPLE ANALYSIS | ||||
| Predictor | Accuracy | Consistency | ||
| F-statistic | P-value | F-statistic | P-value | |
| Group | F(1,192)=4.42 | 0.0369 | F(1,192)=0.17 | 0.0011 |
| Interval duration | F(4,192)=14.45 | <.0001 | F(4,192)=8.10 | <.0001 |
| Age | F(1,192)=11.38 | 0.0009 | F(1,192)=31.31 | <.0001 |
| Working memory | F(1,192)=12.39 | 0.0005 | F(1,192)=30.87 | <.0001 |
| Group*interval | F(4,192)=0.77 | 0.5491 | F(4,192)=0.33 | 0.8513 |
| Group*age | F(1,192)=4.41 | 0.0371 | F(1,192)=0.03 | 0.0026 |
| Working memory*interval | F(4,192)=3.93 | 0.0043 | F(4,192)=4.44 | 0.0014 |
| Age*interval | F(4,192)=10.32 | <.0001 | F(4,192)=3.78 | 0.0044 |
| Age*working memory | F(1,192)=9.72 | 0.0021 | F(1,192)=22.73 | <.0001 |
| (B) | ||||
|---|---|---|---|---|
| ASD-ONLY ANALYSIS | ||||
| Predictor | Accuracy | Consistency | ||
| F-statistic | P-value | F-statistic | P-value | |
| Interval duration | F(4,192)=17.56 | <.0001 | F(4,192)=9.54 | <.0001 |
| Age | F(1,192)=6.60 | 0.0117 | F(1,192)=23.40 | <.0001 |
| Working memory | F(1,192)=8.95 | 0.0035 | F(1,192)=23.44 | <.0001 |
| Inattention/hyperactivity | F(1,192)=0.08 | 0.7841 | F(1,192)=0.73 | 0.3936 |
| Working memory*interval | F(4,192)=5.43 | 0.0006 | F(4,192)=4.17 | 0.0011 |
| Age*interval | F(4,192)=10.43 | <.0001 | F(4,192)=4.98 | 0.0037 |
| Age*working memory | F(1,192)=6.60 | 0.0118 | F(1,192)=15.13 | 0.0002 |
There was one ASD subject who had an outlying average accuracy on the 4 second trials. We therefore re-ran the final model with that observation truncated, as described above. All of the model terms remained significant and of the same order of magnitude except that the working memory by interval duration interaction was attenuated (new p-value = .1350), indicating that that result should be viewed with caution.
Full sample analysis: Consistency
The same modeling procedure as above was repeated with consistency as the dependent variable. As before there were main effects of group (p=.0011), interval duration (p < .0001), age (p < .0001), and working memory (p < .0001), as well as interactions between group and working memory (p = .0026), interval duration and working memory (p = .0014), interval duration and age (p = .0044), and age and working memory (p < .0001) but not between group and interval duration (p = .8513). The working memory by group interaction indicated that individuals with ASD were less consistent than typically developing individuals, with that difference being more pronounced in subjects with poorer working memory. There were also significant interactions between interval duration and working memory and between interval duration and age, whereby individuals became more inconsistent (i.e., variable) when trying to reproduce shorter interval durations, with those effects being magnified in those who had lower auditory working memory scores or were younger. We also found that the effects of age and working memory on consistency were interdependent, with the negative impact of poor auditory working memory being exacerbated in younger children.
There were 5 outlying consistency observations, two in the typically developing group and 3 in the ASD group. When the final model was rerun with those observations truncated, all of the terms remained significant and of the same order of magnitude except that the age by interval duration interaction was attenuated (new p-value = .1520), suggesting that this result should be interpreted with caution.
ASD-only Analysis: Accuracy and consistency
A follow-up analysis was used to determine whether co-morbid inattention/hyperactivity is associated with accuracy and consistency of time reproduction performance in individuals with ASD. Specifically, the same mixed effects repeated measures models were rerun with the ASD-only sample, adding inattention/hyperactivity ratings and removing the diagnostic group variable and associated interactions. Results showed that inattention/hyperactivity ratings were not significantly associated with time reproduction accuracy or consistency for individuals with ASD after controlling for age, working memory and interval duration. The interactions between interval duration and age, age and working memory and interval duration and working memory, remained significant in both models, paralleling the findings in the full sample (see Table 2B). Individuals with ASD underestimated longer durations and less consistently reproduced shorter durations and these effects were more pronounced in individuals who were younger or who had lower auditory working memory scores. Furthermore, the effect of age on time reproduction performance depended on auditory working memory ability. Younger children were even less accurate and more variable in their responses when they also had lower working memory scores. The sensitivity analyses showed the same pattern of attenuation of the working memory by interval duration interaction for accuracy and the age by interval duration interaction for consistency.
Discussion
To our knowledge, this is the first study to evaluate the joint effects of age, auditory working memory, inattention/hyperactivity, and ASD diagnosis on accuracy and consistency of time reproduction. Several novel findings were observed.
The first hypothesis of our study, that we would find group differences in accuracy and consistency, was confirmed. Overall, we find that ASD subjects perform more poorly than typically developing individuals on both accuracy and consistency, with the former effect being moderated by age and the latter by working memory, a novel set of findings. Specifically the gap in accuracy between youth with ASD and those without decreases as age increases, while the gap in consistency increases as a function of working memory deficits.
Our second hypothesis was also confirmed. Consistent with prior research linking auditory working memory (Allman et al., 2011) to temporal processing in children with ASD, auditory working memory was a significant predictor in our study. Across the full sample (individuals with ASD and those without) poorer working memory is associated with worse accuracy and consistency of time reproduction with that effect being magnified at younger ages. Subjects became less consistent as interval durations increased with that effect being more pronounced in those with poorer working memory. Time perception conceivably operates in concert with working memory, which would support the maintenance of temporal representations, to guide more complex behavior. As previous studies have demonstrated (e.g., Baudouin, Vanneste, Pouthas, & Isingrini, 2006), our sense of time, and ability to reproduce our experience of it, is sensitive to other processes that will need to be accounted for when attempting to isolate timing functions.In general we found that accuracy and consistency improve with advancing age, with the degree of effect on the former being moderated by diagnosis, working memory and interval duration and on the latter by working memory and possibly interval duration. This complements prior work showing that the precision of temporal estimates is influenced by developmental changes (Chelonis et al., 2004). Younger children were less accurate and more inconsistent in their temporal reproductions, and these effects were even stronger for individuals with poor working memory. Participants more severely underestimated longer interval durations than shorter ones with this effect being more pronounced in younger subjects. Our age-related findings suggest the potential value of methodological approaches that could more thoroughly examine within-group change from a developmental perspective. For example, future research could model developmental trajectories to examine how the relationship between temporal processing and other cognitive processes (e.g., working memory, attention) changes over time and potentially at different rates for different sub-groups of children with ASD (Thomas, Annaz, Ansari, Scerif, Jarrold, & Karmiloff-Smith, 2009).
Contrary to our hypothesis, parental ratings of inattention/hyperactivity were not associated with the accuracy or consistency of temporal reproductions after adjusting for age, working memory and interval duration. Thus, in our sample, variability in time reproduction performance was not solely attributable to concomitant attention-related symptoms in the ASD group. However, it should be noted that this was a high functioning sample and not necessarily representative of the full spectrum of inattention/hyperactivity that can co-occur with ASD. A possible limitation to this study was the use of the CBCL Attention Problems subscale, which includes symptoms in both the inattentive and hyperactive/impulsive domains, in place of DSM symptom counts from a structured parent interview. As a result, we were not able to assess the differential contributions of inattention and hyperactivity/impulsivity to time reproduction performance. This could be addressed in future studies.
Our study converges with and extends a small but growing body of literature examining temporal processing in ASD (Allman et al., 2011; Maister & Plaisted-Grant, 2011; Martin et al., 2011; Szelag et al., 2004; Gil, Chambres et al., 2012). Research is only just beginning to untangle the relationship between temporal processing and other cognitive and behavioral processes. One of the many questions arising from this line of research is whether temporal processing can be linked to functional deficits in brain development, possibly in frontal-striatal circuitry. To answer this question, future research could use brain morphology within regions relevant to temporal processing (e.g., caudate volume) or connectivity between the basal ganglia and frontal regions to assess associations with time reproduction performance. It is also possible that temporal processing deficits reflect a more general underlying vulnerability in neurobiological systems that are also important for working memory, which would be consistent with integrative theoretical models of oscillatory neuronal firing in frontal-striatal circuits (Lustig, Matell, & Meck, 2005).
From a broader theoretical perspective, deficits in temporal processing could have a highly dispersed impact on other cognitive processes (e.g., language) and social behavior (Boucher et al., 2007; Wimpory et al., 2002); however, there is very little data at present to support this assertion. In one study of temporal processing, Boucher and colleagues (2007) demonstrated that children with high functioning autism fail to show a developmentally appropriate tendency to think “backwards” and “forwards” across time, are impaired in their ability to represent qualitative change across time, and struggle to conceptualize successive temporal events as a unitary whole. Initial findings were replicated in a second group of children and adolescents with ASD, with a series of control tasks employed to rule out the possibility that the early findings were attributable to other task demands (e.g., ability to draw inferences, generate varied responses, understand or have experience with task-specific events) rather than temporal processing per se (Boucher et al., 2007). These results suggest that there are functional implications of an impaired sense of time that are evident in how children with autism perceive chronological sequences and use past and future events to contextualize the present. There is also preliminary evidence from a study by Allman and colleagues (2011) that laboratory measures of temporal processing have ecological validity in predicting parent-ratings of their child’s sense of time, as measured by the “It’s About Time” questionnaire (Barkley, 1998a).
Time is an elemental feature of subjective experience that is potentially relevant to a wide range of activities necessary for daily living. To establish deficits in temporal processing as a useful phenotypic marker in ASD will require additional evidence documenting associated functional impairments and how these evolve over the lifespan. Possible avenues of inquiry might include assessments of temporal processing as it relates to general adaptive function, social skills, language development, or academic performance. A more nuanced view of temporal processing in the context of daily life, and how it evolves over the lifespan, has the potential to revise our approach to behavioral intervention in ASD. For example, environmental contingencies are time-sensitive and may be influenced by a poor perception of elapsed time. Simple temporal adjustments in the delivery of rewards or consequences for certain behaviors could have implications for treatment effectiveness, particularly for younger children.
This study adds to the existing literature by characterizing the effects of chronological age and working memory on the accuracy and consistency of duration reproductions in ASD. Results underscore the importance of modeling age-related change as well as any supportive cognitive functions that may be recruited during temporal processing tasks. A broader objective of this study was to explore temporal processing as a dimensionally-quantifiable trait with a plausible neurobiological substrate that could serve as a clinical correlate of aberrant brain function. Consistency in methodology among studies of temporal processing will help strengthen cross-study comparisons so that current findings can be extended inward to neurobiological systems as well as outward to potential behavioral manifestations of aberrant timing functions in social interaction, patterns of communication, and inflexible motor repertoires.
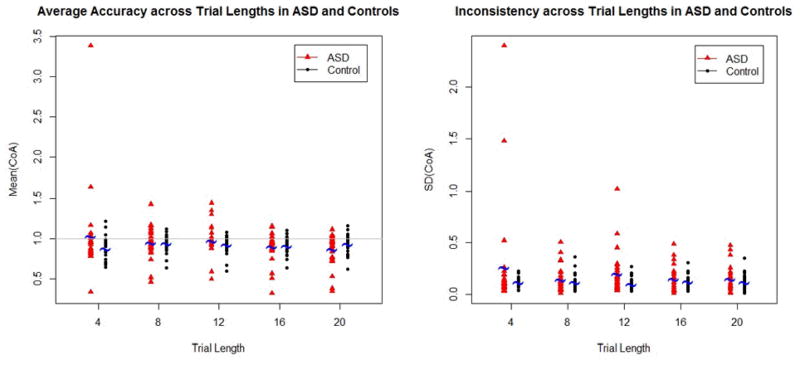
Figure 1.
Time reproduction accuracy (average coefficient of accuracy) and consistency (standard deviation of the coefficient of accuracy) for the five target durations (4, 8, 12, 16, and 20) by diagnosis (ASD and control). For accuracy, values of 1 represent perfect accuracy and anything less than 1 is an under-estimation of the target duration. For consistency, lower values indicate more consistent responding whereas higher values indicate greater variability.
Acknowledgments
This work was supported by National Institutes of Health grants P50HD055784 and 1R01HD065280.
We would like to thank all the families for generously donating their time so that this study could be possible. We are also grateful to the research staff for their dedication to this project.
References
- Achenbach TM. Integrative Guide to the 1991 CBCL/4-18, YSR, and TRF Profiles. Burlington, VT: University of Vermont, Department of Psychology; 1991. [Google Scholar]
- Allman MJ, DeLeon IG, Wearden JH. Psychophysical assessment of timing in individuals with autism. Am J Intellect Dev Disabil. 2011;116(2):165–178. doi: 10.1352/1944-7558-116.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual-text revision. 4. Washington, DC: American Psychiatric Publishing; 2000. [Google Scholar]
- Barkley RA. It’s about time questionnaire. Syracuse; NY: 1998a. [Google Scholar]
- Barkley RA. Time perception application - version 1.0 (computer software) University of Massachussetts Medical Center: Chesapeake Technology; 1998b. [Google Scholar]
- Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. Executive functioning, temporal discounting, and sense of time in adolescents with attention deficit hyperactivity disorder (adhd) and oppositional defiant disorder (odd) J Abnorm Child Psychol. 2001;29(6):541–556. doi: 10.1023/a:1012233310098. [DOI] [PubMed] [Google Scholar]
- Baudouin A, Vanneste S, Pouthas V, Isingrini M. Age-related changes in duration reproduction: involvement of working memory processes. Brain Cogn. 2006;62(1):17–23. doi: 10.1016/j.bandc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Bauermeister JJ, Barkley RA, Martinez JV, Cumba E, Ramirez RR, Reina G, et al. Time estimation and performance on reproduction tasks in subtypes of children with attention deficit hyperactivity disorder. J Clin Child Adolesc Psychol. 2005;34(1):151–162. doi: 10.1207/s15374424jccp3401_14. [DOI] [PubMed] [Google Scholar]
- Boucher J, Pons F, Lind S, Williams D. Temporal cognition in children with autistic spectrum disorders: Tests of diachronic thinking. J Autism Dev Disord. 2007;37(8):1413–1429. doi: 10.1007/s10803-006-0285-9. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Flake RA, Baldwin RL, Blake DJ, Paule MG. Developmental aspects of timing behavior in children. Neurotoxicol Teratol. 2004;26(3):461–476. doi: 10.1016/j.ntt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: Developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gil S, Chambres P, Hyvert C, Fanget M, Droit-Volet S. Children with autism spectrum disorders have “the working raw material” for time perception. PLoS One. 2012;7(11):e49116. doi: 10.1371/journal.pone.0049116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen E, Miall RC. Behavioural aspects of cerebellar function in adults with asperger syndrome. Cerebellum. 2005;4(4):279–289. doi: 10.1080/14734220500355332. [DOI] [PubMed] [Google Scholar]
- Hurks PP, Hendriksen JG. Retrospective and prospective time deficits in childhood adhd: The effects of task modality, duration, and symptom dimensions. Child Neuropsychol. 2011;17(1):34–50. doi: 10.1080/09297049.2010.514403. [DOI] [PubMed] [Google Scholar]
- Kerns KA, McInerney RJ, Wilde NJ. Time reproduction, working memory, and behavioral inhibition in children with adhd. Child Neuropsychol. 2001;7(1):21–31. doi: 10.1076/chin.7.1.21.3149. [DOI] [PubMed] [Google Scholar]
- Levitt P, Campbell DB. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J Clin Invest. 2009;119(4):747–754. doi: 10.1172/JCI37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y, Ebstein RP. Research review: Crossing syndrome boundaries in the search for brain endophenotypes. J Child Psychol Psychiatry. 2009;50(6):657–668. doi: 10.1111/j.1469-7610.2008.01986.x. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. J Autism Dev Disord. 2006;36(7):849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Lustig C, Matell MS, Meck WH. Not “just” a coincidence: Frontal-striatal interactions in working memory and interval timing. Memory. 2005;13(3–4):441–448. doi: 10.1080/09658210344000404. [DOI] [PubMed] [Google Scholar]
- Maister L, Plaisted-Grant KC. Time perception and its relationship to memory in autism spectrum conditions. Dev Sci. 2011;14(6):1311–1322. doi: 10.1111/j.1467-7687.2011.01077.x. [DOI] [PubMed] [Google Scholar]
- Martin JS, Poirier M, Bowler DM. Brief report: Impaired temporal reproduction performance in adults with autism spectrum disorder. J Autism Dev Disord. 2011;40(5):640–646. doi: 10.1007/s10803-009-0904-3. [DOI] [PubMed] [Google Scholar]
- Matson JL, Nebel-Schwalm MS. Comorbid psychopathology with autism spectrum disorder in children: An overview. Res Dev Disabil. 2007;28(4):341–352. doi: 10.1016/j.ridd.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Matson JL, Shoemaker M. Intellectual disability and its relationship to autism spectrum disorders. Res Dev Disabil. 2009 doi: 10.1016/j.ridd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Buonomano DV. The neural basis of temporal processing. Annu Rev Neurosci. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- Meck WH, Benson AM. Dissecting the brain’s internal clock: How frontal-striatal circuitry keeps time and shifts attention. Brain Cogn. 2002;48(1):195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- Mullins C, Bellgrove MA, Gill M, Robertson IH. Variability in time reproduction: Difference in adhd combined and inattentive subtypes. J Am Acad Child Adolesc Psychiatry. 2005;44(2):169–176. doi: 10.1097/00004583-200502000-00009. [DOI] [PubMed] [Google Scholar]
- Plummer C, Humphrey N. Time perception in children with adhd: The effects of task modality and duration. Child Neuropsychol. 2008:1–16. doi: 10.1080/09297040802403690. [DOI] [PubMed] [Google Scholar]
- Radonovich KJ, Mostofsky SH. Duration judgments in children with adhd suggest deficient utilization of temporal information rather than general impairment in timing. Child Neuropsychol. 2004;10(3):162–172. doi: 10.1080/09297040409609807. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. Nimh diagnostic interview schedule for children version iv (nimh disc-iv): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: A systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur Psychiatry. 2008;23(4):289–299. doi: 10.1016/j.eurpsy.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Starr E, Szatmari P, Bryson S, Zwaigenbaum L. Stability and change among high-functioning children with pervasive developmental disorders: A 2-year outcome study. J Autism Dev Disord. 2003;33(1):15–22. doi: 10.1023/a:1022222202970. [DOI] [PubMed] [Google Scholar]
- Szelag E, Kowalska J, Galkowski T, Poppel E. Temporal processing deficits in high-functioning children with autism. Br J Psychol. 2004;95(Pt 3):269–282. doi: 10.1348/0007126041528167. [DOI] [PubMed] [Google Scholar]
- Thomas MS, Annaz D, Ansari D, Scerif G, Jarrold C, Karmiloff-Smith A. Using developmental trajectories to understand developmental disorders. J Speech Lang Hear Res. 2009;52(2):336–358. doi: 10.1044/1092-4388(2009/07-0144). [DOI] [PubMed] [Google Scholar]
- Toplak ME, Dockstader C, Tannock R. Temporal information processing in adhd: Findings to date and new methods. J Neurosci Methods. 2006;151(1):15–29. doi: 10.1016/j.jneumeth.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Happe F. Time perception in autism spectrum disorders. Research in Autism Spectrum Disorders. 2008;2:447–455. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4. The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Wimpory D, Nicholas B, Nash S. Social timing, clock genes and autism: A new hypothesis. J Intellect Disabil Res. 2002;46(Pt 4):352–358. doi: 10.1046/j.1365-2788.2002.00423.x. [DOI] [PubMed] [Google Scholar]
- Yerys BE, Wallace GL, Sokoloff JL, Shook DA, James JD, Kenworthy L. Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Res. 2009;2(6):322–333. doi: 10.1002/aur.103. [DOI] [PMC free article] [PubMed] [Google Scholar]