Abstract
Alzheimer’s disease (AD) is accompanied by an activation of the innate immune system, and many epidemiological studies have shown reduced risk for dementia or AD associated with chronic consumption of non-steroidal anti-inflammatory drugs (NSAIDS). These observations led to animal model studies to test the hypothesis that NSAIDs can be disease-modifying for some aspects of AD pathogenesis. NSAIDS cannot only suppress inflammatory targets, which could contribute to neuroprotection, they also slow amyloid deposition by mechanisms that remain unclear. Several large clinical trials with NSAID therapies with AD subjects have failed, and cyclooxygenase-2 does not appear to be a useful target for disease modifying therapy. However, there may be apolipoprotein E E4 pharmacogenomic effects and a real but delayed positive signal in a large primary prevention trial with naproxen. This encourages researchers to re-address possible mechanisms for a stage-dependent NSAID efficacy, the subject of this review.
Keywords: Alzheimer’s disease, cyclooxygenases, non-steroidal anti-inflammatory drugs, docosahexaenoic acid, curcumin
INTRODUCTION
Non-steroidal anti-inflammatory drugs (NSAIDS) including over-the-counter drugs ibuprofen and naproxen, have a relatively strong rationale and consistent positive epidemiological association for reducing Alzheimer’s disease (AD) risk [1, 2]. In contrast, there is little evidence that NSAIDs can be used to treat established AD. Despite some initial promise from small trials with indomethacin and diclofenac, NSAID trials for 12–18 months with established AD have been disappointing including a mild cognitive impairment (MCI) conversion trial with a selective cyclooxygenase-2 (COX-2) inhibitor (rofecoxib), which was definitively negative and may have even increased pathogenesis [3]. Also, there has not been strong epidemiology supporting selective COX-2 inhibitors. The epidemiology data have consistently shown a duration of use or lagging effect of at least 2 years for reduced risk with conventional NSAIDs [4]. More recent results with nearly 50,000 dementia cases and several hundred thousand control subjects from the Veterans Affairs database have confirmed that chronic use of ibuprofen or naproxen is associated with significantly reduced AD risk [5]. To address whether chronic inflammation is present in animal models and whether NSAIDs may act causally to slow AD pathogenesis, our group initially looked for and found a quantifiable inflammatory glial response in amyloid precursor protein (APP) Tg2576 AD model mice [6]. We then tested ibuprofen, the NSAID with the strongest association with reduced risk from epidemiology, to see whether it could reduce inflammation in APP Tg2576 mice. Ibuprofen not only reduced brain interleukin 1β levels and astrocyte and microglial activation, but also amyloid plaque pathogenesis present in the APP Tg2576 model [7]. Control of inflammation with anti-inflammatory drugs was anticipated, but reduced accumulation of amyloid plaques, a central pathological feature in AD, was an exciting surprise. In general, these anti-amyloid effects have been confirmed and extended [2, 8, 9] (Table 1). However, the mechanisms involved remain unclear. The following year, Weggen et al. [10] demonstrated that a selective subset of NSAIDs, including ibuprofen and indomethacin (but not naproxen), can reduce γ-secretase production of amyloid β-peptide (1–42) (Aβ42). However, both naproxen and ibuprofen [5] and perhaps other NSAIDs, including aspirin that lack “selective Aβ42 lowering agent” activity still appear to reduce AD risk [11]. Therefore, AD risk reduction likely can also derive from other mechanisms, possibly including to their common property of COX inhibition. Consistent with this, we reported that in vivo anti-amyloidogenic dosing with ibuprofen produced central nervous system levels that were in the low µM range, sufficient to inhibit COX-1 and COX-2 as well as interleukin-1 and other inflammatory mediators, but did not seem to be high enough to produce adequate γ-secretase modulation based on the in vitro dosing needed for that activity [12]. In addition, ibuprofen reduced amyloid burden in Aβ-infused rats, arguing against γ-secretase playing an exclusive role to reduce burden [12].
Table 1.
Anti Amyloid Pathology Effects by NSAIDS
| Drug/Dose | Model | Intervention Stage (Duration)* | Main Effect Aβ | Details & Other Effects | References |
|---|---|---|---|---|---|
| ibuprofen 375 ppm | Tg2576 mouse | middle (10–16 mos) | reduction | reduce plaque 56%, reduced SDS insol Aβ:39%, reduced Il-1β, GFAP and microgliosis | [7] |
| ibuprofen 375 ppm | Tg2576 mouse | late (14–17 months) | reduction | soluble Aβ reduced, insoluble Aβ and Aβ42 reduced, but less robust than longer treatments, reduced caspase activation & reduction open field behavior | [13] |
| ibuprofen 50 mg/kg/day | Tg2576 mouse | early (4 months of age, acute for 3 days) | reduction | reduced Aβ42 and increases 1–38, naproxen had no effect | [10] |
| ibuprofen 375 ppm | APPsw × PS1 | middle (from 7 to 12 months of age) | reduction | reduction in plaque load (20–25% but not fibrillar) | [14] |
| NCX-2216, nitro-S-fluorbiprofen (COX-1 specific) | APPsw × PS1 | middle (from 7 to 12 months of age) | reduction | 40–45% reduction in plaques, and 35–40% reduction in Congo Red plaques, increase in plaque associated MHC-II microglia involved in clearance | [14] |
| celecoxib (COX-2 specific) 175 ppm | APPsw × PS1 | middle (from 7 to 12 months of age) | No effect | no effect on plaque burden, increased Aβ42 | [14] |
| indomethacin 2.24 mg/kg in drinking water | Tg2576 (APPsw) | middle (from 12 to 14 months of age) and from 12 to 20 months of age | reduction | reduced hippocampal plaque burden 20% and prostaglandins 90% | [15] |
| ibuprofen 375 ppm | Tg2576 (APPsw) | middle (from 11 to 15 months of age) | reduction | 60% reduction in plaque burden, reduction in SDS soluble Aβ42, reduction in CDllb microglial activation | [8] |
| ibuprofen OR piaglitazone | APPV717I mice | middle (acute, 10 month old for 7 days) | reduction | reduced Aβ42 (both drugs), reduces soluble Aβ (just pioglitazone, reduced β-secretase mRNA and protein levels, reduced COX-2 iNOS mRNA | [16] |
| ibuprofen (50 mg/kg/day), fluorbiprofen (10–25 mg/kg/day) | Tg2576 (APPsw) | early (4 months of age, acute for 3 days) | no effect | overall negative | [17] |
| ibuprofen 375 ppm | rat infusion | middle (from 21 to 22 months of age) | reduction | reduction in diffuse plaques | [12] |
| R-flurbiprofein 10 mg/kg/day | Tg2576 (APPsw) | early (3 months of age) and late | reduction | reduction in plaque at both ages, but corretion of spatial memory deficits only with early intervention | [18] |
| ibuprofen 375 ppm | Tg2576 (APPsw) | Early & acute (from 3 to 4 or from 11.5 to 13 months of age) | No effect | No effect on total brain Aβ levels, (Aβ burden not measured) improved long-term potentiation mediated by COX-2, improved performance in water maze | [19] |
| ibuprofen 375 ppm | 3× Tg AD | early (from 1 to 6 months of age) | reduction | reduces intraneuronal Aβ and phospho tau, corrects memory deficits | [20] |
| ibuprofen (375 ppm) OR celecoxib (COX-2 specific) 120 ppm | APPsw × PS1 | early to late (1 month to 18 months) | reduction | reduction in plaque burden, protects against Noradrenaline and glutamate loss by magnetic resonance spectrocopy | [21] |
| Triflusal (COX-1 Specific) | Tg2576 (APPsw) | middle (from 10 to 13 months age) and (from 1 to 18 months | reduction (Thio S only, not total Aβ load) | longer duration more effective, reducing dense core (Thio S) plaques 67%, No effect on total plaque burden, also reduction in interleukin-1β, tumor necrosis factor-α, astrocytosis and microgliosis, and straightening of dystrophic neurites, correction of defects in spatial learning and contextual fear conditioning | [22] |
Intervention/Duration: Early (pre-plaque) Middle (peri-plaque) or late (post plaque).
RESULTS OF HALTED PRIMARY PREVENTION ADAPT TRIAL: FIRST NEGATIVE, THEN POSITIVE? RELEVANCE TO STAGE-DEPENDENT MECHANISMS
Here we discuss the results of the Alzheimer’s disease anti-inflammatory prevention Trial (ADAPT), questioning the conclusion that NSAIDs simply failed to prevent AD. The issues are complex, requiring an in-depth discussion to interpret and understand the findings. The ADAPT trial enrolled 2,528 patients over 70 with a family history of AD to compare the effects of twice daily 220 mg naproxen, 200 mg COX-2-selective Celebrex or placebo [23]. Although at the start of the trial these subjects were cognitively normal, they were chosen based on a family risk for AD. At the time of enrollment it was understood that some subjects would be well along in the pro-dromal period of AD pathogenesis and therefore at risk of beginning cognitive decline. Unfortunately as will be discussed, there was a failure to exclude some individuals with pre-existing cognitive deficits.
After median time 1.5 years duration on NSAIDs, the ADAPT trial was halted in December 2004. Not only were there slight increases in gastrointestinal bleeds and fatal and non-fatal heart attacks and strokes in the 2 NSAID groups, but also cognitive testing was showing significantly lower global summary cognitive scores in both NSAID groups, and there was already a disturbing non-significant trend in the intent to treat for NSAIDs to increase conversion to AD [23]. As will be explained in the subsequent discussion, extended follow-up on subjects from this seemingly negative study provided a much more complex picture with the emergence of possible stage-dependent protective effects. In the initial data, naproxen may have had more of an adverse effect on subjects with baseline deficits, possibly because of a stage-dependent alteration in the immune response. For example, in patients with cognitive deficits and established AD, a microglial dystrophy with fragmenting of processes has been reported associated with neurofibrillary tangles that is phenotypically distinct from the amyloid-associated microglial activation seen at earlier stages [24]. This novel observation indicates a potential dysfunction of microglial interactions that may explain a failure in responsiveness to any stimuli, including NSAIDs. One molecular mechanism that may help explain a hypothesized loss of responsiveness to anti-inflammatory stimuli could involve the anti-inflammatory microglial cell regulator CD200 and its receptor, which were both recently reported to be reduced in AD brain [25] and suggested to impede normal microglial response to inhibitory stimuli. Consistent with possible stage-dependent efficacy, later stage NSAID interventions were less effective in APP transgenic models (Table 1). NSAIDs could also exert possible negative effects by inhibiting potential protective effects of cytokines. For example, interleukin-6 has been proposed to be neuroprotective and clear plaques in an APP transgenic model [26]. While NSAIDs might therefore increase pathogenesis by reducing interleukin-6, mixed COX inhibitors have not been shown to increase deposition or accelerate pathogenesis (see Table 1).
Adverse effects observed at late intervention (post-MCI) with mixed COX inhibitors may be independent of inflammation, for example by interfering with constitutive neuronal COX-2 required for memory retention [27]. While COX-2 over-expression is implicated in playing some role in early AD, and one trial demonstrated benefits pre-MCI [28] and COX-2 antagonism can protect acute effects of long-term potentiation (LTP) (pre-pathology in an animal models [19] COX-2 antagonism has failed in trials post-MCI and in exerting robust effect on plaque pathogenesis in animal models (see Table 1 and next section). This is in contrast to COX-1 inhibition, where in a recent randomized placebo-controlled NSAID trial with pre-AD intervention, the TRIMCI study, employing a prescription aspirin-like COX-1 inhibitor, Triflusal, to try and delay cognitive decline in MCI patients over an 18-month period. The trial was halted early (median 13 months on drug) even though there were no adverse effects reported because of poor enrollment, but demonstrated a significant delay in the probability of progression to AD [29].
Even though the ADAPT trial had been halted, 2,000 subjects were followed to examine the possibility of a delayed or lagging protective activity. Longer term follow-up at an average of 3.5 years after drug dosing and 2 years after drug was halted, revealed a very different picture than initial findings. Some patients in all groups were determined to have had baseline cognitive deficits, and when these patients were removed from the analysis and only new cases considered, the naproxen group, but not the selective COX-2 inhibitor group had 67% less AD risk, a statistically significant protective effect [30]. A positive result with early intervention (pre-MCI) but not late intervention (post-MCI) is consistent with both the epidemiology and animal model data (Table 1). COX-dependent risk reduction appears relevant because the COX-1/ COX-2 inhibitor naproxen arm, but not the COX-2 specific inhibitor Celebrex arm was positive on follow-up. Furthermore, naproxen use was associated with a 40% reduction in AD cerebrospinal fluid (CSF) tau: Aβ42 ratio compared to placebo or Celebrex in a study of 117 of the subjects randomized to drug in the ADAPT trial [30]. These differences were in samples taken 21–41 months after the drug was discontinued. In summary, AD pathogenesis was apparently suppressed or delayed by the mixed COX-1 and COX-2 inhibitor naproxen, but not by COX-2 selective inhibition at a pre-clinical stage of AD in humans.
There are caveats. The number of subjects converting to AD remains small and could merely be a statistical Type II error. For example, it is possible that this new result represents chance effects obtained by excluding the dementia cases that were accelerated in the earlier report. The patients continue to be followed, and additional data will be needed to clarify the results.
These potentially exciting results suggest that early NSAID intervention may yet prove useful, but powering these studies and recruitment have been problematic. Follow-up on ADAPT is currently being pursued by Breitner and colleagues who will continue to follow and re-evaluate subjects in the ADAPT trial groups as they age. The ADAPT results certainly need confirmation, but they further encourage us to try to understand how and when naproxen or ibuprofen COX inhibition may protect against AD pathogenesis in animal models and in the clinic. That said, long-term naproxen safety concerns led to halting the ADAPT trial after 2 years. At the dose employed, persistent naproxen use for more than 2 years is well known to increase risk for gastrointestinal bleeds. Thus, it would be most practical if lower dosing proved to be efficacious and for a shorter duration (eg. 2 years), particularly since late intervention may have detrimental effects on cognition.
The ADAPT trial raises many questions, for example: did it really show protection and if so, how can we improve upon conventional NSAID safety and efficacy to protect more individuals in at-risk elderly populations? Can alternative NSAIDs, proton pump inhibitors, or lower NSAID doses or shorter courses of therapy or safer NSAIDs be used? If NSAIDs can really work, but only if they are introduced prior to MCI, might that also be true of other potential interventions? Most important, how can we reconcile the new ADAPT clinical trial data and consistent evidence from epidemiology that chronic exposure to an NSAID like naproxen can delay AD onset but clearly fail to arrest decline in clinical trials with established AD? What is the likely mechanism of action?
NSAID MECHANISMS FOR PROTECTING NEURONS AND COGNITIVE FUNCTION
A case can be made for COX-2 based on the theory that increased excitotoxic activation is a major early cause of neurotoxicity in AD and AD models [31]. Increasing COX-2 potentiates excitotoxicity [32], and increased neuronal COX-2 is found both at early stages of AD pathogenesis in humans [33, 34] and with Aβ-induced neurodegeneration in animals [35]. This suggests that COX-2 might be a useful NSAID target to inhibit neurodegeneration related to excitotoxicity in AD.
With Ashe and colleagues (Univ. Minnesota), we have explored how naproxen and ibuprofen can be protect LTP in vitro and cognitive function in vivo, and concluded that NSAIDs with COX-2 inhibitory activity can block Aβ oligomer-induced LTP deficits in vitro and early cognitive deficits in APP transgenic mice [19]. These data support a COX inhibition-dependent mechanism not requiring soluble Aβ lowering activity from γ-secretase modulation. This would be consistent with recent meta-analysis that concluded apparent protective effects in humans was independent of γ secretase modulation and related Aβ42 lowering activity [11]. However, in this Tg2576 mouse study where the model has limited neurodegeneration, a selective COX-2 inhibitor showed some benefits at early time-points or after acute administration to hippocampal slices. This suggested COX-2 as an important target. A role for COX-2 as an important NSAID target at early stages is also consistent with data, which show a cognitive benefit with mild memory complaints and glucose utilization measured by fluorodeoxyglucose(18F)-positron emission tomography [28]. But ultimately, COX-2 inhibitors have not worked in clinical trials and the COX-2 inhibitor rofecoxib was even associated with increased AD in a trial with MCI patients [3]. Since Celebrex also failed to prevent or delay AD in the ADAPT trial, there is limited remaining rationale to pursue selective COX-2 inhibition and its potential neuroprotective role as the primary target sufficient for AD prevention. COX-2 inhibitors have not proved to show major Aβ lowering effects in vivo. In fact, our unpublished data show a major increase in β-amyloid deposition (Frautschy et al., unpublished) and others have reported that some COX-2 inhibitors may increase Aβ production [14].
The more obvious remaining primary target based on failed human and animal model trials is COX-1 [2]. COX-2 shows higher expression in neurons, while COX-1 is the major COX isoform in reactive microglia and appears to be a better target for controlling inflammation [33]. Thus, although COX-1 is generally regarded as a non-inducible “housekeeping” enzyme and targeting it could be considered to have adverse gastrointestinal effects, as with mixed COX inhibitors, COX-2-specific inhibitors may increase cardiovascular risk [36].
It is also possible that NSAIDs might have direct effects on tau pathology. In triple transgenic mice expressing mutant APP, presenilin 1 and tau, ibuprofen not only reduced Aβ (intraneuronal) but also phosphorylated tau pathology [20]. Because ibuprofen slows Aβ accumulation, which is known to drive tau accumulation in this model [37], it remains unclear whether or not these ibuprofen effects on tau are simply secondary to Aβ reduction. However, if NSAIDs really had a significant primary activity that involved preventing the accumulation of tau pathology, one might predict that NSAIDs would prevent progression in established AD. This is because tau pathology is sufficient to drive cognitive decline in other forms of tauopathy, for example frontotemporal dementia with tau mutations, where progression of tau pathology correlates with continuing cognitive decline throughout the progression of AD from mild to severe stages. However, NSAID use is not associated with less tau pathology but with an index of some efficacy, namely fewer reactive glia [38, 39].
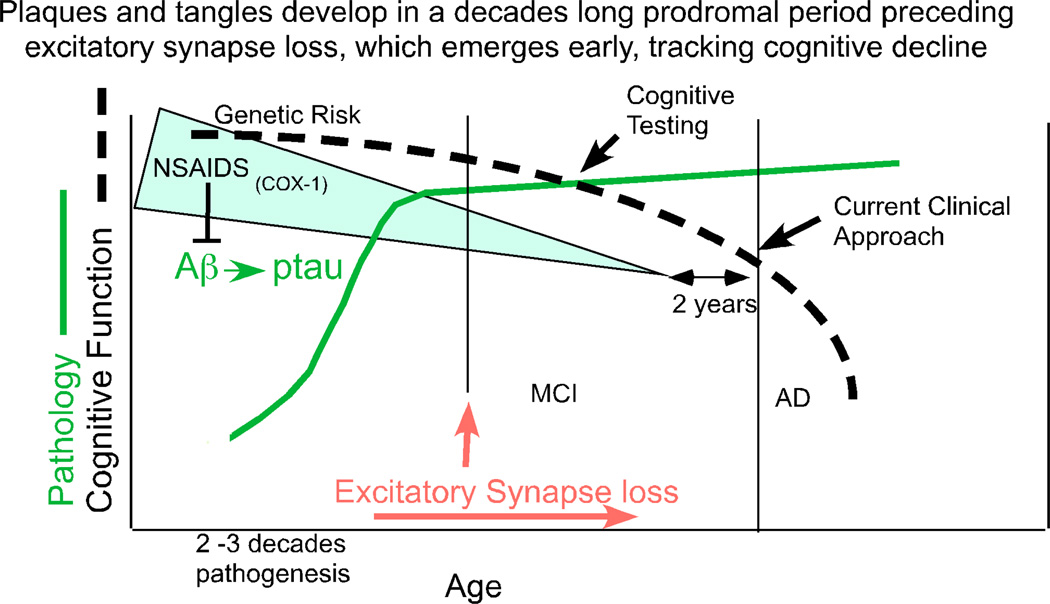
HYPOTHESIS: THE PRIMARY MECHANISM OF CHRONIC NSAID USE IS TO DELAY AMYLOID ACCUMULATION AND AD ONSET (SEE FIG. 1)
Fig. (1). NSAIDs act in the pro-dromal period.
AD pathogenesis begins with genetic or environmental risk factors that accelerate through a prolonged period of at least several decades of Aβ (plaque) and phospho-tau (ptau) (tangle) pathogenesis and later plateau based on studies in Down’s syndrome [51, 52] and cross-sectional studies of cognitively intact individuals dying with AD pathology prior to cognitive deficits [50, 53]. Imaging and cerebrospinal fluid biomarker studies both confirm that amyloid accumulation and tau pathology clearly precede cognitive decline defined as mild cognitive impairment (MCI) by at least several years [54–56]. The transition from cognitively normal to MCI is accompanied by increased ultrastructural and gene expression evidence for increased synapse loss in AD vulnerable regions [57, 58]. This synaptic marker loss includes an early loss of N-methyl-D-aspartic acid receptor subunits [59] and drebrin [60, 61], a marker for excitatory synapses [62].
Genetic data show that mutations which increase Aβ42 production accelerate AD onset prior to age 65. Similarly, Apolipoprotein E (ApoE) ApoE4 accelerates amyloid accumulation and has its most dramatic effect accelerating age of AD onset [40]. Early and chronic NSAID exposure has the opposite effect and delays AD onset [4, 41]. In contrast, chronic NSAID exposure only in the two years prior to AD does not protect in the epidemiology [42, 43]. Consistent with this, large clinical trials with NSAIDs including naproxen have failed to improve cognitive function or slow AD progression in mild to moderate AD [23, 44]. Instead, NSAIDs appear to protect best against very early events including initial cognitive decline and onset, primarily in those with ApoE4 and without reference to γ-secretase modulatory activity [45, 46].
The events leading to AD during the pro-dromal period of plaque and tangle buildup include an initial buildup of amyloid (found in 20–40% of cognitively normal elderly) that is followed first by an increase in CSF tau, reflected in a higher CSF tau/Aβ42 ratio. This is then followed by more pronounced brain atrophy detected by magnetic resonance imaging (and in cross-sectional data by synaptic marker loss), which is fairly proximal to and most predictive of memory decline within a few years [47]. Complex neuroinflammatory changes are intimately related to and proximal to the pathology buildup. NSAIDs could block any or all of these steps. For example, NSAIDs can prevent but cannot reverse the neuronal cell cycle reentry hypothesized to cause neurodegeneration, which provides an intriguing and plausible mechanism for stage-dependent neuroprotection [48]. Based on this evidence one might hypothesize that if NSAIDs really can delay AD, it is most likely due to influencing causal events in the pathogenesis which precede the closer correlates of cognitive decline like tau pathology or synapse loss. The most obvious primary initiating event determining age of onset is amyloid accumulation, and this endpoint is clearly delayed by early intervention with ibuprofen but not selective COX-2 inhibitors in animal models.
One obvious alternative possibility is that NSAIDs with COX-1 inhibitory activity can act to suppress a chronic inflammatory feedback loop involved in early events in the pro-dromal period of AD pathogenesis, for example, Aβ accumulation [7]. NSAIDs may also suppress pro-inflammatory S100β, which is elevated prior to Aβ deposition in Tg2576 where it promotes Aβ accumulation [49].
A delay or reversal in the build-up of amyloid and secondary downstream tangle pathology may significantly extend this decades long process. which lags clinical onset [50]. Further, a delay or reversal of amyloid accumulation might be expected to persist after treatment is halted as in the ADAPT trial, where there appeared to be a lagging effect. In addition, because different cytokines clearly have different effects that can differentially impact amyloidogenic and inflammatory components of the disease, we cannot assume that all NSAIDs will act like naproxen. Some NSAIDs may delay onset but have no effect on progression, while others may accelerate onset but slow progression.
ALTERNATIVE NSAID MECHANISMS FOR AMYLOID REDUCTION
The epidemiology data remain robust for naproxen and ibuprofen (except for the frail elderly on very high doses). Also, the ADAPT trial suggests that naproxen may be disease-modifying. Therefore, protection via alternative mechanisms involving anti-inflammatory pathways acting through COX inhibition (and shared with naproxen) have to be carefully examined [12]. More than one mechanism is likely involved. 1) NSAIDs may reduce Aβ production by lowering pro-inflammatory cytokines that upregulate expression of APP [63] or 2) reduce β-site APP-cleaving enzyme 1 [64] or, 3) reduce aggregation by limiting production of the pro-amyloidogenic co-factor α1ACT [12, 65]. 4) In addition, conventional NSAIDs may increase Aβ clearance by microglia by lowering prostaglandin E2 and its EP2-receptor-mediated suppression of Aβ clearance [66, 67]. 5) NSAIDs may act to protect or favor amyloid clearance, a vaccine-like effect that might reduce pre-existing amyloid deposits. For example, microglial or astrocyte clearance of Aβ deposits is active at early stages of amyloid accumulation and influenced by immunomodulatory cytokines and chemokines [68, 69], which may be enhanced or protected by NSAIDs.
However, the idea that NSAIDs may increase Aβ clearance by engaging phagocytic microglia or astrocytes is a controversial area. Careful 3D reconstruction of microglia around established amyloid plaques has failed to reveal microglial phagocytosis of Aβ in vivo in APP23 mice [70], consistent with earlier reports in AD tissue. However, the role of microglia associated with plaques has long been controversial [71] and may depend on stage, plaque-type and the state of monocytic cell differention [72]. Anti-Aβ antibody can stimulate microglial phagocytosis [73, 74] but antibody may not be required, as microglia clearly recognize plaques without immunization, but phagocytosis is blocked. In contrast, invading monocytic lineage dendritic cells can clear pre-existing deposits with appropriate stimulation [75]. In vivo studies have shown multiple immune factors appear to influence microglial amyloid clearance [76]. Finally, microglia can play a role in soluble Aβ clearance, including ApoE-dependent endolytic peptide clearance [77].
Alternatively NSAIDs may facilitate clearance by invading monocytic lineage cells. For example, NSAIDs may shift the balance of pro- versus anti-inflammatory cytokines (interleukins 4 and 10) and increase amyloid clearance, which was reported to be increased by interleukin-4 [75]. The role of traditional anti-inflammatory cytokines is not straightforward, as stimulating the prototypical anti-inflammatory cytokine transforming growth factor-β can increase amyloid deposition in an Aβ injection model [78] and blocking it can mitigate amyloidosis and increase peripheral macrophage-associated Aβ clearance [79]. It is possible that NSAIDs have similar effects.
AN ApoE4-DEPENDENCE OF NSAID PROTECTION?
Additional mechanistic questions are raised because recent epidemiological studies suggest that although both ApoE genotypes may respond to NSAIDs, the ApoE4 patients may be better responders [45, 46, 80]. This may reflect evidence of greater susceptibility to oxidative damage and inflammation in animals and individuals with ApoE4 [81]. For example, ApoE4 subjects have significantly higher levels of α1 anti-chymotrypsin (ACT) [82], a mixed COX inhibitor NSAID target in APP transgenic mice [12]. Higher plasma levels of ACT and several other inflammatory markers have been associated with increased dementia and AD risk in the Rotterdam study [83]. This may mean that plasma biomarkers like ACT could be used to help predict who is most likely to benefit from an NSAID prevention program. However, NSAIDs alone were insufficient to control oxidative damage in APP transgenic mice, suggesting that NSAIDs should be combined with antioxidants. Indeed, individuals who use both NSAIDs and antioxidants did show slower cognitive decline in the Cache County study, but again only in those with ApoE4 [84]. Further work with NSAIDs in AD animal models expressing human ApoE4 and ApoE3 may help determine whether candidate NSAID/APOE pharmacogenomic interactions related to inflammation and amyloid that can perhaps be observed and better understood in animal models than diverse clinical populations. This is an area of obvious relevance to the design of clinical trials for prevention.
NSAID EPIDEMIOLOGY, ApoE4 RISK AND EFFECTS ON HUMAN PATHOLOGY
Studies of twins and siblings with presumed equivalent genetic AD risk but discordant for NSAID use have shown that NSAID use appears to prevent or delay the onset of AD by roughly a decade [41]. Subsequent positive NSAID epidemiology data have been clearest with earlier onset AD cases [43, 85] but not all studies with later onset AD subjects have shown protection. For example, one recent publication observed that NSAID consumption actually appeared to increase AD risk in a Seattle area cohort, average age 82 [86]. The authors of this study offered one possible explanation, that many of the NSAID users may have been at risk for earlier onset, but delayed their AD onset by NSAID use. This possibility would only make sense if NSAIDs selectively delayed AD more effectively in earlier onset subjects, for example those with ApoE4 risk than those with later onset. Another recent study with a “Religious Orders” cohort concluded there was no protective effect of NSAID use on either AD risk or the development of amyloid and tau pathology [87]. Again, one possible factor explaining lack of NSAID protection would be that the average age at death in this cohort was 86, which is consistent with less ApoE4 risk and possibly fewer subjects who are potentially those best protected by NSAIDs. A hint of clinical trial evidence that this may be true comes from a 12-month randomized, controlled trial in 132 subjects with 800 mg of ibuprofen with a proton pump inhibitor or placebo. They found no overall impact of treatment on the Alzheimer's disease assessment scale-cognitive subscale, but a lack of progression only in the subgroup of 27 ibuprofen-treated ApoE4 subjects [88]. The study is underpowered to draw firm conclusions, but this is consistent with the epidemiology cited above.
Whether NSAIDs better protect ApoE4 subjects has also been raised in a study of AD pathology in NSAID users. Consistent with this possibility, reduction of reactive glia appeared greater in those with ApoE4 genotype, even though NSAIDs did not reduce Aβ accumulation [39]. Reduced reactive glia in the absence of reduced amyloid could be consistent with reduced neurodegeneration, but neurodegeneration was not measured. One caveat to interpreting these correlations with pathology in relation to NSAID risk reduction is that the subjects were all AD cases and typically end-stage. The pathology results may not reflect the impact of pro-dromal/ pre-clinical NSAID use in individuals who may have prevented or delayed AD. Another, perhaps more likely explanation of this discordant negative Religious Orders study is that the participants’ level of NSAID exposure was low. Consistency of NSAID exposure was calculated as an index based on the fraction of annual visits where NSAID use was recorded. The authors reported “for the variable assessing consistency of NSAID use during the follow-up period the mean score was 0.17 (SD 0.26) for the non-aspirin NSAIDs with a value of 0.07 and an SD of 0.16 for ibuprofen … indicating that relatively few subjects had been consistent users…”. In contrast, risk reduction and delayed cognitive decline have been the norm in studies documenting consistent NSAID use.
ALTERNATIVE NSAIDs
Because of the toxicity issues associated with chronic NSAIDs, our group and others have pursued alternatives including plant flavonoids, the polyphenolic NSAID antioxidant curcumin and omega-3 fatty acids. Omega-3 fatty acids found in fish and fish oil are associated with reduced AD risk and limit pathology in our AD animal models [89–91] and similar results have been obtained by others. Like NSAIDs, docosahexaenoic acid (DHA) and omega-3 fatty acids may have major ApoE pharmacogenomic differences (reviewed in [92]). The pharmacogenomic effect of DHA may be more robust than NSAIDs, but the relationship is complex, as unlike NSAIDs ApoE3 patients may respond better to DHA compared to ApoE4 patients [92]. This raises the question as to whether DHA and NSAIDs may synergize to minimize potential differences in ApoE3 vs ApoE4 responses. Some initial clinical trial data indicate omega-3 fatty acids may also potentially slow progression from early stage AD [93], and this result has some support from other suggestive, albeit still inconclusive trials. Because dietary DHA can competitively reduce brain arachidonic acid and its metabolites, it exerts some NSAID-like activity. DHA has a great safety profile but omega-3 fatty acids are usually used as adjuncts because they are not potent anti-inflammatory drugs, and omega-3 fatty acids from fish did not lower inflammatory markers in CSF from patients with established AD [94]. Thus, omega-3 fatty acids, which are pleiotropic and appear likely helpful if given early, are unlikely to be an adequate substitute for conventional NSAIDs.
The curry spice curcumin is another alternative NSAID that can reduce inflammatory markers and amyloid in AD model mice [95]. However, small initial clinical trials in AD patients have not been successful, possibly because of bioavailability problems and the need for NSAIDs to be given pre-clinically. In addition to NSAID activity, curcumin is pleiotropically neuroprotective and also has direct anti-amyloid activity [96, 97]. Because conventional curcumin formulations have poor bioavailability problems in human clinical trial data, we recently compiled animal model data on the blood and brain curcumin levels obtained with various routes of administration, and the levels achieved in relation to levels required to control inflammation and amyloid in AD model mice [98]. Because of bioavailability problems in humans, new oral formulations with better delivery have been developed which appear to deliver anti-inflammatory levels. These formulations are currently in clinical trials for inflammatory diseases and AD. Other plant-derived polyphenols with NSAID activity have been tested in animal models, and may also provide potentially safer alternatives for AD.
CONCLUDING REMARKS
Converging data from epidemiology, clinical trials, and animal models suggest that NSAIDs with COX-1 inhibitory activity can delay AD pathogenesis at the level of amyloid accumulation by mechanisms that are not entirely clear. In contrast, many conventional NSAIDs have toxicity issues with chronic use (depending on efficacious dose) and are not effective in slowing the progression of subjects with mild to moderate AD. They are likely to be most clinically relevant in ApoE4 carriers. NSAIDs may exert multiple mechanisms for slowing amyloid accumulation, some depend on the type of NSAID (γ secretase modulation), but most NSAID mechanisms are likely to include an effect on COX-1. In addition, mechanisms may include immunomodulatory activity, perhaps influencing the innate immune system and amyloid clearance. Safer alternative NSAIDS include the omega-3 fatty acid DHA, which may work better in ApoE3 patients, and the polyphenolic antioxidant/NSAID curcumin. These also lower amyloid accumulation in animal models and may be most useful for prevention. One may speculate that other treatments with favorable safety profiles that delay amyloid accumulation in transgenic mice that model segments of the pro-dromal period of AD may be similar and potentially useful for delaying AD onset, but ineffective in slowing AD progression.
ACKNOWLEDGEMENTS
This work was supported by U01AG28583 (SAF), AG021975 (SAF), NCCAM NIH R01AT3008 (GMC), NIH NIA RO1 AG13471 (GMC), VA Merit (GMC and SAF) and the Mary S. Easton Alzheimer’s Center and Easton Drug Discovery Consortium.
ABBREVIATIONS
- ACT
α1 anti-chymotrypsin
- AD
Alzheimer’s disease
- ADAPT
Alzheimer’s Disease anti-inflammatory prevention Trial
- ApoE
Apolipoprotein E
- APP
Amyloid precursor protein
- Aβ
β-amyloid peptide
- CSF
Cerebrospinal fluid
- COX
Cyclooxygenase
- DHA
Docosahexaenoic acid
- LTP
Long-term potentiation
- MCI
Mild cognitive impairment
- NSAIDs
Nonsteroidal anti-inflammatory drugs
REFERENCES
- 1.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NE, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol. Aging. 2007;28:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Aisen PS, Thal LJ, Ferris SH, Assaid C, Nessly ML, Giuliani MJ, Lines CR, Norman BA, Potter WZ. Rofecoxib in patients with mild cognitive impairment: further analyses of data from a randomized, double-blind, trial. Curr. Alzheimer Res. 2008;5:73–82. doi: 10.2174/156720508783884602. [DOI] [PubMed] [Google Scholar]
- 4.Szekely CA, Thorne JE, Zandi PP, Ek M, Messias E, Breitner JC, Goodman SN. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer's disease: a systematic review. Neuroepidemiology. 2004;23:159–169. doi: 10.1159/000078501. [DOI] [PubMed] [Google Scholar]
- 5.Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frautschy SA, Yang F, Irizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- 7.Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's Disease. J. Neurosci. 2000;20(15):5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, Citron M, Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer's disease. J. Neurosci. 2003;23:7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriksen JL, Sagi SA, Smith TE, Weggen S, Das P, McLendon DC, Ozols VV, Jessing KW, Zavitz KH, Koo EH, Golde TE. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J. Clin. Invest. 2003;112:440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 11.Szekely CA, Green RC, Breitner JC, Ostbye T, Beiser AS, Corrada MM, Dodge HH, Ganguli M, Kawas CH, Kuller LH, Psaty BM, Resnick SM, Wolf PA, Zonderman AB, Welsh-Bohmer KA, Zandi PP. No advantage of A beta 42- lowering NSAIDs for prevention of Alzheimer dementia in six pooled cohort studies. Neurology. 2008;70:2291–2298. doi: 10.1212/01.wnl.0000313933.17796.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morihara T, Teter B, Yang F, Lim GP, Boudinot S, Boudinot FD, Frautschy SA, Cole GM. Ibuprofen suppresses interleukin-1beta induction of pro-amyloidogenic alpha1-antichymotrypsin to ameliorate beta-amyloid (Abeta) pathology in Alzheimer's models. Neuropsychopharmacology. 2005;30:1111–1120. doi: 10.1038/sj.npp.1300668. [DOI] [PubMed] [Google Scholar]
- 13.Lim GP, Yang F, Chu T, Gahtan E, Ubeda O, Beech W, Overmier JB, Hsiao AK, Frautschy SA, Cole GM. Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice. Neurobiol. Aging. 2001;22:983–991. doi: 10.1016/s0197-4580(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 14.Jantzen PT, Connor KE, DiCarlo G, Wenk GL, Wallace JL, Rojiani AM, Coppola D, Morgan D, Gordon MN. Microglial activation and beta -amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J. Neurosci. 2002;22:2246–2254. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn J, Montine T, Morrow J, Woodward WR, Kulhanek D, Eckenstein F. Inflammation and cerebral amyloidosis are disconnected in an animal model of Alzheimer's disease. J. Neuroimmunol. 2003;137:32–41. doi: 10.1016/s0165-5728(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 16.Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O'Banion K, Klockgether T, Van Leuven F, Landreth GE. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- 17.Lanz TA, Fici GJ, Merchant KM. Lack of specific amyloidbeta(1–42) suppression by nonsteroidal anti-inflammatory drugs in young, plaque-free Tg2576 mice and in guinea pig neuronal cultures. J. Pharmacol. Exp. Ther. 2005;312:399–406. doi: 10.1124/jpet.104.073965. [DOI] [PubMed] [Google Scholar]
- 18.Kukar T, Prescott S, Eriksen JL, Holloway V, Murphy MP, Koo EH, Golde TE, Nicolle MM. Chronic administration of R-flurbiprofen attenuates learning impairments in transgenic amyloid precursor protein mice. BMC Neurosci. 2007;8:54. doi: 10.1186/1471-2202-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotilinek LA, Westerman MA, Wang Q, Panizzon K, Lim GP, Simonyi A, Lesne S, Falinska A, Younkin LH, Younkin SG, Rowan M, Cleary J, Wallis RA, Sun GY, Cole G, Frautschy S, Anwyl R, Ashe KH. Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity. Brain. 2008;131:651–664. doi: 10.1093/brain/awn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKee AC, Carreras I, Hossain L, Ryu H, Klein WL, Oddo S, LaFerla FM, Jenkins BG, Kowall NW, Dedeoglu A. Ibuprofen reduces Abeta, hyperphosphorylated tau and memory deficits in Alzheimer mice. Brain Res. 2008;1207:225–236. doi: 10.1016/j.brainres.2008.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi JK, Jenkins BG, Carreras I, Kaymakcalan S, Cormier K, Kowall NW, Dedeoglu A. Anti-inflammatory treatment in AD mice protects against neuronal pathology. Exp. Neurol. 2009 doi: 10.1016/j.expneurol.2009.07.032. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coma M, Serenó L, Da Rocha-Souto B, Scotton TC, Espana J, Sánchez MB, Rodríguez M, Agulló J, Guardia-Laguarta C, Garcia-Alloza M, Agulló J, Guardia-Laguarta C, Garcia- Alloza M, Borrelli LA, Clarimón J, Lleó A, Bacskai BJ, Saura CA, Hyman BT, Gómez-Isla T. Triflusal Reduces Dense-Core Plaque Load, Associated Axonal Alterations and Inflammatory Changes, and Rescues Cognition in a Transgenic Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2010 doi: 10.1016/j.nbd.2010.01.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, Evans D, Green R, Mullan M. Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch. Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker DG, Dalsing-Hernandez JE, Campbell NA, Lue LF. Decreased expression of CD200 and CD200 receptor in Alzheimer's disease: a potential mechanism leading to chronic inflammation. Exp. Neurol. 2009;215:5–19. doi: 10.1016/j.expneurol.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C, Zubair AC, Dickson D, Golde TE, Das P. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2009;24:548–559. doi: 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rall JM, Mach SA, Dash PK. Intrahippocampal infusion of a cyclooxygenase-2 inhibitor attenuates memory acquisition in rats. Brain Res. 2003;968:273–276. doi: 10.1016/s0006-8993(03)02248-0. [DOI] [PubMed] [Google Scholar]
- 28.Small GW, Siddarth P, Silverman DH, Ercoli LM, Miller KJ, Lavretsky H, Bookheimer SY, Huang SC, Barrio JR, Phelps ME. Cognitive and cerebral metabolic effects of celecoxib versus placebo in people with age-related memory loss: randomized controlled study. Am. J. Geriatr. Psychiatry. 2008;16:999–1009. doi: 10.1097/JGP.0b013e31818cd3a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez-Isla T, Blesa R, Boada M, Clarimon J, Del Ser T, Domenech G, Ferro JM, Gomez-Anson B, Manubens JM, Martinez-Lage JM, Martínez-Lage JM, Muñoz D, Peña-Casanova J, Torres F. TRIMCI Study Group. A randomized, double-blind, placebo controlled-trial of triflusal in mild cognitive impairment: the TRIMCI study. Alzheimer Dis. Assoc. Disord. 2008;22:21–29. doi: 10.1097/WAD.0b013e3181611024. [DOI] [PubMed] [Google Scholar]
- 30.Laino C. In follow-up analysis of clinical trial, NSAIDs seem to preserve cognitive function in patients wtih healthy brains. Neurol. Today. 2009;9:21–22. [Google Scholar]
- 31.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley KA, Ho L, Winger D, Freire-Moar J, Borelli CB, Aisen PS, Pasinetti GM. Potentiation of excitotoxicity in transgenic mice overexpressing neuronal cyclooxygenase-2. Am. J. Pathol. 1999;155:995–1004. doi: 10.1016/S0002-9440(10)65199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoozemans JJ, Rozemuller JM, van Haastert ES, Veerhuis R, Eikelenboom P. Cyclooxygenase-1 and -2 in the different stages of Alzheimer's disease pathology. Curr. Pharm. Des. 2008;14:1419–1427. doi: 10.2174/138161208784480171. [DOI] [PubMed] [Google Scholar]
- 34.Lukiw WJ, Bazan NG. Cyclooxygenase 2 RNA message abundance, stability, and hypervariability in sporadic Alzheimer neocortex. J. Neurosci. Res. 1997;50:937–945. doi: 10.1002/(SICI)1097-4547(19971215)50:6<937::AID-JNR4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 35.Jang JH, Surh YJ. Beta-amyloid-induced apoptosis is associated with cyclooxygenase-2 up-regulation via the mitogen-activated protein kinase-NF-kappaB signaling pathway. Free Radic. Biol. Med. 2005;38:1604–1613. doi: 10.1016/j.freeradbiomed.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 36.Rao P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J. Pharm. Pharm. Sci. 2008;11:81s–110s. doi: 10.18433/j3t886. [DOI] [PubMed] [Google Scholar]
- 37.Oddo S, Caccamo A, Tseng B, Cheng D, Vasilevko V, Cribbs DH, LaFerla FM. Blocking Abeta42 accumulation delays the onset and progression of tau pathologyviathe C terminus of heat shock protein70-interacting protein: a mechanistic link between Abeta and tau pathology. J. Neurosci. 2008;28:12163–12175. doi: 10.1523/JNEUROSCI.2464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacKenzie IRA, Munoz DG. Nonsteroidal anti-inflammatory drug use and Alzheimer-type pathology in aging. Neurology. 1998;50:986–990. doi: 10.1212/wnl.50.4.986. [DOI] [PubMed] [Google Scholar]
- 39.Alafuzoff I, Overmyer M, Helisalmi S, Soininen H. Lower Counts of Astroglia and Activated Microglia in Patients with Alzheimer's Disease with Regular Use of Non-Steroidal Anti-Inflammatory Drugs. J. Alzheimers Dis. 2000;2:37–46. doi: 10.3233/jad-2000-2105. [DOI] [PubMed] [Google Scholar]
- 40.Breitner JCS, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, Tschanz JT, Plassman BL, Meyer MR, Skoog I. APOE-epsilon 4 count predicts age when prevalence of AD increases, then declines - The Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 41.Breitner JCS, Welsh KA, Helms MJ, Gaskell PC, Gau BA, Roses AD, Vance MAP, Saunders AM. Delayed onset of Alzheimer's disease with nonsteroidal anti-inflammatory and histamine H2 blocking drugs. Neurobiol. Aging. 1995;16:523–530. doi: 10.1016/0197-4580(95)00049-k. [DOI] [PubMed] [Google Scholar]
- 42.Zandi PP, Anthony JC, Hayden KM, Mehta K, Mayer L, Breitner JC. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 2002;59:880–886. doi: 10.1212/wnl.59.6.880. [DOI] [PubMed] [Google Scholar]
- 43.in t' Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N. Engl. J. Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 44.Aisen PS. The inflammatory hypothesis of Alzheimer disease: dead or alive? Alzheimer Dis. Assoc. Disord. 2008;22:4–5. doi: 10.1097/WAD.0b013e318166ca4c. [DOI] [PubMed] [Google Scholar]
- 45.Hayden KM, Zandi PP, Khachaturian AS, Szekely CA, Fotuhi M, Norton MC, Tschanz JT, Pieper CF, Corcoran C, Lyketsos CG, Breitner JC, Welsh-Bohmer KA. Cache County Investigators. Does NSAID use modify cognitive trajectories in the elderly? The Cache County study. Neurology. 2007;69:275–282. doi: 10.1212/01.wnl.0000265223.25679.2a. [DOI] [PubMed] [Google Scholar]
- 46.Szekely CA, Breitner JC, Fitzpatrick AL, Rea TD, Psaty BM, Kuller LH, Zandi PP. NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology. 2008;70:17–24. doi: 10.1212/01.wnl.0000284596.95156.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varvel NH, Bhaskar K, Kounnas MZ, Wagner SL, Yang Y, Lamb BT, Herrup K. NSAIDs prevent, but do not reverse, neuronal cell cycle reentry in a mouse model of Alzheimer disease. J. Clin. Invest. 2009;119:3692–3702. doi: 10.1172/JCI39716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mori T, Koyama N, Arendash GW, Horikoshi-Sakuraba Y, Tan J, Town T. Overexpression of human S100B exacerbates cerebral amyloidosis and gliosis in the Tg2576 mouse model of Alzheimer's disease. Glia. 2010;58:300–314. doi: 10.1002/glia.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann. Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 51.Rumble B, Retallack R, Hilbich C, Simms G, Multhaup G, Martins R, Hockey A, Montgomery P, Beyreuther K, Masters CL. Amyloid A4 protein and its precursor in Down's syndrome and Alzheimer's disease. N. Engl. J. Med. 1989;320:1446–1452. doi: 10.1056/NEJM198906013202203. [DOI] [PubMed] [Google Scholar]
- 52.Hyman BT, West HL, Rebeck GW, Lai F, Mann DM. Neuropathological changes in Down's syndrome hippocampal formation. Effect of age on and apolipoprotein E genotype. Arch. Neurol. 1995;52:373–378. doi: 10.1001/archneur.1995.00540280059019. [DOI] [PubMed] [Google Scholar]
- 53.Morris JC, Storandt M, McKeel DW, Jr, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in "normal" aging: Evidence for presymptomatic and very mild Alzheimer's disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 54.Rabinovici GD, Jagust WJ. Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo. Behav. Neurol. 2009;21:117–128. doi: 10.3233/BEN-2009-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 56.Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, Marcus D, Morris JC, Holtzman DM. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer's disease. EMBO Mol. Med. 2009;1:371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 58.Liang WS, Dunckley T, Beach TG, Grover A, Mastroeni D, Ramsey K, Caselli RJ, Kukull WA, McKeel D, Morris JC, Hulette CM, Schmechel D, Reiman EM, Rogers J, Stephan DA. Neuronal gene expression in non-demented individuals with intermediate Alzheimer's Disease neuropathology. Neurobiol. Aging. 2010;31(4):549–566. doi: 10.1016/j.neurobiolaging.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sze CI, Bi HK-DBK, Filley CM, Martin LJ. N-Methyl-D-asparate receptor subunit proteins and their phosphorylation status are altered selectively in Alzheimer's disease. J. Neurol. Sci. 2001;182:151–159. doi: 10.1016/s0022-510x(00)00467-6. [DOI] [PubMed] [Google Scholar]
- 60.Harigaya Y, Shoji M, Shirao T, Hirai S. Disappearance of actin-binding protein, drebrin, from hippocampal synapses in Alzheimer's Disease. J. Neurosci. Res. 1996;43:87–92. doi: 10.1002/jnr.490430111. [DOI] [PubMed] [Google Scholar]
- 61.Counts SE, Nadeem M, Lad SP, Wuu J, Mufson EJ. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J. Neuropathol. Exp. Neurol. 2006;65:592–601. doi: 10.1097/00005072-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Aoki C, Sekino Y, Hanamura K, Fujisawa S, Mahadomrongkul V, Ren Y, Shirao T. Drebrin A is a postsynaptic protein that localizesin vivoto the submembranous surface of dendritic sites forming excitatory synapses. J. Comp. Neurol. 2005;483:383–402. doi: 10.1002/cne.20449. [DOI] [PubMed] [Google Scholar]
- 63.Lahiri DK, Nall C. Promoter activity of the gene encoding the beta-amyloid precursor protein is up-regulated by growth factors, phorbol ester, retinoic acid and interleukin-1. Mol. Brain Res. 1995;32:233–240. doi: 10.1016/0169-328x(95)00078-7. [DOI] [PubMed] [Google Scholar]
- 64.Sastre M, Dewachter I, Landreth GE, Willson TM, Klockgether T, van Leuven F, Heneka MT. Nonsteroidal antiinflammatory drugs and peroxisome proliferator-activated receptor-gamma agonists modulate immunostimulated processing of amyloid precursor protein through regulation of beta-secretase. J. Neurosci. 2003;23:9796–9804. doi: 10.1523/JNEUROSCI.23-30-09796.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nilsson LN, Bales KR, DiCarlo G, Gordon MN, Morgan D, Paul SM, Potter H. Alpha-1-antichymotrypsin promotes beta-sheet amyloid plaque deposition in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 2001;21:1444–1451. doi: 10.1523/JNEUROSCI.21-05-01444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koenigsknecht-Talboo J, Landreth GE. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J. Neurosci. 2005;25:8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shie FS, Montine KS, Breyer RM, Montine TJ. Microglial EP2 as a new target to increase amyloid beta phagocytosis and decrease amyloid beta-induced damage to neurons. Brain Pathol. 2005;15:134–138. doi: 10.1111/j.1750-3639.2005.tb00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 69.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-betain vitroand in situ. Nat. Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 70.Stalder M, Deller T, Staufenbiel M, Jucker M. 3DReconstruction of microglia and amyloid in APP23 transgenic mice: no evidence of intracellular amyloid. Neurobiol. Aging. 2001;22:427–434. doi: 10.1016/s0197-4580(01)00209-3. [DOI] [PubMed] [Google Scholar]
- 71.Frackowiak J, Wisniewski HM, Wegiel J, Merz GS, Iqbal K, Wang KC. Ultrastructure of the microglia that phagocytose amyloid and the microglia that produce beta-amyloid fibrils. Acta Neuropathol. (Berl) 1992;84:225–233. doi: 10.1007/BF00227813. [DOI] [PubMed] [Google Scholar]
- 72.D'Andrea MR, Cole GM, Ard MD. The microglial phagocytic role with specific plaque types in the Alzheimer disease brain. Neurobiol. Aging. 2004;25:675–683. doi: 10.1016/j.neurobiolaging.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 73.Bard F, Cannon C, Barbour R, Burke R, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 74.Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, Khan K, Kholodenko D, Lee C, Lee M, Motter R, Nguyen M, Reed A, Schenk D, Tang P, Vasquez N, Seubert P, Yednock T. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer's disease-like neuropathology. Proc. Natl. Acad. Sci. USA. 2003;100:2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Butovsky O, Koronyo-Hamaoui M, Kunis G, Ophir E, Landa G, Cohen H, Schwartz M. Glatiramer acetate fights against Alzheimer's disease by inducing dendritic-like microglia expressing insulin-like growth factor 1. Proc. Natl. Acad. Sci. USA. 2006;103:11784–11789. doi: 10.1073/pnas.0604681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El Khoury J, Luster AD. Mechanisms of microglia accumulation in Alzheimer's disease: therapeutic implications. Trends Pharmacol. Sci. 2008;29:626–632. doi: 10.1016/j.tips.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frautschy SA, Yang F, Calderón L, Cole GM. Rodent models of Alzheimer's disease: rat Aβ infusion approaches to amyloid deposits. Neurobiol. Aging. 1996;17:311–321. doi: 10.1016/0197-4580(95)02073-x. [DOI] [PubMed] [Google Scholar]
- 79.Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, Duman RS, Flavell RA. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat. Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yip AG, Green RC, Huyck M, Cupples LA, Farrer LA. Nonsteroidal anti-inflammatory drug use and Alzheimer's disease risk: the MIRAGE Study. BMC Geriatr. 2005;5:2. doi: 10.1186/1471-2318-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol. Nutr. Food Res. 2008;52:131–145. doi: 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- 82.Licastro F, Mallory M, Hansen LA, Masliah E. Increased levels of alpha-1-antichymotrypsin in brains of patients with Alzheimer's disease correlate with activated astrocytes and are affected by APOE 4 genotype. J. Neuroimmunol. 1998;88:105–110. doi: 10.1016/s0165-5728(98)00096-4. [DOI] [PubMed] [Google Scholar]
- 83.Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch. Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 84.Fotuhi M, Zandi PP, Hayden KM, Khachaturian AS, Szekely CA, Wengreen H, Munger RG, Norton MC, Tschanz JT, Lyketsos CG, et al. Better cognitive performance in elderly taking antioxidant vitamins E and C supplements in combination with nonsteroidal anti-inflammatory drugs: the Cache County Study. Alzheimers Dement. 2008;4:223–227. doi: 10.1016/j.jalz.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.In'T Veld BA, Launer LJ, Hoes AW, Ott A, Hofman A, Breteler MM, Stricker BH. NSAIDs and incident Alzheimer's Disease. The Rotterdam study. Neurobiol. Aging. 1998;19:607–611. doi: 10.1016/s0197-4580(98)00096-7. [DOI] [PubMed] [Google Scholar]
- 86.Breitner JC, Haneuse SJ, Walker R, Dublin S, Crane PK, Gray SL, Larson EB. Risk of dementia and AD with prior exposure to NSAIDs in an elderly community-based cohort. Neurology. 2009;72:1899–1905. doi: 10.1212/WNL.0b013e3181a18691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arvanitakis Z, Grodstein F, Bienias JL, Schneider JA, Wilson RS, Kelly JF, Evans DA, Bennett DA. Relation of NSAIDs to incident AD, change in cognitive function, and AD pathology. Neurology. 2008;70:2219–2225. doi: 10.1212/01.wnl.0000313813.48505.86. [DOI] [PubMed] [Google Scholar]
- 88.Pasqualetti P, Bonomini C, Dal Forno G, Paulon L, Sinforiani E, Marra C, Zanetti O, Rossini PM. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer's disease. Aging Clin. Exp. Res. 2009;21:102–110. doi: 10.1007/BF03325217. [DOI] [PubMed] [Google Scholar]
- 89.Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Jr, Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Calon F, Lim GP, Morihara T, Yang F, Ubeda O, Salem NJ, Frautschy SA G.M.C. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer’s disease. Eur. J. Neurosci. 2005;22:617–626. doi: 10.1111/j.1460-9568.2005.04253.x. [DOI] [PubMed] [Google Scholar]
- 91.Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J. Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cole GM, Ma QL, Frautschy SA. Omega-3 fatty acids and dementia. Prostaglandins Leukot. Essent. Fatty Acids. 2009;81:213–221. doi: 10.1016/j.plefa.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch. Neurol. 2006;63:1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 94.Freund-Levi Y, Hjorth E, Lindberg C, Cederholm T, Faxen-Irving G, Vedin I, Palmblad J, Wahlund LO, Schultzberg M, Basun H, Eriksdotter JM. Effects of omega-3 fatty acids on inflammatory markers in cerebrospinal fluid and plasma in Alzheimer's disease: the OmegAD study. Dement. Geriatr. Cogn. Disord. 2009;27:481–490. doi: 10.1159/000218081. [DOI] [PubMed] [Google Scholar]
- 95.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 97.Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv. Exp. Med. Biol. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, Hu S, Faull KF, Teter B, Cole GM, Frautschy SA. Curcumin Structure-Function, Bioavailability, and Efficacy in Models of Neuroinflammation and Alzheimer’s Disease. JPET. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]