Abstract
The objective of this study was to evaluate the effects of steroid anabolic androgenic hormones use on lean mass gain in elderly men through a systematic review with a meta-analysis of randomized controlled studies. We systematically searched PubMed database until 4th October 2013. We included randomized placebo-controlled trials (RCT) that studied testosterone replacement therapy in men over 60 years of age, with total testosterone levels ≤550 ng/dl, observing gains in weight, lean mass tissue and fat mass as outcome. We excluded duplicated studies, studies which mixed men and women, and studies using weak androgens such as dehydroepiandrosterone or androstenedione. The initial search yielded 2681 articles, of which 26 were selected for full text analysis. In the end, 11 studies were included. However, 3 studies were not included in the meta-analysis. Meta-analysis showed that mean weight increased (lean mass), ranging from 1.65 (95 % CI, 1.61–1.69) to 6.20 (95 % CI, 5.22–7.18) kg, although it was heterogeneous (I2 = 98 %). Effect estimate was 3.59 [2.38–4.81]. Androgen therapy decreased fat mass; effect estimate was −1.78 [−2.57, −0.99] that analysis had also a high level of heterogeneity (I2 = 81 %). The results suggest that testosterone replacement therapy is able to increase muscle mass in elderly men and that is affected by the time that the treatment is carried out and the method of administration of the drug.
Keywords: Androgen therapy, Testosterone, Fat free mass, Elderly men
Introduction
Aging is associated to several physiological changes, progressive lean mass loss (sarcopenia) with consequent decrease of muscular strength, increase in central adiposity, dyslipidemia, fracture risk, and mood problems, and the development of a depression state are among them (Sheffield-Moore et al. 2011; Schroeder et al. 2004; Steidle et al. 2003).
Twenty-five to thirty percent of men with more than 60 years old show low levels of serum testosterone. This event is associated with multiple neuroendocrine-metabolic deficiencies that can explain the progressive change from the anabolic state to the catabolic state in this population (Abbasi et al. 1993; Morales et al. 1998). Besides this, the decreased levels of other hormones like IGF-1 and GH also contribute to the development of these deficiencies (Sattler et al. 2009).
Therapies associated to testosterone and other androgenic hormone treatment favor changes in body composition, especially in lean mass gain and neuromuscular adaptation (Allan et al. 2008; Schroeder et al. 2004; Page et al. 2005; Kenny et al. 2001; Schroeder et al. 2003a). However, the effects of these types of treatment are not fully understood, since their objectives, dosages and duration as well as the association with physical activity adds complexity to the data provided by the studies on this theme (Kenny et al. 2000; Morley et al. 2003; Snyder et al. 1999; Tenover 1998).
Then, the objective of this study was to evaluate the effects of steroid anabolic androgenic hormones use on lean mass gain in elderly through a systematic review with a meta-analysis of controlled and randomized studies.
Methods
Data sources and search method
This meta-analysis was developed according to the PRISMA (preferred reporting items for systematic reviews and meta-analysis) statement guidelines (Liberati et al. 2009).
The systematic search and review was performed on PubMed database until 4th October 2013 using the following Mesh terms, entry terms, and related keywords: “(Randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized controlled trials[mh] OR random allocation[mh] OR double-blind method[mh] OR single-blind method[mh] OR clinical trial[pt] OR clinical trials[mh] OR ("clinical trial"[tw]) OR ((singl*[tw] OR doubl*[tw] OR trebl*[tw] OR tripl*[tw]) AND (mask*[tw] OR blind*[tw])) OR ("latin square"[tw]) OR placebos[mh] OR placebo*[tw] OR random*[tw] OR research design[mh:noexp] OR follow-up studies[mh] OR prospective studies[mh] OR cross-over studies[mh] OR control*[tw] OR prospectiv*[tw] OR volunteer*[tw] AND Aging OR Senescence OR Biological Aging OR Aging, Biological OR aged OR elderly OR Frail Elderly OR Eldef3rly, Frail OR Frail Elders OR Elder, Frail OR Elders, Frail OR Frail Elder OR Functionally-Impaired Elderly OR Elderly, Functionally-Impaired OR Functionally Impaired Elderly OR Frail Older Adults OR Adult, Frail Older OR Adults, Frail Older OR Frail Older Adult OR Older Adult, Frail OR Older Adults, Frail AND Testosterone OR 17-beta-Hydroxy-4-Androsten-3-one OR 17 beta Hydroxy 4 Androsten 3 one OR Androtop OR Dr. Kade Brand of Testosterone OR Histerone OR Hauck Brand of Testosterone OR Sterotate OR Ulmer Brand of Testosterone OR Sustanon OR Androderm OR Watson Brand of Testosterone OR Faulding Brand of Testosterone OR Paladin Brand of Testosterone OR AstraZeneca Brand of Testosterone OR CEPA Brand of Testosterone OR Testoderm OR Ortho Brand of Testosterone OR Ferring Brand of Testosterone OR Testolin OR Pasadena Brand of Testosterone OR Testopel OR Bartor Brand of Testosterone OR Testosterone Sulfate OR AndroGel OR Solvay Brand of Testosterone OR Unimed Brand of Testosterone OR Schering Brand of Testosterone OR 8-Isotestosterone OR 8 Isotestosterone OR 17-beta-Hydroxy-8 alpha-4-Androsten-3-one OR 17 beta Hydroxy 8 alpha 4 Androsten 3 one OR Andropatch OR SmithKline Beecham Brand of Testosterone OR GlaxoSmithKline Brand of Testosterone OR Testim OR Auxilium Pharmaceuticals Inc. Brand of Testosterone OR Testosterone Propionate OR Testosteron propionat Eifelfango OR propionat Eifelfango, Testosteron OR Eifelfango Brand of Testosterone Propionate OR Virormone OR Ferring Brand of Testosterone Propionate OR Agovirin OR testosterone 17 beta-cypionate OR testosterone 17 beta-cyclopentylpropionate OR testosterone cypionate OR testosterone 17 beta cyclopentanepropionate OR Depo-Testosterone OR Pfizer brand of testosterone 17 beta-cypionate OR Depo-Testosterone Cypionate OR deposteron OR Duratest OR Roberts brand of testosterone 17 beta-cypionate OR Testa-C OR Vortech brand of testosterone 17 beta-cypionate OR Testex Elmu OR Byk brand of testosterone 17 beta-cypionate OR Andronate OR Pasadena brand of testosterone 17 beta-cypionate OR Depostomead OR Spencer Mead brand of testosterone 17 beta-cypionate OR testosterone enanthate OR testosterone heptylate OR testosterone heptanoate OR Delatestryl OR Theramed brand of testosterone enanthate OR BTG brand of testosterone enanthate OR Durathate OR Roberts brand of testosterone enanthate OR Theramex OR Testosteron Depot-Rotexmedica OR Rotexmedica brand of testosterone enanthate OR Testosteron-Depot Eifelfango OR Eifelfango brand of testosterone enanthate OR Testosteron-Depot Jenapharm OR Jenapharm brand of testosterone enanthate OR Testrin P.A. OR Pasadena brand of testosterone enanthate OR Andropository OR Rugby brand of testosterone enanthate OR Primoteston Depot OR Schering brand of testosterone enanthate OR testosterone-17-succinate OR testosterone hydrogen succinate OR testosterone-17-hemisuccinate OR T-17-HS OR testosterone hemisuccinate OR testosterone-17-succinate, sodium salt, (17beta)-isomer OR testosterone-17-sulfate OR testosterone 17-sulphate OR testosterone-17-sulfate, sodium salt OR testosterone-17-sulfate, ammonium salt OR testosterone-17-sulfate, (17alpha)-isomer OR testosterone undecanoate OR testosterone undecylate OR Nebido OR Undestor OR Andriol OR Pantestone OR Restandol OR Organon brand of testosterone undecanoate OR Methyltestosterone OR 17 beta-Hydroxy-17-methyl-4-androsten-3-one OR 17 beta Hydroxy 17 methyl 4 androsten 3 one OR 17beta-Methyltestosterone OR 17beta Methyltestosterone OR 17-Epimethyltestosterone OR 17 Epimethyltestosterone OR 17beta-Hydroxy-17-methyl-4-androsten-3-one OR 17beta Hydroxy 17 methyl 4 androsten 3 one OR 17 beta Methyltestosterone OR 17 beta-Methyltestosterone OR Android OR ICN Brand 1 of Methyltestosterone OR Android-10 OR Android 10 OR Android-25 OR Android 25 OR Android-5 OR Android 5 OR Mesteron OR Mesterone OR Methitest OR Global Pharmaceutical Brand of Methyltestosterone OR Oreton OR Schering Brand of Methyltestosterone OR Testoviron OR Testred OR ICN Brand 2 of Methyltestosterone OR Virilon OR Star Brand of Methyltestosterone OR 17-alpha-Methyltestosterone OR 17 alpha OR Methyltestosterone OR 17alpha-Methyltestosterone OR 17alpha Methyltestosterone Or 17alpha-Methyl-Testosterone OR 17alpha Methyl Testosterone OR Metandren OR testosterone 17-phenylpropionate OR testosterone phenylpropionate OR Retandrol OR testosterone decanoate OR testosterone replacement OR androgenic anabolic steroids OR anabolic steroids AND Sarcopenia OR sarcopenias OR hypertrophy OR hypertrophies OR Muscle, Skeletal OR Muscles, Skeletal OR Skeletal Muscles OR Skeletal Muscle OR Muscle, Voluntary OR Muscles, Voluntary OR Voluntary Muscle OR Voluntary Muscles OR Soleus Muscle OR Muscle, Soleus OR Plantaris Muscle OR Muscle, Plantaris OR Anterior Tibial Muscle OR Muscle, Anterior Tibial OR Tibial Muscle, Anterior OR Gastrocnemius Muscle OR Muscle, Gastrocnemius OR Muscle Fibers, Skeletal OR Fiber, Skeletal Muscle OR Fibers, Skeletal Muscle OR Muscle Fiber, Skeletal OR Skeletal Muscle Fiber OR Myotubes OR Myotube OR Skeletal Myocytes OR Myocytes, Skeletal OR Myocyte, Skeletal OR Skeletal Myocyte OR Skeletal Muscle Fibers OR Skeletal Muscle Myosins OR Myosins, Skeletal Muscle OR muscle development OR Development, Muscle OR Muscular Development OR Development, Muscular OR Myogenesis OR Myofibrillogenesis OR lean body mass)”.
Study selection criteria
We included randomized placebo-controlled trials (RCT) that studied testosterone replacement therapy in men over 60 years of age with total testosterone levels ≤550 ng/dl. We excluded non-randomized controlled trials, randomized trials without placebo group, minimal age ≤60 years old, total testosterone levels ≥550 ng/dl, studies which mixed men and women, and studies using weak androgens such as dehydroepiandrosterone or androstenedione. We also checked for duplication based on overlapping authorship, study description, number of participants, and participant characteristics. When duplication occurred, we used the study with the most complete information description. However, when results were duplicated, the study was excluded.
Outcomes
The primary outcome was lean body mass (LBM) because it is usually related to muscle mass and functional levels. Fat mass (FM) was analyzed as secondary outcome.
Data extraction and quality assessment
We extracted the number of participants randomized to each intervention and placebo groups, age, type of androgen and administration method, dosage, therapy duration, and main results. The reviewers (WKN and ECC) independently used the PEDro and Jadad scale to evaluate the quality of each trial.
Sensitivity analysis
We initially planned only to assess whether the effects of testosterone replacement therapy on lean body and fat mass. However, the reporting of many variables opened several interpretations. Due to potential lack of clarity of the therapy method, we also examined whether the effect of testosterone therapy varied with the length of the replacement and administration method.
Data synthesis and analysis
We used the Review manager software 5.2 to calculate heterogeneity by the I2, chi2, and Tau2 values. We used I2 to assess heterogeneity between trials, using random effects models where there was high heterogeneity (I2 = 98 %). We also used inverse variance method and 95 % total confidence interval.
This study is an analysis of published data, which does not require ethics committee approval.
Results
Selected studies
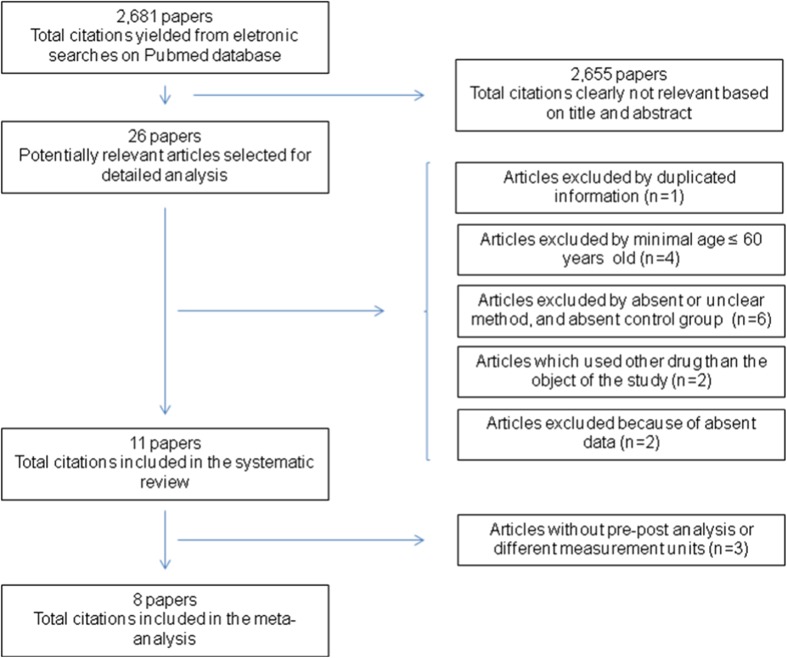
The initial search yielded 2681 articles, of which 26 were selected for full text analysis. At last, 11 studies were included. However, 3 studies were not included in the meta-analysis. These studies did not mention the standard deviation for the pre-pos LBM analysis. This variable is important to calculate the confidence interval. The abstracts of all studies were initially examined by one author (WKN) to determine if they met basic inclusion criteria. A total of 2655 articles were excluded after this procedure. The next step was to examine 26 full articles by two raters (WKN and BR) to determine the appropriateness of the studies for further analysis. At last, 15 articles were excluded, leaving 11 articles for analysis. Of these, 8 articles were included in LBM meta-analysis and only 4 articles for FM analysis. The study selection process is presented in Fig. 1.
Fig. 1.
Fluxogram of the article selection process and exclusion criteria (produced in the review manager 5.2 software)
A total of 651 elderly men participated in the 11 trials included in the systematic review analysis (356 men in intervention groups and 295 in placebo). The mean age of the participants ranged from 65 ± 3 to 77 ± 9 years. The year of publication ranged from 2002 to 2011. Table 1 describes the information for each trial included. The number of participants in intervention groups ranged from 7 to 113 and in placebo groups from 5 to 110. The drug used was testosterone enanthate (Sheffield-Moore et al. 2011; Page et al. 2005; Ferrando et al. 2003; Blackman et al. 2002), oxandrolone (Schroeder et al. 2005; Schroeder et al. 2004; Schroeder et al. 2003a), testosterone undecanoate (Emmelot-Vonk et al. 2008; Wittert et al. 2003), oxymetholone (Schroeder et al. 2003b), and Androgel (Kenny et al. 2010). The administration method used was oral (6 articles), injection (4 articles), and transdermal gel (1 article). There was no pattern about the used dosage. However, the authors used therapeutic dosage for all drug types. Treatment duration ranged from 12 weeks to 36 months.
Table 1.
Data description of all trials included in the systematic review
| Study | Baseline mean age (years) | Intervention (n) | Placebo (n) | Drug name | Administration method | Dosage | Treatment duration | Lean body mass | Fat mass | PEDro score (0–10) | Jadad score (0–5) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blackman et al. 2002 | T = 70 ± 0.7 PL = 70 ± 1.1 | 21 | 17 | Testosterone enanthate | Injection | 100 mg/every 2 weeks | 6 months | T = ↑1.4 kg PL = ↑0.1 kg | T = ↓1.2 kg PL = ↑0.1 kg | 9 | 5 |
| Ferrando et al., 2002 | T = 68 ± 3 PL = 67 ± 3 | 7 | 5 | Testosterone enanthate | Injection | not described | 6 months | T = ↑4.2 ± 0.6 kg PL = ↓2.0 ± 1.0 kg | Not described | 8 | 2 |
| Schroeder et al. 2003a | 72 ± 5 | 21 | 9 | Oxandrolone | Oral | 20 mg/day | 12 weeks | T = ↑3.0 ± 1.5 kg PL = ↑0.1 ± 1.5 kg | Not described | 10 | 4 |
| Schroeder et al. 2003b | 50 mg = 71 ± 4 100 mg = 70 ± 4 PL = 73 ± 4 | 20 | 11 | Oxymetholone | Oral | 50 or 100 mg/day | 12 weeks | 50 mg = ↑3.3 ± 1.2 kg 100 mg = ↑4.2 ± 2.4 kg PL = 0.0 ± 0.6 kg | 50 mg = ↓2.6 ± 1.2 kg 100 mg = ↓2.5 ± 1.6 kg PL = 0.0 ± 1.0 kg | 10 | 3 |
| Wittert et al. 2003 | 68.5 ± 6 | 33 | 25 | Testosterone undecanoate | Oral | 80 mg/day | 6 and 12 months | After 6 months: T = ↑1.04 ± 0.07 kg PL = ↓0.91 ± 0.03 kg After 12 months: T = ↑0.67 ± 0.05 kg PL = ↓0.98 ± 0.08 kg | After 6 months: T = ↓0.2 ± 0.1 kg PL = ↑0.85 ± 0.19 kg After 12 months: T = ↓1.07 ± 1.33 % PL = ↑4.61 ± 1.96 % | 8 | 5 |
| Schroeder et al. 2004 | T = 72.8 ± 6.9 PL = 71.5 ± 3.2 | 20 | 12 | Oxandrolone | Oral | 20 mg/day | 12 weeks | T = ↑ 3.0 ± 1.5 kg PL = ↑0.0 ± 1.4 kg | T = ↓ 1.9 ± 1.0 kg PL = ↓0.2 ± 1.0 kg | 10 | 4 |
| Page et a. 2005 | T = 71 ± 4 PL = 71 ± 5 | 24 | 24 | Testosterone enanthate | Injection | 200 mg/every 2 weeks | 36 months | T = ↑3.7 ± 0.6 kg PL = ↓ 0.21 ± 0.55 | T = ↓5.5 % PL = ↑0.3 % | 8 | 2 |
| Schroeder et al. 2005 | T = 72.8 ± 6.9 PL = 71.5 ± 3.2 | 20 | 12 | Oxandrolone | Oral | 20 mg/day | 12 weeks | T = ↑ 3.0 ± 1.5 kg PL = ↑0.1 ± 1.5 kg | Not described | 10 | 3 |
| Emmelot-Vonk et l. 2008 | T = 67.1 ± 5 PL = 67.4 ± 4.9 | 113 | 110 | Testosterone undecanoate | Oral | 80 mg/day | 6 months | T = ↑1.1 kg PL = ↓0.3 kg | T = ↓1.0 kg PL = ↓0.1 kg | 9 | 5 |
| Kenny et al. 2010 | T = 77.9 ± 7.3 PL = 76.3 ± 8.0 | 69 | 62 | Androgel | Trandermal | 5 mg | 12 months | T = ↑1 kg PL = ↑0.2 kg | T = ↓1.7 % PL = ↓0.8 % | 9 | 5 |
| Sheffield-Moore et al. 2011 | T = 73 ± 8 PL = 65 ± 3 | 8 | 8 | Testosterone enanthate | Injection | 100 mg/week | 5 months | T = ↑ 3.12 ± 2.06 kg PL = ↓1.09 ± 1.64 kg | T = ↓ 1.24 ± 0.95 kg PL = ↑1.2 ± 1.87 kg | 7 | 1 |
Quality assessment
We used PEDro score scale and Jadad as tools to verify the quality assessment of randomized clinical trials. These scales are used with the objective to help the researchers to rapidly identify if these studies contain enough internal and statistical information, and further quality. Our results showed PEDro scores that ranged from 7 to 10 which demonstrated enough internal and statistical information presented by the trials. However, Jadad scale showed a wider score (ranged from 1 to 5).
Data synthesis and sensitivity analysis
Lean body mass
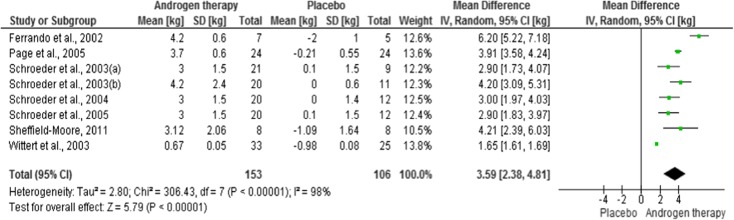
Three studies were not included in our meta-analysis because they did not present the standard deviation information for the mean alteration of LBM. So, 259 participants were included, whose 153 for intervention and 106 for placebo. Androgen therapy increased mean weight difference, ranging from 1.65 (95 % CI, 1.61–1.69) to 6.20 (95 % CI, 5.22–7.18) kg. The forest plot (Fig. 2) shows that the trials were heterogeneous (I2 = 98 %). Effect estimate was 3.59 [2.38–4.81].
Fig. 2.
Forest plots of placebo-controlled randomized trials examining the effect of androgen therapy on lean body mass (produced in the review manager 5.2 software)
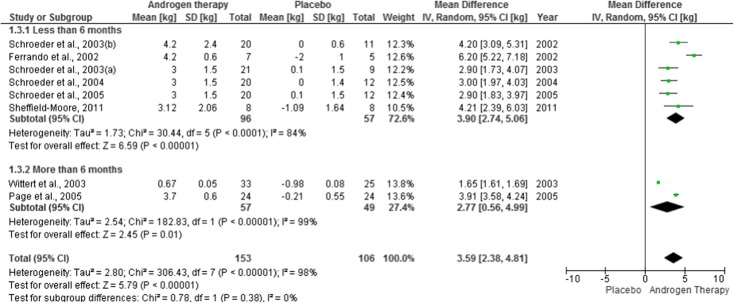
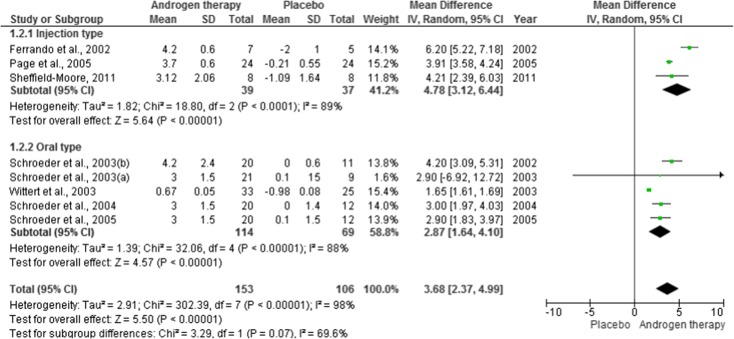
Sensitivity analysis was applied to therapy duration and administration method. Six articles used therapy under 6 months (effect estimate = 3.90 [2.74–5.06]) and two studies above this period (effect estimate = 2.77 [0.56–4.99]). Effect estimate for therapy duration is 3.59 [2.38–4.81]. This analysis also showed a high heterogeneity level (I2 ranged from 84 % [less than 6 months] to 99 % [more than 6 months]. The administration method also showed a high heterogeneity (I2 ranged from 88 % [oral] to 89 % [injection]). Effect estimate was 3.68 [2.37–4.99] for this general analysis, 2.87 [1.64–4.10] for oral and 4.78 [3.12–6.44] for injection. All this data is presented in Figs. 3 and 4.
Fig. 3.
Forest plots of placebo-controlled randomized trials examining the effect of androgen therapy duration on lean body mass (produced in the review manager 5.2 software)
Fig. 4.
Forest plots of placebo-controlled randomized trials examining the effect of administration method on lean body mass (produced in the review manager 5.2 software)
Fat mass
Only four articles presented enough evidence for fat mass meta-analysis. A total of 137 participants were included (81 for intervention and 56 for placebo). Effect estimate was −1.78 [−2.57, −0.99]. Heterogeneity also presented high level (−2 = 81 %). Forest plot (Fig. 5) showed small trials. Androgen therapy decreased fat mass.
Fig. 5.

Forest plots of placebo-controlled randomized trials examining the effect of androgen therapy on fat mass (produced in the review manager 5.2 software)
Discussion
Testosterone used as a hormonal replacement therapy is a popular intervention because it is cheap and it influences several systems at the same time (Liverman and Blazer 2005).
There are some meta-analysis studies that evaluated the effects of hormone replacement therapy in the elderly that showed positive results (Ottenbacher et al. 2006; Yang et al. 2013). Isidori et al. (2005)) in another meta-analysis study reported beneficial effects enough to justify the demand for other interventional studies on this issue. On the other hand, there is the existent, but small risk of side effects, showed in another meta-analysis (Calof et al. 2005).
Although the promising evidence, there are some limitation to the use of hormone replacement therapy in elderly men. General results are positive, but a more specific look will detect that there are important differences between the studies that make it impossible to the reader to reach a more detailed conclusion.
For instance, from several articles available in the literature, there are many different drugs used as replacement; McGill et al. (2012)) states in a review that there are several drugs that can be used, in different forms of application, with different results. The authors also point out that the only form commercially available in the USA is testosterone cypionate, and this drug causes swings in the testosterone concentration.
In the Testosterone Therapy Guideline published by Bhasin et al. (2010)), they also suggest that several types of drugs can be use with different results.
Our systematic review and meta-analysis reinforces the notion that androgen therapy is effective to promote lean body mass in elderly men when compared to placebo. However, when a more detailed observation is made, the heterogeneity of results appears. Although we used a strict methodology for the article inclusion, some aspects of the intervention made the results heterogeneous such as the length of treatment and type of drug administration.
We analyzed only randomized placebo-controlled studies with 11 studies included; the variables analyzed were lean body mass (8 studies included) and fat mass (4 studies included); from the initial results, we included a sensitivity analysis of length of treatment and type of administration, and this analysis strengthened the results.
The search method was on Pubmed (which might be considered as a limitation since it is only one database), and we used Mesh terms. The search strategy was built according to the PICOS orientation, and we followed the PRISMA check list; we also used the PEDro and JADAD scales to ensure that the studies were suitable to the analysis.
Our first results showed increase in lean body mass, although Tau2 and I2 revealed great heterogeneity (98 %) on our data. These results can be reinforced by data found in the literature, as found in Xu et al. (2013)) that conducted a meta-analysis looking for cardiovascular side effects of testosterone replacement and found significant improvements in lean body mass. Ottenbacher et al. (2006)) also found increases in muscle strength in another meta-analysis, although the study was not looking specifically to muscle mass.
Looking at the length of administration, we gathered 6 studies that would meet the criteria of less than 6 months of treatment. That analysis gave us, yet, results with great heterogeneity, although it decreased from 98 to 84 %.
There were 3 studies that had a time of treatment longer than 6 months, and then, I2 level would reach 99 %.
Looking at another sensitivity analysis, we considered the method of administration as an important factor that influenced our results.
Five studies were taken into account when we looked at oral administration of the treatment. That analysis revealed good results with an I2 of 88 %, while for the injection type of administration, only 3 studies would fit and that increased data heterogeneity once again (I2 = 89 %).
There were three different types of drugs used in the studies that composed the meta-analysis. The three studies carried by Schroeder et al. used Oxandrolone as experimental drug and all of them were administered orally. Another study administered the drug orally but used testosterone undecanoate, it was the study conducted by Wittert et al. (2003).
The rest of the three studies used testosterone enanthate, and all of them were intramuscular injections. Bhasin et al. (2005)) in a randomized double blind controlled study used testosterone enanthate and reported that it is the only formulation able to raise its levels to a supraphysiological level.
Other articles that were found in the systematic review, and were not included in the meta-analysis because they did not present the standard deviation of the results, used several drugs, alone or in combination, for example, testosterone with hGH, in a variety of methods, such as, injectable, oral, or transdermal gel.
All these information were taken in consideration, but as we structured the study according to the standards proposed in the PRISMA check list, the studies that were fit for the meta-analysis yielded the heterogeneous results presented.
Prevalence studies suggest that androgen therapy strategies may be of interest in reducing the rate of muscle wasting seen during aging (Iannuzzi-Sucich et al. 2002).
As we have demonstrated, androgen therapy increases lean mass of elderly individuals. So, we did a quick presentation of some of the mechanisms that may explain this phenomenon. The action of androgens on the skeletal muscle involves several pathways of cellular and molecular interaction. Laboratory studies have shown that androgens clearly affect muscular hypertrophy through increasing insulin-like growth factor-1 (IGF-1) expression, controlling forkhead box O (FOXO1), peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1), and p38 mitogen-activated protein kinases (MAPK), and stimulates hypertrophy via intracellular androgen receptor signaling cascade dependent upon Erk and mammalian target of rapamycin (mTOR) (Sculthorpe et al. 2012; Qin et al. 2010; Wu et al. 2010).
Also, anabolic effects of testosterone administration on skeletal muscle are thought to be mediated by myonuclei and satellite cells activation and reduced catabolic state (Urban et al. 2014). Urban et al. (2014)) studied testosterone replacement effect on NF-B-inducing kinase (NIK) of old individuals and showed significant reduction levels after therapy. This protein is known to significantly be elevated in diabetic kidneys, multiple myeloma, and inflammatory arthritis (Aya et al. 2005; Demchenko et al. 2010; Starkey et al. 2006).
Conclusion
We can conclude that testosterone replacement therapy is able to increase muscle mass in elderly men and that is affected by the length of the treatment and the method of administration of the drug.
Surely, the type of drug is also an important element that can be accounted for differences in the clinical results, although this was not considered in our study.
Acknowledgments
Conflict of interest
The authors declare that there is no conflict of interests.
Contributor Information
Walter Krause Neto, Email: wild_krause@hotmail.com.
Eliane Florencio Gama, Email: efgama@pistilli.net.
Leandro Yanase Rocha, Email: lei_yns@hotmail.com.
Carla Cristina Ramos, Email: carlacramos@hotmail.com.
Wagner Taets, Email: wagner.taets.silva@gmail.com.
Katia Bilhar Scapini, Email: katiascapini@yahoo.com.br.
Janaina B. Ferreira, Email: jana.bferreira@yahoo.com.br
Bruno Rodrigues, Email: prof.brodrigues@gmail.com.
Érico Caperuto, Phone: 5511-97337-3012, Email: ericocaperuto@gmail.com.
References
- Abbasi AA, Drinka PJ, Mattson DE, Rudman D. Low circulating levels of insulin-like growth factors and testosterone in chronically institutionalized elderly men. J Am Geriatr Soc. 1993;41(9):975–982. doi: 10.1111/j.1532-5415.1993.tb06764.x. [DOI] [PubMed] [Google Scholar]
- Allan CA, Forbes EA, Strauss BJ, McLachlan RI (2008) Testosterone therapy increases sexual desire in ageing men with low-normal testosterone levels and symptoms of androgen deficiency. Int J Impot Res 20(4):396–401 [DOI] [PubMed]
- Aya K, Alhawagri M, Hagen-Stapleton A, et al (2005) NF-(kappa)B-inducing kinase controls lymphocyte and osteoclast activities in inflammatory arthritis. J Clin Invest 115:1848–54 [DOI] [PMC free article] [PubMed]
- Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O’Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell Em St Clair C, Pabst KM, Harman SM (2002) Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA 288 (18): 2282–92 [DOI] [PubMed]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90(2):678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, Bhasin S. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60(11):1451–1457. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- Demchenko YN, Glebov OK, Zingone A, et al (2010) Classical and/or alternative NFkappaB pathway activation in multiple myeloma. Blood 115:3541–52 [DOI] [PMC free article] [PubMed]
- Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, van der Schouw YT. Effect of testosterone replacement therapy on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299(1):39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab. 2003;88(1):358–62. doi: 10.1210/jc.2002-021041. [DOI] [PubMed] [Google Scholar]
- Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol: Med Sci. 2002;57A(12):M772–M777. doi: 10.1093/gerona/57.12.M772. [DOI] [PubMed] [Google Scholar]
- Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 2005;63(3):280–293. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Prestwood KM, Raisz LG. Short-term effects of intramuscular and transdermal testosterone on bone turnover, prostate symptoms, cholesterol, and hematocrit in men over age 70 with low testosterone levels. Endocr Res. 2000;26(2):153–168. doi: 10.3109/07435800009066159. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56(5):M266–M272. doi: 10.1093/gerona/56.5.M266. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, Judge JO, McGee D. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010;58(6):1134–1143. doi: 10.1111/j.1532-5415.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liverman C, Blazer D. Testosterone in aging: directions for clinical research. Washington, DC: National Academy Press; 2005. [PubMed] [Google Scholar]
- McGill JJ, Shoskes DA, Sabanegh ES. Androgen deficiency in older men: indications, advantages, and pitfalls of testosterone replacement therapy. Cleve Clin J Med. 2012;79(11):797–806. doi: 10.3949/ccjm.79a.12010. [DOI] [PubMed] [Google Scholar]
- Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SS. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf) 1998;49(4):421–432. doi: 10.1046/j.1365-2265.1998.00507.x. [DOI] [PubMed] [Google Scholar]
- Morley JS, Bridson J, Nash TP, Miles JB, White S, Makin MK. Low-dose methadone has an analgesic effect in neuropathic pain: a double-blind randomized controlled crossover trial. Palliat Med. 2003;17(7):576–587. doi: 10.1191/0269216303pm815oa. [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, Acha AA, Ostir GV. Androgen treatment and muscle strength in elderly men: a meta-analysis. J Am Geriatr Soc. 2006;54(11):1666–1673. doi: 10.1111/j.1532-5415.2006.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90(3):1502–1510. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- Qin W, Pan J, Wu Y, et al. Protection against dexamethasone-induced muscle atrophy is related to modulation by testosterone of FOXO1 and PGC-1alpha. Biochem Biophys Res Commun. 2010;403:473–478. doi: 10.1016/j.bbrc.2010.11.061. [DOI] [PubMed] [Google Scholar]
- Sattler FR, Castaneda-Sceppa C, Binder EF, Schroeder ET, Wang Y, Bhasin S, Kawakubo M, Stewart Y, Yarasheski KE, Ulloor J, Colletti P, Roubenoff R, Azen SP. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009;94(6):1991–2001. doi: 10.1210/jc.2008-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder ET, Terk M, Sattler FR. Androgen therapy improves muscle mass and strength but not muscle quality: results from two studies. Am J Physiol Endocrinol Metab. 2003;285(1):E16–E24. doi: 10.1152/ajpendo.00032.2003. [DOI] [PubMed] [Google Scholar]
- Schroeder ET, Singh A, Bhasin S, Storer TW, Azen C, Davidson T, Martinez C, Sinha-Hikim I, Jaque SV, Terk M, Sattler FR. Effects of an oral androgen on muscle and metabolism in older, community-dwelling men. Am J Physiol Endocrinol Metab. 2003;284(1):E120–E128. doi: 10.1152/ajpendo.00363.2002. [DOI] [PubMed] [Google Scholar]
- Schroeder ET, Zheng L, Yarasheski KE, Qian D, Stewart Y, Flores C, Martinez C, Terk M, Sattler FR. Treatment with oxandrolone and the durability of effects in older men. J Appl Physiol (1985) 2004;96(3):1055–1062. doi: 10.1152/japplphysiol.00808.2003. [DOI] [PubMed] [Google Scholar]
- Schroeder ET, Vallejo AF, Zheng L, Stewart Y, Flores C, Nakao S, Martinez C, Sattler FR (2005) Six-week improvements in muscle mass and strength during androgen therapy in older men. J Gerontol A Biol Sci Med Sci 60(12):1586–92 [DOI] [PubMed]
- Sculthorpe N, Solomon AM, Sinanan AC, et al. Androgens affect myogenesis in vitro and increase local IGF-1 expression. Med Sci Sports Exercise. 2012;44:610–615. doi: 10.1249/MSS.0b013e318237c5c0. [DOI] [PubMed] [Google Scholar]
- Sheffield-Moore M, Dillon EL, Casperson SL, Gilkison CR, Paddon-Jones D, Durham WJ, Grady JJ, Urban RJ. A randomized pilot study of monthly cycled testosterone replacement or continuous testosterone replacement versus placebo in older men. J Clin Endocrinol Metab. 2011;96(11):E1831–E1837. doi: 10.1210/jc.2011-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL. Effect of testosterone replacement therapy on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84(8):2647–53. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- Starkey JM, Haidacher SJ, LeJeune WS, et al (2006) Diabetes-induced activation of canonical and noncanonical nuclear factor-kappaB pathways in renal cortex. Diabetes 55:1252–9 [DOI] [PubMed]
- Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R, North American AA2500 T Gel Study Group AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88(6):2673–2681. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- Tenover JL. Male hormone replacement therapy including "andropause". Endocrinol Metab Clin North Am. 1998;27(4):969–87. doi: 10.1016/S0889-8529(05)70050-5. [DOI] [PubMed] [Google Scholar]
- Urban RJ, Dillon EL, Choudhary S, Zhao Y, Horstman AM, Tilton RG, Sheffield-Moore M. Translational studies in older men using testosterone to treat sarcopenia. Trans Am Clin Climatol Assoc. 2014;125:27–44. [PMC free article] [PubMed] [Google Scholar]
- Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE. Oral testosterone treatment increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci. 2003;58(7):618–625. doi: 10.1093/gerona/58.7.M618. [DOI] [PubMed] [Google Scholar]
- Wu Y, Bauman WA, Blitzer RD, et al. Testosterone-induced hypertrophy of L6 myoblasts is dependent upon Erk and mTOR. Biochem Biophys Res Commun. 2010;400:679–683. doi: 10.1016/j.bbrc.2010.08.127. [DOI] [PubMed] [Google Scholar]
- Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Li J, Yuan Z, Liu X. Effect of hormone replacement therapy on cardiovascular outcomes: a meta-analysis of randomized controlled trials. PLoS One. 2013;8(5):e62329. doi: 10.1371/journal.pone.0062329. [DOI] [PMC free article] [PubMed] [Google Scholar]