Abstract
Hand grip strength (GS) is a predictor of mortality in older adults and is moderately to highly heritable, but no genetic variants have been consistently identified. We aimed to identify single nucleotide polymorphisms (SNPs) associated with GS in middle-aged to older adults using a genome-wide association study (GWAS). GS was measured using handheld dynamometry in community-dwelling men and women aged 55–85 from the Hunter Community Study (HCS, N = 2088) and the Sydney Memory and Ageing Study (Sydney MAS, N = 541). Genotyping was undertaken using Affymetrix microarrays with imputation to HapMap2. Analyses were performed using linear regression. No genome-wide significant results were observed in HCS nor were any of the top signals replicated in Sydney MAS. Gene-based analyses in HCS identified two significant genes (ZNF295, C2CD2), but these results were not replicated in Sydney MAS. One out of eight SNPs previously associated with GS, rs550942, located near the CNTF gene, was significantly associated with GS (p = 0.005) in the HCS cohort only. Study differences may explain the lack of consistent results between the studies, including the smaller sample size of the Sydney MAS cohort. Our modest sample size also had limited power to identify variants of small effect. Our results suggest that similar to various other complex traits, many genetic variants of small effect size may influence GS. Future GWAS using larger samples and consistent measures may prove more fruitful at identifying genetic contributors for GS in middle-aged to older adults.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-015-9745-5) contains supplementary material, which is available to authorized users.
Keywords: Hand grip strength, Genetics, GWAS, Ageing
Introduction
The increasing number of aged individuals in the population (United Nations 2002) accentuates the importance of addressing age-related physical decline. Physical function, and more specifically, muscle strength, is known to decrease steadily and significantly from the fifth decade onwards (Frederiksen et al. 2006; Ribom et al. 2011; Stenholm et al. 2012; Werle et al. 2009). Indeed, 20–40 % of skeletal muscle mass and strength is lost between 20 and 80 years old (Semba et al. 2012). This ageing-related process is related to a variety of adverse outcomes, including reduced mobility, increased disability, higher mortality (Alfred et al. 2012), increased rates of institutionalisation (Cesari et al. 2012) and increased risk of falls (Arking et al. 2006; Ribom et al. 2011). Low muscle strength is prevalent late in life and profoundly affects the quality of life (Frederiksen et al. 2002). It is therefore important to identify those at increased risk of age-related decline in muscle strength.
Hand grip strength (GS) can be used as an indicator of overall muscle strength and function (Cesari et al. 2012), as it is strongly correlated with other measures of muscle strength (Frederiksen et al. 2002; Semba et al. 2012). Moreover, a reduction in GS has been strongly associated with increased mortality (Dato et al. 2012a; Deelen et al. 2013), decreased activities of daily living (Taekema et al. 2010) and incident disability (Frederiksen et al. 2002). Thus, GS is not only a marker of physical function and quality of life in old age but also directly relates to the risk of mortality and morbidity.
Heritability studies show that the genetic component of GS ranges from 35 to 65 % (Carmelli and Reed 2000; Frederiksen et al. 2002; Matteini et al. 2010; Murabito et al. 2012; Reed et al. 1991). Moreover, heritability of GS remains stable over the lifetime, as demonstrated in a longitudinal study of Swedish twins (Finkel et al. 2003). Indeed, across a 10-year follow-up of American twins aged 63 years at baseline, 35 % of the stable phenotypic variance was due to genetic influences (Carmelli and Reed 2000). Another study looking at heritability at different age ranges in a middle-aged to elderly Danish twin cohort (45–96 years) found heritability to be approximately 50 % across the different age groups (Frederiksen et al. 2002). Moderate to high heritability measures across different study designs, age groups and populations provide robust evidence for genetic influences on GS across the life span, including in older adults.
In middle-aged to older adults, a wide variety of candidate genes for GS have been examined. They include genes related to muscle catabolism, growth factors, cellular damage, hormonal regulation, vitamin D and collagen. Several studies reported positive association of variants in specific genes, including uncoupling protein 3 (UCP3) (Crocco et al. 2011; Dato et al. 2012b), which is involved in skeletal muscle catabolism; insulin-like growth factor-2 (IGF2) (Sayer et al. 2002), involved in regulating muscle growth; and angiotensin-converting enzyme (ACE) (Yoshihara et al. 2009), whose protein product not only regulates blood pressure but is also a skeletal muscle growth factor. Most of these results, however, have only been reported once, are subject to publication bias (i.e. many candidates are tested but only significant ones are published) and multiple testing error, and cannot be unequivocally associated with grip strength. An alternative to candidate gene studies are non-hypothesis-driven genome-wide association studies (GWAS), which assess the relationships between a large number of common genetic variants across the genome and the phenotype of interest. Over 2000 GWAS have been published to date, and in general, GWAS have been successful in identifying novel genetic polymorphisms for diseases (e.g. Alzheimer’s disease, age-related macular degeneration; Bailey et al. 2014) and traits (e.g. LDL cholesterol; Global Lipids Genetics et al. 2013) that may not have been identified using the candidate gene approach.
Since no genetic variant has been consistently associated with GS despite it having moderate to high heritability, we undertook a GWAS for GS in over 2000 adults aged 55–90 years. Two large community-dwelling cohorts of middle-aged adults and older adults (Hunter Community Study, Sydney Memory and Ageing Study) with both genotyping and grip strength data available were used in the current study. These samples are in general, representative of middle-aged to older adults of the general Australian Caucasian community (McEvoy et al. 2010; Sachdev et al. 2010). Replication of previous GS-associated SNPs was also investigated in our cohorts. To our knowledge, this is the first GS GWAS to be reported.
Methods
Samples
Two Australian community-based cohorts, the Sydney Memory and Ageing Study (Sydney MAS), and the Hunter Community Study (HCS) were analysed, totalling 2629 subjects. All participants provided written informed consent to participate, and approval from the appropriate ethics committees was granted. Both studies collected a wide variety of demographic, health and medical data from older adults recruited from two adjacent cities, Sydney and Newcastle, in Australia.
HCS
More than 7500 people aged 55–85 years residing in Newcastle, Australia, were randomly selected from the electoral roll and were invited to participate in a study aiming to assess the factors important in the health and well-being of older adults. Electoral roll registration is compulsory in Australia. People unable to speak English and residents of aged-care facilities were excluded from the study. The final sample was composed of over 3000 participants. A wide variety of information was collected including anthropometric measures. Blood samples were collected from the majority of participants for DNA extraction. Ethics approval was obtained from the Hunter New England Local Health District and University of Newcastle Human Research Ethics Committees. Further details are provided in McEvoy et al. (2010). There were 2088 (mean age 66.3 years, range 55–86 years) respondents with both GS and genotyping data.
Sydney MAS
Over 8900 people aged 70–90 years old were randomly selected from the electoral rolls of the eastern suburbs of Sydney, Australia, and invited to participate in a longitudinal study on cognition in older adults. Inclusion criteria involved the ability to speak and write English well enough to complete a psychometric assessment. Exclusion criteria included a previous diagnosis of dementia and medical or psychological conditions preventing them from completing assessments. The final sample was composed of 1037 participants. Extensive data was collected including the collection of anthropometric information. Blood samples were collected for genetic and biochemical analyses. Ethics approval for Sydney MAS was granted by the Human Research Ethics Committees of the University of New South Wales and the South Eastern Sydney and Illawarra Area Health Service (for further details, see Sachdev et al. (2010)). Only 541 participants (mean age 78.84, range 70.29–90.80 years) had both grip strength and genotyping data.
Grip strength
GS was measured in a face-to-face assessment with the participant sitting or standing comfortably. The assessor ensured that the arm to be tested was held by their side, and their elbow was at a right angle. The participants were then asked to hold the grip dynamometer in the dominant hand and to squeeze as hard as possible for a few seconds. Participants were given approximately 30 s to 1 min recovery between each trial.
HCS
GS was measured using a calibrated dynamometer (JAMAR, Asimov Engineering Company, Los Angeles, CA, USA) and defined as the best of three trials (highest score).
Sydney MAS
GS measurements were performed in a subset of participants (N = 589), using a calibrated dynamometer (SH5001, SAEHAN Corporation, Changwon, Korea). GS was operationalised as the best trial (highest score) of two or three trials.
Genotyping
DNA was extracted using standard procedures from peripheral blood samples of both HSC and Sydney MAS. For <5 % of the Sydney MAS cohort, DNA was extracted from saliva.
HCS
Genome-wide genotyping used the Affymetrix Axiom Kaiser array following the recommended protocol. Genotyped SNPs were excluded if the call rate was <95 %, the Hardy-Weinberg equilibrium p value was <10−6 or the MAF <0.01 %. Individuals were excluded if the genotype call rate was <95 % or if there were discrepancies between clinical and inferred gender. Relatedness checks were undertaken, and if first- or second-degree relatives were present, only one individual from each family was randomly selected and retained. Individuals with clear evidence of non-European ancestry based on Eigenstrat principal components analysis were excluded. After quality control checks, there were 2088 participants (1036 males, 1052 females) with data for 549,281 SNPs, with a mean genotyping call rate of 99.1 %. Imputation was undertaken using the HapMap2 reference data (release 22, build 36) using MaCH v1.0.16 (Li et al. 2009, 2010).
Sydney MAS
Genome-wide genotyping used the Affymetrix Genome-Wide Human SNP Array 6.0 (CA, USA) at the Ramaciotti Centre, UNSW, Sydney, Australia, following the recommended protocol. The CRLMM package (v1.10.0) in R (v2.12.1) was used to call genotypes. The quality control procedures and filters used were the same as for HCS. After quality control, there were 925 Sydney MAS participants (417 males, 508 females) with data for 734,550 SNPs, with a mean genotyping call rate of 99.5 %. Imputation was performed using the HapMap2 reference data (release 22, build 36) using MaCH (Li et al. 2009, 2010).
Statistical analysis
The distribution of GS measurements was approximately normal in both cohorts. In addition to age and sex, covariates potentially associated with GS were selected from the literature: height, weight, waist-to-hip ratio, waist measurement, body mass index (BMI), sit-to-stand and 6-m walk. The correlations between GS and the available covariates were examined in both cohorts (see Supplementary Tables 1 and 2). Despite often being used as a covariate in the literature, BMI was not significantly correlated with GS in either cohort nor was sit-to-stand test score (available in Sydney MAS only). Height had the strongest correlation with GS in both cohorts (HCS, r = 0.617, p < 0.0001; Sydney MAS, r = 0.618, p = <0.000). Most covariates were significantly correlated with each other. Therefore, only the standard covariates of age and sex were used plus the addition of height.
Replication of prior candidate SNP results
For candidate SNP analyses, two models were assessed: (1) age and sex and (2) age, sex, and height. For replication of prior GS-associated SNPs, the appropriate genetic model was used as previously described in the literature: recessive for SNPs from CNTF (Arking et al. 2006) and UCP3 (Crocco et al. 2011; Dato et al. 2012b) or dominant for IL15 SNPs (Dato et al. 2010). Even though single SNP tests for association within the genes may not be independent due to linkage disequilibrium (LD), a conservative threshold of p = 0.00625 (p = 0.05/8) was set for significance, since we tested eight individual SNPs.
Genome-wide association analyses
Using an additive model and linear regression, a discovery GWAS was undertaken in HCS and replication was performed in Sydney MAS. Model 1 was adjusted for age and sex, and model 2 included the additional covariate, height. By convention, genome-wide significance levels were set to <5 × 10−8 (Barsh et al. 2012) and for suggestive results, a p value cut-off of <1 × 10−5 was used, similar to many other studies reported in the literature (e.g. McDonald et al. 2014). The analyses were undertaken in HCS using GenABEL (Aulchenko et al. 2010) and in Sydney MAS using mach2qtl (Li et al. 2009, 2010). An inverse-weighted meta-analysis was undertaken using METAL (Willer et al. 2010). Manhattan and quantile-quantile (QQ) plots were produced using the R package “GWASTools” (Gogarten et al. 2012). To examine the joint effect of multiple variants within known genes, gene-based tests were performed on the top 10 % of the SNPs within the gene and the p value was assessed based on 1,000,000 permutations using HCS GWAS results and VEGAS software (Liu et al. 2010). For the gene-based tests, a p value threshold of 1.4 × 10−5 for significance was used (Neale and Sham 2004).
Candidate SNP selection
SNPs from prior published studies were selected if they showed a significant association with GS (p < 0.05) and were present in the imputed data set (imputation quality R2 > 0.8). The selected SNPs included two SNPs from UCP3 (rs1800849, rs11235972) (Crocco et al. 2011; Dato et al. 2012b) and two SNPS from IL15 (rs3806798, rs1519551) (Dato et al. 2010). In addition, four SNPs, rs948562, rs1800169, rs550942 and rs4319530, from the CNTF gene were chosen as they covered the variation in a haplotype region previously associated with GS (Arking et al. 2006).
Results
Table 1 reports the general characteristics of the two cohorts. Sydney MAS is substantially older than HCS and, as expected, mean GS is lower in Sydney MAS.
Table 1.
Hunter Community Study (HCS) and Sydney Memory and Ageing Study (MAS) cohort characteristics
| Men | Women | Total | ||||
|---|---|---|---|---|---|---|
| HCS (N = 1036) | MAS (N = 247) | HCS (N = 1052) | MAS (N = 294) | HCS (N = 2088) | MAS (N = 541) | |
| Age mean (SD) (years) | 66.74 (7.66) | 78.07 (4.50) | 65.55 (7.23) | 78.27 (4.74) | 66.31 (7.47) | 78.18 (4.63) |
| Grip strength mean (SD) (kg) | 40.74 (8.12) | 33.98 (7.62) | 25.46 (5.67) | 21.07 (7.19) | 30.06 (9.86) | 26. 96 (9.80) |
| Height mean (SD)a (cm) | 172.9 (7.16) | 171.28 (6.47) | 159.38 (6.30) | 157.80 (6.85) | 166.15 (9.55) | 163.92 (9.47) |
| BMI mean (SD)a | 28.73(4.20) | 27.82 (4.52) | 28.79 (5.63) | 26.84 (4.60) | 28.76 (4.97) | 27.29 (4.59) |
| Weight mean (SD)a | 86.09(13.87) | 81.59 (14.36) | 73.2 (14.76) | 67.30 (13.48) | 79.64 (15.70) | 73.80 (15.60) |
| Education mean (SD) (years) | 14.90 (3.61) | 12.23 (3.75) | 13.99 (3.64) | 11.18 (3.16) | 14.45 (3.64) | 11.66 (3.48) |
BMI body mass index
aFor Sydney MAS, N’s differ due to missing data, for height N = 535; weight N = 536; BMI N = 531
Genome-wide association study
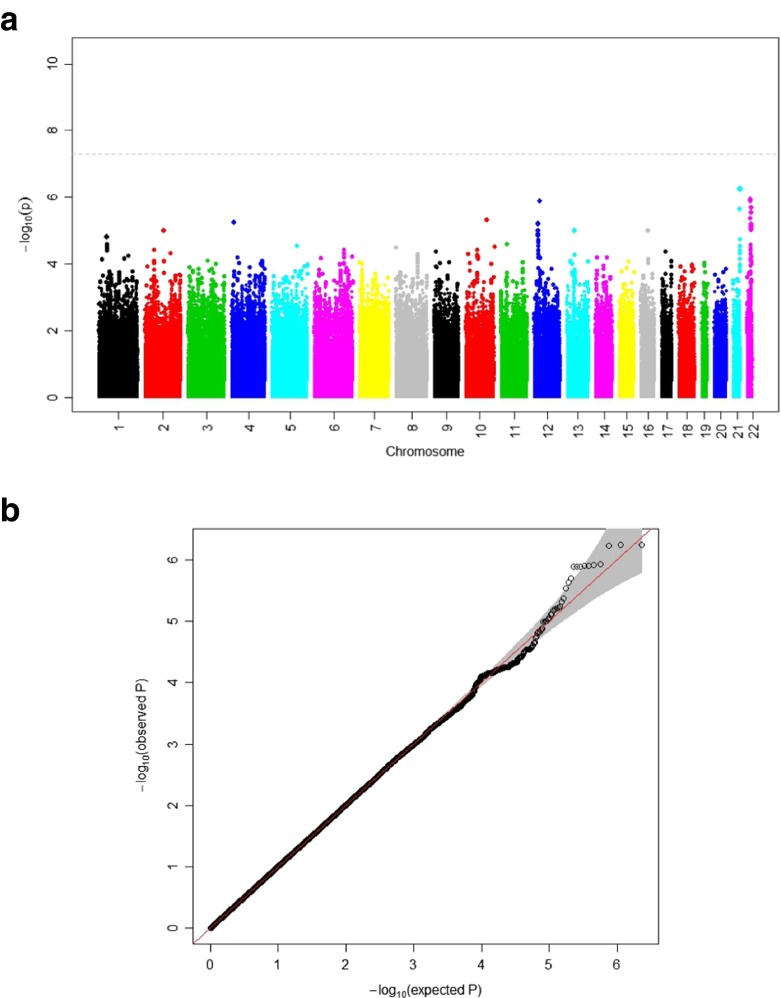
The discovery GWAS found no SNPs achieved genome-wide significance for association with GS in either model (Table 2, Supplementary Table 3). The Manhattan Plots and corresponding QQ plots are shown in Fig. 1 (model 2) and Supplementary Fig. 1 (model 1). None of the suggestively associated SNPs (p < 1 × 10−5) with data available in Sydney MAS was replicated (p > 0.05) although the effects were in the same direction for the majority of the top hits (Table 2, Supplementary Table 3). A meta-analysis including both cohorts identified no genome-wide significant associations with the best p value being observed for rs8133949 on chromosome 21 (beta = −0.9685, p ≤ 1.23 × 10−6); this was also the most strongly associated SNP in the HCS discovery analyses. The effect was in the same direction in both cohorts but not significant in Sydney MAS. We report the meta-analysis results for the top HCS GWAS results in Table 2 and Supplementary Table 3 for the two models.
Table 2.
GWAS results from Hunter Community Study for model 2 (age, sex, height) and replication results in Sydney MAS
| SNP | CHR | BP (hg 18) | Effect allele | Beta | SE | p value | Gene | Function | MAS beta | MAS p value | Meta p value | Meta direction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs8133949 | 21 | 42294183 | T | −1.086 | 0.217 | 5.64E−07 | ZNF295 | Intronic | −0.380 | 0.4341 | 1.23E−06 | −− |
| rs2839434 | 21 | 42291974 | C | −1.085 | 0.217 | 5.73E−07 | ZNF295 | Intronic | −0.366 | 0.4511 | 1.33E−06 | ++ |
| rs871546 | 21 | 42286622 | G | −1.083 | 0.217 | 5.93E−07 | ZNF295 | N-S | −0.360 | 0.4582 | 1.42E−06 | ++ |
| rs6001848 | 22 | 38966745 | G | −1.02 | 0.21 | 1.19E−06 | TNRC6B | Intronic | −0.043 | 0.9278 | 9.03E−06 | ++ |
| rs739182 | 22 | 38945222 | T | −1.022 | 0.211 | 1.24E−06 | TNRC6B | Intronic | −0.054 | 0.9097 | 9.23E−06 | −− |
| rs2143175 | 22 | 38933678 | G | −1.025 | 0.212 | 1.25E−06 | TNRC6B | Intronic | −0.074 | 0.8755 | 8.98E−06 | ++ |
| rs12484438 | 22 | 38888010 | C | −1.04 | 0.215 | 1.26E−06 | TNRC6B | Intronic | −0.092 | 0.8471 | 8.35E−06 | ++ |
| rs17421586 | 22 | 38921258 | A | −1.031 | 0.213 | 1.28E−06 | TNRC6B | Intronic | −0.086 | 0.8563 | 8.43E−06 | −− |
| rs6001832 | 22 | 38922160 | G | −1.030 | 0.213 | 1.29E−06 | TNRC6B | Intronic | −0.082 | 0.8618 | 8.74E−06 | ++ |
| rs2968758 | 12 | 27394222 | G | −1.026 | 0.212 | 1.31E−06 | ARNTL2 | Intronic | 0.062 | 0.8982 | 1.37E−05 | +− |
| rs6001862 | 22 | 39011734 | T | −0.990 | 0.208 | 2.03E−06 | TNRC6B | Intronic | −0.105 | 0.8235 | 1.03E−05 | −− |
| rs2839436 | 21 | 42307670 | T | −1.119 | 0.237 | 2.37E−06 | ZNF295 | 5′ to gene | −0.466 | 0.3278 | 3.70E−06 | −− |
| rs6001877 | 22 | 39042583 | A | −0.977 | 0.209 | 2.98E−06 | TNRC6B | Intronic | −0.097 | 0.8370 | 1.51E−05 | −− |
| rs12628051 | 22 | 38984222 | C | −0.962 | 0.209 | 4.23E−06 | TNRC6B | Intronic | −0.048 | 0.9193 | 2.47E−05 | ++ |
| rs2078059 | 10 | 1.03E+08 | C | 1.368 | 0.299 | 4.82E−06 | AX747408 | 5′ to gene | −1.012 | 0.1410 | 0.00035 | −+ |
| rs16891755 | 4 | 14948707 | T | 4.001 | 0.883 | 5.84E−06 | C1QTNF7 | 5′ to gene | 0.381 | 0.8550 | 2.54E−05 | ++ |
| rs763488 | 12 | 20234741 | T | 1.068 | 0.236 | 6.05E−06 | N/A | N/A | −0.468 | 0.3814 | 0.00017 | +− |
| rs10770619 | 12 | 20235564 | G | 1.065 | 0.236 | 6.29E−06 | N/A | N/A | −0.467 | 0.3822 | 0.00018 | −+ |
| rs5995842 | 22 | 39009308 | G | −0.936 | 0.208 | 6.52E−06 | TNRC6B | Intronic | −0.104 | 0.8247 | 2.99E−05 | ++ |
| rs4821941 | 22 | 39005037 | G | −0.941 | 0.209 | 6.68E−06 | TNRC6B | Intronic | −0.077 | 0.8708 | 3.29E−05 | ++ |
| rs6001872 | 22 | 39033191 | G | −0.926 | 0.207 | 7.79E−06 | TNRC6B | Intronic | −0.119 | 0.8012 | 3.11E−05 | ++ |
| rs2072858 | 22 | 39038625 | C | −0.925 | 0.207 | 7.86E−06 | TNRC6B | Intronic | −0.121 | 0.7968 | 3.14E−05 | ++ |
| rs5995843 | 22 | 39027323 | G | −0.926 | 0.208 | 8.77E−06 | TNRC6B | Intronic | −0.096 | 0.8395 | 3.70E−05 | ++ |
| rs4821943 | 22 | 39052691 | G | −0.916 | 0.207 | 9.36E−06 | TNRC6B | 3′ UTR | −0.171 | 0.7282 | 2.90E−05 | ++ |
SNP annotation information from SNPnexus (Dayem Ullah et al. 2013) or UCSC Genome browser
N-S coding non-synonymous change
Fig. 1.
Results of the discovery GWAS using the Hunter Community Study (N = 2088) for model 2 (adjusted for age, sex, height). a Manhattan plot; b QQ plot (λgc = 1.015)
Two genes showed significant associations in a gene-based based analysis of HCS GWAS results, but none showed significant (p < 0.05) evidence of replication in Sydney MAS (Supplementary Table 4). When the best SNP result in each of these two genes were examined in Sydney MAS, they were significantly associated with GS at the p < 0.05 level but the top SNPs did not overlap with HCS, as shown in Supplementary Table 4.
Replication of prior results
As shown in Supplementary Table 5, only one SNP out of eight, rs550942, was significantly associated with GS, irrespective of the covariates used (T allele associated with higher GS; beta = 0.840, p = 0.005 [model 1]; beta = 0.770, p = 0.009 [model 2]). After correcting for multiple testing (0.05 ÷ 8 = 0.006), this result remained significant (p < 0.006). However, this significant result was found only in the HCS cohort and was not significant in Sydney MAS (p ≥ 0.325); moreover, the effect for Sydney MAS was in the opposite direction.
Discussion
In this study, we examined the genetics of GS in two community-dwelling, dementia-free cohorts of late middle-aged to older adults. Firstly, a GS GWAS was performed although no genome-wide significant results were observed. Further, none of the suggestive results was replicated in an independent cohort. A meta-analysis of the GWAS results from the two cohorts showed no genome-wide significant results. Gene-based analyses of the GWAS results found two significant genes associated with GS in the HCS, but this result was not replicated in the Sydney MAS cohort. We also examined SNPs identified by prior studies and found a significant association for a CNTF SNP with GS, but only in the HCS and not in the smaller Sydney MAS cohort.
To our knowledge, no previous study has undertaken a GWAS examining GS although a number of candidate gene studies have been reported in older adults (see Supplementary Table 6 for a review of prior studies). We did not find any genome-wide significant results nor were any of the suggestive results replicated. Nevertheless, the discovery analysis observed multiple clusters of suggestively associated SNPs in (i) the ZNF295 gene (also known as ZBTB21), which encodes a zinc finger protein implicated in transcriptional regulation and (ii) the TNRC6B gene that plays a role in RNA-mediated gene silencing. Interestingly, given the strong positive correlation between GS and height in our cohorts, variation in the TNRC6B gene has been implicated in a prior GWAS of height (Estrada et al. 2009).
Gene-based analyses showed suggestive results for the two genes, ZNF295 and C2CD2, in HCS with no significant replication in Sydney MAS. Although there was no replication of these results, there were nominally significant SNPs in each of these genes in Sydney MAS but they did not overlap with the top SNPs observed in HCS.
Only one of the previously identified eight candidate SNPs, rs550942, was significantly associated with GS but in the HCS cohort only. This result survived multiple testing correction. The SNP rs550942 is located within one kilobase (downstream) of the ciliary neurotrophic factor gene (CNTF) on chromosome 11. CNTF is an excellent candidate for muscle strength. The CNTF protein is part of the interleukin family, which regulates the expression of cytokines, signalling molecules involved in inflammation. CNTF influences both neuronal and skeletal muscle cells. Moreover, CNTF levels decrease with age and increasing CNTF levels in ageing rats has been shown to improve muscle strength (Arking et al. 2006; Guillet et al. 1999).
In the prior study by Arking et al. (2006), the SNP associated with GS in the HCS, rs550942, was not significant. Instead, another SNP, rs1800169, had the strongest effect; however, this SNP was not significant in any of our cohorts (p ≥ 0.535). These two SNPs are not in LD. In an earlier study by Roth et al. (2001), rs1800169 was also associated with knee muscle strength in a sample aged 20–90 years (N = 494). One reason for the discrepancy in results may be due to study differences. For example, the original Arking et al. (2006) GS results were found in a female-only cohort, whereas the HCS and Sydney MAS cohorts are mixed gender. Moreover, the measurement of GS was different between the original study and ours. Arking et al. (2006) measured GS as the best of three trials in the non-dominant hand, whereas we defined GS as the best trial in the dominant hand. Finally, different covariates were used: in the original study, age, BMI and osteoarthritis of the hands were used, whereas we used age, sex +/−height.
A major limitation of our study is the modest sample size. GWASs are currently performed with tens of thousands of participants to detect the modest effect sizes typical of genetic loci. If we assume that each allele changes grip strength by 1 kg (in line with the estimates seen in our study), in order to have 80 % power to detect this effect at a p value of 5 × 10−8, we would need 14,000 participants if the minor allele frequency was 10 %, reducing to just over 5000 participants if the allele frequency was 40 %. Such large sample sizes were not available to us, but the effect estimates obtained here and the loci highlighted may help guide larger studies. Other limitations of this study include the differences between the two cohorts, Sydney MAS and HCS. Sydney MAS was substantially smaller (N = 541 vs HCS N = 2088) and older (mean 78.22 years vs mean 66.31 years) and had a narrower age range (20.51 vs 31 years), and thus, GS scores were lower in Sydney MAS (mean 26.89 kg vs mean 30.06 kg). There was also a greater proportion of males in HCS than in Sydney MAS (49.6 vs 44.7 %). Another limitation is that we did not have genetic data for all SNPs with a positive result in the literature (e.g. COL1A1) and thus could not comprehensively examine all previously identified candidate genes. Finally, we only looked at GS, and no other measures of muscle strength and function.
In conclusion, in our cohorts, when using a hypothesis-free approach, no genome-wide significant SNPs for GS were identified but two loci, centred on the genes, ZNF295 and TNRC6B, were strongly suggestive. Gene-based analyses suggested that variation in the two genes ZNF295 and C2D2 may also be implicated in GS performance in middle to older aged adults. In our candidate gene analyses, despite having relatively large sample sizes compared to previous studies and two independent cohorts, we observed only one SNP out of eight that showed any evidence of replication that survived multiple testing correction. However, this result was observed in only one of our cohorts. Our results suggest that genetic variants play a role in GS and that further investigation into variation in or nearby the CNTF, ZNF295, C2CD2 and TNRC6B genes in other independent samples, particularly large consortia, may be fruitful.
Future studies should standardise GS measures and perhaps also look at other measures of muscle strength. Given the moderate to high heritability estimates for GS, which suggests that genetic variation plays an important role in GS, and the difficulties of identifying consistent genetic variants, further GWAS using larger samples may prove to be a more productive approach.
Electronic supplementary material
(DOC 506 kb)
Acknowledgments
We would like to acknowledge and thank the HCS and Sydney MAS participants, their supporters and their research teams. This work was supported by various agencies. Sydney MAS is supported by the Australian National Health and Medical Research Council (NHMRC) Program Grants 350833 and 568969. For Sydney MAS, DNA was extracted by Genetic Repositories Australia, an Enabling Facility supported by the NHMRC Grant 401184. The HCS is supported by the University of Newcastle, the Gladys M Brawn Senior Research Fellowship Scheme and the Fairfax Family Foundation. Henry Brodaty is partly supported by the NHMRC-funded Dementia Collaborative Research Centre at the University of New South Wales. Nicola Armstrong is supported by the NHMRC Project Grant 525453, and Karen Mather is supported by an Alzheimer’s Australia Dementia Research Foundation Postdoctoral Fellowship and NHMRC Capacity Building Grant 568940. Amelia Assareh was supported by a PhD scholarship from the Dementia Collaborative Research Centre, UNSW. Elizabeth Holliday is supported by the Australian Heart Foundation and National Stroke Foundation (100071).
References
- Alfred T, et al. A multi-cohort study of polymorphisms in the GH/IGF axis and physical capability: the HALCyon programme. PLoS One. 2012;7:e29883. doi: 10.1371/journal.pone.0029883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, et al. Variation in the ciliary neurotrophic factor gene and muscle strength in older Caucasian women. J Am Geriatr Soc. 2006;54:823–826. doi: 10.1111/j.1532-5415.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- Aulchenko Y, Struchalin M, van Duijn C. ProbABEL package for genome-wide association analysis of imputed data. BMC Bioinforma. 2010;11:134. doi: 10.1186/1471-2105-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JN, Pericak-Vance MA, Haines JL. The impact of the human genome project on complex disease. Genes. 2014;5:518–535. doi: 10.3390/genes5030518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh GS, Copenhaver GP, Gibson G, Williams SM. Guidelines for genome-wide association studies. PLoS Genet. 2012;8:e1002812. doi: 10.1371/journal.pgen.1002812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmelli D, Reed T. Stability and change in genetic and environmental influences on hand-grip strength in older male twins. J Appl Physiol (Bethesda, Md: 1985) 2000;89:1879–1883. doi: 10.1152/jappl.2000.89.5.1879. [DOI] [PubMed] [Google Scholar]
- Cesari M, et al. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J Cachex Sarcopenia Muscle. 2012;3:181–190. doi: 10.1007/s13539-012-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocco P, Montesanto A, Passarino G, Rose G. A common polymorphism in the UCP3 promoter influences hand grip strength in elderly people. Biogerontology. 2011;12:265–271. doi: 10.1007/s10522-011-9321-z. [DOI] [PubMed] [Google Scholar]
- Dato S, Krabbe KS, Thinggaard M, Pedersen BK, Christensen K, Bruunsgaard H, Christiansen L. Commonly studied polymorphisms in inflammatory cytokine genes show only minor effects on mortality and related risk factors in nonagenarians. J Gerontol A Biol Sci Med Sci. 2010;65:225–235. doi: 10.1093/gerona/glp210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dato S, Montesanto A, Lagani V, Jeune B, Christensen K, Passarino G. Frailty phenotypes in the elderly based on cluster analysis: a longitudinal study of two Danish cohorts. Evidence for a genetic influence on frailty. Age (Dordr) 2012;34:571–582. doi: 10.1007/s11357-011-9257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dato S, Soerensen M, Montesanto A, Lagani V, Passarino G, Christensen K, Christiansen L. UCP3 polymorphisms, hand grip performance and survival at old age: association analysis in two Danish middle aged and elderly cohorts. Mech Ageing Dev. 2012;133:530–537. doi: 10.1016/j.mad.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayem Ullah AZ, Lemoine NR, Chelala C. A practical guide for the functional annotation of genetic variations using SNPnexus. Brief Bioinform. 2013;14:437–447. doi: 10.1093/bib/bbt004. [DOI] [PubMed] [Google Scholar]
- Deelen J, Beekman M, Capri M, Franceschi C, Slagboom PE. Identifying the genomic determinants of aging and longevity in human population studies: progress and challenges. Bioessays. 2013;35:386–396. doi: 10.1002/bies.201200148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada K, et al. A genome-wide association study of northwestern Europeans involves the C-type natriuretic peptide signaling pathway in the etiology of human height variation. Hum Mol Genet. 2009;18:3516–3524. doi: 10.1093/hmg/ddp296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL, Reynolds CA, Berg S, de Faire U, Svartengren M. Genetic and environmental influences on decline in biobehavioral markers of aging. Behav Genet. 2003;33:107–123. doi: 10.1023/A:1022549700943. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Gaist D, Petersen HC, Hjelmborg J, McGue M, Vaupel JW, Christensen K. Hand grip strength: a phenotype suitable for identifying genetic variants affecting mid- and late-life physical functioning. Genet Epidemiol. 2002;23:110–122. doi: 10.1002/gepi.1127. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Hjelmborg J, Mortensen J, McGue M, Vaupel JW, Christensen K. Age trajectories of grip strength: cross-sectional and longitudinal data among 8,342 Danes aged 46 to 102. Ann Epidemiol. 2006;16:554–562. doi: 10.1016/j.annepidem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Global Lipids Genetics C, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogarten SM, et al. GWASTools: an R/Bioconductor package for quality control and analysis of genome-wide association studies. Bioinformatics. 2012;28:3329–3331. doi: 10.1093/bioinformatics/bts610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet C, Auguste P, Mayo W, Kreher P, Gascan H. Ciliary neurotrophic factor is a regulator of muscular strength in aging. J Neurosci. 1999;19:1257–1262. doi: 10.1523/JNEUROSCI.19-04-01257.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Willer C, Sanna S, Abecasis G. Genotype imputation. Annu Rev Genomics Hum Genet. 2009;10:387–406. doi: 10.1146/annurev.genom.9.081307.164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34:816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, et al. A versatile gene-based test for genome-wide association studies. Am J Hum Genet. 2010;87:139–145. doi: 10.1016/j.ajhg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteini AM, et al. Heritability estimates of endophenotypes of long and health life: the Long Life Family Study. J Gerontol A Biol Sci Med Sci. 2010;65:1375–1379. doi: 10.1093/gerona/glq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald ML, et al. Common genetic variants associated with resting oxygenation in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2014;51:678–687. doi: 10.1165/rcmb.2014-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy M, et al. Cohort profile: the Hunter Community Study. Int J Epidemiol. 2010;39:1452–1463. doi: 10.1093/ije/dyp343. [DOI] [PubMed] [Google Scholar]
- Murabito JM, Yuan R, Lunetta KL. The search for longevity and healthy aging genes: insights from epidemiological studies and samples of long-lived individuals. J Gerontol A Biol Sci Med Sci. 2012;67:470–479. doi: 10.1093/gerona/gls089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Sham PC. The future of association studies: gene-based analysis and replication. Am J Hum Genet. 2004;75:353–362. doi: 10.1086/423901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed T, Fabsitz RR, Selby JV, Carmelli D. Genetic influences and grip strength norms in the NHLBI twin study males aged 59-69. Ann Hum Biol. 1991;18:425–432. doi: 10.1080/03014469100001722. [DOI] [PubMed] [Google Scholar]
- Ribom EL, Mellstrom D, Ljunggren O, Karlsson MK. Population-based reference values of handgrip strength and functional tests of muscle strength and balance in men aged 70-80 years. Arch Gerontol Geriatr. 2011;53:e114–e117. doi: 10.1016/j.archger.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Roth SM, et al. CNTF genotype is associated with muscular strength and quality in humans across the adult age span. J Appl Physiol (1985) 2001;90:1205–1210. doi: 10.1152/jappl.2001.90.4.1205. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, et al. The Sydney Memory and Ageing Study (MAS): methodology and baseline medical and neuropsychiatric characteristics of an elderly epidemiological non-demented cohort of Australians aged 70-90 years. Int Psychogeriatr. 2010;22:1248–1264. doi: 10.1017/S1041610210001067. [DOI] [PubMed] [Google Scholar]
- Sayer AA, et al. Polymorphism of the IGF2 gene, birth weight and grip strength in adult men. Age Ageing. 2002;31:468–470. doi: 10.1093/ageing/31.6.468. [DOI] [PubMed] [Google Scholar]
- Semba RD, et al. Relationship of low plasma klotho with poor grip strength in older community-dwelling adults: the InCHIANTI study. Eur J Appl Physiol. 2012;112:1215–1220. doi: 10.1007/s00421-011-2072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenholm S, Tiainen K, Rantanen T, Sainio P, Heliovaara M, Impivaara O, Koskinen S. Long-term determinants of muscle strength decline: prospective evidence from the 22-year mini-Finland follow-up survey. J Am Geriatr Soc. 2012;60:77–85. doi: 10.1111/j.1532-5415.2011.03779.x. [DOI] [PubMed] [Google Scholar]
- Taekema DG, Gussekloo J, Maier AB, Westendorp RGJ, de Craen AJM. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing. 2010;39:331–337. doi: 10.1093/ageing/afq022. [DOI] [PubMed] [Google Scholar]
- United Nations (2002) World Population Ageing: 1950-2050. United Nations Department of Economic and Social Affairs: Population Division. http://www.un.org/esa/population/publications/worldageing19502050/index.htm. Accessed 2 Oct 2013
- Werle S, Goldhahn J, Drerup S, Simmen BR, Sprott H, Herren DB. Age- and gender-specific normative data of grip and pinch strength in a healthy adult Swiss population. J Hand Surg Eur. 2009;34:76–84. doi: 10.1177/1753193408096763. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara A, et al. Physical function is weakly associated with angiotensin-converting enzyme gene I/D polymorphism in elderly Japanese subjects. Gerontology. 2009;55:387–392. doi: 10.1159/000222429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 506 kb)