Abstract
The molecular and physiological properties of 2-phenylethanol (2-PE) in the strongly scented genotype (SSG) and a weakly scented genotype (WSG) of damask rose at six floral developmental stages were investigated. The chemical compositions of volatile emissions were determined by gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) analysis of the floral headspace. In both genotypes, the relative percentage of 2-PE increased more in SSG than WSG, as flowers developed. In the petals of damask rose the relative transcript levels of phenyl acetaldehyde reductase (PAR) were higher at stages 3 and 4 in SSG and WSG, respectively. Also, the expression pattern of PAR indicated a significant difference between two genotypes during flower developmental stages. In this study, enzymatic activity leading to the synthesis of 2-PE from the phenyl acetaldehyde (PAld) moderately increased during flower development up to stage 5 in SSG. However, high level of PAR enzymatic activity was observed in stage 3 of WSG. These results indicated that the pattern activity of PAR was different in two used genotypes of damask rose. For SSG, PAR activities were low in early stage of flower development and then gradually increased reaching its highest value at full bloom stage. In WSG, no significant change in enzyme activity was seen after stage 3.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-014-0274-y) contains supplementary material, which is available to authorized users.
Keywords: Damask rose, Scent, 2-Phenylethanol, Genotypes, Gene expression
Introduction
2-Phenylethanol is one of the prominent scent compounds produced by damask rose (Hirata et al. 2012; Sakai et al. 2007), and in various fruits such as strawberry, tomato and grape varieties (Aubert et al. 2005). It is widely applied in the cosmetics, perfumery, and food industries (Hua and Xu 2011). The worldwide use of 2-PE is estimated to be more than 10,000 tons per year (Scognamiglio et al. 2012; Hua and Xu 2011). 2-Phenylethanol is also used as a substrate for the synthesis of other flavors or pharmaceutical compounds such as phenyl ethyl acetate ester (Scognamiglio et al. 2012). 2-Phenylethanol is biosynthesized from L-phenylalanine (L-Phe) with pyridoxal-5’-phosphate (PLP)-dependent aromatic amino acid decarboxylases (AADC) and phenyl acetaldehyde reductases (PAR) in planta (Chen et al. 2011; Sakai et al. 2007). Phenylalanine transformed to phenyl acetaldehyde (PAld) by AADC. Phenylacetaldehyde was also synthesized by plant PAld synthase (PAAS), a member of the AADC family, in Petunia hybrida (Kaminaga et al. 2006) and by AADC in Solanum lycopersicum (Tieman et al. 2006) and Arabidopsis (Gutensohn et al. 2011). Phenylacetaldehyde is converted to 2-PE by the action of PAR (Chen et al. 2011; Tieman et al. 2006). Recent investigations of the biosynthesis of 2-PE in R. hybrida ‘Hoh-Jun’ and R. damascena confirmed the intermediate compound PAld (Hirata et al. 2012; Chen et al. 2011; Sakai et al. 2007). Three enzymes, namely AADC, aromatic amino acid aminotransferase (AAAT) and PAR, were found to be involved in the biosynthesis of 2-PE (Fig. 1) (Hirata et al. 2012).
Fig. 1.
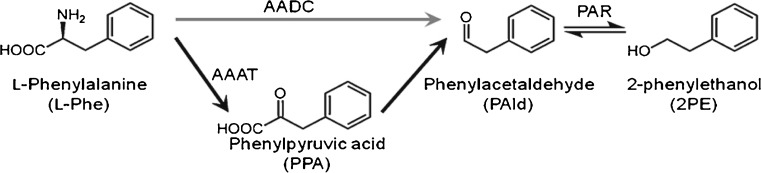
2-PE is biosynthesized from L-phenylalanine (L-Phe) with pyridoxal-5’-phosphate (PLP)-dependent aromatic amino acid decarboxylases (AADC), aromatic amino acid aminotransferase (AAAT) and phenyl acetaldehyde reductase (PAR) in rose (Hirata et al. 2012)
The damask rose (Rosa damascena Mill.) is the most important species used to produce rose water, attar of rose, and essential oils in the perfume and medicinal industry. However, despite the importance of damask rose scent, the molecular and biochemical investigations of flower fragrance of this species are still in its primary stage. To elucidate biochemical functions and molecular biological properties of PAR involved in biosynthesis of 2-PE, a major constituent of rose-like flowers scents, we characterized these properties with transcriptional, metabolite profiling and biochemical analysis during flower development in strongly scented (SSG) and weakly scented (WSG) genotypes of damask rose.
Materials and methods
Plant materials
Flowers of two distinct genotypes of the Iranian Rosa damascena Mill. were harvested throughout six stages of flower development from plants grown at the Estahban Research Center. These two genotypes were identified by genetic markers and other characterization as described previously (Karami et al. 2013; Babaei et al. 2007). The descriptions of Picone et al. (2004) were used as a guideline for these stages as follows: Stage 1: Immature bud with sepals split to reveal dark immature petals. Stage 2: Immature bud, sepals retracting, petal whorl tightly closed, petals beginning to lighten in color. Stage 3: Sepals fully retracted, outer petal whorl beginning to loosen, petal color lightened. Stage 4: Outer petal whorl opened, inner petal whorl closed, reproductive organs invisible. Stage 5: Inner and outer whorls open, reproductive organs visible. Stage 6: Anthers dry and darkened, petals pale and lacking torpor.
The headspace volatiles screening
Three to five branches, displaying the most flowers, were harvested in the early morning from each rose germplasm. The branches were immediately placed in water in a vase, before transporting the samples to the laboratory. Petals of five flowers were randomly selected and mixed to represent each of the floral development stages. About 3 g of each sample was placed, without delay, in a 20 mL headspace vial, which was sealed with a silicone rubber septum and an aluminium cap and transferred to the headspace tray. Sampling of the headspace was done using a Combi PAL System that comprised a headspace auto-sampler, heater and agitator. While being agitated, the content of the vial was heated to a temperature of 45 °C, which was maintained for 20 min. The temperature of the sampling needle and transmission line was 85 °C.
GC-MS analysis of the volatile compounds
The headspace was analysed using an Agilent Technologies gas chromatograph fitted with an HP-5 (5 % phenyl methyl siloxane; 30 m × 0.32 mm i.d. × 0.25 μm) capillary column. An injector temperature of 280 °C and a split ratio of 1: 50 were used. The oven temperature programme was initially ramped from 60 °C to 210 °C at 3 °C min−1, before increasing to 240 °C at 20 °C min−1 (held 8.5 min). Nitrogen, at a flow rate of 1 ml min−1 was used as the carrier gas. A detector temperature of 290 °C was maintained. An Agilent 7890 GC-MS, equipped with a HP-5 MS column (30 m × 0.25 mm i.d. × 25 μm) was used to confirm compound identities. Helium was employed as the carrier gas and the sample was split using a ratio of 1:50. Separated compounds were ionised using an ionisation potential of 70 eV. Retention indices were determined using the retention times of an n-alkane series that was analysed under the same chromatographic conditions as the samples.
Qualitative and quantitative analyses
The compounds were identified by comparison of retention indices (RI, HP-5) with those reported in the literature and by comparison of their mass spectra with the Wiley GC-MS Library, Adams Library, Mass Finder 2.1 Library data published mass spectra data (Adams 2007; McLafferty and Stauffer 1989).
RNA extraction, cDNA synthesis and real-time PCR analyses
Rose petals from each stage were immediately detached and frozen in liquid nitrogen and frozen in −84 °C. High-quality total RNA was successfully isolated from 100 mg of petal tissue using RNeasy Plant Mini kit (Qiagen, Valencia, CA, USA), according to the manufacturers protocol. The purified total RNA was quantified with NanoDrop ND 1000 Spectrophotometer (Wilmington, USA). DNase treatment was carried out using the Fermentas DNase Kit (Fermentas, Hanover, MD) according to the manufacturer’s instructions. 5 μg of DNase-treated RNA was used for first strand cDNA synthesis, using 100 pmol oligo-dT (18 mer), 15 pmol dNTPs, 20 U RNase Inhibitor and 200 U M-Mulv reverse transcriptase (all from Fermentas) in a 20 μl final volume. Primer design was carried out using Allele ID 7 software for the reference gene and gene of interest (rose phenyl acetaldehyde reductase (PAR) (AB426519.2) Gene). The Rose elongation factor α (Ef α) gene (AB370119.1) was used as the reference gene for data normalization (Table 1).
Table 1.
Sequences of primers used for real-time PCR amplification and the resulting product size of damask rose
| Primer | Sequence | Amplicon length (bp) | Ta |
|---|---|---|---|
| PAR-F | ACAGACCCAAAGGCAGAAC | 164 | 54 |
| PAR-R | TCATCAACCACTACATCAGGAG | 164 | 54 |
| EF-α-1 F | TTTCACTCTTGGAGTGAAGCAGAT | 103 | 55 |
| EF-α -1R | GACCTCCTTGACAATTTCTTCATAA | 103 | 55 |
Real-time PCR was performed on RNA obtained from three independent experiments. All samples included equal quantities of RNA. Relative Real-time PCR was performed in a total volume of 20 μL containing 1 μl of cDNA, 1 × Syber Green buffer and 4 pmol of each primer. The amplification reactions were carried out in a Line Gene K thermal cycler (Bioer, China) under the following conditions: 2 min at 94 °C, 40 cycles of 94 °C for 10 s, Ta °C (annealing temperature) for 15 s and 72 °C for 30 s. After 40 cycles, the amplification products were heated to 95 °C to determine their melting curves and confirm their specificity. All amplification reactions were repeated three times under identical conditions and included a negative control and five standard samples. To ensure that the PCR products were generated from cDNA and not genomic DNA, proper control reactions were carried out without the presence of reverse transcriptase. For the Quantitative Real Time PCR data, the relative expression for the gene of interest was calculated using the threshold cycle (CT) method. The CT for each sample was calculated using the Line- Gene K software and the method of Larionov et al. (2005). Accordingly, the amount of expression of target mRNAs over reference values was calculated by the equation 2-ΔΔCT, where ΔCT is determined by subtracting the corresponding Ef α CT value (reference gene) from the specific CT of the target (gene of interest) and ΔΔCT is obtained by subtracting the ΔCT of each experimental sample from that of the calibrator sample (Livak and Schmittgen 2001).
PAR activity assay
Activities of PAR were assayed at 30 °C by measuring the decrease in absorbance of NADPH at 340 nm (Chen et al. 2011; Larroy et al. 2002). The reaction mixture (200 μL) (100 mM potassium phosphate (pH 7.0), PAR solution, 10 mM PAld, and 2.5 mM NADPH) was incubated at 30 °C for 10 min. The reaction was centrifuged at 3000 × g for 5 min. The relative activities of the PAR with selected substrates were determined by measuring the decrease in absorbance of NADPH at 340 nm using 10 mM of substrate. One unit of enzyme activity was defined as the oxidation of 1 μmol NADPH min−1 at 30 °C. Specific activity described as enzyme activity divided by total protein content and was shown as unit of activity per gram of protein.
Statistical analysis
All of data were analyzed as a completely randomized design with three replications. Data were expressed as means ± standard deviation (SD). The statistical significance of differences between treatments were determined by analysis of variance (ANOVA) and then testing for differences between means was by Duncan’s new multiple range test and SPSS software version 16 at P ≤ 0.05.
Results
Headspace analysis of floral scent in damask rose
The applied headspace GC-MS metabolite profiling resulted in the identification of a total of 72 compounds, based on comparison with MS library consisting of compounds from rose volatile oils (data was not shown). The main floral headspace components identified in two R. damascena germplasms were as follows: 2-Phenylethanol, β-citronellol, α-pinene, benzyl alcohol and geranyl acetate.
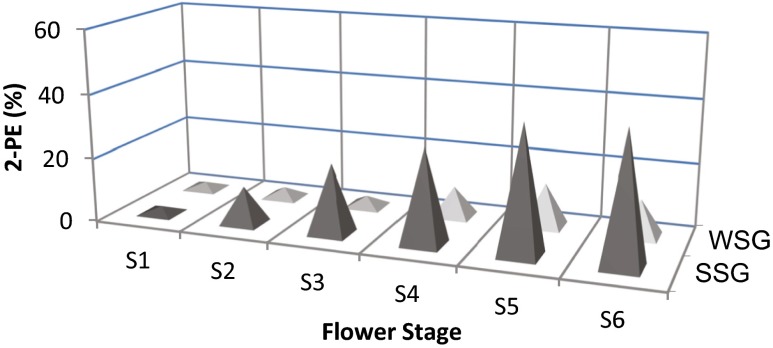
2-Phenylethanol was highest and a major component in SSG and increased with floral development stages (Fig. S1 and S2). The relative percentage of 2-PE was significantly increased to peak (40.63 %) at the last stage of the floral development in SSG, but in WSG the highest quantity (13.42 %) was at the stage 5. In the both SSG and WSG flower development stages, the relative percentage of 2-PE were increased up to stage 5 (Fig. 2).
Fig. 2.
The relative percentage of 2-PE in the volatile oils from two genotypes (SSG and WSG) of Rosa damascena at six flower developmental stages (S) used in this study. The mean relative percentage of 2-PE was calculated with three replications
Expression of PAR transcripts in petals of damask rose at six floral developmental stages
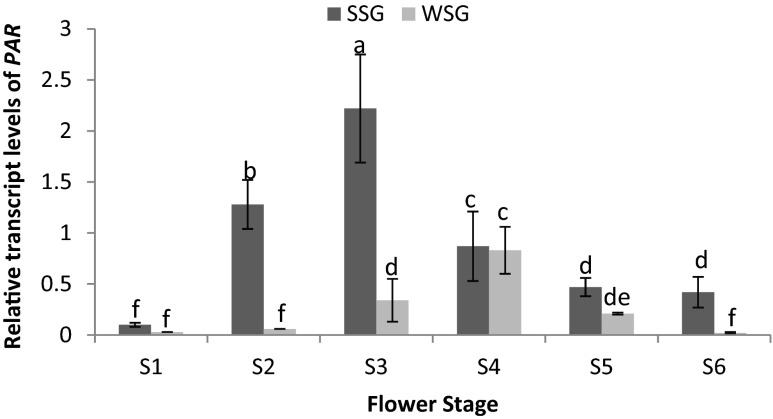
To further substantiate PAR’s involvement in the biosynthesis of 2-PE, expression of PAR transcripts in petals of two distinct genotypes (SSG and WSG) of damask rose at six floral developmental stages were investigated by real time RT-PCR (Fig. 3). In the petals of damask rose the relative transcript levels of PAR were higher at stage 3 and 4 in SSG and WSG, respectively. As shown in Fig. 3, relative expression level of PAR gene has been significantly (P ≤ 0.05) increased during stage 3 and 4 (full bloom) stages compared to early stage of flower development (bud stage) and during senescence. Comparison of expression level of PAR indicated that in the flowers of SSG, the expressions of PAR were about 2.67 fold more than WSG. Although expression of the gene was observed throughout floral development, PAR mRNA levels significantly (P ≤ 0.05) decreased during senescence. This highest expression in flowers was consistent with 2-PE emissions, which were highest on a per gram tissue basis from damask rose flowers.
Fig. 3.
Relative expression level of PAR during flower developmental stages (S) from two distinct genotypes (SSG and WSG) of Rosa damascena. Error bars are standard deviation of the mean. Relative expression for PAR was calculated based on the threshold cycle (CT) method. All data were normalized to the Ef α expression level. The mean expression value was calculated with three replications. The different letters denote a statistically significant difference at P ≤ 0.05, as determined by Duncan’s new multiple range tests
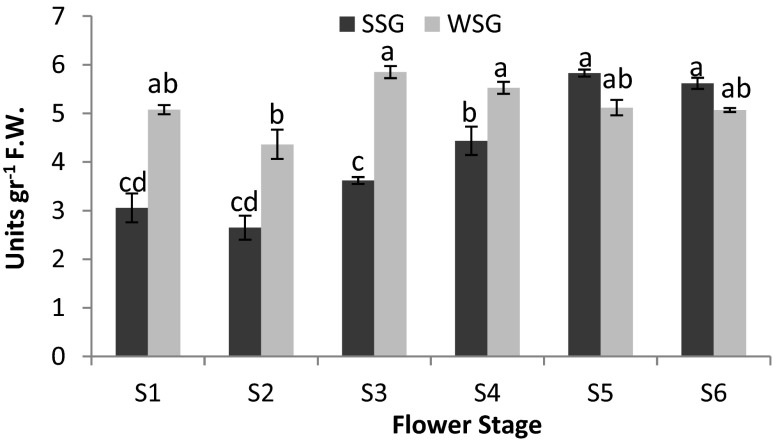
PAR assay
To test for changes in PAR enzymatic activities in two distinct genotypes of damask rose petals during flower development, cell-free extracts of petals of the six flowering stages were prepared and tested for potential enzymatic reductase activity with its substrates. Specific activity described as enzyme activity divided by total protein content and was shown as unit of activity per gram of protein (Fig. S3). Enzymatic activity leading to the synthesis of phenyl ethyl alcohol from the phenyl acetaldehyde moderately increased during flower development up to stage 5 in SSG. However, high level of PAR enzymatic activity was seen in stage 3 of WSG. As shown in Fig. 4 the pattern activity of phenyl acetaldehyde reductase was different in two distinct genotypes of damask rose. For SSG, PAR activities were low in early stage of flower development and then gradually increased reaching its highest value at full bloom stage. However, in WSG enzyme activity remained without significant (P ≤ 0.05) changes after stage 3.
Fig. 4.
Comparison of phenyl acetaldehyde reductase (PAR) enzyme activity during flower developmental stages (S) from two distinct genotypes (SSG and WSG) of Rosa damascena. Error bars are standard deviation of the mean. The mean enzyme activity value was calculated with three replications. The different letters denote a statistically significant difference at P ≤ 0.05, as determined by Duncan’s new multiple range tests
Discussion
Volatiles have been investigated most extensively in the airspace (headspace) surrounding above-ground plant parts. Headspace sampling is a non-destructive method for collecting volatiles. Compared with solvent extractions of volatiles from plant tissues, headspace analysis gives a more realistic picture of the volatile profile emitted by plants and detected by insects that respond to plant volatiles, making this method most suitable for many ecologically relevant applications (Tholl et al. 2006). The headspace analysis of volatiles from our study indicated that the relative percentage of 2-PE increased significantly at the floral developmental stages in both the genotypes. Similar evolutions of 2-PE were reported during the development of the flower of R. hybrid, Rosa canina and other genotypes of R. damascena. These observations indicate that the main component in all oil samples was 2-PE, and its content increased gradually during the floral development process (Hosni et al. 2011; Rusanov et al. 2011; Shalit et al. 2004). 2-PE is a prominent scent compound released from flowers of damask rose and some hybrid roses (Rosa ‘Hoh-Jun’ and Rosa ‘Yves Piaget’). 2-PE is biosynthesized from L-Phe via the intermediate PAld by three key enzymes, AADC, AAAT and PAR. The transcripts of PAR were higher in petals than calyxes and leaves and peaking at the unfurling stage 4 (Hirata et al. 2012; Chen et al. 2011; Sakai et al. 2007). The accessions from Fars province of Iran formed two distinct clusters in the presented dendrogram (Karami et al. 2013; Karami et al. 2012; Babaei et al. 2007). In this study, variability between two distinct genotypes of Fars province was observed as far as levels of volatile oils contents and composition are concerned. Interestingly, whereas the levels of volatiles in both genotypes of damask rose increased during flower development, the ratios of the major volatiles remained constant during the different stages, suggesting that emission of the different classes of major volatiles is regulated by similar mechanisms (Caissard et al. 2006). In general, the present investigation showed that the early stage of flowers (stages 1 and 2) differed in fragrance characteristics compared to fully opened flowers (stages 3, 4 and 5). This may suggest that the final process is developmentally regulated and the production of volatile compounds is related to their possible function in attracting and guiding pollinators or in protecting the reproductive parts of the plant from their enemies (Dudareva et al. 2004). In this way, several reports have revealed that the floral scents are orchestrated during the life span of the flower development to ensure successful pollination (Hosni et al. 2011; Baldermann et al. 2009; Dudareva et al. 2004). To further substantiate PAR’s involvement in the biosynthesis of 2-PE, expression of PAR transcripts in petals of two distinct genotypes (SSG and WSG) of damask rose at six floral developmental stages were investigated by real time RT-PCR. In the petals of damask rose the relative transcript levels of PAR were higher at stage 3 and 4 in SSG and WSG respectively. Also, the expression pattern of PAR analyzed by real-time RT-PCR indicated a significant difference between two genotypes during flower developmental stages. The GC-MS validated functional analysis of both PAR and recombinant PAR confirmed that the PARs catalyze the conversion of PAld to 2-PE (Chen et al. 2011). It was shown that PAR can contribute to the production of important scent molecules on molecular level (Chen et al. 2011). The highest transcripts have been observed at stage 4, suggesting a correlation to the maximum emission of 2-PE at stage 4 of R. damascena as already reported (Chen et al. 2011; Oka et al. 1999). Other rose scent-related genes exhibited the highest transcripts at the same unfurling stage, where the emission of volatile compounds was the highest (Guterman et al. 2002; Lavid et al. 2002). Several reductases as well as the PAR may be involved in the emission of other alcoholic volatile compounds such as (S)-(β)-citronellol and geraniol. It might be reasonable to elucidate if PAR plays an essential part for the production of several main rose scents. In this study, enzymatic activity leading to the synthesis of 2-PE from PAld moderately increased during flower development up to stage 5 in SSG. However, high level of PAR enzymatic activity was seen in stage 3 of WSG. These results indicated that the pattern activity of PAR was different in two distinct genotypes of damask rose. For SSG, PAR activities were low in early stage of flower development then gradually increased reaching its highest value at full bloom stage. However, in WSG no significant change in enzyme activity was observed after stage 3. Some rose species like R. rugosa and R. canina are probably partly self-incompatible and pollinated by generalist insects like bees (Dobson et al. 1999). In the wild roses, from which R. hybrida is derived, floral odors are thought to be chemical signals between the plant and insects, the latter including both pollinators and predators (Pichersky and Gershenzon 2002). In R. damascena, in this study it was shown that petal odors are dominated by 2-PE, which are known insect attractants (Shalit et al. 2004; Dobson et al. 1999). Chemical analyses in the two distinct genotypes of damask rose (SSG and WSG) clearly showed that the emission of most scent compounds reached to peaks during the late stages of flower development and decreases afterwards. This is a very general feature, which has been observed in a wide range of species including Clarkia breweri (Pichersky and Gershenzon 2002), A. majus (Dudareva et al. 2004) and different rose cultivars (Bergougnoux et al. 2007; Picone et al. 2004; Shalit et al. 2004; Guterman et al. 2002). This link between emission of scent and the opening of the flower is generally related to the capacity of the flower to attract pollinators (Negre et al. 2003). Volatile emissions in SSG and WSG of damask rose were investigated in present experiment. It is interesting to note that WSG generally had a lowest amount of 2-PE, typical of heavily scented roses. This lack of scent in modern hybrids is not restricted to roses but has also been observed in other flowers such as carnation (Clery et al. 1999). In roses, most scentless flowers are found in the hybrid tea groups which were bred for the cut flower market and it is not yet clear why this particular type of rose should lack scent. The content of starch was higher in young petals and decreases as the development proceeded but no clear link could be made between starch content and the capability to make volatiles in different rose varieties (Bergougnoux et al. 2007). Hence, although it is likely that the starch present in the petal is necessary to provide carbon and energy for the biosynthesis of scent compounds (Sood et al. 2006), this does not appear to be the limiting factor in the WSG of damask rose that we studied. The pattern of total protein content was not different in two distinct genotypes of damask rose. In both of genotypes, the total protein contents were low in early stage of flower development then gradually increased reaching its highest value at full bloom stage. Although total protein level of damask rose has been increased during flower development up to stage 4, it was decreased during senescence in both of genotypes. Finally, pioneering research on C. breweri and A. majus has shown that scent production and emission is often regulated at the level of transcription (Dudareva et al. 2004). Some rose cultivars that were studied are almost devoid of scent molecules, belonging to different biosynthetic pathways. Isolation of scent gene in roses has only begun recently and clearly more research is needed to characterize in detail the various pathways leading to the enormous diversity of compounds synthesized by rose flowers (Vainstein et al. 2006). The major volatile of the R. damascena floral headspace, 2-PE, exhibits rhythmic emission with a peak during the light period (Picone et al. 2004). In our study, the inhibition of 2-PE accumulation and emission in early and late stage of flower development is probably determined by the level of its substrate under light. 2-PE emission from R. damascena flowers is regulated by the endogenous circadian oscillator. Previous study indicated that the diurnal accumulation and emission of 2-PE coincides with the expression of its biosynthetic gene PAR and, therefore, is expected to be regulated at the level of gene transcription (Picone et al. 2004).
Conclusions
Genetically and temporal factors affect volatile oil composition in damask rose. During the lifespan of the flower, production and emission of 2-PE is developmentally regulated. Volatile emission in flowers of different plant species follows similar developmental patterns, increasing during the early stages of flower development, peaking when the flowers are ready for pollination, and decreasing thereafter. During flower development, expression of genes encoding scent biosynthetic enzymes peaks when the flowers are at full bloom stage and ready for pollination. The concurrent temporal changes in activities of PAR enzyme responsible for the final steps of volatile formation in SSG and the expression of corresponding structural gene (PAR) in both the genotypes suggested that the developmental biosynthesis of 2-PE is regulated largely at the level of gene expression. Finally, there was high variation in 2-PE levels in the different damask rose genotypes suggesting a key role of the genotype in the biosynthesis of this metabolite.
Electronic supplementary material
(DOC 187 kb)
References
- Adams RP. Identification of essential oil components by gas chromatography-mass spectroscopy. Illinois: Allured Publishing Corporation; 2007. [Google Scholar]
- Aubert C, Baumann S, Arguel H. Optimization of the analysis of flavor volatile compounds by liquid-liquid microextraction (LLME). Application to the aroma analysis of melons, peaches, grapes, strawberries, and tomatoes. J Agric Food Chem. 2005;53:8881–8895. doi: 10.1021/jf0510541. [DOI] [PubMed] [Google Scholar]
- Babaei A, Tabaei-Aghdaei SR, Khosh-Khui M, Omidbaigi R, Naghavi MR, Esselink GD, Smulders MJM. Microsatellite analysis of damask rose (Rosa damascena Mill.) accessions from various regions in Iran reveals multiple genotypes. BMC Plant Biol. 2007;7:12. doi: 10.1186/1471-2229-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldermann S, Yang Z, Sakai M, Fleischmann P, Watanabe N. Volatile constituents in the scent of roses. Floricul Ornament Biotechnol. 2009;3:89–97. [Google Scholar]
- Bergougnoux V, Caissard JC, Jullien F, Magnard JL, Scalliet G, Cock JM, Hugueney P, Baudino S. Both the adaxial and abaxial epidermal layers of the rose petal emit volatile scent compounds. Planta. 2007;226:853–866. doi: 10.1007/s00425-007-0531-1. [DOI] [PubMed] [Google Scholar]
- Caissard JC, Bergougnoux V, Martin M, Mariat M, Baudino S. Chemical and histochemical analysis of ‘Quatre Saisons Blanc Mousseux’, a moss rose of the Rosa damascena group. Ann Bot. 2006;97:231–238. doi: 10.1093/aob/mcj034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XM, Kobayashi H, Sakai M, Hirata H, Asai T, Ohnishi T, Watanabe N. Functional characterization of rose phenyl acetaldehyde reductase (PAR), an enzyme involved in the biosynthesis of the scent compound 2-phenylethanol. J Plant Physiol. 2011;168:88–95. doi: 10.1016/j.jplph.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Clery RA, Owen NE, Chambers SF, Thornton-Wood SP. An investigation into the scent of carnations. J Essent Oil Res. 1999;11:355–359. doi: 10.1080/10412905.1999.9701153. [DOI] [Google Scholar]
- Dobson HEM, Danielson EM, Van-Wesep ID. Pollen odor chemicals as modulators of bumble bee foraging on Rosa rugosa Thunb. (Rosaceae) Plant Spectrum Biol. 1999;14:153–166. doi: 10.1046/j.1442-1984.1999.00020.x. [DOI] [Google Scholar]
- Dudareva N, Pichersky E, Gershenzon J. Biochemistry of plant volatiles. Plant Physiol. 2004;135:1893–1902. doi: 10.1104/pp.104.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutensohn M, Klempien A, Kaminaga Y, Nagegowda DA, Negre-Zakharov F, Huh JH. Role of aromatic aldehyde synthase in wounding/herbivory response and flower scent production in different Arabidopsis ecotypes. Plant J. 2011;66:591–602. doi: 10.1111/j.1365-313X.2011.04515.x. [DOI] [PubMed] [Google Scholar]
- Guterman I, Shalit M, Menda N, Piestun D, Dafny-Yelin M, Shalev G, et al. Rose scent: genomics approach to discovering novel floral fragrance-related genes. Plant Cell. 2002;14:2325–2338. doi: 10.1105/tpc.005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Ohnishi T, Ishida H, Tomida K, Sakai M, Hara M, Watanabe N. Functional characterization of aromatic amino acid aminotransferase involved in 2-phenylethanol biosynthesis in isolated rose petal protoplasts. J Plant Physiol. 2012;169:444–451. doi: 10.1016/j.jplph.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Hosni K, Zahed N, Chrif R, Brahim NB, Kallel M, Sebei H. Volatile oil constituents of Rosa canina L.: differences related to developmental stages and floral organs. Plant Biosyst. 2011;145:627–634. doi: 10.1080/11263504.2011.586378. [DOI] [Google Scholar]
- Hua D, Xu P. Recent advances in biotechnological production of 2-phenylethanol. Biotechnol Adv. 2011;29:654–660. doi: 10.1016/j.biotechadv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Kaminaga Y, Schnepp J, Peel G, Kish CM, Ben-Nissan G, Weiss D. Plant phenyl acetaldehyde synthase is a bifunctional homo tetrameric enzyme that catalyzes phenylalanine decarboxylation and oxidation. J Biol Chem. 2006;281:23357–23366. doi: 10.1074/jbc.M602708200. [DOI] [PubMed] [Google Scholar]
- Karami A, Khosh-Khui M, Salehi H, Saharkhiz MJ, Rowshan V. Headspace analysis of floral scent from two distinct genotypes of Iranian Damask Rose (Rosa damascena Mill) J Essent Oil Bear Plants. 2013;16:489–498. doi: 10.1080/0972060X.2013.813266. [DOI] [Google Scholar]
- Karami A, Zandi P, Khosh-Khui M, Salehi H, Saharkhiz MJ. Analysis of essential oil from nine distinct genotypes of Iranian damask rose (Rosa damascena Mill) J Med Plants Res. 2012;6:5495–5498. [Google Scholar]
- Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinforma. 2005 doi: 10.1186/1471-2105-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larroy C, Fernandez MR, Gonzalez E, Pares X, Biosca JA. Characterization of the Saccharomyces cerevisiae YMR318C (ADH6) gene product as a broad specificity NADPH-dependent alcohol dehydrogenase: relevance in aldehyde reduction. Biochem J. 2002;361:163–172. doi: 10.1042/0264-6021:3610163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavid N, Wang J, Shalit M, Guterman I, Bar E, et al. O-Methyltransferases involved in the biosynthesis of volatile phenolic derivatives in rose petals. Plant Physiol. 2002;129:1899–1907. doi: 10.1104/pp.005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McLafferty FW, Stauffer DB. The Wiley/NBS registry of mass spectral data. New York: Wiley and Sons; 1989. [Google Scholar]
- Negre F, Kish CM, Boatright J, Underwood B, Shibuya K, Wagner C, Clark DG, Dudareva N. Regulation of methylbenzoate emission after pollination in snapdragon and petunia flowers. Plant Cell. 2003;15:2292–3006. doi: 10.1105/tpc.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka N, Ohishi H, Hatano T, Hornberger M, Sakata K, Watanabe N. Aroma evolution during flower opening in Rosa damascena Mill. Z Naturforsch. 1999;54:889–895. [Google Scholar]
- Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol. 2002;5:237–243. doi: 10.1016/S1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Picone JM, Clery RA, Watanabe N, MacTavish HS, Turnbull CGN. Rhythmic emission of floral volatiles from Rosa damascena semperflorens cv. ‘Quatre Saisons’. Planta. 2004;219:468–478. doi: 10.1007/s00425-004-1250-5. [DOI] [PubMed] [Google Scholar]
- Rusanov K, Kovacheva N, Rusanova M, Atanassov I. Traditional Rosa damascena flower harvesting practices evaluated through GC-MS metabolite profiling of flower volatiles. Food Chem. 2011;129:1851–1859. doi: 10.1016/j.foodchem.2011.05.132. [DOI] [Google Scholar]
- Sakai M, Hirata H, Sayama H, Sekiguchi K, Itano H, Asai T, Dohra H, Hara M, Watanabe N. Production of 2-phenylethanol in roses as dominant floral scent compound from L-phenylalanine by two key enzymes; a PLP-dependent decarboxylase and a phenyl acetaldehyde reductase. Biosci Biotechnol Biochem. 2007;71:2408–2419. doi: 10.1271/bbb.70090. [DOI] [PubMed] [Google Scholar]
- Scognamiglio J, Jones L, Letizia CS, Api AM. Fragrance material review on phenylethyl alcohol. Food Chem Toxicol. 2012;50:224–239. doi: 10.1016/j.fct.2011.10.028. [DOI] [PubMed] [Google Scholar]
- Shalit M, Guterman I, Volpin H, Bar E, Tamari T, Menda N, Adam Z, Zamir D, Vainstein A, Weiss D, Pichersky E, Lewinsohn E. Volatile ester formation in roses: identification of an acetyl-coenzyme A. Geraniol/Citronellol acetyltransferase in developing rose petals. Plant Physiol. 2004;131:1868–1876. doi: 10.1104/pp.102.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood S, Vyas D, Nagar PK. Physiological and biochemical studies during flower development in two rose species. Sci Hortic. 2006;108:390–396. doi: 10.1016/j.scienta.2006.02.012. [DOI] [Google Scholar]
- Tholl D, Boland W, Hansel A, Loreto F, Rose USR, Schnitzler JP. Practical approaches to plant volatile analysis. Plant J. 2006;45:540–560. doi: 10.1111/j.1365-313X.2005.02612.x. [DOI] [PubMed] [Google Scholar]
- Tieman D, Taylor M, Schauer N, Fernie AR, Hanson AD, Klee HJ. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc Natl Acad Sci U S A. 2006;103:8287–8292. doi: 10.1073/pnas.0602469103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainstein A, Lewinsohn E, Weiss D. An integrated genomics approach to identifying floral scent genes in rose. In: Dudareva N, Pichersky E, editors. Biology of floral scent, section II. Biochemistry and molecular biology of floral scent. Boca Raton: CRC Press Taylor and Francis Group; 2006. pp. 91–102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 187 kb)