Abstract
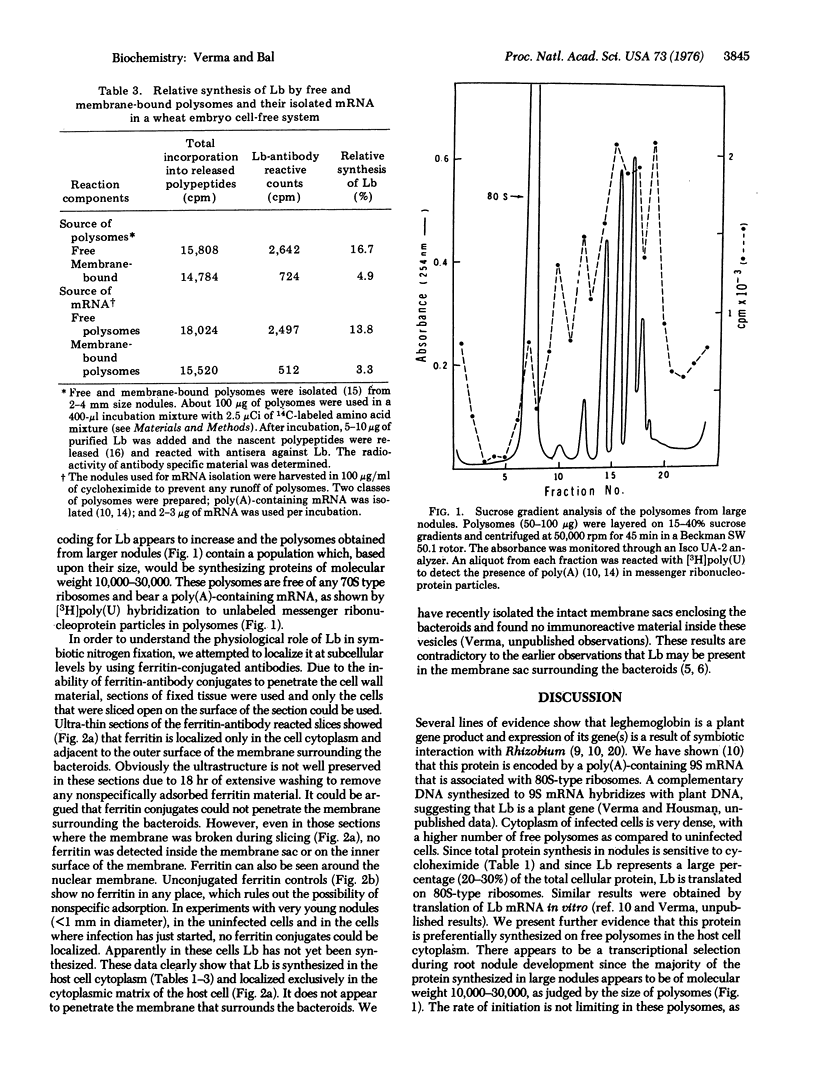
A majority of the total protein synthesis in host cell cytoplasm is inhibited by cycloheximide. Because leghemoglobin represents a large proportion of total cellular protein in the nodule, this observation suggests that leghemoglobin may be translated on the 80S-type ribosomes. Analysis of the nascent peptides isolated from free and membrane-bound polysomes showed that free polysomes contain more immunoreactive material against antibodies to leghemoglobin as compared to that of membrane-bound polysomes. When free and membrane-bound polysomes were incubated in a wheat embryo cell-free protein-synthesizing system, a larger percentage of the released polypeptides from free polysomes was found to be immunoreactive. Similar results were obtained by translation in vitro of the poly(A)-containing mRNA isolated from free and membrane-bound polysomes. For immunocytochemical localization of leghemoglobin, the antibodies were conjugated with ferritin. Antibody conjugates were strictly localized in the host cell cytoplasm and adjacent to the outer surface of the membrane surrounding the bacteroids. Ferritin was not found on the inner surface of the membrane or within the membrane sac. These data suggest that leghemoglobin is synthesized preferentially on the free polysomes in the host cell cytoplasm, directed by a poly(A)-containing 9S mRNA that is most likely of plant origin, and that its location is restricted to the host cell cytoplasm.
Keywords: free and membrane-bound polysomes, poly(A), translation in vitro, immunocytochemistry
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bal A. K., Verma D. P., Byrne H., Maclachlan G. A. Subcellular localization of cellulases in auxin-treated pea. J Cell Biol. 1976 Apr;69(1):97–105. doi: 10.1083/jcb.69.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne H., Christou N. V., Verma D. P., Maclachlan G. A. Purification and characterization of two cellulases from auxin-treated pea epicotyls. J Biol Chem. 1975 Feb 10;250(3):1012–1018. [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The biogenesis of leghemoglobin. The determinant in the Rhizobium-legume symbiosis for leghemoglobin specificity. Biochim Biophys Acta. 1971 Jan 19;229(1):58–62. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dilworth M. J., Kidby D. K. Localization of iron and leghaemoglobin in the legume root nodule by electron microscope autoradiography. Exp Cell Res. 1968 Jan;49(1):148–149. doi: 10.1016/0014-4827(68)90527-2. [DOI] [PubMed] [Google Scholar]
- Dilworth M. J. The plant as the genetic determinant of leghaemoglobin production in the legume root nodule. Biochim Biophys Acta. 1969 Jul 30;184(2):432–441. doi: 10.1016/0304-4165(69)90047-6. [DOI] [PubMed] [Google Scholar]
- Godfrey C. A., Dilworth M. J. Haem biosynthesis from ( 14 C)- -aminolaevulinic acid in laboratory-grown and root nodule Rhizobium lupini. J Gen Microbiol. 1971 Dec;69(3):385–390. doi: 10.1099/00221287-69-3-385. [DOI] [PubMed] [Google Scholar]
- Goodchild D. J., Bergersen F. J. Electron microscopy of the infection and subsequent development of soybean nodule cells. J Bacteriol. 1966 Jul;92(1):204–213. doi: 10.1128/jb.92.1.204-213.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN D. C., GRINYER I., COULTER W. H. ELECTRON MICROSCOPY OF INFECTION THREADS AND BACTERIA IN YOUNG ROOT NODULES OF MEDICAGO SATIVA. J Bacteriol. 1963 Jul;86:125–137. doi: 10.1128/jb.86.1.125-137.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D. The concentration and distribution of haemoglobin in the root nodules of leguminous plants. Biochem J. 1949;44(5):585–591. doi: 10.1042/bj0440585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Maclachlan G. A., Byrne H., Ewings D. Regulation and in vitro translation of messenger ribonucleic acid for cellulase from auxin-treated pea epicotyls. J Biol Chem. 1975 Feb 10;250(3):1019–1026. [PubMed] [Google Scholar]
- Verma D. P., Marcus A. Activation of Protein Synthesis upon Dilution of an Arachis Cell Culture from the Stationary Phase. Plant Physiol. 1974 Jan;53(1):83–87. doi: 10.1104/pp.53.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]
- Wittenberg J. B. Facilitated oxygen diffusion. The role of leghemoglobin in nitrogen fixation by bacteroids isolated from soybean root nodules. J Biol Chem. 1974 Jul 10;249(13):4057–4066. [PubMed] [Google Scholar]




