Abstract
Guggulsterone is an aromatic steroidal ketonic compound obtained from vertical rein ducts and canals of bark of Commiphora wightii (Arn.) Bhandari (Family - Burseraceae). Owing to its multifarious medicinal and therapeutic values as well as its various other significant bioactivities, guggulsterone has high demand in pharmaceutical, perfumery and incense industries. More and more pharmaceutical and perfumery industries are showing interest in guggulsterone, therefore, there is a need for its quantitative determination in existing natural populations of C. wightii. Identification of elite germplasm having higher guggulsterone content can be multiplied through conventional or biotechnological means. In the present study an effort was made to estimate two isoforms of guggulsterone i.e. E and Z guggulsterone in raw exudates of 75 accessions of C. wightii collected from three states of North-western India viz. Rajasthan (19 districts), Haryana (4 districts) and Gujarat (3 districts). Extracted steroid rich fraction from stem samples was fractionated using reverse-phase preparative High Performance Liquid Chromatography (HPLC) coupled with UV/VIS detector operating at wavelength of 250 nm. HPLC analysis of stem samples of wild as well as cultivated plants showed that the concentration of E and Z isomers as well as total guggulsterone was highest in Rajasthan, as compared to Haryana and Gujarat states. Highest concentration of E guggulsterone (487.45 μg/g) and Z guggulsterone (487.68 μg/g) was found in samples collected from Devikot (Jaisalmer) and Palana (Bikaner) respectively, the two hyper-arid regions of Rajasthan, India. Quantitative assay was presented on the basis of calibration curve obtained from a mixture of standard E and Z guggulsterones with different validatory parameters including linearity, selectivity and specificity, accuracy, auto-injector, flow-rate, recoveries, limit of detection and limit of quantification (as per norms of International conference of Hormonization). Present findings revealed the role of environmental factors on biosynthesis of guggulsterone isomers under natural conditions.
Keywords: Biochemical characterization, Burseraceae, Endangered species, Guggulsterone, Oleo-gum resin
Introduction
Commiphora wightii (Arn.) Bhandari (Burseraceae) is a gum bearing (Greek word kommi means ‘gum’ and pheros means ‘to bear’) plant species indigenous to the Indian subcontinent and growing in rocky tracts of arid and semi-arid lands of India, Bangladesh, Pakistan, China, Ethiopia, Arabia, Tropical and Northern Africa, and many other countries (Deng 2007; Kant et al. 2010). It is commonly known as Guggulu in Sanskrit and guggul in Hindi due to the presence of E and Z isomers of aromatic steroidal ketonic compound guggulsterone in vertical rein ducts and canals of bark. Oleo-gum resin of C. wightii is widely used in traditional medicines to treat various afflictions including rheumatism, arthritis, arterosclerosis, obesity, hypercholestermia, inflammation and cancer (Samudio et al. 2005; Kulhari et al. 2012; Harish et al. 2014). Various successful clinical studies on effectiveness of herbal formulations containing guggulsterone made it popular in the pharmacy world. Destructive and unscientific harvesting for economic benefits with negligible conservation efforts led to dwindling of natural population of this species making it endangered and led to its categorization in ‘data deficient’ category ver. 2.3 (1994) of the Red Data Book of IUCN (International Union for the Conservation of Nature) assemblage. However, the Government of India has included it under RET (Rare, Endangered, Threatened) category and now only few wild populations exists in the states of Rajasthan and Gujarat (Haque et al. 2007; Samantaray et al. 2011; Kulloli and Kumar 2014). Lack of organized cultivation strategies, over exploitation, increasing desertification and environmental vulnerabilities, slow growth, poor seed setting (16 %), very poor seed germination (5 %), and long dormant phase has hampered its natural regeneration and led to scarcity of raw as well as finished products of this valuable drug in the country. According to an estimate, the demand of gum guggul is 1000 MT but India produces only 100 MT against its requirement (Maheshwari 2010). Even the price of gum guggul has increased manifold (from Rs. 100–600 kg−1) in the last 10– years and the deficiency is being met through imports from Pakistan and Afghanistan. India spends approximately Rs 45 crores on its import mainly from Afghanistan indicating many fold increase in its demand with decreasing availability of natural sources in the country.
Guggul is the dried form of oleo-gum resin obtained primarily from the bark of the guggul plant. It is a complex mixture of resin (61 %), gum (29.3 %) and other chemicals (6.1 %) including several plant sterols, steroids, esters, diterpenes and higher alcohols. However, its main active compounds are inter-convertible isomeric forms (E and Z) of guggulsterone which are steroidal in nature (Agrawal et al. 2004a). Two different arrangements of CH3 at C20 in three-dimensional space and the hindered rotation about the carbon–carbon double bond at C17 and C20 classifies guggulsterone into Z-{4,17(20)-cis-pregnadiene-3,16-dione} and E-{4,17(20)-trans-pregnadiene-3,16-dione} isomers.
Genetic variability of this species is facing a great threat since more and more pharmaceutical and perfumery industries and showing interest in this wonder plant, thereby severely increasing pressure on natural wild populations. Therefore, there is an urgent need for reliable and consistent quantitative determination of bioactive ingredients in existing natural populations of C. wightii for identification of elite germplasm having higher oleo-gum resin yielding ability which can be multiplied and mass propagated through tissue culture (Kumar and Nadgauda 2014) and vegetative propagation (Tripathi et al. 2014) methods for afforestation purposes. Kulhari et al. (2013) recently determined guggulsterone content in 11 samples of C. wightii using High Performance Thin Layer Chromatography (HPTLC). Mesorb et al. (1998) validated gradient HPLC method for quantification of E and Z stereoisomers in oleo-gum resin exudates of C. mukul Engl. Soni et al. (2010) estimated the content of guggulsterone isomers in resin using HPLC while Dass and Ramawat (2009) used reverse phase column HPLC to determine guggulsterone content in cell and callus cultures of C. wightii. Verma et al. (1998) and Singh et al. (1995) quantified the two isomeric forms of guggulsterone, simultaneously by HPLC in rat serum after administration of a single dose (50 mg/kg). Agrawal et al. (2004a) determined the concentration of E (Rf 0.38) and Z (Rf 0.46) guggulsterone in pharmaceutical dosage forms while Agrawal et al. (2004b) carried out stress degradation studies on guggulsterone using HPTLC. Recently, Kulhari et al. (2012) elaborately and exhaustively reviewed the status of pharmacological, biochemical and biotechnological progress made in the genus Commiphora with emphasis on C. wightii.
Keeping in view the endangered status and importance of C. wightii, the present investigation was designed to develop a HPLC based quick and validated procedure, conforming to ICH recommendations, for simultaneous estimation of bio-active constituents, E and Z guggulsterone, in wild as well as cultivated C. wightii samples collected from diverse geographical regions (75 locations from 26 districts of 3 states namely Rajasthan, Gujarat and Haryana) of North-western India for identification of elite genotypes. A total of 75 samples from eleven agro-climatic regions of India were screened to determine the nature and extent of variability in these two steroidal components among C. wightii accessions.
Materials and methods
Procurement of samples
Stem samples of wild as well as cultivated C. wightii plants were collected from Rajasthan (19 districts), Haryana (4 districts) and Gujarat (3 districts), the three hot spot states for guggul occurrence in India.
Preparation of raw material
Collected plant material was washed with running tap water followed by deionized autoclaved water to remove the dust particles and possible parasites. Stem samples (10 g) were shade dried, pulverized and finally coarsely powdered before subjecting to extraction with petroleum ether (60–80 °C) in Soxhlet apparatus for 8–10 h. Extracted samples were concentrated under vacuum using rotary evaporator at high temperature to near dryness. These concentrated sticky samples were reconstituted quantitatively in acetone, filtered through syringe filters (size 0.45 μ, Axiva) and the final volume was made to 5 ml in volumetric flasks. Purified samples were stored at 4 °C and subjected for sonication prior to HPLC analysis.
Chemicals and reagents
For quantitative estimation of guggulsterone, reference compounds (isomeric form of E and Z guggulsterone) were procured from Chromadex, USA. HPLC grade solvents (methanol, petroleum ether, acetone, water, acetonitrile and trifluroacetic acid) were obtained from Sigma, Hi- Media, Fisher Scientific and Qualigens.
Preparation of stock solution
Accurately weighed (5 mg) standards of E and Z guggulsterone were transferred to 5 ml volumetric flask and volume was made using methanol. This stock standard solution was further diluted to obtain working standard solution of different concentrations ranging from 20 to 100 μg ml−1 and stored at 4 °C.
HPLC instrumentation and chromatographic conditions
HPLC analysis was performed on Rapid Separation LC (RSLC) system (Shimadzu, Japan), equipped with auto sampler, LC- 2010 pump (low pressure gradient mode), with a Sentry C18 guard column, degasser, column oven and a UV/VIS detector. Chromatographic separation of analytes was carried out using a reverse phase Nucleosil C18 column (5 μm, 4.6 × 250 mm). Mobile phase (pH 3.0+/−0.2), a binary gradient system consisting of eluent A (0.05 % trifluroacetic acid in water) and eluent B (0.03 % trifluroacetic acid in acetonitrile), was properly filtered and degassed in ultrasonic bath for 20 min prior to use. Injected volume (20 μl) was maintained at a constant flow rate (0.6 ml/min) and column temperature (35 °C). The spectral data was collected at 250 nm detection wavelength (LC- 2010 UV detector with Duterium D2 lamp) and data acquisition was performed by LC- Solution software version 1.25. All the samples were analyzed in triplicate and data was subjected to standard error calculation using SAS 9.3 software.
Method validation
The described method was validated according to the ICH guidelines (ICH 1993) including following validation characteristics: linearity, selectivity and specificity, accuracy, repeatability, precision (Intraday and Interday), limit of detection and quantification (LOD and LOQ).
Linearity
The calibration graphs were obtained for each individual compound (E and Z) by plotting the peak area versus the concentration. Regression analysis calibration curves demonstrated linearity in the range of 20–100 μg ml−1 after evaluating five concentrations of standards. Linearity of the developed method was presented in terms of regression coefficient (R2) and it was > 0.99 in the two reference compounds, E (0.994) and Z (0.993) guggulsterone, at 250 nm wavelength.
Selectivity and specificity
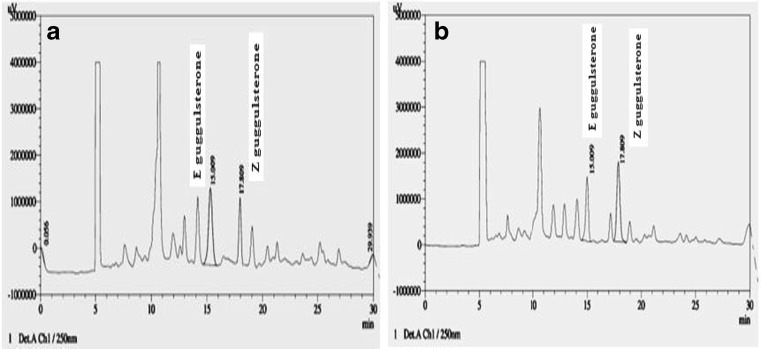
The selectivity of the method was determined by comparing the retention time of representative chromatogram of ultraviolet–visible (UV/Vis) spectra of sample extracts with the reference compounds of E and Z guggulsterone. E and Z guggulsterone reference compounds were eluted at 15.009 and 17.809 min, respectively.
Accuracy
Three standard concentrations (20, 60 and 100 μg ml−1) were used. The negative values of % bias in the recovery of standard samples verified the accuracy of the analytical method.
Repeatability
Accuracy data was used to determine the repeatability of the method. For total 9 determinations for each component (E and Z isomers) the values of mean % recovery and mean relative standard deviation (% RSD) were 99.6387 and 0.601 respectively. The value of mean recovery as well as mean and individual RSD (<2.0) verified the repeatability of the method.
Precision
The intraday and interday precision was determined in terms of % RSD (n = 2). The 3 standard concentration samples were analyzed in triplicate on 2 different sessions to determine the intraday precision. The data obtained, indicated that % RSD for any sample was not more than 2 % and thus verified the precision.
Limit of detection (LOD) and limit of quantification (LOQ)
The LOD and LOQ were calculated on the basis of slope of the calibration curve and standard deviation of the response using the following formula;
Results and discussion
Various chemical fingerprinting means are employed for quantification and also for quality control of herbal remedies including chromatographic, spectroscopic, thermo-gravimetric analysis, capillary electrophoresis and polarography techniques (Choudhary and Sekhon 2011). Among them HPLC is a highly efficient, robust and quick analytical method for quantitative estimation of desirable components with optimum resolution. It can also be exploited as a regular investigative method for testing purity of drugs in marketed products as well as for detection of resinous adulterants from related plants like Mangifera indica L., Acacia nilotica (L) Willd. Ex Delile, Ficus religiosa L., while trading pharmacologically important oleo-gum resins. No reports are available regarding quantitative estimation of guggulsterone isomers in wild collections of C. wightii using HPLC. However, the technique has been used for estimation of E and Z isomers of guggulsterone in gum resin exudates of C. wightii and C. mukul, and dietary supplements containing C. mukul guggulipids (Soni et al. 2010; Nagarajan et al. 2001; Musharraf et al. 2011). Preliminary results indicated that negligible amount of guggulsterone isomers were detectable in the leaf and root samples of C. wightii, therefore, only the stem samples showing presence of substantial quantity of guggulsterone were taken up for further studies. The oleo-gum resin is mainly found in the stem only therefore, selective distribution of the resin and its components is expected (Kulhari et al. 2013).
The E and Z isomers of guggulsterone, individual as well as mixture, depicted a clear peak during separation at a retention time of 15.009 and 17.809 min., respectively. A calibration plot was obtained by plotting peak area against concentration of guggulsterone. A linear straight line was observed for guggulsterone E and Z standards using the regression equation Y = 14296x–69744 and Y = 11278x + 43499 respectively. LOD and LOQ values for E isomer were 0.214 μg ml−1 and 0.649 μg ml−1 respectively, while for Z isomer they were 2.789 μg ml−1 and 8.454 μg ml−1. The correlation coefficients for E and Z guggulsterones were 0.994 and 0.993 with a linear calibration graph (linearity range of 20–100 μg/ml) (Table 1). The value of mean recovery as well as mean and individual RSD (<2.0) verified the repeatability, accuracy and precision of the method (Tables 2, 3 and 4).Chemical profiling of all the 75 samples collected from various locations could elucidate the difference in guggulsterone content without interference of any other constituents at 250 nm wavelength (Table 5). Figure 1 depicts a representative HPLC chromatogram showing separation of guggulsterone isomers as well as other constituents in stem extracts of C. wightii samples. Concentration of guggulsterone E varied from 120.82 μg g−1 to 487.45 μg g−1 while that of guggulsterone Z varied from 111.74 μg g−1 to 487.68 μg g−1. Concentration of guggulsterone was highest in Rajasthan, as compared to Haryana and Gujarat, due to water deficiency and adverse climatic conditions. Among different geographical locations highest concentration of E guggulsterone (487.45 μg g−1) and Z guggulsterone (487.68 μg g−1) was found in the samples collected from Devikot (Jaisalmer - Western Rajasthan) and Palana (Bikaner - North-Western Rajasthan) respectively, both belonging to hyper-arid agro-climatic region (Fig. 1). Least concentration of guggulsterone isomers viz. 118.35 ± 0.28 μg g−1 (E isomer) and 111.74 ± 0.28 μg g−1 (Z isomer) was found in wild accessions collected from Banswara (Southern Rajasthan). Central part of Rajasthan, such as Ajmer district, also reflected a significantly higher amount of E (270.43 ± 0.33 μg g−1) and Z (211.27 ± 0.55 μg g−1) guggulsterone. Total guggulsterone was also highest in sample collected from Devikot, Jaisalmer (960.33 μg g−1) followed by that collected from Palana, Bikaner (955.33 μg g−1), the two hyper-arid districts of Rajasthan. Major difference in concentration of the two isomeric forms was found in the sample collected from Jodhpur {E guggulsterone (343.10 μg g−1); Z guggulsterone (173.11 μg g−1)} followed by the samples collected from Swai Madhopur, Ajmer and Chittorgarh districts of Rajasthan. While minor variation was observed in concentration of both the isomers in the samples gathered from Nakhatrana, Gujarat {E guggulsterone (160.00 μg g−1); Z guggulsterone (161.83 μg g−1)} followed by Jawantpura (Jalore), (Kushalgarh, Dungra and Sarwa Kalan), Banswara, Rajasthan. Among the 26 districts having diverse (eleven) agro-climatic conditions, collections made from Trans-Gangetic plains (Mahendergarh), semi-arid-rocky tracts (Rajsamand and Ajmer), sub-humid (Chittorgarh, Bhilwara and Udaipur), humid (Swai Madhopur) and hyper-arid regions (Jaisalmer) were found to contain higher concentration of E isomer compared to Z guggulsterone. During quantification both the isomers exhibited a wide disparity which can be discerned on the basis of geographical inequality as well as on environmental factors, unambiguously. The recoveries of both E and Z isomers from different samples were found greater than 98 % in the present study, whereas it was >96 % in serum samples (Verma et al. 1998) and >90 % in C. mukul extract and dietary supplements (Nagarajan et al. 2001) while Akhade et al. (2013) reported 103.84 % recovery of Z guggulsterone in tablet formulations.
Table 1.
Validatory parameters for E and Z guggulsterone - Linear regression equation, R2, LOD and LOQ values
| Compound | Wavelength | Regression | R2 | Retention time | LOD (3.3*SD)/S (μg ml−1) | LOQ (10*SD)/S (μg ml−1) |
|---|---|---|---|---|---|---|
| E guggulsterone | 250 nm | Y = 14296x − 69744 | 0.994 | 15.009 min | 0.214 | 0.649 |
| Z guggulsterone | 250 nm | Y = 11278x + 43499 | 0.993 | 17.809 min | 2.789 | 8.454 |
Table 2.
Validatory parameters for E and Z guggulsterone - Accuracy and recovery for quality consistency evaluation
| Conc. (μg ml−1) | 20 | 60 | 100 | |||
|---|---|---|---|---|---|---|
| Component | E | Z | E | Z | E | Z |
| Conc. Determined 1 | 19.43 | 19.73 | 59.59 | 59.89 | 100.12 | 100.02 |
| Conc. Determined 2 | 19.79 | 19.99 | 59.68 | 59.68 | 99.98 | 99.92 |
| Conc. Determined 3 | 19.96 | 19.96 | 60.49 | 60.29 | 99.92 | 99.87 |
| Conc. Determined Mean | 19.72 | 19.89 | 59.92 | 59.95 | 100.00 | 99.93 |
| Std. deviation (SD) | 0.27 | 0.14 | 0.49 | 0.30 | 0.10 | 0.076 |
| RSD | 1.37 | 0.71 | 0.82 | 0.519 | 0.105 | 0.07 |
| Recovery 1 (%) | 97.15 | 98.65 | 99.32 | 99.82 | 100.12 | 100.02 |
| Recovery 2 (%) | 98.95 | 99.95 | 99.47 | 99.47 | 99.98 | 99.92 |
| Recovery 3 (%) | 99.8 | 99.80 | 100.82 | 100.48 | 99.92 | 99.87 |
| Recovery mean (%) | 98.63 | 99.46 | 99.86 | 99.92 | 100.00 | 99.93 |
| Std. deviation (SD) | 1.35 | 0.71 | 0.82 | 0.51 | 0.10 | 0.076 |
| RSD | 1.371 | 0.71 | 0.82 | 0.51 | 0.10 | 0.076 |
| % Bias | −1.366 | −0.533 | −0.133 | −0.077 | 0.006 | −0.063 |
Table 3.
Method precision for E and Z guggulsterones – (A) Intra-day precision
| Session | 1 | 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conc. | 20 | 60 | 100 | 20 | 60 | 100 | ||||||
| Component | E | Z | E | Z | E | Z | E | Z | E | Z | E | Z |
| Conc. Determined 1 | 19.63 | 19.43 | 59.89 | 59.59 | 100.12 | 100.08 | 19.33 | 19.93 | 59.99 | 59.89 | 100.62 | 100.22 |
| Conc. Determined 2 | 19.89 | 19.79 | 59.67 | 59.68 | 99.98 | 99.88 | 19.89 | 19.79 | 59.58 | 59.88 | 99.48 | 99.98 |
| Conc. Determined 3 | 19.76 | 19.96 | 60.19 | 60.49 | 99.92 | 99.97 | 19.86 | 19.66 | 60.19 | 60.09 | 99.62 | 99.54 |
| Mean | 19.76 | 19.73 | 59.92 | 59.92 | 100.01 | 99.98 | 19.69 | 19.79 | 59.92 | 59.95 | 99.91 | 99.9 |
| Standard deviation | 0.13 | 0.27 | 0.26 | 0.50 | 0.08 | 0.10 | 0.30 | 0.14 | 0.26 | 0.12 | 0.51 | 0.34 |
| RSD | 0.65 | 1.37 | 0.44 | 0.83 | 0.08 | 0.10 | 1.52 | 0.68 | 0.43 | 0.20 | 0.51 | 0.34 |
Table 4.
Method precision for E and Z guggulsterones – (B) Inter-day precision
| Conc. | 20 | 60 | 100 | |||
|---|---|---|---|---|---|---|
| Component | E | Z | E | Z | E | Z |
| Day 1 | ||||||
| Conc. Determined 1 | 19.79 | 19.79 | 59.68 | 59.68 | 99.98 | 99.88 |
| Conc. Determined 2 | 19.96 | 19.96 | 60.49 | 60.49 | 99.92 | 100.08 |
| Conc. Determined 3 | 19.93 | 19.88 | 59.89 | 59.89 | 100.12 | 99.88 |
| Mean | 19.89 | 19.87 | 60.02 | 60.02 | 100.01 | 99.94 |
| Standard deviation | 0.09 | 0.09 | 0.42 | 0.42 | 0.10 | 0.11 |
| RSD | 0.46 | 0.43 | 0.70 | 0.70 | 0.10 | 0.11 |
| Day 2 | ||||||
| Conc. Determined 1 | 19.71 | 19.33 | 59.78 | 59.67 | 99.78 | 99.67 |
| Conc. Determined 2 | 19.69 | 19.89 | 60.69 | 60.19 | 99.97 | 99.44 |
| Conc. Determined 3 | 19.70 | 19.61 | 60.24 | 59.93 | 99.88 | 98.97 |
| Mean | 19.7 | 19.61 | 60.235 | 59.93 | 99.875 | 99.36 |
| Standard deviation | 0.01 | 0.28 | 0.46 | 0.26 | 0.09 | 0.36 |
| RSD | 0.05 | 1.43 | 0.76 | 0.43 | 0.10 | 0.36 |
| Day 3 | ||||||
| Conc. Determined 1 | 19.33 | 19.93 | 59.99 | 59.89 | 100.62 | 100.22 |
| Conc. Determined 2 | 19.89 | 19.79 | 59.58 | 59.88 | 99.48 | 99.98 |
| Conc. Determined 3 | 19.86 | 19.66 | 60.19 | 60.09 | 99.62 | 99.54 |
| Mean | 19.69 | 19.79 | 59.92 | 59.95 | 99.91 | 99.91333 |
| Standard deviation | 0.30 | 0.14 | 0.26 | 0.12 | 0.51 | 0.344867 |
| RSD | 1.52 | 0.68 | 0.43 | 0.20 | 0.51 | 0.345166 |
Table 5.
Analysis of E and Z guggulsterones in oleo-gum resin exudates of different accessions of Commiphora wightii
| Code | District | Specific location | Agroecological region | Latitude | Longitude | Status | Rainfall (mm/yr) | Height (m) | Girth (cm) | Canopy E-W (cm) | Canopy N-S (cm) | E Guggulsterone (μg g−1) | Z Guggulsterone (μg g−1) | Total guggulsterone (μg g−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haryana | ||||||||||||||
| H1 | Fatehabad | Herbal farm | Trans-Gangetic Plains Region | 29º31 N | 75º27 E | C | 260.45 | 1.63 | 6.2 | 48 | 53 | 143.68 ± 0.35 | 155.37 ± 0.32 | 299.05 |
| H2 | Hisar | HAU Campus | Trans-Gangetic Plains Region | 28º84 N | 75º54 E | C | 325.85 | 1.9 | 6.4 | 52 | 57 | 148.23 ± 0.34 | 163.26 ± 0.24 | 311.49 |
| H3 | Rewari | Mandola | Trans-Gangetic Plains Region | 28º24 N | 76º58 E | W | 474.45 | 3.39 | 8.4 | 67 | 61 | 153.10 ± 0.36 | 163.10 ± 0.25 | 316.20 |
| H4 | Rewari | Nursery | Trans-Gangetic Plains Region | 28º24 N | 76º58 E | C | 474.45 | 1.10 | 4.7 | 49 | 32 | 145.14 ± 0.39 | 169.98 ± 0.26 | 315.12 |
| H5 | Mahendergarh | Narnaul | Trans-Gangetic Plains Region | 28º09 N | 76º07 E | W | 416.95 | 3.26 | 6.7 | 70 | 62 | 195.36 ± 0.07 | 190.08 ± 0.33 | 385.44 |
| H6 | Mahendergarh | Khudana | Trans-Gangetic Plains Region | 28º02 N | 76º07 E | W | 416.95 | 3.19 | 6.2 | 58 | 43 | 190.92 ± 0.32 | 187.80 ± 0.29 | 378.72 |
| H7 | Mahendergarh | Kultajpur | Trans-Gangetic Plains Region | 28º02 N | 76º07 E | W | 416.95 | 2.89 | 5.1 | 61 | 47 | 188.11 ± 0.47 | 183.69 ± 0.46 | 371.80 |
| Gujarat | ||||||||||||||
| G8 | Aanand | GAU | Plains & Hills Region | 22º57 N | 72º93 E | C | 610.30 | 2.04 | 4.6 | 54 | 42 | 138.49 ± 0.07 | 142.44 ± 0.45 | 280.93 |
| G9 | Jamnagar | Nursery | Plains & Hills Region | 22º47 N | 70º07 E | C | 516.00 | 1.93 | 4.8 | 57 | 49 | 160.00 ± 0.44 | 161.83 ± 0.06 | 321.83 |
| G10 | Kachchh | Nakhatrana | Plains & Hills Region | 23º62 N | 71º20 E | C | 350.00 | 1.93 | 4.8 | 57 | 49 | 145.86 ± 0.34 | 147.55 ± 0.30 | 293.41 |
| Rajasthan | ||||||||||||||
| R11 | Udaipur | MLSU Campus | Sub-humid | 24º62 N | 73º68 E | C | 724.45 | 2.78 | 5.4 | 46 | 49 | 178.21 ± 0.13 | 173.87 ± 0.44 | 352.08 |
| R12 | Udaipur | Neemach Mata | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 2.78 | 5.4 | 46 | 49 | 182.50 ± 0.10 | 176.21 ± 0.32 | 358.71 |
| R13 | Udaipur | Moti Magri | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 3.2 | 6.7 | 69 | 52 | 182.73 ± 0.14 | 174.42 ± 0.57 | 357.15 |
| R14 | Udaipur | Kaler ka jungle | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 3.39 | 6.7 | 58 | 56 | 188.65 ± 0.18 | 164.24 ± 0.31 | 352.89 |
| R15 | Udaipur | Kaler ka jungle | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 3.40 | 6.7 | 78 | 69 | 187.47 ± 0.18 | 170.85 ± 0.52 | 358.32 |
| R16 | Udaipur | Sajangarh forest | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 3.38 | 7.9 | 54 | 56 | 186.22 ± 0.09 | 192.32 ± 0.17 | 378.54 |
| R17 | Udaipur | Brahmino Ki Hunder | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 3.318 | 8.7 | 69 | 62 | 183.43 ± 0.03 | 188.15 ± 0.09 | 371.58 |
| R18 | Udaipur | Bagdhara Nature park | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 4.11 | 7.6 | 56 | 55 | 181.12 ± 0.35 | 186.50 ± 0.07 | 367.62 |
| R19 | Udaipur | Bagdhara Nature park | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 3.80 | 6.4 | 52 | 50 | 202.47 ± 0.12 | 205.68 ± 0.54 | 408.15 |
| R20 | Udaipur | Bagdhara Nature park | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 3.49 | 8.6 | 61 | 56 | 208.80 ± 0.28 | 212.08 ± 0.34 | 420.88 |
| R21 | Udaipur | Baanki forest | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 3.48 | 6.5 | 67 | 63 | 200.81 ± 0.31 | 205.97 ± 0.30 | 406.78 |
| R22 | Udaipur | Baanki forest | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 3.31 | 5.8 | 69 | 64 | 207.19 ± 0.67 | 211.78 ± 0.32 | 418.97 |
| R23 | Udaipur | Sisaram | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 3.19 | 5.4 | 70 | 62 | 206.39 ± 0.67 | 212.48 ± 0.13 | 418.87 |
| R24 | Udaipur | Gogunda | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 3.19 | 8.2 | 58 | 49 | 183.84 ± 0.44 | 191.84 ± 0.32 | 375.68 |
| R25 | Udaipur | Chirwaghata forest | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 2.89 | 8.1 | 61 | 55 | 188.98 ± 0.50 | 192.34 ± 0.28 | 381.32 |
| R26 | Udaipur | Chirwaghata forest | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 2.16 | 8.8 | 72 | 67 | 186.98 ± 0.17 | 195.83 ± 0.17 | 382.81 |
| R27 | Udaipur | Balicha | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 2.28 | 8.1 | 75 | 68 | 185.11 ± 0.48 | 191.14 ± 0.49 | 376.25 |
| R28 | Udaipur | Adamagra | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 2.39 | 8.4 | 67 | 61 | 182.74 ± 0.23 | 188.06 ± 0.32 | 370.80 |
| R29 | Udaipur | Macchlamgra | Sub-humid | 24º62 N | 73º68 E | W | 724.45 | 2.78 | 8.9 | 65 | 64 | 183.89 ± 0.33 | 189.14 ± 0.32 | 373.03 |
| R30 | Rajsamand | Dhermata mines | Semi-arid, Rocky tract | 25º17 N | 73º51 E | W | 540 | 3.18 | 8.7 | 78 | 69 | 220.46 ± 0.37 | 202.12 ± 0.41 | 422.58 |
| R31 | Rajsamand | National Highway-8 | Semi-arid, Rocky tract | 25º17 N | 73º51 E | W | 540 | 3.22 | 8.7 | 70 | 72 | 238.12 ± 0.27 | 219.51 ± 0.35 | 457.63 |
| R32 | Rajsamand | Haldighati Area | Semi-arid, Rocky tract | 25º17 N | 73º51 E | W | 540 | 3.61 | 8.9 | 84 | 82 | 252.94 ± 0.37 | 257.03 ± 0.37 | 509.97 |
| R33 | Rajsamand | Haldighati Area | Semi-arid, Rocky tract | 25º17 N | 73º51 E | W | 540 | 3.65 | 8.8 | 81 | 82 | 252.61 ± 0.28 | 259.62 ± 0.32 | 512.23 |
| R34 | Rajsamand | Piplantri green belt | Semi-arid, Rocky tract | 25º06 N | 73º88 E | W | 540 | 3.51 | 8.9 | 65 | 64 | 265.28 ± 0.37 | 272.15 ± 0.32 | 537.43 |
| R35 | Rajsamand | Puthol village | Semi-arid, Rocky tract | 25º06 N | 73º88 E | W | 540 | 3.54 | 8.4 | 70 | 74 | 269.23 ± 0.40 | 276.97 ± 0.31 | 546.20 |
| R36 | Chittorgarh | Bhadesar | Sub-humid | 24º88 N | 76º63 E | W | 708 | 3.78 | 7.4 | 73 | 66 | 201.80 ± 0.40 | 246.82 ± 0.24 | 448.62 |
| R37 | Chittorgarh | Bhadsoda | Sub-humid | 24º88 N | 76º63 E | W | 708 | 3.73 | 7.6 | 77 | 69 | 215.04 ± 0.37 | 281.33 ± 0.02 | 496.37 |
| R38 | Chittorgarh | Chittorgarh fort | Sub-humid | 24º88 N | 76º63 E | W | 708 | 3.82 | 7.3 | 73 | 65 | 274.41 ± 0.33 | 247.02 ± 0.13 | 521.43 |
| R39 | Chittorgarh | Chhoti sadadi | Sub-humid | 24º88 N | 76º63 E | W | 708 | 3.86 | 7.6 | 77 | 69 | 294.82 ± 0.59 | 229.16 ± 0.44 | 523.98 |
| R40 | Chittorgarh | Nimbahera | Sub-humid | 25º75 N | 71º38 E | W | 708 | 3.71 | 7.4 | 72 | 65 | 217.92 ± 0.35 | 268.05 ± 0.03 | 485.97 |
| R41 | Bhilwara | Bhillo ki jhopadi | Sub-humid | 25º35 N | 74º63 E | W | 633.90 | 3.22 | 7.6 | 77 | 76 | 164.24 ± 0.48 | 176.54 ± 0.41 | 340.78 |
| R42 | Bhilwara | Mandavgarh | Sub-humid | 25º35 N | 74º63 E | W | 633.90 | 3.02 | 7.3 | 73 | 70 | 173.14 ± 0.23 | 178.50 ± 0.09 | 351.64 |
| R43 | Bhilwara | Det village | Sub-humid | 25º35 N | 74º63 E | W | 633.90 | 3.13 | 7.4 | 75 | 74 | 170.53 ± 0.24 | 176.08 ± 0.44 | 346.61 |
| R44 | Ajmer | Mangliawas herbal farm | Semi-arid, Rocky tract | 26º25 N | 74º51 E | W | 550 | 3.9 | 8.9 | 77 | 79 | 259.07 ± 0.46 | 209.56 ± 0.32 | 468.63 |
| R45 | Ajmer | Pitamber-ki-gal | Semi-arid, Rocky tract | 26º25 N | 74º51 E | W | 550 | 3.81 | 8.7 | 89 | 86 | 270.43 ± 0.33 | 211.27 ± 0.55 | 481.70 |
| R46 | Ajmer | Nasirabad valley | Semi-arid, Rocky tract | 26º25 N | 74º51 E | W | 550 | 3.82 | 8.7 | 779 | 82 | 267.52 ± 0.33 | 208.46 ± 0.33 | 475.98 |
| R47 | Ajmer | Nagpahar | Semi-arid, Rocky tract | 26º25 N | 74º51 E | W | 550 | 3.88 | 8.6 | 76 | 56 | 260.88 ± 0.28 | 209.92 ± 0.48 | 470.80 |
| R48 | Jaipur | Neem Ka Thana | Semi-arid | 27º73 N | 75º78 E | W | 565 | 2.78 | 6.1 | 72 | 65 | 244.09 ± 0.16 | 285.22 ± 0.21 | 529.31 |
| R49 | Jaipur | Jhalana | Semi-arid | 27º73 N | 75º78 E | W | 565 | 2.82 | 6.4 | 65 | 62 | 236.22 ± 0.30 | 240.03 ± 0.23 | 476.25 |
| R50 | Jodhpur | CAZRI | Arid | 26º11 N | 73º42 E | C | 301.5 | 1.44 | 5.9 | 43 | 47 | 162.96 ± 0.34 | 179.26 ± 0.34 | 342.22 |
| R51 | Jodhpur | Kailana lake | Arid | 26º11 N | 73º42 E | W | 301.5 | 2.88 | 6.7 | 64 | 58 | 343.10 ± 0.40 | 173.11 ± 0.40 | 516.21 |
| R52 | Barmer | Mungeria hills | Arid, Desert | 25º75 N | 71º38 E | W | 277 | 3.44 | 7.6 | 78 | 73 | 382.02 ± 0.41 | 324.26 ± 0.04 | 706.28 |
| R53 | Jaislmer | Dabra | Hyper- arid | 26º92 N | 70º90 E | W | 209 | 3.56 | 7.8 | 65 | 63 | 455.21 ± 0.30 | 450.15 ± 0.41 | 905.36 |
| R54 | Jaislmer | Devikot | Hyper- arid | 26º92 N | 70º90 E | W | 209 | 3.49 | 7.9 | 76 | 74 | 487.45 ± 0.19 | 472.88 ± 0.19 | 960.33 |
| R55 | Jaislmer | Akal Wood fossil forest | Hyper- arid | 26º92 N | 70º90 E | W | 209 | 3.40 | 7.4 | 67 | 65 | 480.85 ± 0.37 | 447.22 ± 0.18 | 928.07 |
| R56 | Jaislmer | Pithla | Hyper- arid | 26º92 N | 70º90 E | W | 209 | 3.48 | 7.6 | 69 | 67 | 475.82 ± 0.29 | 470.02 ± 0.21 | 945.84 |
| R57 | Bikaner | Palana | Hyper- arid | 28º01 N | 73º18 E | W | 266 | 3.12 | 6.7 | 74 | 69 | 467.65 ± 0.19 | 487.68 ± 0.18 | 955.33 |
| R58 | Churu | Rajgarh | Hyper- arid | 28º38 N | 72º02 E | W | 331.5 | 3.00 | 6.6 | 71 | 74 | 358.74 ± 0.52 | 364.69 ± 0.27 | 723.43 |
| R59 | Jhunjhunu | Khetri | Rocky tract, Inland drainage | 27°52 N | 75º46 E | W | 500 | 3.56 | 6.5 | 67 | 63 | 373.98 ± 0.35 | 388.62 ± 0.31 | 762.6 |
| R60 | Jhunjhunu | Khetri | Rocky tract, Inland drainage | 27°52 N | 75º46 E | W | 500 | 3.82 | 5.8 | 69 | 64 | 364.90 ± 0.36 | 351.91 ± 0.05 | 716.81 |
| R61 | Jhunjhunu | Khetri | Rocky tract, Inland drainage | 27º52 N | 75º46 E | W | 500 | 3.90 | 6.1 | 78 | 64 | 328.88 ± 0.39 | 318.78 ± 0.06 | 647.66 |
| R62 | Jalore | Govindgarh | Arid, Desert | 27º35 N | 72º62 E | W | 420 | 3.49 | 7.8 | 82 | 80 | 427.14 ± 0.42 | 447.24 ± 0.40 | 874.38 |
| R63 | Jalore | Jaswantpura | Arid, Desert | 27º35 N | 72º62 E | W | 420 | 3.80 | 8.1 | 87 | 83 | 422.80 ± 0.19 | 424.12 ± 0.36 | 846.92 |
| R64 | Alwar | Prithripura | Flood prone | 27º57 N | 76º60 E | W | 724 | 3.42 | 6.4 | 55 | 56 | 181.13 ± 0.49 | 185.67 ± 0.08 | 366.80 |
| R65 | Dungarpur | Baroda | Humid | 28º83 N | 73º72 E | W | 701 | 3.12 | 6.4 | 56 | 59 | 171.23 ± 0.36 | 182.99 ± 0.26 | 354.22 |
| R66 | Dungarpur | Mandli | Humid | 28º83 N | 73º72 E | W | 701 | 3.00 | 6.6 | 58 | 56 | 172.61 ± 0.15 | 181.24 ± 0.42 | 353.85 |
| R67 | Dungarpur | Balota | Humid | 28º83 N | 73º72 E | W | 701 | 3.29 | 6.9 | 62 | 64 | 166.82 ± 0.08 | 179.17 ± 0.17 | 345.99 |
| R68 | Banswara | Bagidora | Humid | 23º55 N | 74º45 E | W | 781 | 3.22 | 6.5 | 55 | 57 | 160.35 ± 0.16 | 172.44 ± 0.19 | 332.79 |
| R69 | Banswara | Kushalgarh | Humid | 23º55 N | 74º45 E | W | 781 | 3.12 | 6.6 | 57 | 58 | 128.19 ± 0.22 | 125.23 ± 0.19 | 253.42 |
| R70 | Banswara | Dungra | Humid | 23º55 N | 74º45 E | W | 781 | 3.04 | 6.4 | 43 | 48 | 120.82 ± 0.44 | 116.99 ± 0.19 | 237.81 |
| R71 | Banswara | Sarwa Kalan | Humid | 23º55 N | 74º45 E | W | 781 | 3.11 | 6.3 | 42 | 43 | 118.35 ± 0.28 | 111.74 ± 0.28 | 230.09 |
| R72 | Sawai Madhopur | Ranthambor | Humid | 25º98 N | 76º36 E | W | 800 | 3.44 | 7.5 | 76 | 74 | 347.10 ± 0.32 | 282.60 ± 0.34 | 629.70 |
| R73 | Hanumangarh | Kohla farm house | Trans-Gangetic Plains Region | 29º58 N | 74º32 E | C | 225 | 2.28 | 6.3 | 43 | 39 | 132.51 ± 0.21 | 140.55 ± 0.19 | 273.06 |
| R74 | Hanumangarh | Nursery | Trans-Gangetic Plains Region | 29º58 N | 74º32 E | C | 225 | 1.98 | 6.1 | 40 | 37 | 135.02 ± 0.29 | 143.89 ± 0.28 | 278.91 |
| R75 | Ganganagar | Nursery | Trans-Gangetic Plains Region | 29º92 N | 73º88 E | C | 200 | 1.90 | 6.2 | 42 | 41 | 121.19 ± 0.27 | 128.73 ± 0.07 | 249.92 |
C – Cultivated; W - Wild
Fig. 1.
Chromatogram of oleo-gum resin of C. wightii collected from (a) Devikot (Jaisalmer) and (b) Palana (Bikaner); two hyper-arid regions showing separation of various components including guggulsterone E (Rt : 15.009) and Z (Rt : 17.809)
Both the isomeric forms of guggulsterone are inter-convertible as was reported in callus and cell cultures of guggul (Ramawat et al. 2008). Agrawal et al. (2004a) also reported that significant variations are likely to occur in the component content of guggul oleo-gum resin depending upon the climatic conditions under which the plants are grown and the resin is harvested. Concentration of the bio-active agents has been found to be influenced by atmospheric factors (seasonal variation, geographical variation, average rainfall, and temperature), agriculture practices (planting strength, genotype of plant, time of sowing and harvesting period) and laboratory factors (chromatographic conditions, mobile phase composition, extraction solvents). Along with these factors guggul gum yield also depend on the age of the plant (Jain and Nadgauda 2013). Influence of these factors has also been monitored in other medicinal plants like Lepidium sativum L. (Nayak et al. 2009, 2012), Plantago ovata Forsk (Mann and Vyas 1996) and Andrographis paniculata (Burm. f.) Wall. ex Nees (Saxena et al. 2000). Quantitative estimation of methanolic seed extract of L. sativum through HPTLC exhibited an apparent variation in sinapic acid concentration owing to difference in date of sowing and harvesting period (Nayak et al. 2009). Highest efficiency of extraction of andrographolide derivatives was gained in methanol as compared to chloroform, ethyl acetate and ethanol extracts of A. paniculata (Saxena et al. 2000). Maceration under sonication was the most effective extraction method compared to maceration alone and its infusion with supercritical fluid extraction (after consideration of extraction yield/extraction time ratio) in HPLC analysis of coumarin in hydroalcoholic extracts of Mikania glomerata Spreng (Celeghini et al. 2001).
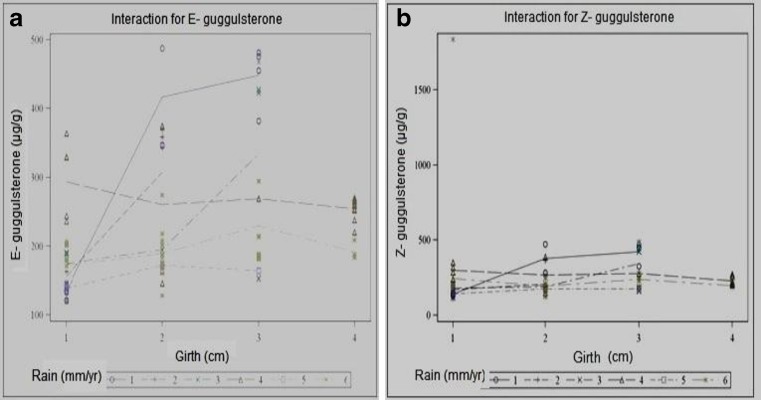
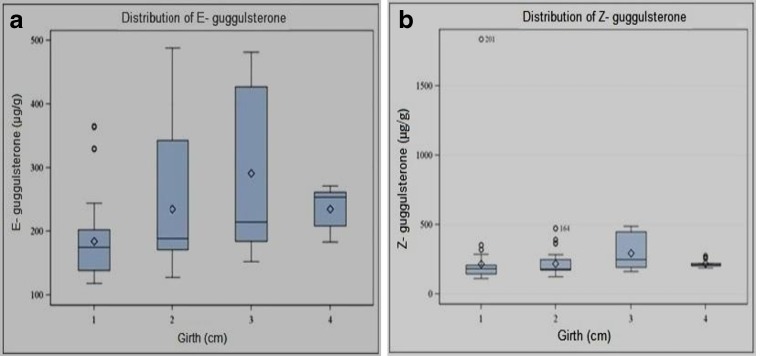
Production of oleo-gum resin is a stress induced phenomenon. Both biotic and abiotic factors including ecological (geographical and seasonal), individual plant performance (genotypes and morphotypes), pathogens and elicitors (methyl jasmonate, ethrel and salicyclic acid) as well as cultivation practices affect the production of secondary metabolite guggulsterone in intact plants as well as in tissue culture (see Kulhari et al. 2012; Suthar and Ramawat 2010). In the present investigation, a strong correlation was seen between average rainfall of the area and guggulsterone content; regions with lower rainfall exhibited higher amount of guggulsterone (Fig. 2). Duncan’s multiple range test revealed a relationship between developmental stage of the plant and guggulsterone content. Mature plants with thick trunk produced higher guggulsterone content against nursery raised smaller plants (Fig. 3). These outcomes depicted that wild mature guggul plants (more than eight years of age) growing in adverse conditions or in rocky tracts had more guggulsterone content. Variation in guggulsterone content among accessions has also been reported by Kulhari et al. (2013); Soni et al. (2010) and Yadav et al. (1999). Soni et al. (2010) attributed the discrepancy in guggulsterone content to environmental factors like temperature and rainfall of the concerned geographical region; higher guggulsterone content was obtained during summer (May-July, highest in May) which gradually decreased in the rainy season (Aug-Oct) and was lowest in winter (Nov-March). In case of geographical locations northern, western and central part of Rajasthan showed maximum amount of guggulsterone whereas southern part of the state produced lower amount. They also reported that Z isomeric form was dominating over E guggulsterone however the same trend was not found in the present study. E and Z isomers were found in an approximate constant ration of 4:1 by Mathur and Ramawat (2007) while Kumar et al. (2006) reported 46.3 μg g−1 and 104.3 μg g−1 respectively of E and Z isomers in stem samples of C. wightii, and Musharraf et al. (2011) reported their concentration as 51.042 ng μL−1 and 28.399 ng μL−1 respectively in C. mukul extracts. Total guggulsterone yield was variable in different regions of same agroclimatic provinces (hyper-arid) viz. 2.291 w/w in Churu; 2.088 w/w in Bikaner and 1.871 w/w in Jaiselmer (Soni et al. 2010). Agrawal et al. (2004a) also reported that the content of guggulsterone produced in winters was very limited due to dormant phase of the plant while its production enhanced in summers.
Fig. 2.
Correlation between rainfall and concentration of guggulsterone (a) E isomer and (b) Z isomer
Fig. 3.
Correlation between girth and distribution of guggulsterone (a) E isomer, and (b) Z isomer
The developed technique is a precise, specific and accurate method for estimation of guggulsterone content within a 17-min run using single reverse phase HPLC in C. wightii extracts. Present finding revealed the role of environmental factors on biosynthesis of guggulsterone isomers under natural conditions. By HPLC based fingerprinting the botanical identity of the plant can be linked with its biochemical profile as well as it allows for the determination of variations in the guggul resin component’s content in different collected accessions grown and harvested at different climatic conditions. These methods would also be useful for comparing the resinous analysis in related plant species. The present work is unique in terms of wide collection from three hot spot biodiversity rich Indian states for quantification of these bioactive agents in stem samples. The given method is gainful as it showed good reproducibility with higher resolution, competence as well as separation of marker compounds. Additionally, no peaks of other constituents present in the extracts were found to interfere with that of the marker compounds, indicating no hindrance.
Identification of high guggulsterone producing lines will play an important role in designing mass propagation as well as conservation strategies. Reverse phase HPLC analysis evaluated that embryogenic callus is the best alternate for guggulsterone production in vitro (Kumar et al. 2006; Dass and Ramawat 2009). Tissue culture is the only available method for production of secondary metabolites via cell, callus culture and cloning of selected high yielding guggul varieties. However, cytodifferentiation in callus and cell cultures is a prerequisite for the production of secondary metabolites, which are produced in complex tissue systems like laticifers and resin canals (Ramawat et al. 2008). The superior genotypes identified can also be used in forward genetics for isolation of gene(s) responsible for guggulsterone production which can be integrated in to other plants or micro organisms for production of higher quality and quantity of guggulsterone. This in turn would reduce the pressure to undertaken commercial cultivation of Commiphora wightii, and will also reduce over-exploitation of this plant in the wild and will thereby complement the conservation process.
Acknowledgments
AK thankfully acknowledges for the financial assistance provided by DBT, Government of India, New Delhi, under the project sanctioned vide order no- BT/PR 10526/ NDB/51/164/ 2008.
References
- Agrawal H, Kaul N, Paradkar AR, Mahadik KR. HPTLC method for guggulsterone I. Stress degradation studies on guggulsterone. J Pharm Biomed Anal. 2004;36:23–31. doi: 10.1016/j.jpba.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Agrawal H, Kaul N, Paradkar AR, Mahadik KR. HPTLC method for guggulsterone II. Quantative determination of E- and Z- guggulsterone in herbal extract and pharmaceutical dosage form. J Pharm Biomed Anal. 2004;36:33–41. doi: 10.1016/j.jpba.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Akhade MS, Agrawal PA, Laddha KS. Development and validation of RP-HPLC method for simultaneous estimation of picroside I, plumbagin, and Z-guggulsterone in tablet formulation. Indian J Pharm Sci. 2013;4:476–482. doi: 10.4103/0250-474X.119835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeghini RMS, Vilegas JHY, Lanças FM. Extraction and quantitative HPLC analysis of coumarin in hydroalcoholic extracts of Mikania glomerata Spreng. (“guaco”) leaves. J Brazilian Chem Soc. 2001;12:706–709. doi: 10.1590/S0103-50532001000600003. [DOI] [Google Scholar]
- Choudhary N, Sekhon BS. An overview of advances in the standardization of herbal drugs. J Pharm Educ Res. 2011;2:55–70. [Google Scholar]
- Dass S, Ramawat KG. Studies on somatic cell variability in Commiphora wightii (Arnott.) Bhandari for guggulsterone production. Nat Prod Radiance. 2009;8:532–536. [Google Scholar]
- Deng R. Therapeutic effects of guggul and its constituent guggulsterone: cardiovascular benefits. Cardiovasc Drug Rev. 2007;25:375–390. doi: 10.1111/j.1527-3466.2007.00023.x. [DOI] [PubMed] [Google Scholar]
- Haque S, Farooqi AHA, Gupta MM, Sangwan RS, Khan A. Effect of ethrel, chlormequat, chloride and paclobutrazol on growth and pyrethrin accumulation in Chrysanthemum cinerariaefolium Vis. Plant Growth Regul. 2007;51:263–269. doi: 10.1007/s10725-007-9170-6. [DOI] [Google Scholar]
- Harish GAK, Phulwaria M, Rai MK, Shekhawat NS. Conservation genetics of endangered medicinal plant Commiphora wightii in Indian Thar Desert. Gene. 2014;535:266–272. doi: 10.1016/j.gene.2013.11.018. [DOI] [PubMed] [Google Scholar]
- ICH . Q1A stability testing of new drug substances and products. Geneva: International Conference on Harmonization, IFPMA; 1993. [PubMed] [Google Scholar]
- Jain N, Nadgauda RS. Commiphora wightii (Arnott) Bhandari - a natural source of guggulsterone: facing a high risk of extinction in its natural habitat. Am J Plant Sci. 2013;4:57–68. doi: 10.4236/ajps.2013.46A009. [DOI] [Google Scholar]
- Kant T, Prajapati S, Parmar A. Efficient micropropagation from cotyledonary node cultures of Commiphora wightii (Arn.) Bhandari, an endangered medicinally important desert plant. J Plant Dev. 2010;17:37–48. [Google Scholar]
- Kulhari A, Sheorayan A, Kalia S, Chaudhury A, Kalia RK. Problems, progress and future prospects of improvement of Commiphora wightii (Arn.) Bhandari, an endangered herbal magic, through modern biotechnological tools: a review. Genet Resour Crop Evol. 2012;59:1223–1254. doi: 10.1007/s10722-012-9854-2. [DOI] [Google Scholar]
- Kulhari A, Sheorayan A, Saxena N, Mohan C, Mangal M, Chaudhury A, Dhawan AK, Kalia RK. HPTLC analysis of guggulsterone isomers in Commiphora wightii (Arn.) Bhandari - an endangered oleo-gum resin species heading towards extinction. Genet Resour Crop Evol. 2013;60:1173–1180. doi: 10.1007/s10722-012-9947-y. [DOI] [Google Scholar]
- Kulloli RN, Kumar S (2014) Commiphora wightii (Arnott.) Bhandari in the Indian Desert: Biology, Distribution and Threat Status. In Nandwani D (ed.). Sustain Hortic Syst: Issues Technol Innov 2: 301–313. doi:10.1007/978-3-319-06904-3_13
- Kumar S, Nadgauda R. Control of morphological aberrations in somatic embryogenesis of Commiphora wightii (Arnott) Bhandari (Family: Bursaraceae) through secondary somatic embryogenesis. Proc Natl Acad Sci, India, Sec B Biol Sci. 2014 [Google Scholar]
- Kumar S, Mathur M, Jain AK, Ramawat KG. Somatic embryo proliferation in Commiphora wightii and evidence for guggulsterone production in culture. Indian J Biotechnol. 2006;5:217–222. [Google Scholar]
- Maheshwari DV (2010) Guggul plantation shows good success in Kutch. Find Articles / Business / DNA: Daily News & Analysis; Mumbai
- Mann PS, Vyas AK. Effect of sowing date and nitrogen levels on yield, quality and net returns of blonde psyllium (Plantago ovata Forsk.) Ann Agric Res. 1996;22:425–428. [Google Scholar]
- Mathur M, Ramawat KG. Guggulsterone production in cell suspension cultures of the guggul tree, Commiphora wightii, grown in shake-flasks and bioreactors. Biotechnol Lett. 2007;29:979–982. doi: 10.1007/s10529-007-9342-5. [DOI] [PubMed] [Google Scholar]
- Mesorb B, Nesbitt MR, Pandey CR. High-performance liquid chromatographic method for fingerprinting and quantative determination of E- and Z-guggulsterones in Commiphora mukul resin and its products. J Chromatogr B: Biomed Sci Appl. 1998;720:189–196. doi: 10.1016/S0378-4347(98)00433-2. [DOI] [PubMed] [Google Scholar]
- Musharraf SG, Iqbal N, Ahmed MA, Mazhar S, Choudhary MI. Screening of E- and Z- guggulsterones in the gum-resin exudates of some common plants and method validation in raw, extracted, and pharmaceutical formulations of Commiphora mukul by HPLC. J Liq Chromatogr Relat Technol. 2011;34:2103–2117. doi: 10.1080/10826076.2011.585481. [DOI] [Google Scholar]
- Nagarajan M, Waszkuc Ted W, Sun J. Simultaneous Determination of E- and Z-guggulsterones in dietary supplements containing Commiphora mukul extract (Guggulipid) by liquid chromatography. J AOAC Int. 2001;84:24–28. [PubMed] [Google Scholar]
- Nayak PS, Upadhyaya SD, Upadhyaya A. A HPTLC densitometeric determination of sinapic acid in Chandrasur (Lepidium sativum) J Sci Res. 2009;1:121–127. [Google Scholar]
- Nayak PS, Upadhyaya A, Dwivedi SK, Rao S. HPLC analysis of sinapic acid in Lepidium sativum. Electron J Environ Agric Food Chem. 2012;11:156–162. [Google Scholar]
- Ramawat KG, Mathur M, Dass S, Suthar S (2008) Guggulsterone: a potent natural hypolipidemic agent from Commiphora wightii- Problems, preservence, and prospects. In: Ramawat KG, Merillon JM (eds.). Bioact Mol Med Plants 101–121
- Samantaray S, Bishoyi A, Geetha KA, Satyabrata M. Assessment of genetic diversity using RAPD and ISSR markers in guggul (Commiphora wightii) J Med Aromat Plant. 2011;1:TS2–P33. [Google Scholar]
- Samudio I, Konopleva M, Safe S, McQueen T, Andreeff M. Guggulsterones induce apoptosis and differentiation in acute myeloid leukemia: identification of isomer-specific antileukemic activities of the pregnadienedione structure. Mol Cancer Ther. 2005;4:1982–1992. doi: 10.1158/1535-7163.MCT-05-0247. [DOI] [PubMed] [Google Scholar]
- Saxena S, Jain DC, Gupta MM, Bhakuni RS, Mishra HO, Sharma RP. High-Performance Thin-Layer Chromatographic analysis of hepatoprotective diterpenoids from Andrographis paniculata. Phytochem Anal. 2000;11:34–36. doi: 10.1002/(SICI)1099-1565(200001/02)11:1<34::AID-PCA487>3.0.CO;2-V. [DOI] [Google Scholar]
- Singh SK, Verma N, Gupta RC. Sensitive high-performance liquid chromatographic assay method for the determination of guggulsterone in serum. J Chromatogr B: Biomed Sci Appl. 1995;670:173–176. doi: 10.1016/0378-4347(95)00149-D. [DOI] [PubMed] [Google Scholar]
- Soni V, Sawarnkar PL, Tyagi V, Pareek LK. Variation in E and Z guggulsterone of Commiphora wightii. South Afr J Bot. 2010;76:421–424. doi: 10.1016/j.sajb.2009.10.004. [DOI] [Google Scholar]
- Suthar S, Ramawat KG. Growth retardants stimulate guggulsterone production in the presence of fungal elicitor in fed-batch cultures of Commiphora wightii. Plant Biotechnol Rep. 2010;4:9–13. doi: 10.1007/s11816-009-0110-y. [DOI] [Google Scholar]
- Tripathi A, Shukla JK, Gehlot A, Mishra DK. Standarization of cloning in Commiphora wightii. Adv For Sci. 2014;1:19–25. [Google Scholar]
- Verma N, Singh SK, Gupta RC. Simultaneous determination of the stereoisomers of guggulsterone in serum by high-performance liquid chromatography. J Chromatogr B: Biomed Sci Appl. 1998;708:243–248. doi: 10.1016/S0378-4347(97)00626-9. [DOI] [PubMed] [Google Scholar]
- Yadav BBL, Billore KV, Joseph JG, Chaturvedy DD. Cultivation of Guggulu. New Delhi: Central Council for Research in Ayurveda and Siddha; 1999. [Google Scholar]