Abstract
A rapid analytical method has been developed to determine xanthone and secoiridoid glycoside in in vitro and in vivo Swertia chirayita extracts. Ultra performance liquid chromatography–electrospray ionization mass spectrometry (LC-ESI/MS) was applied and validated for the analysis of xanthone and secoiridoid glycoside a potential active component isolated from methanolic extracts of in vitro and in vivo Swertia chirayita plantlets. Chromatographic separation was achieved on a RP-C18 column using gradient elution. Mangiferin (Xanthone), Amarogentin and Swertiamarin (Secoiridoid glycosides) were identified in both the extracts. In the LC/ESI-MS spectra, major [M + H] + and [M + Na] + ions were observed in positive ion mode and provided molecular mass information. An ultra-performance liquid-chromatography in combination with electrospray ionization tandem mass spectrometry involving metal cationisation was successfully utilized for the rapid identification of xanthone and secoiridoid glycosides. This method is suitable for the routine analysis, as well as for the separation and identification of known and novel secoiridoid glycoside and xanthone.
Keywords: Swertia chirayita, LC-ESI/MS, Xanthone, Secoiridoid glycoside
Introduction
S. chirayita (Roxb. ex Fleming. H. Karst.) is used as herbal medicine for various health ailments and it has been used in Unani medicine (Joshi and Dhawan 2005). Extracts of S. chirayita have been shown to possess antioxidative, antihepatotoxic and hypoglycemic, anti-inflammatory, antimalarial, anticarcinogenic, and antimicrobial activities (Kar et al. 2003; Saha et al. 2004; Tripathi et al. 2005). Previous reports documented the presence of flavonoids, xanthones, terpenoids, iridoid and secoiridoid glycoside, that are responsible for therapeutic properties in S. chirayita (Pant et al. 2000). The bioactive constituents include the xanthone and secoiridoid glycosides consisting of mangiferin, amarogentin and swertiamarin. Mangiferin has been reported to possess various biological activities like antitumour, antiviral, antioxidant, antidiabetic and immunomodulatory activity (Guha et al. 1996; sanchez et al. 2000; Garcia et al. 2003). Swertiamarin, and Amarogentin compounds also possesses various biological activities such as chemopreventive, antibacterial, anticholinergic and antihepatitis activity and served as important chemotaxonomic markers (Saha et al. 2006; Yamahara et al. 1991; EI-Sedawy et al. 1989). Mass spectrometry (MS), especially coupled with electrospray ionization (ESI), is an important physiochemical method for the analysis of bioactive compounds. Protonated ions are usually generated by ESI-MS in positive ion mode (He et al. 2013). Identification of compounds in botanical extracts is established by a combination of useful online system liquid chromatography (LC) photodiode array UV–vis detection and electrospray ionisation mass spectrometry (ESI-MS) (He et al. 1998; Cubbon et al. 2010). Swertiamain, Gentiopicrin and Mangiferin were identified by TLC in Swertia chirayita and its content was determined by HPLC (Yuan-can et al. 2010). Determination of five active components in Swertia chirayita by HPLC is also reported by Lei et al. (2010). Very few reports have been documented the analysis of xanthone and secoiridoid glycosides composition of wild S. chirayita by using liquid chromatography/tandem mass spectrometry (Suryawanshi et al. 2006, 2007). Hence, the present investigation was undertaken with the objective of to present a LC-ESI/MS based technique for the identification and analysis of xanthone (mangiferin) and secoiridoid glycosides (swertiamarin, amarogentin) in methanolic extracts of in vitro regenerated and in vivo S. chirayita. The study was performed in positive ion mode.
Experimental
Reagents and standards
Acetonitrile (HPLC grade) was purchased from Thomas Baker (Mumbai, India). Ultra-pure water was obtained from a MilliQ PLUS purification system (Millipore, USA). All the three standards (Mangiferin, Amarogentin and Swertiamarin) were procured from Chromadex™.
Plant material
Young and mature S. chirayita plants were collected from natural habitat of Darjeeling, West Bengal during the month of November. Following the procedure described by Kumar et al. (2014) leaf explants excised from young plants were decontaminated in 0.2% Bavastin solution followed by (5-6drops/100 mL) Tween 20 (20 min) and 0.1% mercuric chloride (8 min). The leaf explants were rinsed three times in sterile distilled water, cut into approximately 1 cm3 cubes and inoculated onto Murashige and Skoog (MS) (1962) medium supplemented with 30 g L−1 sucrose, 0.1 g L−1 myo inositol and 1.0 mg L−1 6- Benzyladenine (BA), 100 mg L−1 Adenine sulphate (Ads), 0.1 mg L−1 Indole-3-acetic acid (IAA), and 0.8 % agar, pH 5.8. In vitro shoots were maintained in a growth chamber under a 16 h-photoperiod at the light intensity of 40 μmol s−1 m−2 provided by white fluorescent tubes and temperature 25 ± 2 °C. The shoots were subcultured every 4 weeks.
Preparation of plant extracts
In vitro regenerated and wild plants were freeze-dried and lyophilized. The ground materials (100 mg/mL) were homogenized with methanol. The resultant extracts were centrifuged for 10 min at 3000 rpm and the supernatant was retained for analysis.
Separation of secondary metabolites using UPLC
Analytes were separated using a Waters Acquity UPLC system (Waters ACQUITY QSM) consisting of an Acquity UPLC binary systems manager, an Acquity UPLC sample manager, a PDA detector and an H/T column heater containing an Acquity UPLC BEH C18 reverse phase column (2.1 mm × 50 mm; 1.7 μm particle size). Several mobile phases (acetonitrile, methanol, water with and without additives like 0.1% formic acid and 5 mM ammonium acetate pH 4.2) and different gradient elution programs were tested to choose and optimise the best analytical conditions for simultaneous detection of mangiferin, amarogentin and swertiamarin. The binary mobile phase optimised consisted of (A) acetonitrile and (B) 5 mM ammonium acetate pH4.2. A linear gradient (curve no. 6) elution program was applied as follows: Initial: 10% A, 90% B; 0–4 min: 40% A, 60% B; 4–6 min: 70%A, 30%B; 6–8: 80%A, 20%B; 8–10: 10%A, 90%B; 10–15: 10%A, 90%B. The flow rate was maintained at 0.5 ml min−1 and the temperature of the column and sample manager were set at 40 and 5 °C respectively. Injection volumes were 2 μl for standards as well as for samples. The pressure limits were set at 0 psi low and 15,000 psi high, the highest pressure observed was 9346 psi during the elution process. The PDA (Photo Diode Array) detector was set at 254 nm and instrument operations, data acquisition and processing were performed using EmPower2 chromatographic data software. The Mangiferin, Amarogentin and Swertiamarin peaks from samples were identified by the comparison of retention times with the corresponding retention times of standards.
Mass spectrometry conditions
To confirm the identification of secondary metabolites in in vitro and in vivo plantlets of S. chirayita, samples were subjected to UPLC and separations were performed. The TUV eluent was sent to Electrospray Ionisation Mass Spectrometer (ESI-MS) operated in positive ion mode. Probe and source conditions included capillary voltage 3.50 kV, 350 °C desolvation temperature, 50 L h−1 cone gas, 650 L h−1 desolvation gas and 110 °C block temperature. Mangiferin, Swertiamarin and Amarogentin were identified based on comparison of the mass fragmentation tandem MS analysis data with standard compounds.
Results and discussion
The capacity of in vitro cultures to accumulate secondary metabolites is an interesting biochemical phenomenon in plant cell biotechnology applications. S. chirayita plants has a diverse chemical profile (Joshi and Dhawan 2005). The xanthone (Mangiferin) and secoiridoid glycosides (Amarogentin and Swertiamarin) profiles of in vitro regenerated and wild plant extracts were analysed using LC-ESI/MS (Figs. 1 and 2; Table 5). For analysis, three reference compounds procured from chromadex™ viz. swertiamarin, mangiferin and amarogentin were used in the experiment. The results indicate similarity in presence of mangiferin, amarogentin and swertiamarin between in vitro and wild plant extracts. There was a variation in the concentration of mangiferin, amarogentin and swertiamarin between both the extracts. The concentration of Amarogentin was higher in wild plant extracts compared to a in vitro regenerated plant extracts (Table 5). The concentration of mangiferin and swertiamarin were higher in in vitro regenerated compared to wild plants (Table 5). This finding is concordant with the results of a similar study in Centaurium erythraea (Jankovic et al. 2011). He also reported higher amounts of xanthone compounds in in vitro raised plantlets in comparison to naturally growing plantlets. Similarly, higher concentration of mangiferin and swertiamarin in in vitro compared to wild plant extracts has been recently reported by Kumar et al. (2014).
Fig. 1.

a. UPLC chromatogram of Mangiferin (Standard compound). b. UPLC chromatogram of Swertiamarin (Standard compound). c. UPLC chromatogram of Amarogentin (Standard compound). D. Toatl ion chromatogram of in vitro plant extract of S. chirayita. E. Total ion chromatogram of in vivo plant extract of S. chirayita
Fig. 2.


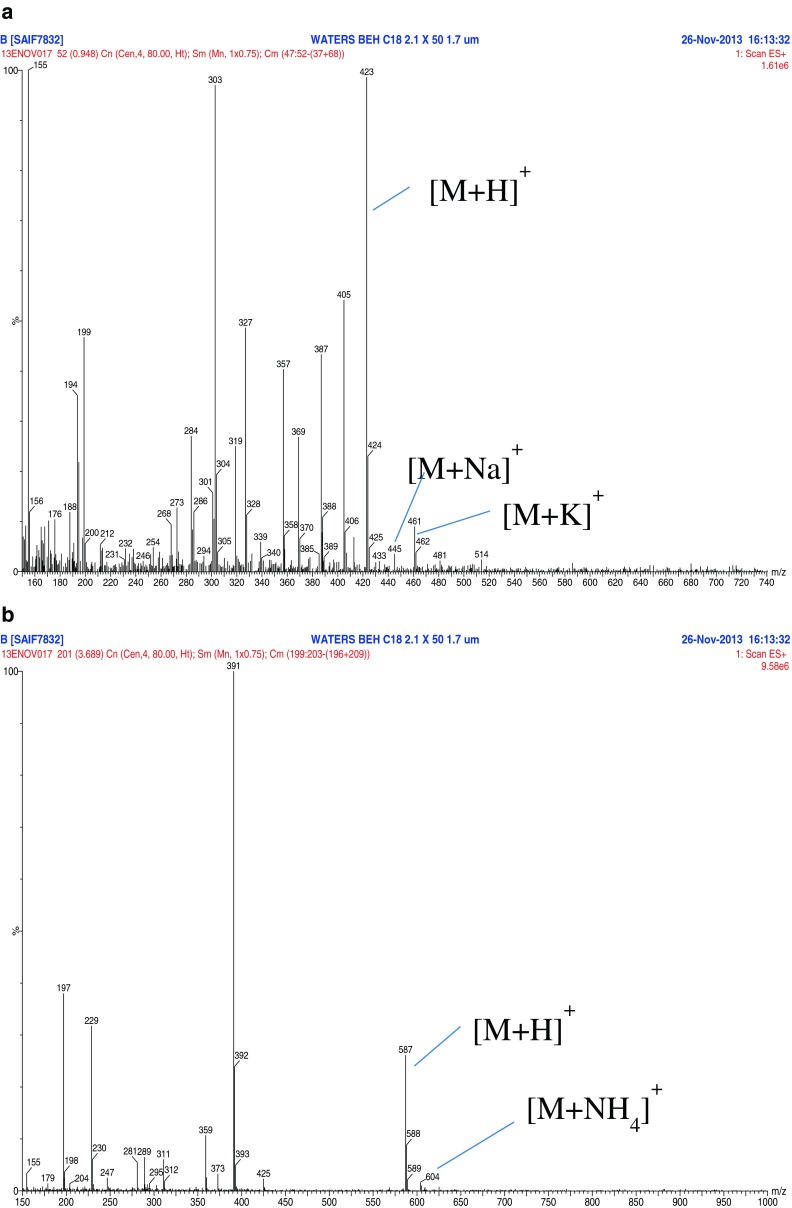
a. LC/ESI-MS/MS spectra of Mangiferin (m/z 423) in in vitro S. chirayita extract. b. LC/ESI-MS/MS spectra of Amarogentin (m/z 587) in in vitro S. chirayita extract. c. LC/ESI-MS/MS spectra of Swertiamarin (m/z 375) in in vitro S. chirayita extract. d. LC/ESI-MS/MS spectra of Mangiferin (m/z 423) in in vivo S. chirayita extract. e. LC/ESI-MS/MS spectra of Amarogentin (m/z 587) in in vivo S. chirayita extract. f. LC/ESI-MS/MS spectra of Swertiamarin (m/z 375) in in vivo S. chirayita extract
Table 5.
Mangiferin, Amarogentin and Swertiamarin profile (μg/mL) of in vitro and in vivo S. chirayita plant extracts
| Plant extract | Mangiferin | Amarogentin | Swertiamarin |
|---|---|---|---|
| In vitro plantlets | 11.48 ± 8.41 | 28.96 ± 10.38 | 15.16 ± 0.44 |
| In vivo plantlets | 4.63 ± 0.39 | 30.45 ± 0.68 | 9.94 ± 4.19 |
Initially, full scan LC/ESI-MS spectra of in vivo and in vitro S. chirayita methanolic extracts was acquired in positive ion mode utilizing the LC gradient condition as described earlier with a mobile phase consisting of acetonitrile and 5 mM ammonium acetate pH4.2. LC/ESI-MS spectral data of in vivo and in vitro S. chirayita methanolic extracts are presented in Tables 1 and 2 and shows a series of peaks between m/z 200–1000. In positive ion mode of LC/ESI-MS experiments, all components in in vitro extracts showed highly abundant proton and sodium ion adducts but a relatively lower proportion of potassium and ammonium adducts was also detected (Table 1). In case of in vivo extracts all three components showed highly abundant proton ion adducts but a relatively lower proportion of sodium, potassium and ammonium adducts were detected (Table 2). Similar results were obtained by Suryawanshi et al. (2006, 2007) in in vivo methanolic extracts of S. chirayita. The Collision induced dissociation (CID) spectra and mass spectral data of in vivo and in vitro extracts of fragment ions of all three compounds in positive ion mode are given in Tables 3 and 4. The CID spectra of the [M + H] + ion at m/z 423 (Mangiferin) in both extracts (in vivo and in vitro) showed fragment ions at m/z 405 and 387 corresponding to subsequent loss of two water units, [M + H–18] + and [M + H–36] +, respectively. A similar result was found by Suryawanshi et al. (2006). The CID spectrum of the [M + H]+ ion of Amarogentin (see Fig. 2b and e) in in vitro and in vivo extracts contains fragment ions at m/z 391, 373, 359, 311, 289, 247, 229, 197 and m/z 391, 373, 359, 311, 281, 247, 229, 197 respectively. Highly abundant [M + Na]+ ions completely replaced the protonated ion formation. Usually, [M + Na]+ ions are found to be very stable and produce only negligible amounts of fragment ions (Suryawanshi et al. 2006). Similarly this unfavourable phenomenon did not occur with swertiamarin. As we observed LC/ESI-MS spectra in positive ion mode for swertiamarin in in vivo and in vitro extracts. The CID spectrum of the [M + H] + ion of swertiamarin (see Fig. 2c and f) in in vitro and in vivo extracts contains fragment ions at m/z 357, 327, 303, 284, 256, 247, 223, 213, 195, 183, 177 and 167. All three compounds (mangiferin, amarogentin and swertiamarin) were analyzed in in vitro and in vivo plantlets of S. chirayita. Table 5 shows the total mangiferin, amarogentin and swertiamarin, contents in in vitro and in vivo plantlets of S. chirayita. The amount of mangiferin (11.48 ± 8.41 μg/mL−1) and swertiamarin (15.16 ± 0.44 μg/mL−1) was higher in in vitro plantlets of S. chirayita whereas amarogentin (30.45 ± 0.68 μg/mL−1) was higher in a in vivo plantlets.
Table 1.
The ions detected (m/z) in LC/ESI-MS spectra with positive ion mode at constant cone voltage in in vitro regenerated S. chirayita extract
| Mode | Compound | [M + H]+ | [M + Na]+ | [M + K]+ | [M + NH4]+ |
|---|---|---|---|---|---|
| ESI+ | Mangiferin | 423 | 445 | 461 | – |
| Amarogentin | 587 | – | – | 604 | |
| Swertiamarin | 375 | – | 413 | – |
Table 2.
The ions detected (m/z) in LC/ESI-MS spectra with positive ion mode at constant cone voltage in in vivo S. chirayita extract
| Mode | Compound | [M + H]+ | [M + Na]+ | [M + K]+ | [M + NH4]+ |
|---|---|---|---|---|---|
| ESI+ | Mangiferin | 423 | _ | 461 | _ |
| Amarogentin | 587 | _ | _ | 604 | |
| Swertiamarin | 375 | 397 | 413 | _ |
Table 3.
LC/ESI-MS/MS characterization of components in in vitro regenerated Swertia chirayita extract
| Compound | M.W. | Precursor ion | Product ion (m/z) |
|---|---|---|---|
| Mangiferin | 422 | 423 | 405, 387, 369, 357, 327, 303, 284, 273, 199 |
| Amarogentin | 586 | 587 | 391, 373, 359, 311, 289, 247, 229, 197 |
| Swertiamarin | 374 | 375 | 357, 339, 303, 284, 273, 213, 195, 177, 167 |
Table 4.
LC/ESI-MS/MS characterization of components in in vivo Swertia chirayita extract
| Compound | MW | Precursor ion | Product ion (m/z) |
|---|---|---|---|
| Mangiferin | 422 | 423 | 405, 387, 369, 357, 327, 303, 273, 239, 223, 209, 201 |
| Amarogentin | 586 | 587 | 391, 373, 359, 311, 281, 247, 229, 197 |
| Swertiamarin | 374 | 375 | 357, 327, 303, 284, 256, 247, 223, 213, 195, 183, 177, 167 |
In conclusion, a rapid and convenient LC/ESI-MS technique was developed and validated for the extraction and identification of secoiridoid glycosides (Amarogentin and Swertiamarin) and xanthone (Mangiferin) from in vitro and in vivo methanolic extracts of S. chirayita, an important medicinal plant. Major [M + H] + and [M + Na] + ions were observed in LC/ESI-MS with positive ion mode and provided molecular mass information. More detailed study in relation of component identification and analysis is still awaited.
Acknowledgments
This work is financially supported by University Grants Commission (UGC), GOI, New Delhi for the major research project [F. No. 37-111/2009 (SR)]. The authors are grateful to Sophisticated Analytical Instrument Facility (SAIF), CDRI, Lucknow for LC-MS/MS analysis of S. chirayita samples. SAIF-CDRI Communication Number 7832. V. Kumar gratefully acknowledges the Centre of Excellence (TEQIP), Department of Bio-Engineering, Birla Institute of Technology, Mesra, Ranchi, for providing the fellowship. The authors also wish to thanks to anonymous reviewers for their suggestions which help to improve the manuscript.
Conflict of interest
Authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Contributor Information
Vijay Kumar, Email: vijay.srm23@gmail.com.
Sheela Chandra, Phone: +91-651-2276223, Email: schandra@bitmesra.ac.in.
References
- Cubbon S, Antonio C, Wilson J, Thomas‐Oates J. Metabolomic applications of HILIC‐LC‐MS. Mass Spectrom Rev. 2010;29:671–684. doi: 10.1002/mas.20252. [DOI] [PubMed] [Google Scholar]
- EI-Sedawy AI, Shu YZ, Hattori M, Kobashi K, Namba T. Metabolism of Swertiamarin from Swertia japonica by Human Intestinal Bacteria. Planta Med. 1989;55:147–15. doi: 10.1055/s-2006-961909. [DOI] [PubMed] [Google Scholar]
- Garcia D, Leiro J, Delgado R, Sanmartin ML, Ubeira FM. Mangifera indica L. extract (Vimang) and mangeferin modulate mouse humoral immune responses. Phytother Res. 2003;17:1182–1187. doi: 10.1002/ptr.1338. [DOI] [PubMed] [Google Scholar]
- Guha S, Ghosal S, Chattopadhyay U. Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a naturally occuring glucosylxanthone. Chemotherapy. 1996;42:443–451. doi: 10.1159/000239478. [DOI] [PubMed] [Google Scholar]
- He XG, Lin LZ, Lian LZ, Lindenmaier M. Liquid chromatography electrospray mass spectrometric analysis of curcuminoids and sesquiterpenoids in turmeric (Curcuma longa) J Chromatogr A. 1998;818:127–132. doi: 10.1016/S0021-9673(98)00540-8. [DOI] [Google Scholar]
- He GY, Xu XY, Fang DM, Luo SW, Wang LX, Zhang GL, et al. Unexpected [M-H]+ ions in cyclopenta [b] indoles detection by electrospray ionization mass spectrometry. J Mass Spectrom. 2013;48:1266–1269. doi: 10.1002/jms.3285. [DOI] [PubMed] [Google Scholar]
- Joshi P, Dhawan V. Swertia chirayita an overview. Curr Sci. 2005;89:635–640. [Google Scholar]
- Kar A, Choudhary BK, Bandopadhyay NG. Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J Ethnopharmacol. 2003;84:105–108. doi: 10.1016/S0378-8741(02)00144-7. [DOI] [PubMed] [Google Scholar]
- Kumar V, Singh SK, Bandopadhyay R, Sharma MM, Chandra S. In vitro organogenesis secondary metabolite production and heavy metal analysis in Swertia chirayita. Cent Eur J Biol. 2014;9:686–698. doi: 10.2478/s11535-014-0300-7. [DOI] [Google Scholar]
- Lei S, Jingai T, Hongyu J, Ruichao L. Determination of five active components in Swertia chirayita by HPLC. Chin Pharma Affairs. 2010;24:687–689. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Pant N, Jain DC, Bhakmi RS. Phytochemicals from genus Swertia and their biological activities. Ind J Chem. 2000;39:565–586. [Google Scholar]
- Saha P, Mandal S, Das A, Das PC, Das S. Evaluation of the anticarcinogenic activity of Swertia chirata Buch-Ham, an Indian medicinal plant, on DMBA induced mouse skin carcinogenesis model. Phytother Res. 2004;18:373–378. doi: 10.1002/ptr.1436. [DOI] [PubMed] [Google Scholar]
- Saha P, Mandal S, Das A, Das S. Amarogentin can reduce hyperproliferation by down regulation of Cox-II and upregulation of apoptosis in mouse skin carcinogenesis model. Cancer Lett. 2006;244:252–259. doi: 10.1016/j.canlet.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Sanchez GM, Re L, Guiliani A, Nunez-Selles AJ, Davison GP, Leon-Fernandez OS (2000) Protective effects of Mangifera indica L. extract, mangiferin and selected antioxidants against TPA-induced biomolecules oxidation and peritoneal macrophage activation in mice. 42:565–573 [DOI] [PubMed]
- Suryawanshi S, Mehrotra N, Asthana RK, Gupta RC. Liquid chromatography/tandem mass spectrometric study and analysis of xanthone and secoiridoid glycoside composition of Swertia chirata, a potent antidiabetic. Rapid Commun Mass Spectrom. 2006;20:3761–3768. doi: 10.1002/rcm.2795. [DOI] [PubMed] [Google Scholar]
- Suryawanshi S, Asthana RK, Gupta RC. Simultaneous estimation of mangiferin and four secoiridoid glycosides in rat plasma using liquid chromatography tandem mass spectrometry and its application to pharmacokinetic study of herbal preparation. J Chromatog B. 2007;858:211–219. doi: 10.1016/j.jchromb.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Tripathi R, Mohan H, Kamat JP. Modulation of oxidative damage by natural products. Food Chem. 2005;100:81–90. doi: 10.1016/j.foodchem.2005.09.012. [DOI] [Google Scholar]
- Yamahara J, Kobayashi M, Matsuda H, Aoki S. Anticholinergic action of Swertia japonica and an active constituent. J Ethnopharmacol. 1991;33:31–35. doi: 10.1016/0378-8741(91)90157-9. [DOI] [PubMed] [Google Scholar]
- Yuan-can X, Li-xin W, Hong-xia Y, Yu-zhi D. Quality control of traditional Tibetan medicine Swertia chirayita. Chin Pharma J. 2010;45:255–258. [Google Scholar]