Abstract
Protein adsorption on material surfaces is a common phenomenon that is of critical importance in many biotechnological applications. The structure and function of adsorbed proteins are tightly interrelated and play a key role in the communication and interaction of the adsorbed proteins with the surrounding environment. Because the bioactive state of a protein on a surface is a function of the orientation, conformation, and accessibility of its bioactive site(s), the isolated determination of just one or two of these factors will typically not be sufficient to understand the structure–function relationships of the adsorbed layer. Rather a combination of methods is needed to address each of these factors in a synergistic manner to provide a complementary dataset to characterize and understand the bioactive state of adsorbed protein. Over the past several years, the authors have focused on the development of such a set of complementary methods to address this need. These methods include adsorbed-state circular dichroism spectropolarimetry to determine adsorption-induced changes in protein secondary structure, amino-acid labeling/mass spectrometry to assess adsorbed protein orientation and tertiary structure by monitoring adsorption-induced changes in residue solvent accessibility, and bioactivity assays to assess adsorption-induced changes in protein bioactivity. In this paper, the authors describe the methods that they have developed and/or adapted for each of these assays. The authors then provide an example of their application to characterize how adsorption-induced changes in protein structure influence the enzymatic activity of hen egg-white lysozyme on fused silica glass, high density polyethylene, and poly(methyl-methacrylate) as a set of model systems.
I. INTRODUCTION
The interaction of proteins with material surfaces constitutes one of the most prominently studied areas within the field of biomaterials and is of considerable interest for many applications of biotechnology and biomedical engineering, including biosensors,1,2 enzyme based technologies,3,4 tissue engineering and regenerative medicine,5,6 implants,7–9 and biodefense.10,11 These interactions typically occur spontaneously as a protein-containing solution contacts a solid material surface, which can result in a substantial shift in the protein's structure as well as changes in the solvent accessibility of its amino acid residues,12–15 often leading to a reduction in bioactivity.13–15
The biological function of a protein is imparted by the binding strength, as well as the correct alignment of the ligand or receptor to the protein's active site, which results from its folded structure. A protein's folded structure is composed of up to four hierarchical levels. The primary structure is determined by the specific amino acid sequence along its polypeptide chain, which is numbered from the chain's N- to C-terminus. The amino acids of this sequence are typically composed of the 20 naturally occurring l-amino acids, which are classified by their side-group as having nonpolar, polar, or charged character.14 The polypeptide chain formed by the primary sequence is then organized into three basic types of secondary structure: helices (α, 310, and π), β-sheets, and random loops that connect the helix and sheet elements. The secondary structural elements are further organized together to form the tertiary protein structure. Finally, if the overall protein is made up of more than one polypeptide chain, the interchain associations define the protein's quaternary structure, with each of the individual polypeptide chains having its own distinct N- and C-terminus. In the native-state of a given protein, these three or four levels of structure create the geometry and reactive functional groups that combine together to form the bioactive sites of the protein.
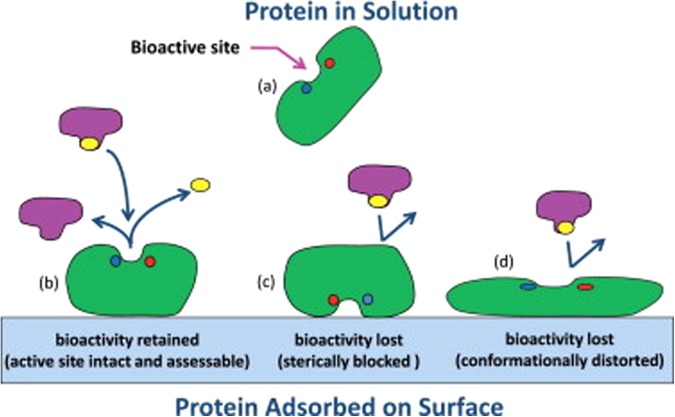
When a protein adsorbs from solution onto a material surface, the interactions between the amino acid residues of the protein and the functional groups of the surface typically result in a situation where the native state of the protein no longer represents the low free energy state of the combined protein–surface–solution system. This situation can lead to substantial shifts in the protein away from its native-state structure. If these changes influence the structure of the bioactive site in the protein such that its intended ligand or receptor can no longer bind to that site, bioactivity will be lost. Likewise, if the protein adsorbs to a surface such that accessibility to the binding site is sterically blocked, bioactivity can be lost as well. These effects are depicted in Fig. 1.16 Unfortunately, bioactivity assays are unable to distinguish between these two factors when adsorption causes a loss in bioactivity (i.e., loss due to conformational distortion versus steric hindrance of the bioactive site), thus making it difficult to determine how a given system should be redesigned to correct the problem. To address this situation, experimental methods are needed to characterize how adsorption influences both the orientation and the structure of protein on a surface in addition to adsorption-induced changes in bioactivity. With these combined datasets, assessment can then be made regarding the actual factor(s) that are responsible for a loss in bioactivity if it occurs.
Fig. 1.
Illustration of the influence of adsorption on the bioactive state of an enzyme. (a) The enzyme in its native-state structure in solution, and (b) when adsorbed with its bioactive site accessible and conformationally intact, thus providing native-state-like bioactivity. (c) Enzyme adsorbed with its bioactive site sterically blocked by the surface, thus inhibiting substrate binding with subsequent loss in bioactivity due to adsorbed orientation. (d) Enzyme adsorbed with its bioactive site accessible but conformationally distorted with subsequent loss in bioactivity due to structural changes of the bioactive site. Reproduced with permission from R. A. Latour, Colloids Surf., B 124, 25 (2014). Copyright 2014 Elsevier.
Relatively few methods have been developed to probe the orientation and structure of adsorbed proteins.15,17 Some of the methods that can provide information on adsorbed protein orientation and tertiary (and quaternary) structure include fluorescence,18–21 time-of-flight secondary-ion mass spectrometry,22–24 nuclear magnetic resonance spectroscopy (NMR),25,26 and amino acid labeling/mass spectrometry (AAL/MS).27–31 Methods for the determination of secondary structure of adsorbed proteins include Fourier transform infrared spectroscopy,32,33 surface enhanced Raman scattering,34,35 and circular dichroism spectropolarimetry (CD).27,36–39 Unfortunately, as the size of the protein increases, many of the spectral signatures that are needed for tertiary structure determination using fluorescence and NMR overlap, introducing much subjectivity into the analyses, thus making it difficult to accurately interpret the configuration of the adsorbed protein. In contrast, MS has shown great promise in characterizing the adsorbed configuration of both large and small proteins at a molecular level, especially when used with AAL.27–31 After evaluating several of these types of methods, our group has specifically focused on the development and adaptation of CD and AAL/MS for the characterization of adsorbed protein orientation and structure, and we will therefore focus on these methods for this paper. Readers are encouraged to refer to the above cited references for the application of the other types of methods that are noted above.
CD spectropolarimetry has been extensively used to spectroscopically study the structure of biomolecules in solution and when absorbed to surfaces due to its characteristics of being nondestructive, relative easy to perform, requirement of small sample volume, and providing fast, reliable data analysis.40–42 In particular, CD provides a very convenient experimental method for quantifying the secondary structure and environmentally induced structural changes in proteins since the different forms of the main secondary structural elements found in proteins (e.g., α-helix, β-sheet, and random loop) each exhibits a distinctly different CD spectrum.42,43 Though, CD has been usually reported with proteins in solution or with proteins adsorbed to colloidal particles, its use with flat transparent material surfaces was reported as early as 1974 by McMillin and Walton.44 However, unlike then, the modern versions of CD spectropolarimeters are equipped with photoelastic modulators instead of Pockel's cells, which have much improved signal-to-noise ratio and place less stringent requirement on the minimum amount of protein that is needed for adsorption studies on planar surfaces.38,45,46 In addition to quantifying the secondary structural elements, the shifts in the near UV CD spectral range (260–320 nm) have been used to qualitatively determine the “molten globule” states in many adsorbed proteins, which reflect the protein's tertiary structure.40,42,46,47 Similarly, in many proteins containing cofactors as an important functional part of the bioactive site, such as metal ions, shifts in the spectral features in the visible light related to the cofactor's position have also been used as indicators of the integrity of the binding site.48,49 However, since the shape and magnitude of near-UV CD spectra are influenced by the type of protein, and strategies to quantify the tertiary structural shifts using CD techniques are lacking, CD is primarily used for secondary structural determination. Alternative techniques, such as AAL/MS, are thus required to provide insight into higher order structure.42
The AAL/MS technique combines the use of side-chain selective chemical modification of amino acids within the proteins (i.e., AAL) along with MS to provide a readout of the sites susceptible to covalent modifications upon reaction with a labeling reagent.50,51 The chemical labeling is conducted under mild reaction conditions to minimize any possible alterations to the protein structure, which can be confirmed by CD. The proteins are separately labeled in solution and after adsorption, following which the labeled proteins are digested to peptide fragments and the specific sites that are labeled are identified by MS. The amino acid residues that are found to be labeled in solution but unlabeled following adsorption indicate regions of the protein that are sterically blocked by the surface (i.e., indicative of adsorbed orientation) or by neighboring proteins (i.e., indicative of protein–protein interactions). Although it is not possible to distinguish between these two causes, protein–protein interactions can be expected to have a higher probability of causing a loss in amino acid residue solvent accessibility when the protein is adsorbed from high solution concentration where the protein adsorbs with high packing density on the surface. In contrast to this, when a protein is adsorbed from low solution concentration, it generally undergoes a greater degree of unfolding and spreading out on the surface with lower adsorbed surface density, with a loss in solvent accessibility subsequently having greater probability for being caused by steric hindrance from the surface. Alternatively, amino acids that are unlabeled in solution but become labeled following adsorption are indicative of the sites in the protein that underwent adsorption-induced tertiary unfolding, thus exposing the side-chains of amino acids that are not solvent accessible in the protein's native-state. Thus, by the application of AAL/MS to multiple different amino acid types that are distributed throughout a protein, a fairly comprehensive picture can be generated regarding the distribution of sites in the protein that are tightly adsorbed to the surface (or blocked by neighboring proteins) and the sites that undergo adsorption-induced tertiary unfolding.
In this paper, we present the experimental methods that we have developed and/or adapted to gain amino-acid-residue-level information on adsorbed protein orientation and adsorption-induced changes in a protein's secondary and tertiary structure using CD and AAL/MS. When coupled with measurements of adsorption-induced changes in bioactivity, these data can provide insights regarding whether a measured loss in bioactivity is due to adsorbed protein orientation or adsorption-induced changes in protein structure. Following the presentation of our CD and AAL/MS methods, we demonstrate their application to characterize the adsorption behavior of hen egg-white lysozyme (HEWL) on materials exhibiting three distinctly different types of surface chemistry [silica glass, poly(methyl methacrylate), and high-density polyethylene] to show how these methods can be used to provide insights into the cause of adsorption-induced changes in bioactivity.
II. EXPERIMENTAL SET UP AND METHODOLOGY
This section contains the overview of the protocols and the critical considerations taken by the authors when using CD,36,38,52 AAL/MS,51,52 and spectrophotometric techniques to gain quantitative information on the adsorbed structure and bioactivity of proteins on flat material surfaces with relatively low surface area.36,37,52 The reader is referred to our referenced original papers for additional details on the specific application of each of these methods to specific protein adsorption systems.27,36–38,51–53
A. Material surface characterization and protein adsorption
Characterization of the chemical, physical, and morphological properties of a material surface is important prior to structure determination of the adsorbed protein in order to validate and document that the material surface is of the type expected and to provide a basis for evaluating how various surface properties influence the adsorption processes. Because of the need for a material that exhibits negligible absorbance over the wavelengths that we use for CD (i.e., 190–250 nm), we use fused silica glass slides as our base substrate for each of our material surfaces. The glass slides can subsequently be modified by coating them with thin films of polymer by spin coating, metal/metal oxide by vapor deposition, or self-assembled monolayers (SAMs) to present different surface functional groups. We typically characterize each type of surface for chemical composition via x-ray photoelectron spectroscopy (NESAC/BIO, University of Washington), surface roughness by atomic force microscopy (Asylum Research, MFP–3D), surface wettability by static contact angle (Krüss, DSA–20 E), film thickness by variable angle spectroscopic ellipsometry (Sopra Inc., GES–5), and surface charge density using a streaming potential method (SurPASS, Anton Paar GmbH, Austria).54 Prior to surface analysis and protein adsorption, standard cleaning procedures are followed, which vary depending on the type of surface being used,36,51,52,55 and the respective substrates are then stored under buffered aqueous solution at room temperature until use.
We typically purchase protein for our studies from commercial vendors in crystallized form with at least 95% purity. The proteins are subsequently dissolved in a low-salt buffer to minimize absorbance (e.g., 10 mM phosphate buffered saline), with the protein concentration verified by the biuret method (Thermo Scientific, 23225) or absorbance at 205 nm (A205).36,51,52 Prior to protein adsorption, the surfaces are equilibrated in buffer for several hours and then protein solution is added to the buffer as necessary to obtain the desired net solution concentration. The placement of the surface samples under pure buffer (i.e., in the absence of proteins) prior to adding the protein solution is very important. Since the air–water interface effectively acts as a hydrophobic surface, proteins tend to adsorb to this interface to form a relatively thick film of denatured protein. Therefore, if the adsorbent surface is placed in the buffer solution after the protein is added, the surface will pass through this denatured film of protein at the air–water interface, which will subsequently coat the surface with a layer of this denatured protein. This adsorbed layer of protein will thus be very different from what would adsorb from the solution itself. Similarly, when pipetting the protein solution into the pure buffer solution, it is important to position the pipette tip beneath the air–water interface. This step thus minimizes the exposure of the stream of protein solution to an air–water interface, which can also induce protein denaturation.
B. Quantification of the secondary structure in adsorbed protein using CD spectropolarimetry
We use a Jasco J-810 spectrophotometer to determine the secondary structures of protein both in solution and after adsorption on surfaces. Prior to spectral scans, cuvettes are calibrated and the performance of the instrument is evaluated using freshly prepared 1 S-(+)-10-camphorsulphonic acid as the calibration standard over the spectral range of 190–320 nm and the ratio of the absolute signals at 192.5 and 290.5 nm is checked to ensure a value of ≥2, thereby confirming that CD instrument is performing within recommended standards.42
1. Cuvette and scan settings for acquiring the spectra
The scan settings for acquiring the spectra of proteins in solution or in the adsorbed state are determined based on the total absorbance of the test sample, which in turn is directly related to the high-tension voltage (HTV, the voltage applied to the photomultiplier). In all our studies with the protein in solution or in its adsorbed state, the HTV is kept <700 V.42,56 In the event, that the spectrum is too noisy or too weak, the concentration of protein and/or the path length of the cuvettes are adjusted. Generally, for determining the solution structure of protein we use protein concentrations of 1.00 mg/ml for a 0.01 cm path length cuvette (Starna Cells), or 0.1 mg/ml for a cuvette of 0.1 cm path length (Starna Cells). But the protein structure determination in more dilute solutions should be avoided, as a significant proportion of the protein structure could be altered by adsorption on the surfaces of the cuvette.
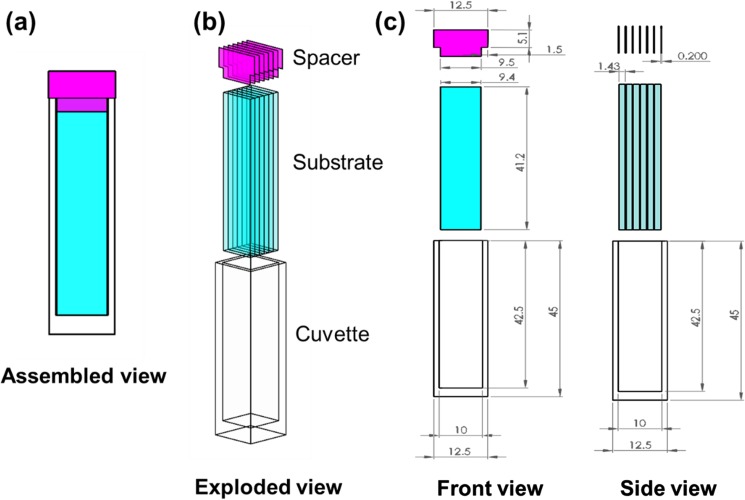
For the determination of the structure of adsorbed proteins, we had previously used a custom designed cuvette that was capable of supporting four individual slides by gluing two quartz windows to a poly(ether–ether–ketone) (PEEK) polymer holder.38 However, technical difficulties imposed by gluing the quartz windows to PEEK, and the limited reusability of these cuvettes, prompted us to improve the cuvette design used for our adsorption studies. The assembled setup of the improved cuvette design that we currently use for protein adsorption studies is shown in Fig. 2.
Fig. 2.
Improved experimental setup to investigate the effect of bulk surface properties on the structure of adsorbed proteins is shown in (a)–(c). The assembled view of the improved setup is shown in panel (a) with the individual components within the setup [one standard spectroscopic grade quartz cuvette (21-Q-10, Starna Cells), six custom-cut fused silica substrates (Custom order CU-1005-041JS, ChemGlass Life sciences), and seven vinyl polymeric spacers] being shown in the exploded view of (b) and (c). The dimensions of each individual component: the standard spectroscopic grade quartz cuvette of path length 1 cm (external dimension: 12.5 mm × 12.5 mm × 45 mm; internal dimension: 42.5 mm × 10 mm × 10 mm), fused silica substrates (9.4 mm × 1.43 mm × 41.2 mm), and T-spacers (base: 1.5 mm × 9.5 mm × 0.2 mm; head: 5.1 mm × 12.5 mm × 0.2 mm) are shown in the front and side views of the setup.
The relative advantages of this new construct over our old one lies in its relative simplicity, lower absorbance of the incident beam, and the prolonged reusability of the cuvettes. For example, the background absorbance at 190 nm for the old and new cuvettes at their full capacity of loading of six fused silica glass slides is about 2.1 and 1.7, respectively. In terms of percent transmission (%T), these absorbance values correspond to about 0.79% [%T = 10(2-A190)] and 2.00%, respectively, of the deep UV-spectra for the old and new designs. These values result in 153% improvement in beam transmission as well as decreased path length of the new cuvette (0.14 cm as opposed to 0.16 cm). These changes provide the new design with an enhanced limit of detection that is equivalent to the absorbance of 0.03 mg/ml of protein solution concentration in a 1 cm cuvette at 195 nm.
Because of the differences in the light throughput in the cuvettes used for solution and adsorbed studies, we typically use two types of settings in our CD studies.42,56 When the light throughput from the instrument is high (%T > 5), the CD spectra are recorded from 190 to 300 nm at a scan rate of 100 nm/min, bandwidth of 0.5 nm, with a response time of 0.25 s. But, for all other cases, the CD spectra are recorded from 190 to 300 nm at a scan rate of 10 nm/min with a response time of 2 s, and a bandwidth of 0.5 nm. In each case, a spectrum of protein in solution or in the adsorbed state represents the data averaged over six (n = 6) accumulations. These scan parameters are set so as to optimize the contribution of shot and systematic noise in the acquired spectra. For example, lengthening the response time of the instrument will increase the number of photons reaching the detector (minimizing the shot noise), but require a longer time to complete a scan, thereby increasing the effect of baseline drift (systematic noise). Similarly, severely shortening the response time will minimize the systematic noise, but increase the shot noise. Therefore, the noise from these two sources is kept minimal by optimizing the response time and scan rates, and any residual effects on the spectra are off-set by averaging the spectra over multiple scans (n), which will reduce the signal error due to noise as a function of the square root of n.
2. Scaling of CD spectra
The CD spectrum (θ, in mdeg) is dependent on the path length and the molar concentration of the proteins and needs to be appropriately scaled to its molar elliptical units ([θ], deg·cm2/dmol) using Eq. (1) (for proteins in solution) and Eq. (2) (for adsorbed proteins) prior to quantifying the secondary structural elements within the protein of interest
| (1) |
| (2) |
where M0 is the mean residue molecular weight of protein (≈112 g/mol) and is obtained by averaging the molecular weight of the protein over the overall sequence length, Csoln is concentration of protein in solution (g/ml), L is the path length of cuvette (cm), and Qads is the surface coverage of adsorbed protein (g/cm2). Values for Csoln and Qads are calculated from
| (3) |
| (4) |
where Aw is the absorbance of the protein containing solution at wavelength (w) and εw [units of cm2 g−1 or (g/ml)−1 cm−1] is the molar extinction coefficient corresponding to wavelength, w.
The unknown concentration of proteins is typically determined using the absorbance at 280 nm (Ref. 42) and, the extinction coefficient at 280 nm for 1% (w/v) protein solutions [ε280 (1%), units of (g/100 ml)−1 cm−1] are usually reported by the commercial vendors. However, if proteins in solution are denatured (e.g., by chemical additives, or by thermally or pH-induced unfolding), the red shifts associated with the protein can introduce significant errors in these estimates.57 As a result, the extinction coefficient at far UV wavelengths, like those of 195 or 205 nm, are preferred due to the lesser influence of red-shifts and better sensitivity due to peak absorbance of the protein at 205 nm.36,51 Additionally, the protein–protein variability with the 205 nm technique is also minimal. The extinction coefficients ɛ205 or ɛ195 are determined from the slope of the calibration curve for A205 or A195 plotted versus solution concentration (Csoln, units of g/ml) using serial dilutions of fresh stock solutions of protein, with the concentration verified using ε280 (1%) or the biuret method. Alternatively, the use of absorbance at 230 nm has also been shown better than 280 nm in determining the unknown concentration of proteins, when in presence of strongly absorbing chemical additives like urea or guanidium hydrochloride.57
Once a spectrum is appropriately scaled, the quality of spectra for designated proteins in their native state in solution obtained over the 190–260 nm range can be verified by comparison to reference spectra for the respective proteins using online databases like the Protein Circular Dichroism Data bank (PCDDB), which contains synchrotron CD spectra (higher resolution spectra) for many proteins in their native state.58 Additionally, these spectra can be used to provide indicators of the structural integrity of the proteins in solution.
As a cautionary note regarding the determination of the structure of adsorbed proteins, it is important that the surfaces with the adsorbed protein are gently rinsed to remove loosely bound protein before they are mounted in the cuvette and that the remaining protein is effectively irreversible adsorbed. Otherwise, protein may desorb from the surface into the surrounding solution after the slides are placed under pure buffer (protein-free buffer) solution in the cuvette, with the contribution of any desorbed protein in solution causing artifacts in the measured adsorbed-state structure. To insure that protein did not desorb from the surfaces during CD measurement, the amount of protein on the surface should be determined both before and after CD measurement, with the buffer solution replaced with fresh buffer (protein-free buffer) for the after-scan measurement.
3. Quantification of secondary structure
To quantify the relative proportion of each associated secondary structure contained in a protein sample, the resulting CD spectrum acquired between wavelengths of 190–240 nm is typically empirically interpreted as a sum of fractional multiples of the reference spectrum for each type of secondary structure.43 This deconvolution process is conducted using a variety of mathematical tools (such as CONTIN/LL, SELCON3, and CDSStr methods provided with the CDPro software package)59 that fit the acquired spectra with the spectra of reference datasets of highly resolved protein structures (i.e., the protein structures within the SP43 and SP48 datasets obtained using x-ray crystallography and NMR spectroscopy).46 The quality of the fit is typically assessed by analyzing the R-fit using nonlinear regression. With different algorithms and different reference datasets obtained over a larger wavelength range, such as 175–260 nm (SP-175 or MP-180, provided with Dichroweb), there can be slight variations in the estimated secondary structure content, and thus some discrepancies in the fitting parameters can be expected.59–63 The analysis is usually considered reliable if different mathematical tools give similar results, at which point the values obtained from the different fitting algorithms can be averaged and confidently reported.42
One of the limitations of the deconvolution algorithms is that they depend on the availability of ellipticity values over the full range of wavelengths from 190 to 240 nm. Thus, these methods cannot be used in the presence of additives in solution that strongly adsorb wavelengths below 220 nm. In this case, alternative methods like the 222, 225, 228, or 230–240 nm slope method can be used to quantify the helical content of proteins in solution or in the adsorbed state.42,64 Unfortunately, similar alternative methods are not available for quantifying the β-sheet structures in a protein.
C. Protein structure determination using amino acid side-chain modification with mass spectrometry
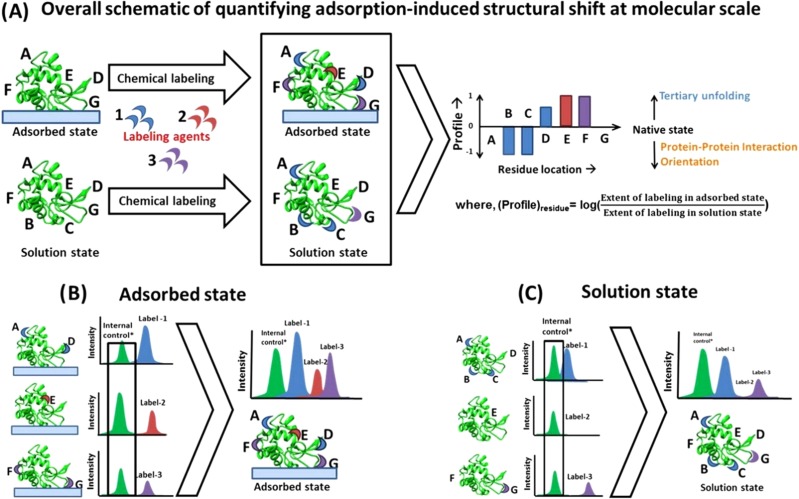
The amino acid side-chain modification with mass spectrometry (AAL/MS) technique is used to identify the sites susceptible to covalent modifications upon reaction with a labeling reagent and, in turn, assess the solvent exposure of these sites.51 Although, the applicability of this technique on adsorbed protein has been previously investigated by many groups, its use has been restricted to determining the labeling profile of just one type of amino acid because of problems related to differences in MS intensities that result from each of the different labeling processes.27–30 However, the labeling profile for a single amino acid type provides very limited information for the assessment of the orientation and conformational changes of an adsorbed protein. Therefore, to provide more complete coverage for a given protein, our group recently developed methodology to combine labeling results from multiple target amino acid types applied to a single protein.51 In our approach, we label an individual amino acid residue type for the protein in solution and after adsorption to a surface. Samples are then prepared for MS by digesting the protein using various proteolytic enzymes and then analyzed to identify which of the targeted amino acids are labeled and which are not. For this purpose, we use the Mass Spectrometry Center at Auburn University (Dr. Yonnie Wu, Director), which uses electrospray ionization quadrupole time-of-flight mass spectrometry (Q-ToF MS, Waters) for MS analysis. An overview of our AAL/MS method is provided in Fig. 3, with further details provided in the following paragraphs.
Fig. 3.
Quantification of adsorption-induced structural shifts at a molecular level using the AAL/MS technique (Ref. 51). (a) The overall scheme of the methodology while the specific approach to directly compare the labeling from multiple sites within the adsorbed and solution state are shown in (b) and (c), respectively. The extent of amino acid labeling by a labeling agent is directly related to its solvent exposure. After being labeled for each individual amino acid residue type, the proteins are digested off of the surface, and the mass spectrum from each labeling process is acquired. The mass spectra from different batch labeling processes are then directly compared after normalizing them with the mass spectra of an internal control peptide fragment that does not contain one of the targeted amino acids to adjust for batch-to-batch differences in MS intensities. Subsequently, the profile of a residue relates to the extent of its solvent exposure in the adsorbed state relative to the solution state. When the protein is adsorbed from a very low solution concentration, a negative shift in an amino acid profile can be considered to be primarily related to adsorbed orientation, while a positive shift infers areas of tertiary unfolding. When the protein is adsorbed from high solution concentration, the negative shift in an amino acid's profile can also be due to protein–protein interaction effects on the surface. Reproduced with permission from Thyparambil et al., Acta Biomater. 10, 2404 (2014). Copyright 2014 by Elsevier.
1. Batch labeling of target amino acids
Side-chain selective chemical modification of amino acids within proteins has been widely used for more than 40 years as a quick, simple tool to gain information on the role of particular amino acids in proteins.50 To this end, variety of chemical agents have been used or developed for side-chain modification of Arg, carboxylic acids (Asp, Glu, and carboxyl terminal of protein), Cys, His, Lys, Trp, and Tyr.50 However, side-chain modification by any label can influence the protein's proteolytic digestion pattern and kinetics, and can also alter the hydrophobicity and ionization efficiency of the peptide-fragment digests, all of which can affect the signal intensities of the target peptides in ways that are difficult to predict.50,65,66 This problem is even more pronounced when different amino acid types are labeled on the same peptide, which further complicates the quantification of modified peptide fragments by MS. Additionally, when the labeling of each type of amino acid is done under its respective optimal conditions (e.g., temperature, pH, and concentration of reactants), the resulting labeling profile is a function of not only the configuration of adsorbed protein, but also of reaction kinetics.50 Because of these complications, AAL/MS has typically been limited to the labeling of only a single type of amino acid residue for a given protein.
To minimize these complications, we chose a straightforward approach of labeling different amino acid types in a given protein, in solution and in its adsorbed state, under a common set of reactive conditions by targeting only one single type of amino acid at a time.51,52 CD analysis is also then used to verify that the applied reactive conditions do not alter the protein's structure. Then, to overcome the inherent problem of different MS intensities occurring for each of the different labeling schemes, the combined MS results are analyzed to identify a common peptide fragment that does not contain one of the targeted residues. The MS intensities of each sample are then normalized by the respective intensity of that untargeted fragment, thus adjusting the MS profiles to a common intensity level so that the MS results from each labeling process can be combined on an equal basis.51
2. Digestion of labeled proteins and mass spectrometric analysis
The digestion of labeled proteins to peptide fragments is an essential step in identifying the sites of modification and quantifying the extent of labeling in the protein. This procedure is usually done after reducing and acetylating any disulfide bonds in the protein using iodoacetamide.51 Currently, the two main strategies to cleave proteins in solution or in their adsorbed state are using either a sequence-grade proteolytic agent (typically, trypsin) and/or chemical agents like cyanogen bromide (CNBr), all of which are site-specific. Peptide digestion for the adsorbed protein can be done directly on the surface, or the protein can first be desorbed off the surface and then digested into peptide fragments in solution. However, since desorption of proteins from most surfaces requires the use of surfactants, which can interfere with the ionization process of the peptides, digestion directly from the surface is usually used as the preferred method.
Most of the specific recognition sites within a protein that can be recognized by proteolytic agents are susceptible to alteration by the chemical labeling process, resulting in missed cleavages by the otherwise site-specific peptide digesting agents. Additionally, the specificity of cleaving agents can be altered or missed when the target site are sterically blocked by the adsorbent surface. Online resources like PeptideMass67 are available to help with these issues, which contain a repository of different protein cleaving/digesting agents and their preferences to a recognition site within the protein. These resources provide valuable tools for optimizing the selection of the types of amino acid labeling to be used, the number of modifications per peptide fragment (preferably 1–2 of the target residues within a peptide fragment), the type of cleaving agent, and suggested settings for the mass spectrometry scan procedures.67
When possible, it is best to use a cleaving agent that has multiple recognition sites as opposed to those digestive agents that are specific to limited sites, as this eliminates the usage of different cleaving agents for different batch labeling processes, and also minimizes the occurrence of peptide fragments with widely varying lengths. For example, enzymes like trypsin are specific to the carboxyl side of both Lys and Arg (except when either one is followed by Pro) in any protein, and can be used for proteolytic cleavage when either of these amino acids are targeted.65 Also, since each of these amino acids are relatively abundant within a protein sequence, approximately equal sized peptide fragments can be obtained following trypsin treatment. In contrast, CNBr is very specific to Met, which is very sensitive to oxidation and occurs much less frequently in proteins, resulting in higher probabilities of missed cleavages and unequally sized peptide fragments.65 Peptide fragments that are too short result in difficulty in distinguishing the modification-induced mass increase from background noise. In contrast, the modification process on the peptides that are too long results in longer retention in the chromatographic columns and increased difficulty to be ionized by the ionization unit of the MS.50,65
Once the protein is digested into peptide fragments, the sites of labeling are identified by measuring the mass-to-charge (m/z) ratios of the proteolytic fragments. The fragments whose m/z ratios differ from the theoretical mass of the unlabeled peptide by the mass of the applied label (within 0.1% precision) are then identified using software typically provided by the MS system. The intensities obtained from mass matching are subsequently used to quantify the extent of labeling for each individual targeted amino acid.
3. Correlating mass spectra to protein configuration
The sample-to-sample variation in the ionization process, even within unmodified peptide digests, is typically high, and this variability is further compounded when amino acids within the peptide fragments are modified by chemical labels.50,65 These problems result in differences in the MS peptide intensity values from sample to sample, especially between proteins subjected to different treatments to label different targeted amino acid residue types. To overcome these problems, we have adapted a method to normalize out the differences in MS intensities between different datasets, thus enabling the MS results for multiple targeted amino acids within a given protein to be combined on an equivalent basis. This is accomplished by normalizing the MS intensities of each of the peptide fragments of a given protein sample by the MS intensity of an unlabeled peptide fragment of the protein that is present in the protein dataset for each of the different labeling processes applied. When selecting the unlabeled peptide fragment for MS-intensity normalization, care should be taken to select a fragment that is as identical as possible (i.e., same amino acid sequence) between each of the batch-labeled datasets to provide a common basis for MS intensity normalization. After this normalization procedure, we determine a labeling intensity parameter for each of the targeted amino acids. The labeling intensity is calculated by dividing the number of times the designated targeted amino acid residue is present in the MS spectrum in its modified state divided by the total number of times the amino acid is present in all of the identified fragments (i.e., including both its labeled and unlabeled states). We then calculate a relative ratio of the extent of modification for each targeted amino acid residue of the protein by dividing its labeling intensity in its adsorbed state (Iads) by its labeling intensity in its solution state (Isoln). Finally, we take the base-10 logarithm of this ratio to represent what we refer to as the amino acid's “residue profile” as indicated in
| (5) |
If the value of either Isoln or Iads is found to be less than 0.10 (which is considered to be the limit of detection), a low ceiling threshold value of 0.10 is designated for the respective intensity value instead of zero to avoid the mathematical problems of dividing by zero or taking the log(0) in Eq. (5).51 Similarly, the maximum value for Isoln and Iads is 1.0, which occurs when the targeted amino acid residue it found to be modified in every peptide fragment in the MS results.51 Thus, the range of possible Iads/Isoln values is from 0.1 to 10, with the values of the residue profiles for each targeted amino acid, thus ranging from −1.0 to +1.0.51 Accordingly, a given residue's profile is representative of the ensemble average change in its solvent accessibility when it is adsorbed on a given surface relative to its state in solution.
By sequentially mapping the positive and negative profile shifts of the multiple residues, the effect of adsorption on the protein structure can be analyzed. The orientation of the protein on an adsorbent surface and the effect of neighboring proteins on the protein's structure can be inferred by hierarchically mapping the negative shift in residues' profiles. Similarly, indications of the sites in the protein's structure undergoing adsorption-induced tertiary unfolding are then determined from the locations of the amino acids with positive profile values. Accordingly, the profile values for the combined set of targeted amino acids for a given protein can be mapped onto the native-state structure of the protein for visualization, which can be represented by an appropriate tertiary structure model from the Protein Data Bank;68 preferably using a model that is provided within the PCDDB,58 as these share similarity in the predicted and theoretical secondary structural content of the protein.
D. Spectrophotometric assay to assess the bioactive state of adsorbed proteins
There are a variety of ways in which assays for assessing the bioactivity of proteins, especially enzymes, can be carried out.69 These methods can be broadly classified as continuous or discontinuous assays. For most of our studies, spectrophotometric assays (a type of continuous assay) have been used to measure the changes in the absorbance of an incident beam following the addition of a ligand to the protein in solution or in its adsorbed state.36,37 For this purpose, a working mass range of the protein (based on an estimate of the adsorbed amount of protein on a given surface) is determined for a fixed substrate concentration over which the change in absorbance for a designated wavelength is expected to be linear over time. The specific activity for the protein in solution is then determined under these conditions. Similarly for the adsorbed protein, the same general procedures are followed to determine the change in absorbance for the same substrate-to-protein stoichiometry over time to determine its specific activity. Since the bioactivity of enzymes are critically affected by the environmental conditions, identical conditions (preferably those used in the adsorption and equilibration of the adsorbed protein) should be maintained while assaying the bioactivity of the protein in both its solution and adsorbed states to avoid any influence of the reaction kinetics on the bioactivity assay. The amount of adsorbed protein should be quantified before and after the bioactivity assays to ensure that the applied assay does not cause a measureable amount of the protein to be desorbed from the adsorbent surface. We typically measure this using the method of absorbance at 205 nm (A205).36,51,52
The bioactive state of the proteins can be expressed in either enzymatic units or in terms of its kinetic activity, whichever method suits the intended application. For example, while the specific activity of the proteins are generally used to express the enzyme's purity in a mixture, the Michaelis constant (KM) is used as an indicator of the binding strength of the protein to its natural ligand. In our studies, we have preferred to express the bioactivity of the proteins in terms of its specific activity, as an indicator of the loss of its native-state activity rather than the KM because of the use of highly purified one-component protein systems. Additionally, the specific activity of the native-state protein at a given purity is constant. Therefore, the relative shifts in the specificity of these highly purified enzymes following adsorption can be directly related to the adsorption-induced changes in protein structure. As part of these assays, it is important to ensure that the adsorbed systems are not diffusion-limited, which can be verified when determining the working range for the study from preliminary measurements of the reaction rate as a function of substrate concentration. The relative bioactivity (units of%) are subsequently calculated, which we represent as the ratio of the protein's specific activity in its adsorbed state relative to its specific activity in solution. The resulting relative bioactivity values can then be used as indicators of either the amount of adsorbed proteins that retain their native-state-like activity or, equivalently, the average degree of adsorption-induced loss in bioactivity.
III. SYNERGISTIC APPLICATION OF TECHNIQUES TO ASSESS CAUSES OF ADSORPTION-INDUCED LOSS OF BIOACTIVITY
The adsorption of HEWL has been extensively investigated by our group as a model system for the development and demonstration of the above-presented methods.27,36,37,51 Readers are referred to our previous publications regarding the details of the specific methodology applied in these studies to characterize the structure and bioactivity of HEWL on different surfaces.36,51 In this section, we present a summary of some of the results from these studies involving the adsorption of HEWL to glass, HDPE, and PMMA surfaces from two different solution concentrations (0.03 and 1.00 mg/ml) to obtain different degrees of surface coverage and associated different degrees of protein–protein interaction effects on the surface. Each surface was exposed to HEWL in solution for 2 h to form the adsorbed layer of protein, gently rinsed to remove loosely bound protein, following which adsorbed layer of HEWL on each surface was placed under protein-free buffer solution for 15 h to allow the adsorbed protein to further equilibrate on the surface. Subsequently, CD, AAL/MS, and bioactivity assays were applied to characterize the adsorbed orientation and conformation of HEWL on these surfaces and these analyses were then used to provide insights into the causes of the measured loss of HEWL bioactivity following adsorption.
A. Impact of secondary structural shifts on the enzyme activity of adsorbed HEWL
The influence of the different solution concentrations for similar exposure time (2 h in protein solution and 15 h in protein-free buffers) on the surface coverage, secondary structure, and relative enzymatic activity of adsorbed HEWL on glass, HDPE, and PMMA is presented in Table I.36,51
Table I.
Secondary structure content, surface coverage, and relative enzymatic activity for adsorbed HEWL from two different protein solution concentrations (0.03 and 1.00 mg/ml) on (a) glass, (b) HDPE, and (c) PMMA (N = 3; average ± 95% C.I. values). For comparison, the helical and β-sheet content of HEWL in solution was found to be ∼38% (±2%) and 16% (±2%), respectively, which is very close to the reported solution-state secondary structure from the protein data bank (PDB ID: 193L: 40% helix, 10% sheet). The theoretical full surface coverage of HEWL for adsorption in “side-on” and “end-on” orientations are τside (0.17 μg/cm2) and τend (0.26 μg/cm2), respectively. Reprinted with permission from Thyparambil et al., Acta Biomater. 10, 2404 (2014). Copyright 2014 Elsevier.
| Surface | Solution conc. (mg/ml) | Surface coverage (μg/cm2) | Helices (%) | Sheets (%) | Relative enzymatic activity (%) |
|---|---|---|---|---|---|
| Glass | 0.03 | 0.05 ± 0.03 | 4 ± 2 | 42 ± 3 | 12 ± 5 |
| 1.00 | 0.14 ± 0.03 | 22 ± 4 | 30 ± 4 | 31 ± 14 | |
| HDPE | 0.03 | 0.07 ± 0.02 | 22 ± 3 | 28 ± 3 | 39 ± 9 |
| 1.00 | 0.09 ± 0.04 | 12 ± 3 | 33 ± 4 | 17 ± 8 | |
| PMMA | 0.03 | 0.05 ± 0.01 | 39 ± 3 | 16 ± 4 | 54 ± 22 |
| 1.00 | 0.17 ± 0.03 | 22 ± 4 | 28 ± 3 | 66 ± 9 |
1. Effect of protein crowding and surface chemistry on the secondary structure of HEWL
As shown in Table I, adsorption of HEWL resulted in a significant shift in its secondary structure on each surface and for each solution concentration. These results reflect the combined influences of protein–surface interactions, protein–protein interactions (PPI), and internal protein stability effects.36
When HEWL was adsorbed from a 1.00 mg/ml solution concentration, the resulting surface coverage of adsorbed protein on each surface was within 53% of a saturated, close-packed monolayer with side-on protein orientation. In contrast to this, when adsorbed from 0.03 mg/ml solution and equilibrated under pure buffer conditions, the surface coverage of the HEWL was about one third of that was adsorbed from 1.00 mg/ml solution conditions (e.g., 0.05 μg/cm2, nearly 3× less than the closed-packed side-on arrangement of 0.17 μg/cm2). These differences suggest that PPI effects have a much greater influence on the adsorbed state of the HEWL when it was adsorbed from the 1.00 mg/ml solution compared to 0.03 mg/ml.
As clearly evident from the results presented in Table I, the surface coverage of the HEWL and the type of surface that it was adsorbed on had a profound influence on both its secondary structure and bioactivity. Interestingly, the degree of surface coverage had a completely different effect on the adsorbed-state bioactivity on each of these three different surfaces, with increased surface coverage enhancing bioactivity on glass, decreasing bioactivity on HDPE, and having little effect on PMMA.
2. Correlation between the secondary structure and the enzyme activity of adsorbed HEWL
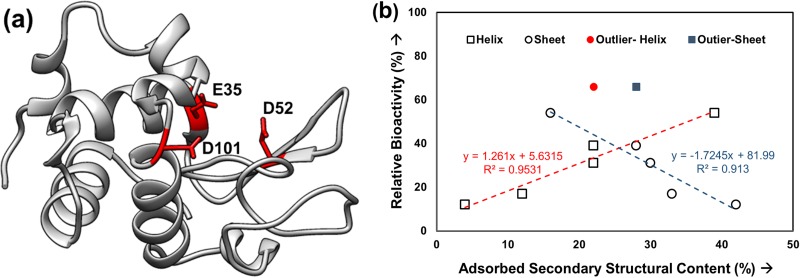
The key items of interest for an adsorbed enzyme are its activity and the factors influencing its activity. The native-state structure of the HEWL resembles a kidney shape, with three of the primary active site residues (E35, D52, and D101) laying in the concave cleft of the enzyme [Fig. 4(a)]. As indicated by its PDB structure, one of the three residues involved in catalysis lies within a α-helix in the protein structure [Fig. 4(a); E35]. Many studies have indicated that the loss in secondary structural content, especially helices, is associated with the loss in native-state activity. Figure 4(b) plots the data from Table I to investigate the relationships between the adsorbed secondary structures (i.e., helix and the sheets) and the observed enzymatic activity of HEWL on our three different surfaces.
Fig. 4.
(a) Ribbon diagram of the three-dimensional structure of HEWL (PDB ID: 193L) (Ref. 70). The three residues most important for catalysis: E35, D52, and D101 are marked in red, and (b) % relative bioactivity (y-axis) vs % secondary structural content (helix and sheet) (x-axis) in the adsorbed HEWL layers on different surfaces. The helix and β-sheet content of HEWL in solution was found to be ∼38% (±2%) and 16% (±2%) (N = 3, averaged 95% C.I. values = ±4% helicity for each data point, averaged 95% C.I. values = ±9% for bioactivity).
As indicated from the results presented in Fig. 4(b), the loss in native enzymatic activity of HEWL is strongly correlated to the secondary structural content within the protein, with the enzyme tending to lose its native-state bioactivity as the adsorbed-state of the enzyme deviated more and more away from its native-state structure (i.e., loss in helicity, gain in β-sheet). These results suggest that the enzymatic activity of HEWL on these three surfaces is primarily caused by conformational changes in the enzyme's bioactive site as opposed to adsorbed orientation, with the outlier data points possibly indicating an exception where the bioactivity is influenced by orientation or protein–protein interactions. While these data provide important structural-level insights into the cause of the adsorption-induced loss of HEWL bioactivity, they do not provide any direct information regarding how these structural changes may influence the actual active site of the enzyme. If conformational changes of the active site are indeed primarily causing this loss in bioactivity, then it can be expected that these changes may be detectable by measuring changes in the solvent accessibility of the three key amino acids that are involved in HEWL's catalytic site, which can be probed using AAL/MS.
B. Conformational distortion of the bioactive site within HEWL probed by AAL/MS
AAL/MS provides an approach to identify areas in a protein that undergo adsorption-induced conformational changes and protein orientation as reflected in changes in the solvation profile of targeted amino acid residues. In particular, an increase in the solvent accessibility of amino acid residues that are present within the active site of a protein indicate conformational unfolding of the binding site as a likely cause of adsorption-induced loss in bioactivity. Alternatively, a decrease in solvent accessibility of the residues within the active site indicates loss of bioactivity due to steric hindrance from either the surface, neighboring adsorbed proteins.
In this section, we present an example of the application of AAL/MS to provide additional insights into the loss of HEWL bioactivity when adsorbed on surfaces to complement the data presented in Sec. III A. Readers are referred to our published paper for specific details on the methods and analysis of the results from the larger study that these example data are abstracted from.36,51
A total of 32 residues from five different amino acid types [Lys (K), Arg (R), Asp (D), Glu (E), and Trp (W)] that are distributed throughout HEWL were targeted in solution and following adsorption on glass, HDPE, and PMMA surfaces. As presented in Fig. 4(a), amino acid residues E35, D52, and D101 primarily form the catalytic site in HEWL, which are utilized by this enzyme to hydrolyze glycosidic bonds within the peptidoglycan layer of the gram-positive bacteria. The residues E35 and D52 acts as an acid or a base to both accept and donate electrons while D101 stabilizes the transition state of the catalytic reaction.71
In Sec. III A, a comparison between CD and bioactivity results provided evidence that the loss in bioactivity of HEWL following adsorption on our set of surfaces was due to adsorption-induced conformational changes. In this section, we present data from AAL/MS studies for the same adsorption systems to provide further evidence of this behavior by evaluating the effect of adsorption on the solvent accessibility of the three key amino acids (i.e., E35, D52, and D101) that form the catalytic site of this enzyme.71,72
1. Solvent accessibility of amino acid residues in solution phase.
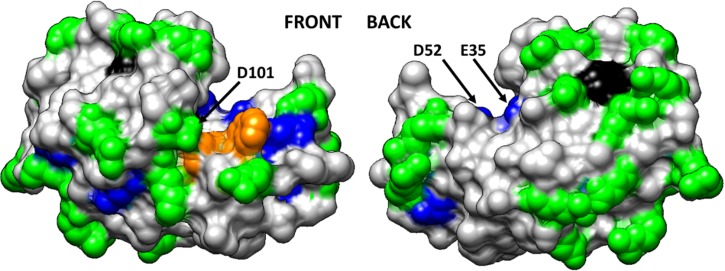
Figure 5 illustrates the solvent accessibility of each of the targeted amino acid types for HEWL in solution using a space–filled model of HEWL with the targeted amino acid residues color-coded by their degree of solvent exposure as determined by AAL/MS. Based on these results, two of the catalytic residues (E35 and D52) are partially buried with limited solvent accessibility, while the third (D101) is fully solvent exposed in HEWL's solution-state structure.
Fig. 5.
Space-filled model of HEWL (PDB ID: 193L) with amino acid residues color coded by their solvent accessibility as determined from targeted amino acid labeling in solution (Ref. 70). Color coding: charged amino acid residues (Asp, Glu, Lys, Arg) with high solvent accessibility (green) and moderate solvent accessibility (blue), Trp residues with high solvent accessibility (orange) and low solvent accessibility (black). Nontargeted amino acid residues are color coded in light gray. This figure is illustrated using UCSF Chimera (Ref. 73). The arrows point out the location of the three key amino acid residues that provide the catalytic function of the enzyme (E35, D52, and D101). Reproduced with permission from Thyparambil et al., Acta Biomater. 10, 2404 (2014). Copyright 2014 Elsevier.
2. Solvent accessibility of amino acid residues in adsorbed state
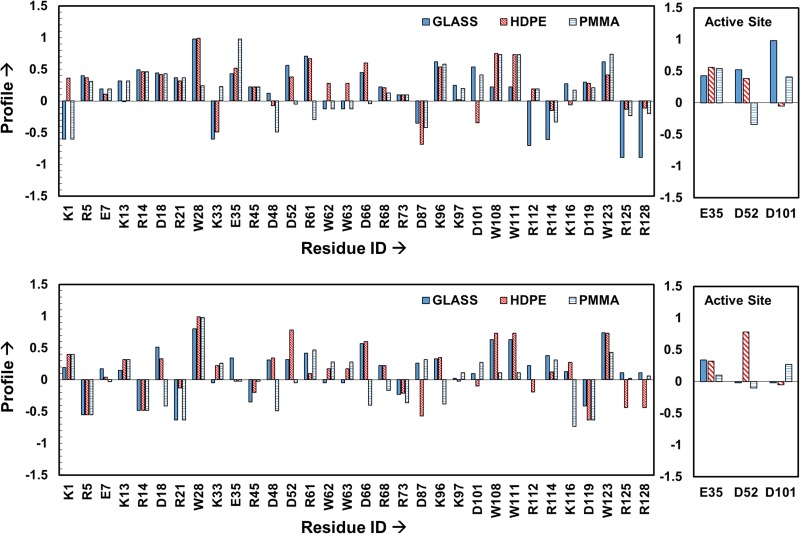
The resulting profiles for each of the targeted amino acids were determined as described in Sec. II C and are shown in Fig. 6 for each of the three different surfaces when adsorbed from 0.03 to 1.00 mg/ml protein solutions. As clearly evident from these plots, the solvent accessibility of each targeted amino acid residue is influenced both by the type of surface that HEWL is adsorbed to and the solution concentration from which it was adsorbed.
Fig. 6.
Labeling profile of the targeted residues in HEWL on glass, PMMA and HDPE surfaces when adsorbed from 0.03 mg/ml (top plot) to 1.00 mg/ml (bottom plot) protein solutions. The residues within the active site of HEWL are shown separately in the right-hand plot to more clearly show their response. Profiles within about ±0.1 of zero can be considered to be not significantly different from the solution state (n = 3). Profiles beyond ±0.5 represent greater than threefold change to the native-state solvent exposure, which is a highly significant difference (p < 0.0001). Residues showing no difference in their solvation between solution and the adsorbed states have profile values equal to 0. Reproduced with permission from Thyparambil et al., Acta Biomater. 10, 2404 (2014). Copyright 2014 Elsevier.
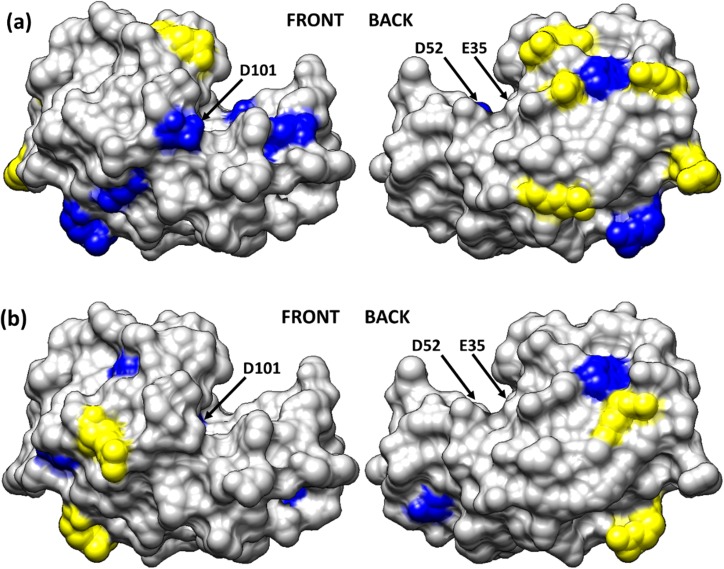
As an example of how these results can be used to provide insight into the amino acid-level mechanisms influencing the effect of adsorption on the activity of the enzyme, Fig. 7 presents the native-state structure of HEWL with the targeted amino acids color-coded to designate areas of both high and low profile values when HEWL was adsorbed on glass. Amino acid residues exhibiting more than a threefold increase in solvent accessibility compared to the solution-state accessibility are colored-coded in blue while residues exhibiting more than a threefold decrease in solvent accessibility are color-coded in yellow. As evident from the solvation profiles shown in Fig. 6, when HEWL was adsorbed on glass with low surface coverage from the 0.03 mg/ml solution, the solvent accessibility of the catalytic site residues E35 and D101 are shown to greatly increase, thus indicating adsorption-induced conformational unfolding of the active site. In contrast to this, these same residues are shown to maintain a degree of solvent accessibility much closer to the solution-state structure when HEWL was adsorbed with high surface coverage from a 1.00 mg/ml solution concentration. In addition, the fact that the profiles for the three key amino acids in the active site are all positive for HEWL on glass indicate that access to the active site was not sterically blocked by either the surface or neighboring proteins.
Fig. 7.
Solvation profile of the residues in HEWL adsorbed from (a) 0.03 and (b) 1.00 mg/ml on the glass surface. Residue color code: yellow (>threefold decrease in solvent accessibility), blue (>threefold increase in solvent accessibility) and light gray (other residues). The arrows point to the location of the three key amino acid residues that provide the catalytic function of the enzyme (E35, D52, and D101). Reproduced with permission from Thyparambil et al., Acta Biomater. 10, 2404 (2014). Copyright 2014 Elsevier.
The results presented in Fig. 7 for HEWL on glass both complement and support the CD-bioactivity results presented in Sec. III A. These combined results indicate that the greater loss in HEWL activity on the glass surface when adsorbed with low surface coverage compared to high surface coverage is caused by greater adsorption-induced conformational unfolding of the active site. The AAL/MS results further indicates that the loss in bioactivity under both the low and high surface coverage conditions is not due to steric hindrance of the active site due to adsorbed orientation or PPI on the surface.
These results are directly in line with the observations obtained from many other experimental and theoretical studies by other groups on the adsorption behavior of HEWL on negatively charged hydrophilic surfaces similar to glass, which have identified conformational loss74–76 and an influence of electrostatic interactions in orienting the protein on a surface.15,77,78 However, previous studies have not quantitatively connected the role of these individual molecular-level events with the bioactive state of HEWL. As we have demonstrated in our combined set of studies, the synergistic use of CD, AAL/MS, and adsorbed-state spectrophotometric assays provide a powerful approach to investigate how adsorption influences the molecular structure of proteins and how this subsequently causes changes in bioactivity. Obviously these combined methods do not provide a fully comprehensive description of the influence of adsorption on protein structure and bioactivity and continued development work is certainly called for to provide further understanding of these complex issues.
IV. CONCLUSIONS
In this paper, we present an overview of the methodology that we have developed and/or adapted to quantitatively characterize the orientation, structure, and bioactivity of adsorbed protein using CD, AAL/MS, and adsorbed-state spectrophotometric assays. CD provides the ability to document the effect of adsorption on the secondary structure of a protein, while AAL/MS provides the ability to probe adsorption-induced changes in amino acid residue solvent accessibility, which can be related to adsorbed orientation, protein–protein interactions, and tertiary unfolding.
As demonstrated for HEWL adsorption, when these methods are used in combination with bioactivity assays, they can synergistically provide insights into the underlying adsorption processes, which can be used to determine whether adsorption-induced loss in bioactivity of a protein is due to steric blockage of the bioactive site by the surface or neighboring proteins, or is due to conformational unfolding of the active site. The molecular-level understanding of adsorption processes that these methods provide can thus be very helpful to guide the design and development of surfaces for a broad range of applications in biotechnology and biomedical engineering. Examples include the design of substrate surfaces for biosensors to optimally preserve the bioactivity of enzymes that are either adsorbed or tethered to the surface; or, alternatively, the design of filters and decontamination systems for biodefense to purposely deactivate adsorbed protein toxins or the protein capsid of virus particles by surface-induced protein unfolding or steric blockage of bioactive sites. These methods are extremely important as well to provide the kinds of data that is needed for the evaluation and validation of molecular modeling methods that are being developed to predict protein adsorption behavior through molecular simulation.
While our applications have primarily involved the investigation of protein adsorption on flat surfaces, these methods are certainly not limited to these types of substrates, but can be readily extended to other types of substrates as well, including micron-to-nanoscale sized particles, fibers, and meshes. However, the presented techniques do have the limitation of not being capable of resolving the full atomistic structure of adsorbed protein, thus emphasizing the need for the further development of new methods to provide greater detail for the characterization of the effect of adsorption on the structure–bioactivity relationships of proteins on surfaces.
ACKNOWLEDGMENTS
This project received support from the Defense Threat Reduction Agency–Joint Science and Technology Office for Chemical and Biological Defense (Grant No. HDTRA1‐10‐1‐0028). The facilities used in this research were also supported by National Institutes of Health Grants 5P20RR021949 and 8P20GM103444. The authors also would like to thank Megan Grobman, Lara Gamble, and David Castner of NESAC/BIO at the University of Washington for assistance with surface characterization with XPS under the funding support by NIBIB (Grant No. EB002027). The authors also thank Nick Erdman (Clemson University) for assistance in drafting the designs for the cuvette.
References
- 1.Itamar W., Azalia R., Benjamin S., Dalia R., and Eugenii K., Adv. Mater. 5, 912 (1993). 10.1002/adma.19930051206 [DOI] [Google Scholar]
- 2.Larsson A., Ekblad T., Andersson O., and Liedberg B., Biomacromolecules 8, 287 (2007). 10.1021/bm060685g [DOI] [PubMed] [Google Scholar]
- 3.Czeslik C., Jackler G., and Royer C., Spectroscopy 16, 139 (2002). 10.1155/2002/473035 [DOI] [Google Scholar]
- 4.Knowles J. R., Nature 350, 121 (1991). 10.1038/350121a0 [DOI] [PubMed] [Google Scholar]
- 5.Shard A. G. and Tomlins P. E., Regener. Med. 1, 789 (2006). 10.2217/17460751.1.6.789 [DOI] [PubMed] [Google Scholar]
- 6.Tsapikouni T. S. and Missirlis Y. F., Mater. Sci. Eng., B 152, 2 (2008). 10.1016/j.mseb.2008.06.007 [DOI] [Google Scholar]
- 7.Kasemo B. and Gold J., Adv. Dent. Res. 13, 8 (1999). 10.1177/08959374990130011901 [DOI] [PubMed] [Google Scholar]
- 8.Latkany R., Tsuk A., Sheu M. S., Loh I. H., and Trinkaus-Randall V., J. Biomed. Mater. Res. 36, 29 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Wisniewski N., Moussy F., and Reichert W. M., Fresenius' J. Anal. Chem. 366, 611 (2000). 10.1007/s002160051556 [DOI] [PubMed] [Google Scholar]
- 10.Bramwell V. W., Eyles J. E., and Oya Alpar H., Adv. Drug Delivery Rev. 57, 1247 (2005). 10.1016/j.addr.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 11.Herr A. E., Protein Microarrays for the Detection of Biothreats, edited by Dill K., Liu R. H., and Grodzinski P. ( Springer, New York, 2009), p. 169. [Google Scholar]
- 12.Norde W., Macromolecular Symposia 103, 5 (1996) 10.1002/masy.19961030104. [DOI] [Google Scholar]
- 13.Nakanishi K., Sakiyama T., and Imamura K., J. Biosci. Bioeng. 91, 233 (2001). 10.1016/S1389-1723(01)80127-4 [DOI] [PubMed] [Google Scholar]
- 14.Latour R. A., “ Biomaterials: Protein-surface interactions,” in The Encyclopedia of Biomaterials and Bioengineering, 2nd ed., edited by Bowlin G. E. W. a. G. L. ( Informa Healthcare, New York, 2008), Vol. 1, pp. 270–284. [Google Scholar]
- 15.Rabe M., Verdes D., and Seeger S., Adv. Colloid Interface Sci. 162, 87 (2011). 10.1016/j.cis.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 16.Latour R. A., Colloids Surf., B 124, 25 (2014). 10.1016/j.colsurfb.2014.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horbett T., Brash J. L., and Norde W., Proteins at Interfaces III State of the Art ( American Chemical Society, Washington DC, 2012), Vol. 1120. [Google Scholar]
- 18.Lynch I. and Dawson K. A., Nano Today 3, 40 (2008). 10.1016/S1748-0132(08)70014-8 [DOI] [Google Scholar]
- 19.Benesch J., Hungerford G., Suhling K., Tregidgo C., Mano J. F., and Reis R. L., J. Colloid Interface Sci. 312, 193 (2007). 10.1016/j.jcis.2007.03.016 [DOI] [PubMed] [Google Scholar]
- 20.Kastantin M., Langdon B. B., and Schwartz D. K., Adv. Colloid Interface Sci. 207, 240 (2014). 10.1016/j.cis.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai D.-H., DelRio F. W., Keene A. M., Tyner K. M., MacCuspie R. I., Cho T. J., Zachariah M. R., and Hackley V. A., Langmuir 27, 2464 (2011). 10.1021/la104124d [DOI] [PubMed] [Google Scholar]
- 22.Baio J. E., Weidner T., Baugh L., Gamble L. J., Stayton P. S., and Castner D. G., Langmuir 28, 2107 (2011). 10.1021/la203907t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apte J. S., Collier G., Latour R. A., Gamble L. J., and Castner D. G., Langmuir 26, 3423 (2010). 10.1021/la902888y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weidner T. and Castner D. G., Phys. Chem. Chem. Phys. 15, 12516 (2013). 10.1039/c3cp50880c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goobes G.et al. , Magn. Reson. Chem. 45, S32 (2007). 10.1002/mrc.2123 [DOI] [PubMed] [Google Scholar]
- 26.Roehrich A. and Drobny G., Acc. Chem. Res. 46, 2136 (2013). 10.1021/ar300321e [DOI] [PubMed] [Google Scholar]
- 27.Fears K. P., Sivaraman B., Powell G. L., Wu Y., and Latour R. A., Langmuir 25, 9319 (2009). 10.1021/la901885d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J. S. and Bowden E. F., J. Am. Chem. Soc. 128, 6813 (2006). 10.1021/ja054219v [DOI] [PubMed] [Google Scholar]
- 29.Ovod V., Scott E. A., Flake M. M., Parker S. R., Bateman R. J., and Elbert D. L., J. Biomed. Mater. Res., Part A 100, 622 (2012). 10.1002/jbm.a.33285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott E. A. and Elbert D. L., Biomaterials 28, 3904 (2007). 10.1016/j.biomaterials.2007.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan X., Watson J., Ho P. S., and Deinzer M. L., Mol. Cell. Proteomics 3, 10 (2004). 10.1074/mcp.R300010-MCP200 [DOI] [PubMed] [Google Scholar]
- 32.Roach P., Farrar D., and Perry C. C., J. Am. Chem. Soc. 127, 8168 (2005). 10.1021/ja042898o [DOI] [PubMed] [Google Scholar]
- 33.Krishnan C., “ Proteins on surfaces,” in Biopolymers at Interfaces, 2nd ed. ( CRC, Boca Raton, FL, 2003). [Google Scholar]
- 34.Chandra G., Ghosh K. S., Dasgupta S., and Roy A., Int. J. Biol. Macromol. 47, 361 (2010). 10.1016/j.ijbiomac.2010.05.020 [DOI] [PubMed] [Google Scholar]
- 35.Huang H.et al. , ChemPhysChem 12, 3642 (2011). 10.1002/cphc.201100398 [DOI] [PubMed] [Google Scholar]
- 36.Wei Y., Thyparambil A. A., and Latour R. A., Colloids Surf., B 110, 363 (2013). 10.1016/j.colsurfb.2013.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fears K. P. and Latour R. A., Langmuir 25, 13926 (2009). 10.1021/la900799m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sivaraman B., Fears K. P., and Latour R. A., Langmuir 25, 3050 (2009). 10.1021/la8036814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivaraman B. and Latour R. A., Biomaterials 32, 5365 (2011). 10.1016/j.biomaterials.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C. H., Nguyen X., Narhi L., Chemmalil L., Towers E., Muzammil S., Gabrielson J., and Jiang Y., J. Pharm. Sci. 100, 4642 (2011). 10.1002/jps.22695 [DOI] [PubMed] [Google Scholar]
- 41.Sreerama N. and Woody R. W., Anal. Biochem. 287, 252 (2000). 10.1006/abio.2000.4880 [DOI] [PubMed] [Google Scholar]
- 42.Kelly S. M., Jess T. J., and Price N. C., Biochim. Biophys. Acta 1751, 119 (2005). 10.1016/j.bbapap.2005.06.005 [DOI] [PubMed] [Google Scholar]
- 43.Greenfield N. J., Nat. Protoc. 1, 2876 (2006). 10.1038/nprot.2006.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMillin C. R. and Walton A. G., J. Colloid Interface Sci. 48, 345 (1974). 10.1016/0021-9797(74)90170-2 [DOI] [Google Scholar]
- 45.Arteaga O., Freudenthal J., Wang B., Nichols S., and Kahr B., Chim. Oggi 30, 6 (2012). [Google Scholar]
- 46.Kelly S. M. and Price N. C., Curr. Protein Pept. Sci. 1, 349 (2000). 10.2174/1389203003381315 [DOI] [PubMed] [Google Scholar]
- 47.Engel M. F. M., Visser A., and van Mierlo C. P. M., Proc. Natl. Acad. Sci. U.S.A. 101, 11316 (2004) 10.1073/pnas.0401603101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ronda L., Bruno S., Viappiani C., Abbruzzetti S., Mozzarelli A., Lowe K. C., and Bettati S., Protein Sci. 15, 1961 (2006). 10.1110/ps.062272306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devineau S., Zanotti J.-M., Loupiac C., Zargarian L., Neiers F., Pin S., and Renault J. P., Langmuir 29, 13465 (2013). 10.1021/la4035479 [DOI] [PubMed] [Google Scholar]
- 50.Mendoza V. L. and Vachet R. W., Mass Spectrom. Rev. 28, 785 (2009). 10.1002/mas.20203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thyparambil A. A., Wei Y., Wu Y., and Latour R. A., Acta Biomater. 10, 2404 (2014). 10.1016/j.actbio.2014.01.027 [DOI] [PubMed] [Google Scholar]
- 52.Wei Y., Thyparambil A. A., Wu Y., and Latour R. A., Langmuir 30, 14849 (2014). 10.1021/la503854a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sivaraman B. and Latour R. A., Biomaterials 31, 832 (2010). 10.1016/j.biomaterials.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ladner D. A., Steele M., Weir A., Hristovski K., and Westerhoff P., J. Hazard. Mater. 211, 288 (2012). 10.1016/j.jhazmat.2011.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thyparambil A. A., Wei Y., and Latour R. A., Langmuir 28, 5687 (2012). 10.1021/la300315r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DiNitto J. M. and Kenney J. M., Appl. Spectrosc. 66, 180 (2012). 10.1366/11-06417 [DOI] [PubMed] [Google Scholar]
- 57.Liu P.-F., Avramova L. V., and Park C., Anal. Biochem. 389, 165 (2009). 10.1016/j.ab.2009.03.028 [DOI] [PubMed] [Google Scholar]
- 58.Whitmore L., Woollett B., Miles A. J., Klose D. P., Janes R. W., and Wallace B. A., Nucleic Acids Res. 39, D480 (2011). 10.1093/nar/gkq1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wallace B. A., Lees J. G., Orry A. J. W., Lobley A., and Janes R. W., Protein Sci. 12, 875 (2003). 10.1110/ps.0229603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdul-Gader A., Miles A. J., and Wallace B., Bioinformatics 27, 1630 (2011). 10.1093/bioinformatics/btr234 [DOI] [PubMed] [Google Scholar]
- 61.Whitmore L. and Wallace B., Nucleic Acids Res. 32, W668 (2004). 10.1093/nar/gkh371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitmore L. and Wallace B. A., Biopolymers 89, 392 (2008). 10.1002/bip.20853 [DOI] [PubMed] [Google Scholar]
- 63.Lees J. G., Miles A. J., Wien F., and Wallace B., Bioinformatics 22, 1955 (2006). 10.1093/bioinformatics/btl327 [DOI] [PubMed] [Google Scholar]
- 64.Wei Y., Thyparambil A. A., and Latour R. A., Biochim. Biophys. Acta 1844, 2331 (2014). 10.1016/j.bbapap.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duncan M. W., Aebersold R., and Caprioli R. M., Nat. Biotechnol. 28, 659 (2010). 10.1038/nbt0710-659 [DOI] [PubMed] [Google Scholar]
- 66.Iwasaki Y., Nakano Y., Mochizuki K., Nomoto M., Takahashi Y., Ito R., Saito K., and Nakazawa H., J. Chromatogr. B 879, 1159 (2011). 10.1016/j.jchromb.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 67.Wilkins M. R., Lindskog I., Gasteiger E., Bairoch A., Sanchez J.-C., Hochstrasser D. F., and Appel R. D., Electrophoresis 18, 403 (1997). 10.1002/elps.1150180314 [DOI] [PubMed] [Google Scholar]
- 68.Berman H. M., Westbrook J., Feng Z., Gilliland G., Bhat T. N., Weissig H., Shindyalov I. N., and Bourne P. E., Nucleic Acids Res. 28, 235 (2000). 10.1093/nar/28.1.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lundblad R. L., “ The chemical cleavage of peptide bonds,” in Chemical Reagents for Protein Modification, 3rd ed. ( CRC, Boca Raton, FL, 2004). [Google Scholar]
- 70.Vaney M. C., Maignan S., Ries-Kautt M., and Ducriux A., Acta Crystallogr., Sect. D: Biol. Crystallogr. 52, 505 (1996). 10.1107/S090744499501674X [DOI] [PubMed] [Google Scholar]
- 71.Vocadlo D. J., Davies G. J., Laine R., and Withers S. G., Nature 412, 835 (2001). 10.1038/35090602 [DOI] [PubMed] [Google Scholar]
- 72.Lundblad R. L., “ The modification of histidine residues,” in Chemical Reagents for Protein Modification, 3rd ed. ( CRC, Boca Raton, FL, 2004). [Google Scholar]
- 73.Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., and Ferrin T. E., J. Comput. Chem. 25, 1605 (2004). 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- 74.Vertegel A. A., Siegel R. W., and Dordick J. S., Langmuir 20, 6800 (2004). 10.1021/la0497200 [DOI] [PubMed] [Google Scholar]
- 75.Wu X. and Narsimhan G., Biochim. Biophys. Acta 1784, 1694 (2008). 10.1016/j.bbapap.2008.06.008 [DOI] [PubMed] [Google Scholar]
- 76.van der Veen M., Stuart M. C., and Norde W., Colloids Surf., B 54, 136 (2007). 10.1016/j.colsurfb.2006.08.017 [DOI] [PubMed] [Google Scholar]
- 77.Kubiak-Ossowska K. and Mulheran P. A., Langmuir 26, 15954 (2010). 10.1021/la102960m [DOI] [PubMed] [Google Scholar]
- 78.Kim D. T., Blanch H. W., and Radke C. J., Langmuir 18, 5841 (2002). 10.1021/la0256331 [DOI] [Google Scholar]