Abstract
The present study was aimed to test the efficacy of adding vitamins C or E to Tris-fructose-egg yolk diluent to increase Awassi ram sperm storage period at 5 ˚C. Semen samples from six mature Awassi rams were used in this study. The semen samples were diluted by Tris-glucose-egg yolk. Diluted semen sample was divided into three parts. The first part was added with 0.9 mg mL-1 vitamin C, the second part was added with 1 mg mL-1 vitamin E and the third part was considered as a control without any addition. The diluted semen samples were cooled gradually and preserved at 5 ˚C for five days. Sperms in cooled diluted semen samples were examined for motility, vitality, abnormalities and acrosomal defects every 24 hr for five days. Results of the present study showed an increase in the viability of spermatozoa diluted in the Tris diluent containing vitamins C or E stored at 5 ˚C for 120 hr compared with the control group. There were significant (p < 0.05) effects of vitamins C and E addition to semen diluents on sperm motility as well as the sperm viability in different times of preservation at 5 ˚C. Significant (p < 0.05) higher sperm abnormalities and acrosomal defects values (37.6 ± 1.3% and 71.5 ± 1.1%, respectively) were found after 120 hr incubation in Tris free vitamin C (Control) at 5 ˚C compared with those of containing vitamin C (18.8 ± 1.8% and 52.8 ± 4.3%, respectively). From the results of the present study, it could be concluded, that the addition of antioxidants such as vitamins C and vitamin E to semen preservation media could improve longevity and quality of cooled sperm in Awassi ram semen.
Key Words: Awassi ram, Semen, Tris, Vitamin C, Vitamin E
Introduction
Sperm cells have a high content of unsaturated fatty acids in their membranes, while lacking a significant cytoplasmic component containing antioxidants. Therefore, sperm cells are very susceptible to lipid peroxidation by free radicals such as hydrogen peroxide, superoxide anion, and hydroxyl radical, which could later lead to the structural damage of sperm membranes during the aerobic storage of sperm.1 The cooling and aerobic preservation processes produce physical and chemical stress on the sperm membrane which sequentially reduces sperm viability and fertilizing ability. Cold shock of sperm cells during the cooling process is associated with oxidative stress induced by free radicals.2,3 Free radicals seek stability by “stealing” electrons from nucleic acids, lipids, and proteins leading to the damage of cells.4 Free radicals are mostly eliminated by antioxidant systems. Antioxidants play an important role in scavenging free radicals which otherwise may cause lipid peroxidation of sperm plasma membranes.5
Vitamin C (ascorbic acid or ascorbate) and vitamin E (tocopherol) are non-enzymatic antioxidants.6 The addition of anti-oxidants is well known method to improve viability and motility of liquid storage or cryopreserved ram sperm cells.7 The most important antioxidants in seminal fluid seem to be vitamins C and E.8,9 The concentration of vitamin C in seminal plasma is 10 times greater than in blood plasma (364 vs. 40 μmol L-1).10 Hughes et al. found that in vitro treatment of sperm with antioxidants (300 and 600 μM ascorbic acid with 3 and 60 μM alpha-tocopherol) reduced the magnitude of DNA damage.11 Vitamin E inhibits lipid peroxidation in membranes by scavenging peroxyl (ROO-) and alkoxyl (RO-) radicals.12 However, the ability of α-tocopherol to maintain a steady-state rate of peroxyl radical reduction in the plasma membrane depends on the recycling of α-tocopherol by external reducing agents such as ascorbate or thiols.13,14 Although much work has been done by the addition of antioxidants to cattle semen, information on their use in sheep especially Iraqi Awassi rams are scares. Therefore, the present study was undertaken to test the efficacy of adding vitamins C or E to Tris-fructose-egg yolk diluent on sperm quality of the Awassi ram semen stored at 5 ˚C.
Materials and Methods
Animals and semen collection. Semen samples from six mature Awassi rams (2-3 years of age) were used in this study. The rams were fed, housed and lit, conventionally. The study was carried out from September 2010 to June 2011. Animals were housed at the Animal Research and Practice Farm of College of Veterinary Medicine, University of Mosul (36o20' N, 43o 8' E), Iraq. All the rams were apparently in good health. They were maintained in equal nutritional and managerial condition throughout the period of the study. Throughout the experimental period, the animals were kept in open front barrens, were fed individually with concentrated mixture (Containing 65% barley, 33% bran, and 2% minerals and salt mixed in the farm) of 1 kg per ram per day, and water was given ad libitum. A total number of 60 ejaculates were collected from the rams using an artificial vagina once a week. For collecting ejaculates, rams were penned with ewes in estrus, in the presence of a handler with an artificial vagina. Ejaculates were evaluated and included in this study if the following criteria were met: volume of 0.5-2 mL; sperm concentration of 1-2 × 109 sperm per mL; the progressive motile sperms percentage higher than 70% and abnormal sperm of less than 10%.
Semen analysis. The volume of each ejaculate was recorded and sperm concentration was determined using semen diluted with 3% NaCl, the diluted semen was placed on a hemocytometer with the sperm counted in five squares of one chamber. Sperm motility was identified as those sperm cells that demonstrated progressive motility. Sperm motility was scored from zero to 100% by a qualified and experienced investigator. Semen was placed on a heated glass slide, and scoring was performed at microscopic magnification of 200×. Each sample was evaluated twice. The mean value was used for data analysis. Assessment of abnormal and normal spermatozoa was performed using an eosin–nigrosin staining method15. A fast green stain was used for the estimation of spermatozoa with abnormal acrosomes.16
Dilution of semen and addition of vitamins C and E. Semen samples were diluted by Tris-fructose-egg yolk (Hydroxy methyl amino methane-Tris 3.6 g, glucose 0.5 g, citric acid 1.7 g, streptomycin sulphate 100 mg, crystalline penicillin 100000 IU, double distilled water 85 mL and egg yolk 15 mL). Semen quality was re-evaluated to ensure that the dilution had not affected the semen quality. Diluted semen sample was divided into three parts. The first part was added with 0.9 mg mL-1 vitamin C, the second part was added with 1 mg mL-1 vitamin E and the third part was considered as a control without any addition. The diluted semen samples were cooled gradually and preserved at 5 ˚C for five days. Cooled diluted semen samples were examined for individual sperm motility, vitality, abnormalities and acrosomal defects every 24 hr for five days.
Statistical analysis. Data for the percentages of motility, live sperm, abnormal sperm and acrosomal defects were arcsine transformed. Statistical analyses were performed with Sigma Stat (Version 2.0, Jandel scientific software, Richmond, CA, USA). Data were expressed as mean ± SEM. Variance of homogeneity of samples was examined by Levene's test. The differences between means of the same parameter were tested by the ANOVA and LSD tests.
Results
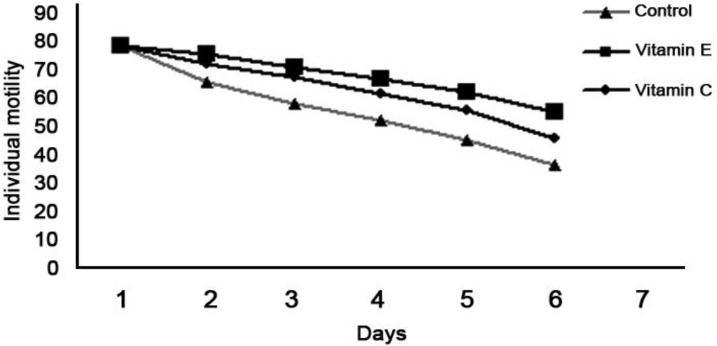
In this experiment no significant differences (p > 0.05) were found between individual rams in the evaluated parameters. Ram effect was eliminated from the model. Results of the present study showed an increased viability of spermatozoa diluted in Tris diluent containing vitamins C or E stored at 5 ˚C for 120 hr compared with the control group. The effect of adding vitamins C and E to Awassi ram semen diluted with Tris on sperm characteristics following the preservation at 5 ˚C for 120 hr are presented in Table 1. Spermatozoa motility declined gradually in vitamins C and E Tris containing diluents when preserved at 5 ˚C from 0 to 120 hr to 54.7 ± 1.0% and 45.5 ± 1.4%, respectively, compared to Tris diluent without vitamins which was declined markedly to 35.9 ± 1.9% (Fig. 1). There were significant major effects of vitamins C and E addition to semen diluents on sperm motility as well as sperm viability in different times of preservation at 5 ˚C (p < 0.05). A significant (p < 0.05) higher sperm abnormalities and acrosomal defects values (37.6 ± 1.3% and 71.5 ± 1.1%, respectively) were found after 120 hr incubation in vitamin C free Tris (Control) at 5 ˚C compared with those obtained in Tris diluent containing vitamin C (18.8 ± 1.8% and 52.8 ± 4.3%, respectively). Adding vitamin C to Tris diluent showed more protective effect than vitamin E. Moreover, addition of vitamin C resulted in a statistically significant decrease (p < 0.05) in sperm abnormalities and acrosomal defects of Awassi ram semen after 120 hr of incubation at 5 ˚C.
Table 1.
Values of individual motility, live sperms, abnormal sperm and abnormal acrosomes of Awassi ram semen diluted and stored at 5 ˚C for 120 hr with Tris diluent containing vitamins C and E in contrast to the control group. Data are presented as mean ± SEM.
| Groups | Individual motility (%) | Live sperms (%) | Abnormal sperms (%) | Abnormal acrosomes (%) |
|---|---|---|---|---|
| Tris with vitamin C | 54.7 ± 1.1a | 59.7 ± 1.3a | 18.8 ± 1.8a | 52.8 ± 4.3a |
| Tris with vitamin E | 45.5 ± 1.4a | 50.5 ± 1.5a | 35.3 ± 1.9b | 62.7 ± 2.9b |
| Control | 35.9 ± 1.9b | 40.9 ± 1.8b | 37.6 ± 1.3b | 71.5 ± 1.1b |
Mean values for each parameter in the same row, with different superscript (a, b) are significantly different (p < 0.05).
Fig. 1.
Percent of progressive individual motility of Awassi ram semen diluted with Tris diluent containing vitamin C, E and control groups stored at 5 ˚C for 120 hr.
Discussion
This study is the first report on the effects of adding vitamins C or E to Tris-fructose-egg yolk diluent for sperm storage at 5 ˚C of Iraqi Awassi rams. Free radicals are mostly eliminated by antioxidant systems. Thuwanuta et al. found an improvement of ram sperm after the addition of antioxidants (vitamins C and E) to semen diluents. The results of the present study showed that Awassi ram semen diluted with Tris diluent containing vitamin C or E could improve sperm motility and viability. The addition of vitamin C not only improved viability but it also protected acrosome and membrane integrity. Our results showed that sperm motility and viability of Awassi ram semen diluted with Tris containing vitamin C could be maintained and improved for 120 hr of preservation at 5 ˚C. These results were in agreement with Hughes11 of human semen; Maia13, 14 of ram semen and Singh17 in buffalo semen. The beneficial effect of adding vitamin C to diluted ram semen was in accordance with the results obtained by Asghari.18 Previous studies in humans showed that ascorbate in the range of 0.02-0.6 mM adversely affected sperm motility.19 Higher concentrations of vitamin C (2.5 mM) proved harmful to sperm motility in frozen-thawed bull semen.20 Vitamin C represents the major water-soluble antioxidant in blood plasma and seminal plasma.10 Most animals can synthesize ascorbic acid from glucose via the glucuronic acid pathway. Ascorbic acid is required as a cofactor for at least eight enzymes21 and can also act as an antioxidant by reacting with free radicals (e.g. O2-, OH-). However, in the presence of transition metal ions (e.g. Fe3+, Cu2+) high concentrations of ascorbic acid can act as a pro-oxidant by donating an electron that reduces such ions to forms that, in turn, can react with oxygen molecules to form oxygen radicals. Beneficial effect of supplementation sturgeon sperm with ascorbic acid was demonstrated by Mirzoyan22. These authors detected an increase in the motility rate of Russian sturgeon sperm added with 10 mM ascorbic acid. This favorable effect of adding vitamin C to diluted semen seems to relate to a reduction in DNA damage of spermatozoa.23 Vitamin C can protect membrane integrity of sperm cells from heat shock during dilution-cooling-storage process of spermatozoa.10 A probable improvement in semen quality by addition of vitamin C in ram semen diluent is more likely related to an inhibition of lipid peroxidation of the sperm plasma membrane as was revealed by Barati.24 The beneficial effect of vitamin C or vitamin E in improving fertilization rate as found by Hsu,25 was possibly due to a reduction in lipid peroxidation of ram sperm.14 Vitamin E can inhibit lipid peroxidation reaction in the membrane by eliminating peroxyl (ROO-), alkoxyl (RO-), and other lipid-derived radicals.26 Askasi reported vitamin E was more efficacious than vitamin C in improving post-thaw motility of human spermatozoa.27
However, the present study showed vitamin C was more efficient in protecting ram spermatozoa viability and acro-somal integrity. This could be due to the vitamin C capability of neutralizing H2O2 produced in a hydrophilic environment. It could be concluded that the addition of antioxidants such as vitamins C and E to the preservation media could improve longevity and quality of cooled sperm in Awassi ram semen.
Acknowledgements
Authors wish to thank College of Veterinary Medicine, University of Mosul for their support and providing facilities.
References
- 1.Sinha MP, Sinha AK, Singh BK, et al. The effect of glutathione on the motility, enzyme leakage and fertility of frozen goat semen. Theriogenology. 1996;41:237–243. [Google Scholar]
- 2.Sanocka DM, Kurpisz M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol. 2004;2:12–18. doi: 10.1186/1477-7827-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thuwanuta P, Chatdarongb K, Bergqvista AS, et al. The effects of antioxidants on semen traits and in vitro fertil-izing ability of sperm from flat-headed cat (Prionailurus planiceps) Theriogenology. 2011;76:115–125. doi: 10.1016/j.theriogenology.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Yue D, Yan L, Luo H, et al. Effect of vitamin E supplementation on semen quality and testicular cell membranal and mitochondrial antioxidant abilities in Aohan fine-wool sheep. Anim Reprod Sci. 2010;118:217–222. doi: 10.1016/j.anireprosci.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Baumber J, Ball BA, Gravance CG, et al. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential and membrane lipid peroxidation. J Androl. 2000;21:895–902. [PubMed] [Google Scholar]
- 6.Al-Guborya KH, Fowlerb PA, Garrelc C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol . 2010;42:1634–1650. doi: 10.1016/j.biocel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Maxwell WMC, Stojanov T. Liquid storage of ram semen in the absence or presence of some antioxidants. Reprod Fert Develop. 1996;32:353–360. doi: 10.1071/rd9961013. [DOI] [PubMed] [Google Scholar]
- 8.Chow CK. Vitamin E and oxidative stress. Free Radic Biol Med. 1991;11:215–232. doi: 10.1016/0891-5849(91)90174-2. [DOI] [PubMed] [Google Scholar]
- 9.Niki E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am J Clin Nutr. 1991;54 (suppl):1119S–1124S. doi: 10.1093/ajcn/54.6.1119s. [DOI] [PubMed] [Google Scholar]
- 10.Lewis S EM, Sterling ESL, Young IS, et al. Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. Fertility and Sterility. 1997;67:142–147. doi: 10.1016/s0015-0282(97)81871-7. [DOI] [PubMed] [Google Scholar]
- 11.Hughes CM, Lewis SE, McKelvey-Martin VJ, et al. The effects of antioxidant supplementation during Percoll preparation on human sperm DNA integrity. Hum Reprod. 1998;13:1240–1247. doi: 10.1093/humrep/13.5.1240. [DOI] [PubMed] [Google Scholar]
- 12.Almeida J, Ball BA. Effect of (alpha)-tocopherol and tocopherol succinate on lipid peroxidation in equine spermatozoa. Anim Reprod Sci. 2005;87:321–337. doi: 10.1016/j.anireprosci.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Maia MS, Bicudo SD, Azevedo HC, e al. Motility and viability of ram sperm cryopreserved in a Tris-egg yolk extender supplemented with anti-oxidants. Small Ruminant Res. 2009;85:85–90. [Google Scholar]
- 14.Maia MS, Bicudo SD, Sicherle CC, et al. Lipid peroxidation and generation of hydrogen peroxide in frozen-thawed ram semen cryopreserved in extenders with anti-oxidants. Small Ruminant Res. 2010;122:118–123. doi: 10.1016/j.anireprosci.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Salisbury GM, Van Denmark NL, Lodge JR. Semen evaluation. Physiology of reproduction and artificial insemination of cattle. 2nd ed. WH Freeman & Co., San Francisco, USA: 1978:326–353. [Google Scholar]
- 16.Wells ME, Awa OA. New technique for assessing acrosomal characteristics of spermatozoa. J Dairy Sci. 1970;53:227–232. doi: 10.3168/jds.S0022-0302(70)86184-7. [DOI] [PubMed] [Google Scholar]
- 17.Singh B, Chand D, Singh P, et al. Effect of vitamin C addition in the diluent on the quality of deep frozen Murrah buffalo bull (Bubalus bubalis) semen. Int J Anim Sci. 1996;11:131–132. [Google Scholar]
- 18.Asghari SR. Reproductive characteristics of Ghezel and Mehraban rams and effect of vitamin C on semen parameters and fertility [MSc. Thesis] Iran: Shiraz University; 1999. p. 155. [Google Scholar]
- 19.Donnelly ET, Mc Clure N, Lewis SE. The effect of ascorbate and alpha-tocopherol supplementation in vitro on DNA integrity and hydrogen peroxide induced DNA damage in human spermatozoa. Mutagenesis. 1999;14:505–512. doi: 10.1093/mutage/14.5.505. [DOI] [PubMed] [Google Scholar]
- 20.Beconi MT, Francia CR, Mora NG, et al. Effect of natural antioxidants in frozen bovine semen preservation. Theriogenology. 1993;40:841–851. doi: 10.1016/0093-691x(93)90219-u. [DOI] [PubMed] [Google Scholar]
- 21.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3rd ed. . New York, USA: Oxford University Press. pp. 122–167. [Google Scholar]
- 22.Mirzoyan AV, Nebesikhina NA, Voynova NV, et al. Preli-minary results on ascorbic acid and lysine suppression of clastogenic effect of deep-frozen sperm of the Russian sturgeon. Int J Refrigerat. 2006;29:374–378. [Google Scholar]
- 23.Cabritaa E, Mab S, Diogob P, et al. The influence of certain amino acids and vitamins on post-thaw fish sperm motility, viability and DNA fragmentation. Anim Reprod Sci. 2011;125:189–195. doi: 10.1016/j.anireprosci.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Barati F, Papahn AA, Afrough M, et al. Effects of Tyrode's solution osmolarities and milk on bull sperm storage above zero temperatures. Iran J Reprod Med. 2011;9:25–30. [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu PC, Liu MY, Hsu CC, et al. Effects of vitamin E and/ or C on reactive oxygen species-related lead toxicity in the rat sperm. Toxicology. 1998;28:169–179. doi: 10.1016/s0300-483x(98)00068-7. [DOI] [PubMed] [Google Scholar]
- 26.Silva PFN. Physiology of peroxidation process in mammalian sperm [PhD Thesis] Ridderkerk: Utrecht University Ridderprint ; 2006. pp. 35–36. [Google Scholar]
- 27.Askasi HA, Check JH, Peymer N, et al. Effect of natural anti-oxidants tocopherol and ascorbic acids in maintenance of sperm activity during freeze-thaw process. Arch Androl. 1994;33:11–15. doi: 10.3109/01485019408987797. [DOI] [PubMed] [Google Scholar]