Abstract
Viroids are uncoated infectious RNA molecules pathogenic to certain higher plants. Four different highly purified viroids were studied. By ultracentrifugation, thermal denaturation, electron microscopy, and end group analysis the following features were established: (i) the molecular weight of cucumber pale fruit viroid from tomato is 110,000, of citrus exocortis viroid from Gynura 119,000, of citrus exocortis viroid from tomato 119,000 and of potato spindle tuber viroid from tomato 127,000. (ii) Viroids are single-stranded molecules. (iii) Virods exhibit high thermal stability, cooperativity, and self-complementarity resulting in a rod-like native structure. (iv) Viroids are covalently closed circular RNA molecules.
Full text
PDF

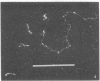
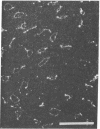
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agol V. I., Romanova L. I., Cumakov I. M., Dunaevskaya L. D., Bogdanov A. A. Circularity and cross-linking in preparations of replicative form of encephalomyocarditis virus RNA. J Mol Biol. 1972 Dec 14;72(1):77–89. doi: 10.1016/0022-2836(72)90069-1. [DOI] [PubMed] [Google Scholar]
- Bockstahler L. E., Kaesberg P. Isolation and properties of RNA from bromegrass mosaic virus. J Mol Biol. 1965 Aug;13(1):127–137. doi: 10.1016/s0022-2836(65)80084-5. [DOI] [PubMed] [Google Scholar]
- Chaconas G., Van de Sande J. H., Church R. B. End group labelling of RNA and double stranded DNA by phosphate exchange catalyzed by bacteriophage T4 induced polynucleotide kinase. Biochem Biophys Res Commun. 1975 Oct 6;66(3):962–969. doi: 10.1016/0006-291x(75)90734-2. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Kaesberg P., Diener T. O. Potato spindle tuber viroid. XII. An investigation of viroid RNA as a messenger for protein synthesis. Virology. 1974 Sep;61(1):281–286. doi: 10.1016/0042-6822(74)90262-1. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Potato spindle tuber "virus". IV. A replicating, low molecular weight RNA. Virology. 1971 Aug;45(2):411–428. doi: 10.1016/0042-6822(71)90342-4. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Viroids: the smallest known agents of infectious disease. Annu Rev Microbiol. 1974;28(0):23–39. doi: 10.1146/annurev.mi.28.100174.000323. [DOI] [PubMed] [Google Scholar]
- Dingman C. W., Peacock A. C. Analytical studies on nuclear ribonucleic acid using polyacrylamide gel electrophoresis. Biochemistry. 1968 Feb;7(2):659–668. doi: 10.1021/bi00842a022. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C., Wepprich R. K., Davies J. W., Weathers L. G., Semancik J. S. Functional distinctions between the ribonucleic acids from citrus exocortis viroid and plant viruses: cell-free translation and aminoacylation reactions. Virology. 1974 Oct;61(2):486–492. doi: 10.1016/0042-6822(74)90284-0. [DOI] [PubMed] [Google Scholar]
- Helinski D. R., Clewell D. B. Circular DNA. Annu Rev Biochem. 1971;40:899–942. doi: 10.1146/annurev.bi.40.070171.004343. [DOI] [PubMed] [Google Scholar]
- Henley D. D., Lindahl T., Fresco J. R. Hydrodynamic changes accompanying the thermal denaturation of transfer ribonucleic acid. Proc Natl Acad Sci U S A. 1966 Jan;55(1):191–198. doi: 10.1073/pnas.55.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Vinograd J. Replication of circular DNA in eukaryotic cells. Annu Rev Biochem. 1974;43(0):695–719. doi: 10.1146/annurev.bi.43.070174.003403. [DOI] [PubMed] [Google Scholar]
- Krakauer H. A calorimetric investigation of the heats of binding of Mg ++ to poly A, to poly U, and to their complexes. Biopolymers. 1972;11(4):811–828. doi: 10.1002/bip.1972.360110406. [DOI] [PubMed] [Google Scholar]
- Krauss G., Pingoud A., Boehme D., Riesner D., Peters F., Maas G. Equivalent and non-equivalent binding sites for tRNA on aminoacyl-tRNA synthetases. Eur J Biochem. 1975 Jul 15;55(3):517–529. doi: 10.1111/j.1432-1033.1975.tb02189.x. [DOI] [PubMed] [Google Scholar]
- Pearce T. C., Rowe A. J., Turnock G. Determination of the molecular weights of RNAs by low-speed sedimentation equilibrium: 16 S ribosomal RNA as a model compound. J Mol Biol. 1975 Sep 15;97(2):193–201. doi: 10.1016/s0022-2836(75)80034-9. [DOI] [PubMed] [Google Scholar]
- Perrault J. Cross-linked double-stranded RNA from a defective vesicular stomatitis virus particle. Virology. 1976 Apr;70(2):360–371. doi: 10.1016/0042-6822(76)90278-6. [DOI] [PubMed] [Google Scholar]
- Prunell A., Bernardi G. Fractionation of native and denatured deoxyribonucleic acid on agarose columns. J Biol Chem. 1973 May 25;248(10):3433–3440. [PubMed] [Google Scholar]
- RajBhandary U. L. Studies on polynucleotides. LXXVII. The labeling of end groups in polynucleotide chains: the selective modification of diol end groups in ribonucleic acids. J Biol Chem. 1968 Feb 10;243(3):556–564. [PubMed] [Google Scholar]
- Römer R., Riesner D., Coutts S. M., Maass G. The coupling of conformational transitions in alanine specific transfer ribonucleic acid from yeast studied by a modified differential absorption technique. Eur J Biochem. 1970 Jul;15(1):77–84. doi: 10.1111/j.1432-1033.1970.tb00978.x. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Morris T. J., Weathers L. G., Rodorf B. F., Kearns D. R. Physical properties of a minimal infectious RNA(viroik) associated with the exocortis disease. Virology. 1975 Jan;63(1):160–167. doi: 10.1016/0042-6822(75)90381-5. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Weathers L. G. Exocortis disease: evidence for a new species of "infectious" low molecular weight RNA in plants. Nat New Biol. 1972 Jun 21;237(77):242–244. doi: 10.1038/newbio237242a0. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Weathers L. G. Exocortis virus: an infectious free-nucleic acid plant virus with unusual properties. Virology. 1972 Feb;47(2):456–466. doi: 10.1016/0042-6822(72)90281-4. [DOI] [PubMed] [Google Scholar]
- Singh R. P., Clark M. C. Infectious low-molecular weight ribonucleic acid from tomato. Biochem Biophys Res Commun. 1971 Sep;44(5):1077–1083. doi: 10.1016/s0006-291x(71)80195-x. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Koller T., Diener T. O. Potato spindle tuber viroid. X. Visualization and size determination by electron microscopy. Virology. 1973 Sep;55(1):70–80. doi: 10.1016/s0042-6822(73)81009-8. [DOI] [PubMed] [Google Scholar]
- Vollenweider H. J., Sogo J. M., Koller T. A routine method for protein-free spreading of double- and single-stranded nucleic acid molecules. Proc Natl Acad Sci U S A. 1975 Jan;72(1):83–87. doi: 10.1073/pnas.72.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Poly (A) and poly (U) in poliovirus double stranded RNA. Nat New Biol. 1973 Apr 11;242(119):171–174. doi: 10.1038/newbio242171a0. [DOI] [PubMed] [Google Scholar]