Abstract
The field of drug discovery is ever growing and excipients play a major role in it. A novel class of amphiphiles has been discussed in the review. The review focuses on natural as well as synthetic bolaamphiphiles, their chemical structures and importantly, their ability to self assemble rendering them of great use to pharmaceutical industry. Recent reports on their ability to be used in fabrication of suitable nanosized carriers for drug as well as genes to target site, has been discussed substantially to understand the potential of bolaamphiphiles in field of drug delivery.
Keywords: Bolaamphiphiles, Nanocarriers, Archaesomes, Self Assembly
Introduction
Formulation development has undergone stupendous improvement with respect to effective drug delivery as a result of ever increasing efforts of formulation scientists across the globe, either to improve permeation for enhancing oral/ topical/ rectal/ nasal bioavailability or to render system more target specific. The improvement in formulation development has been witnessed over the past few years due to development of various novel formulation techniques in conjunction with availability of versatile and smart excipients. Many novel drug delivery systems available commercially today, viz. Liposomes – Doxil®, Myocet®; Nanosuspension – Emmend®, Rappamune®; Microemulsion – NeOral®, is by virtue of stabilization provided by an important class of excipients, namely, surfactants or colloidal stabilizers.
Amphiphilic surfactants constitute an important class of different categories of surfactants available for pharmaceutical research. Amphiphiles, a term coined by Paul Winsor more than 50 years ago, consist of water loving (hydrophile, polar) and lipid loving (lipophile, apolar) fractions in one molecular structure. Both, low molecular weight conventional amphiphilic surfactants and high molecular weight amphiphilic polymers, display characteristic molecular self-assembly behavior in solutions, at interfaces and in bulk, generating nanoscale structures that hold promise for use in pharmaceuticals and biomedical sciences.1,2 Of the amphiphiles studied and evaluated till date, bolaamphiphiles (BA) are a novel class, being less investigated so far. However, many research papers and few reviews explaining success of bolaamphiphiles in delivery of bioactives have already surfaced up, in the past few years making it an interesting research area for formulators worldwide (Table 1).
Table 1. List of studies exploring bolaamphiphiles and its uses worldwide.
| Authors/ Year | Applications | Type of Bolaamphiphile | Reference |
| Marc Hebrant et al. | Micellar extraction of europium (III) | As bolaform extractant: HP-10-PH | 56 |
| Stéphanie Sistach et al. | As gold Nanoparticle stabilizers | L-Alanine terminated bolaamphiphile | 57 |
| Jain, N., et al., | As nonviral vectors for efficient gene delivery | Lactose ornithine BA, gluconic acid ornithine BA | 53,54 |
| Volkmar Weissig et al. | As mitochondria-specific DNA delivery systems | Dequalinium - cationic BA | 58 |
| Sudipta Ray et al. | As Dye-Adsorbing Agent, Water Purifier, and Vit. B12 carrier | Phenylalanine-based BA | 38 |
| Moira Ambrosi et al. | For the production of redox active nanostructures | Vit.C based BA | 37 |
| Yiguang Jin et al. | As nanomedicine : for Combination Anti-HIV Therapy | Asymmetric BA : Zidovudine/Didanosine Prodrug; Dual zidovudine | 35,36 |
| M. Popov et al. ; George R. Dakwar et al. ; S. Grinberg et al. | As cationic vesicles for targetted brain delivery | Vernonia oil based BA | 34,39,41 |
Bolaamphiphiles, also referred to as bolaform detergents, two-headed amphiphiles and bipolar amphiphiles, consist of two hydrophilic headgroups connected to the ends of a hydrophobic skeleton3,4 (Figure 1). The name has its origin in the word ‘bola’ which stands for a weapon made up of a long cord or thong with heavy metal balls at the end used for throwing at and entangling cattle. Fuoss and Edelson coined the term ‘bolaform electrolyte’ for a hydrophobic chain connecting two ionic head groups.5,6 Bolaamphiphiles are composed of two polar head groups separated by one, two or three long hydrophobic spacers that are mostly alkyl chains, and can even be steroids or porphyrins.7-10 The two hydrophilic groups on a bolaamphiphile can be ionic or non-ionic and they can be identical to or different from one another. The most commonly known natural bolaamphiphiles are tetraether lipids present in archaebacterial membranes4,5,11-14 conferring them ability to survive under unfriendly conditions viz. high salt concentrations, extreme temperatures or anaerobic conditions. Their lipids are composed of two biphytanyl chains, which are attached to two glycerol moieties by ether linkages, with additional cyclopentane rings in the chains in some cases, responsible for the adaptation of the organism to higher growth temperature providing the necessary rigidity and stability and maintaining membrane integrity.4,5,15 In general, membranes composed of bolaamphiphiles are known to possess less permeability and better durability as against mono – polar lipids.11,16 By themselves, bolaamphiphiles form monolayer membranes in contrast to phospholipids known to form bilayer vesicles.5 Bolaamphiphiles possess increased aqueous solubility and therefore increased Critical Aggregation Concentration (CAC) in range of 10-4 to 10-6 M much higher in comparison to phospholipids (10-8 M).16
Figure 1.
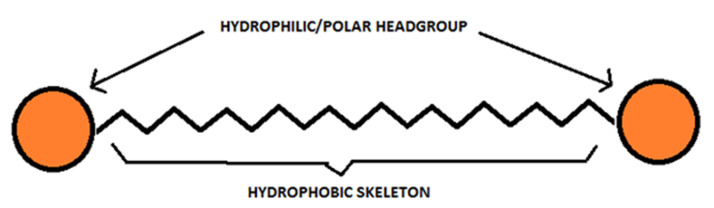
Schematic representation of Bolaamphiphile
Various advantages offered by bolaamphiphiles are related to its chemical structure. Chemical structure of bolaamphiphiles can dictate their arrangement into parallel or antiparallel sheets resulting in formation of either unsymmetrical or symmetrical monolayer membranes (MLMs).3,4,17 Bolaamphiphiles have been used in formulating stable nanocarriers systems and have already demonstrated enough potential in effective drug as well as gene delivery. In the current review, an attempt has been made to describe chemical properties of bolaamphiphiles, their effect on self assembly pattern and applications of bolaamphiphiles in drug and gene delivery.
Chemical structure and Self assembly pattern
Bolaamphiphiles present in MLMs of archaebacteria consist of ether bonds, instead of ester bonds commonly present in synthesized bolaamphiphiles, which are comparatively less susceptible to breakdown under high acidic degradation, conferring archaebacteria ability to survive in extreme conditions such as hostile sulfuric acid environment. Their bolaamphiphiles possess greater stiffness due to helix formation within macrocycles due to presence of several chiral methine groups with methyl substituents in its hydrophobic core. Introduction of cis configured -C=C- bond in hydrophobic region can make bolaamphiphile based membranes more fluidic. Synthesis of macrocyclic tetraethers as present in archaebacteria has been attempted but without any success so far. Combination of two bolas with two cationic/ two anionic headgroups or by combination of a dianionic bola and a cationic polymer or vice versa can be employed to form multilayered membranes. Bolaamphiphiles aqueous dispersion when subjected to high energy in form of sonic waves yields spherical lipid particles made of MLMs. Long-chain and short-chain bolaamphiphiles produce vesicles and micelles respectively.5
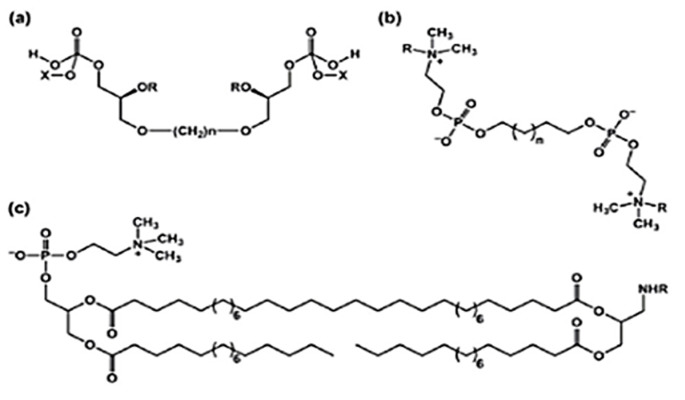
Bolaamphiphiles can be divided in two main categories (Figure 2): symmetric (with same polar headgroups at both ends) and asymmetric ones (possessing different polar headgroups at both ends), however both capable of self assembling to yield interesting nano-assemblies. In 1980s, the work on synthesis of bolaamphiphiles was initiated and aimed at development of asymmetric ones, that are of special interest to formulators and biotechnologists to design several nano-architectures with unsymmetrical membranes.5
Figure 2.
Symmetrical (a), (b) and Assymetrical Bolaamphiphiles (c)
Bolaamphiphile family consist of bolalipids, bolapeptides, carbohydrate based bolaamphiphiles and aminoacid bolaamphiphiles. Bolalipids can further be classified as bipolar phospholipids or bipolar glycolipids. The headgroup in former would generally consist of phosphocholine or phosphoethanolamine, whereas bipolar glycolipids possess a large variety of carbohydrate moieties with six or five-membered cyclic or acyclic forms as headgroups.4 The hydrophobic chain length may contain 22 – 32 carbon atoms in case of bipolar phospholipids or 6 – 32 carbon atoms in case of bipolar glycolipids.4 Branched isoprenyl units, cyclopentane rings, fluorinated alkyl chain, bicycloheptene rings and sulphur bridges may also be a part of hydrophobic region of bolalipids.4,18,19 Glycerol is generally present as linker, however, sometimes, the headgroup and hydrophobic chain is directly attached via oxygen, nitrogen, sulfur or amide bridges.4,18,20-23
Condensation and substitution reactions are employed mainly for synthesis of most bolaamphiphiles made from commercial α,ω-diols, -dihalides, -diamines, and dicarboxylates. For e.g. α,ω-diiodides have been useful for synthesizing single chain bolaamphiphiles; Stille reaction was employed between two polyene iodide molecules and a bis(tributylstannyl)- ethane and is reasonably successful in synthesis of long chain (C-28) bolaamphiphiles.5,24,25 Various researchers have put in efforts to synthesize different type of bolaamphiphiles such as α,ω – phosphate bolas, ones possessing tetraester cyclophane groups, or asymmetric bolas, to name a few.5 An interesting example is synthesis of Vitamin C based bolas in the search for the production of redox active nanostructures. Amphiphilic ascorbyl derivatives were synthesized to extend their reducing properties to encompass hydrophobic environments, since Vitamin C possesses poor lipid solubility. Also, considerable effort has been dedicated to the synthesis of bolaamphiphiles containing aromatic rings viz. phenyl, biphenyl, azobenzene, pyridinium and aromatic dye groups that can be either a part of hydrophilic headgroup or hydrophobic chain.4,26-31
Dhasaiyan et al.32 have recently synthesized various sophorolipid derivatives and studied the effect of unsaturation of the fatty acid chain on their self assembly properties using in vitro and in silico techniques. Sophorolipids are glycolipids belonging to the class of asymmetric bolaamphiphiles, wherein a glucose-sophore unit is attached to one end of the fatty acid tail while the other end bears a carboxylic group. Oleic acid bolaamphiphiles were observed to form ribbon like structures, whereas linoleic acid derivatives failed to form any self-assembled structures. Linolenic acid bolaamphiphiles, in contrast formed vesicle like structures of size 3 – 15 µm on mixing with water (pH around 3), confirmed by imaging techniques like TEM, SEM and AFM and Confocal Laser Scanning Microscopy (CLSM). In order to understand the mechanism that gives rise to self assemblage, authors performed self assemble molecular dynamic simulations. Solvated bolaamphiphiles were randomly placed in water in simulation box and allowed to self assemble for 700 ns. Spherical aggregates were observed to be formed after 300 ns run time. MD studies also revealed greater disruption in the head-tail-tail-head bilayer with more penetration of water as compared to tail-head-head-tail bilayer displaying greater stability and therefore assuming preferred conformation of molecular arrangement within the bilayer.
Thus, a large variety of bolaamphiphiles with diverse structures have been synthesized and reported in literature. A substantial broadening of the range of experimental techniques applied for the characterization of the lyotropic and interfacial properties of bolaamphiphiles (Table 2) has lead to a better understanding of the structure-property relationship. Efforts are now being directed to study the self assembly pattern and the molecular arrangement in order to design feasible drug delivery systems.
Table 2. Methods to determine lyotropic properties of bolaamphiphiles.
| Physico-chemical methods | Interfacial behavior |
|
|
Applications
In drug delivery
Bolaamphiphiles have been employed in drug delivery to design vesicular systems, hydrogels, and micellar systems to name a few. Molecular parameters are the dictating factors determining the geometrical shape assumed by bolaamphiphiles like micelles, rods, tubes, fibers and so on. With double strands bolaform surface active agents forming spherical vesicles and symmetrical ones with minimum chain length yielding spherical micelles as depicted by Polidori et al,33,34 they have demonstrated promising potential in targeting brain on suitable chemical modifications.
Zidovudine is a polar molecule with water solubility of ~ 20 mg/mL and is used in treatment of HIV AIDS. Jin Y. et al synthesized novel symmetric bolaamphiphilic prodrug named PDDZ composed of long alkyl chain as hydrophobic part with polar zidovudine molecules as head groups at both its ends. PDDZ exhibited capability of forming self assemblies with size restricted to 156 nm and improved stability with aid of Polysorbate 20 and HPMC. It was observed that PDDZ was converted to active moiety (Zidovudine) in plasma. Most of the zidovudine was taken up by MPS (mononuclear phagocyte system) rich target organs viz. liver, spleen and testis known to be HIV reservoirs, with no free zidovudine present in plasma after 1 hr.35 Another bolaamphiphilic prodrug ZPDD (zidovudine phosphoryl-deoxycholyl didanosine) composed of zidovudine and didanosine as two polar head groups on either side of hydrophobic chain has been reported to improve efficacy of both anti-HIV drugs. The bioavailability of ZPDD was 90.5% and 30.8% for i.p. and oral routes in comparison to i.v. route. Didanosine was released slowly as compared to zidovudine during breakdown of ester bonds in ZPDD, which was available immediately to inhibit virus. As PDDZ, ZPDD also concentrated in macrophage rich organs serving as HIV reservoirs. The remarkable advantage of ZPDD was its capability to form nanosized self assemblies and combining two different types of antivirals so that they could simultaneously be delivered to the targeted cells.36
Few researchers have reported ability of bolaamphiphiles to form hydrogels that can serve as carriers for vitamin C and Vit B12. Ambrosi M. et al synthesized 1,12-diascorbyl dodecanedioate bolaamphiphiles with ascorbic acid as its two polar head groups and characterized it to demonstrate its potential to form nanotubes at concentration above 0.5% w/w.37 Synthesis of pH responsive hydrogels (phenylalanine based bolaamphiphile) loaded with vitamin B12 that could modulate its release profile with respect to pH of medium has been described.38 These results suggest bolaamphiphiles’ promising ability to yield novel hydrogel based carriers for bioactives.
Bolaamphiphile based monolayered vesicles are thought to possess several advantages over phospholipid based vesicles. Grinsberg S. et al has reported synthesis of novel bolaamphiphiles derived from vernonia oil with choline ester head groups capable of forming vesicles that can be enzymatically disrupted by acetylcholine esterases.34,39 These bola-based vesicles were reported to possess potential of site specific delivery with decapsulation taking place at enzymatically active anatomical region. Few reports claim superiority of bolaamphiphilic vesicles over liposomes with respect to their stability and cell membrane permeability due to lesser fusion of lipid exchange observed with former system.16,40 In agreement to notion of their ability to cross cell membrane intact unlike liposomes, and better stability, Popov M. designed cationic nanovesicles fabricated using bolas synthesized with ACh (Acetycholine) as their headgroups.41 Dakwar et al39 have further evaluated them for their potential to cross the Blood Brain Barrier (BBB) to enable brain targeted drug delivery of proteins. Vesicles comprising of cholesterol, cholesterol hemisuccinate (CHEMS) and bolaamphiphiles were prepared by lipid film hydration method which possessed greater serum stability than cationic liposomes. Also, greater cell uptake of encapsulated Bovine Serum Albumin (BSA) was observed as against BSA solution in HeLa (tumor cells) and b.End3 cells (cells constituting blood brain barrier), thus establishing greater efficiency of bolaamphiphiles to cross the BBB. Once internalized, bolaamphiphiles are postulated to be decapuslated by the cholinesterease present in the brain thereby triggering drug release. In vivo study in mice demonstrated intensely florescent cells in the brain when administered with BSA encapsulated in vesicles as opposed to solution, thereby firmly establishing their potential for brain delivery.
Another study evaluates the ability of bolaamphiphiles to deliver anti-HIV drugs to brain for treatment of neuro-HIV. Heldman et al42 have developed novel V-Smart vesiclesTM for delivery of tenofovir to brain. V-Smart is a platform technology and is claimed to have greater advantages over liposomes in terms of stability, encapsulation efficiency and targeting potential. Vesicles encapsulating 20 – 40% tinofovir were prepared using bolaamphiphiles containing chitosan head groups to increase brain penetration. A single iv. injection of tinofovir in patients resulted in concentration of tinofovir in brain higher than required therapeutic concentration, thus establishing the efficiency of system for anti-retroviral treatment of neural HIV.
In yet another recently reported interesting study, Popov et al43 evaluated bolavesicles comprising of bolalipids (single or combining two different bolalipids), CHEMS and cholesterol; bolavesicles containing chitosan-vernolic acid conjugate and liposomes containing either DSPC or DOTAP combined with cholesterol. The detailed study demonstrated that the bolavesicles were superior to DSPC as well as DOTAP liposomes in delivering analgesic peptides, enkephalin and kyotorphin across the BBB and releasing them sufficiently to elicit efficient and prolonged analgesic activity. The analgesic effect was enhanced by using bolavesicles made from a mixture of the bolas GLH-19 (that possessed non-hydrolyzable acetylcholine head group) and GLH-20 (that possessed hydrolysable acetylcholine head group) and by incorporating chitosan pendants into the formulation. The release of the encapsulated analgesic peptides also appeared to be dependent on the choline esterase (ChE) activity in the brain vs. other organs and tissues. It was observed that vesicles of GLH 20 or combination of GLH 19 and GLH 20 with chitosan pendants along with pretreatment with pyridostigmine (the BBB-impermeable ChE inhibitor) were most promising for enhanced brain delivery of encapsulated analgesics. Bolaamphiphiles are being extensively explored for their promising potential to cross the blood brain barrier and thereby enable brain targeted drug delivery.44
Stern et al45 investigated the effect of length of alkyl chain pendant near the polar head group of bolaamphiphile on the rate of hydrolysis of head group and consequently the release of active encapsulated in bolaamphiphilic vesicles. In an attempt to examine the same, they designed two bolaamphiphiles, with different alkyl chain lengths near the polar head groups comprising mainly of acetylcholine, capable of releasing encapsulated active only upon breakdown by enzyme acetylcholine esterase, thus making system site specific. Bolaamphiphilic vesicles fabricated using both short alkyl chain bearing and long alkyl chain bearing bolaamphiphiles possessed size of ~125 nm and ~140 nm, respectively. It was observed that the bolaamphiphile with 5 methylene groups in its alkyl chain pendant possessed higher zeta potential value (~55 mV ) as against one possessing 8 methylene groups (~42 mV) which was correlated to slightly larger outer surface area possessed by the later. The larger alkyl chain length bearing vesicles demonstrated lower rate of hydrolysis of acetylcholine headgroup when exposed to acetylcholinesterase as compared to vesicle comprising of short alkyl chain pendant bearing bolaamphiphile. This was possibly due to the steric hindrance offered by the larger alkyl chain in comparison to the shorter one. Consequently, the release of encapsulated active from vesicles comprised of larger alkyl chain length bearing bolas was slower than their counterparts possessing shorter alkyl chain length. The study clearly highlighted the potential of newly designed bolaamphiphiles in obtaining controlled and / or different release profiles for encapsulated actives. Philosof-Mazor et al,46 designed bolavesicles encapsulating iron oxide nanoparticles (IONP) for magnetically assisted targeted drug delivery. The findings were very promising indicating that IONPs contained in bolavesicles interacted better with membrane bilayers in model systems as compared to IONPs free bolavesicles. It was also demonstrated that bolavesicle formulation was endocytosed by b.End3 brain endothelial cells efficiently even in absence of magnetic field. Thus, bolavesicles encapsulating IONPs were considered effective enough in improving the accumulation/interaction of IONPs with the cells. Reports on use of bolaamphiphiles for effective drug delivery are continuously increasing, indicative of its potential as promising carrier for loaded actives.
In gene delivery
Of considerable interest are the efforts being made worldwide to rectify diseases/disorders at cell/molecular level by genetic manipulation in form of gene/DNA/siRNA/shRNA delivery.47-49 Both, viral and non viral vectors have been explored for ensuring effective transfection of gene. Viral vectors are known to offer better gene transfection than non-viral vectors generally composed of cationic lipids/surfactants such as DOPE, DODAB, DOTMA, DOTAP and their combinations such as Lipofectamine (1:3 DOPE:DOSPA) or cationic polymers. Non-viral vectors are yet preferred due to lack of pathogenic and immunogenic property, better scalability and large scale production unlike viral vectors. Non-viral vectors also possess their own limitations. Most of these cationic agents are known to exert their own genomic and biological effects at cellular level, and can hinder with the activity of siRNA/shRNA/gene and exert their own toxic effects. Complexes between cationic lipids used as non-viral vectors and DNA/siRNA is thought to be affected by plasma proteins. Besides no significant protection is offered to complexed genetic material against plasma enzyme mediated (DNAase, RNAase, etc.) degradation before the gene reaches target site.48,50-52
Bolaamphiphiles comprised of one head group being neutral and other being positively charged offer a suitable alternative to cationic lipids for effective gene delivery (Figure 3). Such bolas give rise to asymmetric membranes with outer neutral surface and inner positively charged surface complexed to negatively charged genetic material. The DNA/siRNA being encapsulated inside is offered significant protection against enzyme mediated degradation before it reaches target site. Few reports already suggest bolas to be useful candidates for effective gene delivery. Klymchenko A.S. and co-workers53,54 synthesized bolas with dicationic ornithine as one head group that would associate with DNA and the other head group being neutral, either gluconic acid or lactose was used. Both bolas, bearing either lactose or gluconic acid were extensively characterized with respect to changes in size based on N/P ratio upon DNA complexation, microscopic (AFM) evaluation, DNA condensation studies based on electrophoresis studies, transfection and cell toxicity studies. Lactose based bolas showed DNA binding and condensation at higher N/P ratios than ones with gluconic headgroups, reason being larger size of lactose as compared to gluconic acid. With their previous experience with gluconic acid based bolas, it was realized that DOPE and chloroquine facilitate better transfection indicating endosomal escape as key obstacle in cell internalization of bolaplexes (DNA-bola complex). As a result, DOPE was included in lactonic head group based bolas for transfection studies. Bolas without DOPE did not reveal appreciable transfection, though the ones with DOPE showed transfection comparable to PEI in HeLa, COS-7 and HepG2 cell lines. Bolaplexes with both head groups demonstrated low cytotoxicity.53,54 Fluorescence imaging suggested endocytosis as major mechanism of cell binding and internalization of bolaplexes. Kim et al55 demonstrated potential of bolaamphiphiles in delivery of siRNA after thorough evaluation performed during in silico , in vitro and importantly in vivo studies. Two cationic bolaamphiphiles (labeled as GLH-19 and GLH-20) with acetylcholine headgroups, attached to an alkyl chain in two distinct configurations were tested for their ability to complex with siRNA and deliver it to cells. The cationic micelles resulting from GLH-19 during MD (molecular dynamic) simulations predicted better protection offered to complexed siRNA against nucleases. Gel electrophoresis and computational studies demonstrated GLH-19 / siRNA complexes possess stronger binding and better interaction as compared to GLH-20/ siRNA complexes. In vitro studies were performed in MDA-MB 231 (breast cancer) cells capable of stably expressing enhanced green fluorescence protein (eGFP). Transfection was performed with siRNA against eGFP complexed with bolaamphiphiles. Again, fluorescence microscopy and FACS study after 3 days of exposure demonstrated better cell uptake of siRNA complexed with GLH-19 micelles resulting in increased silencing of eGFP. In vivo studies were performed in athymic nude mice which were injected fluorescently labeled siRNA complexed with GLH-19. Whole body fluorescence imaging demonstrated higher uptake of siRNA in tumors within 3 hour time course, as compared to major organs (heart, spleen, brain and lungs). The study can be considered a positive leap forward in field of gene delivery, demonstrating that bolaamphiphiles could offer chemical stability to complexed siRNA as well as better cellular uptake resulting in specific gene silencing. Favorable results from studies so far, present a new opportunity to formulation scientists and chemists to design such bolas that could render effective gene delivery in vivo possible.
Figure 3.
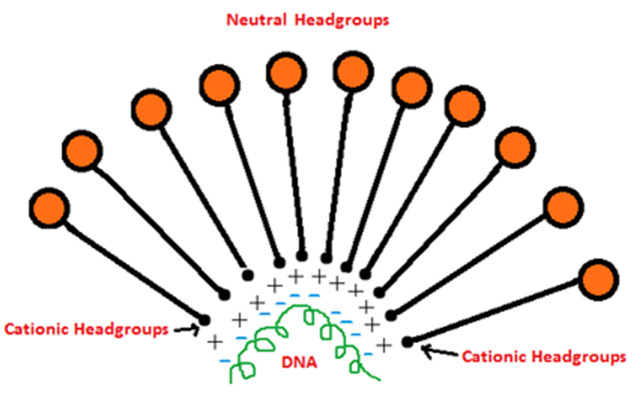
Hypothetical schematic of Bolaplex
Conclusion
After three decades of initial development, bolaamphiphiles have presented a new area of research to formulation scientists. Bolaamphiphiles have demonstrated their ability to serve as important structural blocks of vesicles and/or micelles for drug delivery as depicted by archaeosomes. They are potential candidates for the formation of nanosized systems for efficient drug and gene delivery to target sites. This is evident from the numerous reports that have been published so far on bolaamphiphiles and the numbers are escalating further.
However, their safety profile remains unexplored yet with few reports available in literature on toxicity evaluation. Scientists have studied their cytotoxicity profile in vitro on various cell lines and their efficiency in vivo has been evaluated. In contrast, acute, sub-chronic and chronic toxicity potential and bio-compatibility remains largely unknown hampering their use in drug delivery. Ample reports have sprung up on synthesis of bolaamphiphiles which have greatly reduced in vitro toxicity as compared to PEI, a cationic polymer widely employed in transfection experiments. However, with exception of few reports, not much data is available on the pharmacokinetics, biodistribution and in vivo fate of these self assembled structures. Extensive research should now be focused on assessing their safety profile to establish them as safe excipients for drug delivery applications.
Further, since bolaamphiphiles are synthetic in nature and are intended for intravenous use, there is need for establishment of guidelines suggesting the nature and acceptable levels of impurities and apyrogenicity. Another area of concern is sterilization of bola-complexes. While few reports employ filtration as a means of sterilization, it would be worthwhile to investigate terminal sterilization by autoclaving as a feasible alternative for ease of large scale manufacturing. Stability of bolaamphiphiles over long term periods also needs to be considered for successful commercialization.
Computational techniques such as molecular dynamics along with experimental techniques is proven powerful tool in undermining and understanding the mechanism of self assemblage and molecular orientation of self assembled bolaamphiphiles. Following better understanding, it should be possible to witness a few bolaamphiphile based products available commercially for effective drug and / or gene delivery.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Schramm LL, Stasiuk EN, Marangoni DG. 2 Surfactants and their applications. Annu Rep Prog Chem Sect C: Phys Chem. 2003;99:3–48. [Google Scholar]
- 2.Xu JP, Ji J, Chen WD, Shen JC. Novel biomimetic surfactant: synthesis and micellar characteristics. Macromol Biosci. 2005;5(2):164–71. doi: 10.1002/mabi.200400139. [DOI] [PubMed] [Google Scholar]
- 3.Mao G, Tsao YH, Tirrell M, Davis HT, Hessel V, Van Esch J. et al. Monolayers of Bolaform Amphiphiles: Influence of Alkyl Chain Length and Counterions. Langmuir. 1994;10(11):4174–84. [Google Scholar]
- 4.Meister A, Blume A. Self-assembly of bipolar amphiphiles. Curr Opin Colloid Interface Sci. 2007;12(3):138–47. [Google Scholar]
- 5.Fuhrhop JH, Wang T. Bolaamphiphiles. Chem Rev. 2004;104(6):2901–37. doi: 10.1021/cr030602b. [DOI] [PubMed] [Google Scholar]
- 6.Fuoss RM, Edelson D. Bolaform Electrolytes. I. Di-(ß-trimethylammonium Ethyl) Succinate Dibromide and Related Compounds. J Am Chem Soc. 1951;73(1):269–73. [Google Scholar]
- 7.Escamilla GH, Newkome GR. Bolaamphiphiles: From Golf Balls to Fibers. Angew Chem Int Ed Engl. 1994;33(19):1937–40. [Google Scholar]
- 8.Fuhrhop JH, Fritsch D. Bolaamphiphiles form ultrathin, porous and unsymmetric monolayer lipid membranes. Acc Chem Res. 1986;19(5):130–7. [Google Scholar]
- 9.Fuhrhop JH, Endisch C. Molecular and Supramolecular Chemistry of Natural Products and Their Model Compounds. CRC Press; 2000. [Google Scholar]
- 10.Zana R. Bolaform and dimeric (gemini) surfactants. In: Robb I, editor. Specialist surfactants. London: Chapman & Hall; 1996. p. 81. [Google Scholar]
- 11.Arakawa K, Eguchi T, Kakinuma K. 36-Membered Macrocyclic Diether Lipid is Advantageous for Archaea to Thrive under the Extreme Thermal Environments. Bull Chem Soc Jpn. 2001;74(2):347–56. [Google Scholar]
- 12.Arakawa K, Eguchi T, Kakinuma K. Highly thermostable liposome from 72-membered macrocyclic tetraether lipid: Importance of 72-membered lipid for archaea to thrive under hyperthermal environments. Chem Lett. 2001;30(5):440–1. [Google Scholar]
- 13.Chang EL. Unusual thermal stability of liposomes made from bipolar tetraether lipids. Biochem Biophys Res Commun. 1994;202(2):673–9. doi: 10.1006/bbrc.1994.1983. [DOI] [PubMed] [Google Scholar]
- 14.Rethore G, Montier T, Le Gall T, Delepine P, Cammas-Marion S, Lemiegre L. et al. Archaeosomes based on synthetic tetraether-like lipids as novel versatile gene delivery systems. Chem Commun (Camb) 2007;(20):2054–6. doi: 10.1039/b618568a. [DOI] [PubMed] [Google Scholar]
- 15.Gabriel JL, Chong PL. Molecular modeling of archaebacterial bipolar tetraether lipid membranes. Chem Phys Lipids. 2000;105(2):193–200. doi: 10.1016/s0009-3084(00)00126-2. [DOI] [PubMed] [Google Scholar]
- 16.Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E. et al. Lipid-Based Nanoparticles as Pharmaceutical Drug Carriers: From Concepts to Clinic. Crit Rev Ther Drug Carrier Syst. 2009;26(6):523–80. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuda M, Shimizu T. Multilayer structure of an unsymmetrical monolayer lipid membrane with a 'head-to-tail' interface. Chem Commun (Camb) 2001;(23):2442–3. doi: 10.1039/b106581p. [DOI] [PubMed] [Google Scholar]
- 18.Denoyelle S, Polidori A, Brunelle M, Vuillaume PY, Laurent S, Elazhary Y. et al. Synthesis and preliminary biological studies of hemifluorinated bifunctional bolaamphiphiles designed for gene delivery. New J Chem. 2006;30(4):629–46. [Google Scholar]
- 19.Halter M, Nogata Y, Dannenberger O, Sasaki T, Vogel V. Engineered lipids that cross-link the inner and outer leaflets of lipid bilayers. Langmuir. 2004;20(6):2416–23. doi: 10.1021/la035817v. [DOI] [PubMed] [Google Scholar]
- 20.Gerber S, Garamus VM, Milkereit G, Vill V. Mixed micelles formed by SDS and a bolaamphiphile with carbohydrate headgroups. Langmuir. 2005;21(15):6707–11. doi: 10.1021/la050439a. [DOI] [PubMed] [Google Scholar]
- 21.Lopez O, Maya I, Fuentes J, Fernandez-Bolanos JG. Simple and efficient synthesis of O-unprotected glycosyl thiourea and isourea derivatives from glycosylamines. Tetrahedron. 2004;60(1):65–76. [Google Scholar]
- 22.Schuur B, Wagenaar A, Heeres A, Heeres EH. A synthetic strategy for novel nonsymmetrical bola amphiphiles based on carbohydrates. Carbohydr Res. 2004;339(6):1147–53. doi: 10.1016/j.carres.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Soussan E, Pasc-Banu A, Consola S, Labrot T, Perez E, Blanzat M. et al. New Catanionic Triblock Amphiphiles: Supramolecular Organization of a Sugar-Derived Bolaamphiphile Associated with Dicarboxylates. Chemphyschem. 2005;6(12):2492–4. doi: 10.1002/cphc.200500273. [DOI] [PubMed] [Google Scholar]
- 24.Quesada E, Acuña AU, Amat-Guerri F. New Transmembrane Polyene Bolaamphiphiles as Fluorescent Probes in Lipid Bilayers Thanks are given to Prof. E. Gratton and Dr. L. A. Bagatolli, University of Illinois at Urbana-Champaign, USA, for the experiments on the orientation of the probes in POPC vesicles, and to P. P. Garcia Alvarez for his help in the synthesis. This work was financed by the Spanish D.G.I. (Projects PB96-852 and BQU2000/1500). E.Q. acknowledges a predoctoral fellowship from the same source. Angew Chem Int Ed Engl. 2001;40(11):2095–7. [PubMed] [Google Scholar]
- 25.Quesada E, Acuña AU, Amat-Guerri F. Synthesis of Carboxyl-Tethered Symmetric Conjugated Polyenes as Fluorescent Transmembrane Probes of Lipid Bilayers. Eur J Org Chem. 2003;2003(7):1308–18. [Google Scholar]
- 26.Guo P, Liu M, Nakahara H, Ushida K. Controllable growth of straight nanorods and nanowires in the Langmuir films of a bolaamphiphilic par derivative. Chemphyschem. 2006;7(2):385–93. doi: 10.1002/cphc.200500268. [DOI] [PubMed] [Google Scholar]
- 27.Han F, He X, Huang J, Li Z, Wang Y, Fu H. Surface Properties and Aggregates in the Mixed Systems of Bolaamphiphiles and Their Oppositely Charged Conventional Surfactants. J Phys Chem B. 2004;108(17):5256–62. [Google Scholar]
- 28.Mizoshita N, Seki T. Flat orientation of hydrophobic cores induced by two-dimensional confinement of flexible bolaamphiphiles at the air-water interface. Langmuir. 2005;21(23):10324–7. doi: 10.1021/la052150z. [DOI] [PubMed] [Google Scholar]
- 29.Mizoshita N, Seki T. Organised structures of flexible bolaamphiphiles with trisiloxane spacers: three- and two-dimensional molecular assemblies with different molecular conformation. Soft Matter. 2006;2(2):157–65. doi: 10.1039/b516768j. [DOI] [PubMed] [Google Scholar]
- 30.Song B, Wang Z, Chen S, Zhang X, Fu Y, Smet M. et al. The introduction of pi-pi stacking moieties for fabricating stable micellar structure: formation and dynamics of disklike micelles. Angew Chem Int Ed Engl. 2005;44(30):4731–5. doi: 10.1002/anie.200500980. [DOI] [PubMed] [Google Scholar]
- 31.Toyota T, Tsuha H, Yamada K, Takakura K, Yasuda K, Sugawara T. Fluorescence microscopic investigation on morphological changes of giant multilamellar vesicles induced by amphiphilic additives. Langmuir. 2006;22(5):1976–81. doi: 10.1021/la0529198. [DOI] [PubMed] [Google Scholar]
- 32.Dhasaiyan P, Pandey PR, Visaveliya N, Roy S, Prasad BL. Vesicle structures from bolaamphiphilic biosurfactants: experimental and molecular dynamics simulation studies on the effect of unsaturation on sophorolipid self-assemblies. Chemistry. 2014;20(21):6246–50. doi: 10.1002/chem.201304719. [DOI] [PubMed] [Google Scholar]
- 33.Polidori A, Wathier M, Fabiano AS, Olivier B, Pucci B. Synthesis and aggregation behaviour of symmetric glycosylated bolaamphiphiles in water. ARKIVOC. 2006;2006(4):73–89. [Google Scholar]
- 34.Grinberg S, Kolot V, Linder C, Shaubi E, Kas'yanov V, Deckelbaum RJ. et al. Synthesis of novel cationic bolaamphiphiles from vernonia oil and their aggregated structures. Chem Phys Lipids. 2008;153(2):85–97. doi: 10.1016/j.chemphyslip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Jin Y, Qi N, Tong L, Chen D. Self-assembled drug delivery systems. Part 5: self-assemblies of a bolaamphiphilic prodrug containing dual zidovudine. Int J Pharm. 2010;386(1-2):268–74. doi: 10.1016/j.ijpharm.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 36.Jin Y, Xin R, Tong L, Du L, Li M. Combination anti-HIV therapy with the self-assemblies of an asymmetric bolaamphiphilic zidovudine/didanosine prodrug. Mol Pharm. 2011;8(3):867–76. doi: 10.1021/mp100457d. [DOI] [PubMed] [Google Scholar]
- 37.Ambrosi M, Fratini E, Alfredsson V, Ninham BW, Giorgi R, Lo Nostro P. et al. Nanotubes from a vitamin C-based bolaamphiphile. J Am Chem Soc. 2006;128(22):7209–14. doi: 10.1021/ja057730x. [DOI] [PubMed] [Google Scholar]
- 38.Ray S, Das AK, Banerjee A. pH-Responsive, Bolaamphiphile-Based Smart Metallo-Hydrogels as Potential Dye-Adsorbing Agents, Water Purifier, and Vitamin B12 Carrier. Chem Mater. 2007;19(7):1633–9. [Google Scholar]
- 39.Dakwar GR, Abu Hammad I, Popov M, Linder C, Grinberg S, Heldman E. et al. Delivery of proteins to the brain by bolaamphiphilic nano-sized vesicles. J Control Release. 2012;160(2):315–21. doi: 10.1016/j.jconrel.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 40.Zana R, Yiv S, Kale KM. Chemical relaxation and equilibrium studies of association in aqueous solutions of bolaform detergents. 3. Docosane-1,22-bis(trimethylammonium bromide) J Colloid Interface Sci. 1980;77(2):456–65. [Google Scholar]
- 41.Popov M, Linder C, Deckelbaum RJ, Grinberg S, Hansen IH, Shaubi E. et al. Cationic vesicles from novel bolaamphiphilic compounds. J Liposome Res. 2010;20(2):147–59. doi: 10.3109/08982100903218900. [DOI] [PubMed] [Google Scholar]
- 42.Heldman E, Linder C, Grinberg S, Popov M, Hollander I. Delivery of Tenofovir to the Brain by Novel Nano-Vesicles for the Treatment of Neuro-HIV (S10.003) Neurology. 2014;82(10 Supplement):S10.003. [Google Scholar]
- 43.Popov M, Abu Hammad I, Bachar T, Grinberg S, Linder C, Stepensky D. et al. Delivery of analgesic peptides to the brain by nano-sized bolaamphiphilic vesicles made of monolayer membranes. Eur J Pharm Biopharm. 2013;85(3 Pt A):381–9. doi: 10.1016/j.ejpb.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Hwang SR, Kim K. Nano-enabled delivery systems across the blood-brain barrier. Arch Pharm Res. 2014;37(1):24–30. doi: 10.1007/s12272-013-0272-6. [DOI] [PubMed] [Google Scholar]
- 45.Stern A, Guidotti M, Shaubi E, Popov M, Linder C, Heldman E. et al. Steric environment around acetylcholine head groups of bolaamphiphilic nanovesicles influences the release rate of encapsulated compounds. Int J Nanomedicine. 2014;9:561–74. doi: 10.2147/IJN.S53563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Philosof-Mazor L, Dakwar GR, Popov M, Kolusheva S, Shames A, Linder C. et al. Bolaamphiphilic vesicles encapsulating iron oxide nanoparticles: new vehicles for magnetically targeted drug delivery. Int J Pharm. 2013;450(1-2):241–9. doi: 10.1016/j.ijpharm.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 47.Akhtar S. Opportunities and challenges for therapeutic gene silencing using RNAi and microRNA technologies. Adv Drug Deliv Rev. 2007;59(2-3):73–4. [Google Scholar]
- 48.Rao DD, Vorhies JS, Senzer N, Nemunaitis J. siRNA vs. shRNA: similarities and differences. Adv Drug Deliv Rev. 2009;61(9):746–59. doi: 10.1016/j.addr.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Sato A, Takagi M, Shimamoto A, Kawakami S, Hashida M. Small interfering RNA delivery to the liver by intravenous administration of galactosylated cationic liposomes in mice. Biomaterials. 2007;28(7):1434–42. doi: 10.1016/j.biomaterials.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Akhtar S, Benter I. Toxicogenomics of non-viral drug delivery systems for RNAi: potential impact on siRNA-mediated gene silencing activity and specificity. Adv Drug Deliv Rev. 2007;59(2-3):164–82. doi: 10.1016/j.addr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Ogris M, Wagner E. Targeting tumors with non-viral gene delivery systems. Drug Discov Today. 2002;7(8):479–85. doi: 10.1016/s1359-6446(02)02243-2. [DOI] [PubMed] [Google Scholar]
- 52.Weber N, Ortega P, Clemente MI, Shcharbin D, Bryszewska M, De La Mata FJ. et al. Characterization of carbosilane dendrimers as effective carriers of siRNA to HIV-infected lymphocytes. J Control Release. 2008;132(1):55–64. doi: 10.1016/j.jconrel.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 53.Jain N, Arntz Y, Goldschmidt V, Duportail G, Mely Y, Klymchenko AS. New unsymmetrical bolaamphiphiles: synthesis, assembly with DNA, and application for gene delivery. Bioconjug Chem. 2010;21(11):2110–8. doi: 10.1021/bc100334t. [DOI] [PubMed] [Google Scholar]
- 54.Jain N, Goldschmidt V, Oncul S, Arntz Y, Duportail G, Mely Y. et al. Lactose-ornithine bolaamphiphiles for efficient gene delivery in vitro. Int J Pharm. 2012;423(2):392–400. doi: 10.1016/j.ijpharm.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 55.Kim T, Afonin KA, Viard M, Koyfman AY, Sparks S, Heldman E. et al. In Silico, In Vitro, and In Vivo Studies Indicate the Potential Use of Bolaamphiphiles for Therapeutic siRNAs Delivery. Mol Ther Nucleic Acids. 2013;2:e80. doi: 10.1038/mtna.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hebrant M, Provin C, Brunette JP, Tondre C. Micellar extraction of europium (III) by a bolaform extractant and parent compounds derived from 5-pyrazolone. Colloids Surf Physicochem Eng Aspects. 2001;181(1-3):225–36. [Google Scholar]
- 57.Sistach S, Rahme K, Pérignon N, Marty JD, Viguerie NLD, Gauffre F. et al. Bolaamphiphile surfactants as nanoparticle stabilizers: application to reversible aggregation of gold nanoparticles. Chem Mater. 2008;20(4):1221–3. [Google Scholar]
- 58.Weissig V, Torchilin VP. Cationic bolasomes with delocalized charge centers as mitochondria-specific DNA delivery systems. Adv Drug Deliv Rev. 2001;49(1-2):127–49. doi: 10.1016/s0169-409x(01)00131-4. [DOI] [PubMed] [Google Scholar]