Abstract
Purpose: To investigate, for the first time, the chemical composition of essential oil of the tubers and leaves of Jerusalem artichoke (Helianthus tuberosus L.), a species of sunflower native to eastern North America, growing in Ukraine.
Methods: A hydrodistillation apparatus was used for the extraction of volatile components and then it was analysed by gas chromatography equipped with a split-splitless injector (split ratio, 1:50) and flame ionization detector (FID). The oil was analyzed under linear temperature programming applied at 4°C/min from 50°C - 340°C. Temperatures of the injector and FID detector were maintained at 280°C and 300°C, respectively. The chemical analysis of the oil was carried out using gas chromatography coupled to mass spectrometry (GC-MS), to determine the chemical composition of the volatile fraction.
Results: The essential oils content ranged from 0.00019 to 0.03486 and 0.00011 to 0.00205 (g/100g), in leaves and tubers, respectively. The qualitative and quantitative analysis led to the identification of 17 components in both species samples. The major component found in leaves and tubers was (-)-β-bisabolene with 70.7% and 63.1%, respectively.
Conclusion: Essential oil profile of Jerusalem artichoke species showed significant differences between leaves and tubers species. Additionally, the leaves of Jerusalem artichoke are a promising source of natural β-bisabolene.
Keywords: Essential oil, Helianthus tuberosus L., Jerusalem artichoke, Leaves, Tubers, Gas chromatography
Introduction
Jerusalem artichoke (Helianthus tuberosus L.), a plant of the Asteraceae family that grows in cool to warm climates, also cultivated widely in China for its highly adaptability and multiple tuber usability options, and its tubers can be produced world-wide, including Asia, Europe, and North America.1,2 A recent study also suggested the use of the tubers in the diet of patients, due to the fact that tubers contain low amounts of polyamines.3 Helianthus tuberosus L. contains many compounds, including coumarins, unsaturated fatty acids, polyacetylenic derivatives,4 and sesquiterpenes.5 Therefore, it has various pharmacological activities, such as cholagogue, aphrodisiac, aperient, stomachic, diuretic, and tonic effects. Moreover its tuber, a potential source of biomass, is used as food and folk medicine for the treatment of diabetes and rheumatism due to the presence of inulin, which can be converted into fructose.6 Moreover, it was found that the extracts of the aerial part were also possess antifungal, antimicrobial, and anticancer activities.6 Additionally, Helianthus tuberosus L leaf is a natural medicine for the treatment of skin wound, bone fracture and swelling.5,7
Plant essential oils and its extracts have been used for many thousands years, particularly in pharmaceuticals, food preservation, and natural therapies.8 Moreover, it has been long acknowledged that some plant essential oils exhibit antimicrobial properties, especially against bacterial pathogens, and thus it is necessary to investigate those plants. Essential oils also are potential sources of novel antimicrobial compounds especially against bacterial pathogens.9
Several methods have been reported in literature for the analysis of Jerusalem artichoke plant including carbohydrates analysis in tubers,10-12 or for the analysis of sugars using high-performance liquid chromatography (HPLC) whether coupled with mass spectrometry detector (MS),13 or with refractive index (RI) in dried chips in tubers,14 or with ultraviolet (UV) detection for the analysis of total phenolic in leaves.15 Moreover, high performance anion exchange coupled with pulsed amperometric detection (HPAE-PAD) has been reported for the analysis of carbohydrates.16,17 Gas chromatography-mass spectrometry (GC-MS) is a powerful analytical chemistry tool not only for the quantification purposes but also for the identification of chemical components in essential oils, and used in many applications, because it combines the ability of physical separation of gas chromatography with the mass analysis of mass spectrometry.18 Additionally, mass spectrometry offers major advantages due to its high sensitivity and mass selectivity.13
The volatile compounds in tubers of Jerusalem artichoke have been investigated previously.19 Hence, an investigation of the volatile compounds present in tubers and leaves species can give a better understanding of the compounds present. Therefore, this paper reports, for the first time, the investigation of the chemical composition of essential oils of the tubers and leaves species of Jerusalem artichoke growing in Ukraine. Additionally, increased knowledge on volatile components in Jerusalem artichoke as a raw material can be used to help the consumption of this vegetable in the human diet, and provide consumers with a larger choice of vegetables for human consumption purposes.
Materials and Methods
Plant material
Leaves and tubers of Jerusalem artichoke growing in Ukraine were collected from healthy trees, harvested during flowering period in summer and autumn in 2010. Leaves were separated from the stem. Plant species were air-dried, ground, sifted through 0.5 mm mesh screen to obtain a uniform particle size. After collected, the samples were immediately fractioned in two parts and then submitted to hydrodistillation.
Hydrodistillation
Twenty grams of each tubers and leaves samples were submitted to water-distillation in a Clevenger type apparatus for 4 hours (until no more essential oil was recovered), and used for the isolation of the essential oils from leaves and tuber of Jerusalem artichoke according to the British Pharmacopoeia.20 The hydrodistillation was also performed by the use of Deryng apparatus with 400 mL after 4 hours as described in the European Pharmacopoeia.21 Subsequently, received essential oil was dried over anhydrous sodium sulfate and refrigerated prior analysis at 4°C until analyzed by gas chromatographic and determination of its composition. The obtained essential oil was separated from water and the yield was determined in terms of dry basis yield, as g/100g of dry herb. Tridecane was used as internal standard.
GC-MS analysis
Quantitative analysis was carried out using gas chromatography system (Agilent Technologies, model 6890, Waldbronn, Germany). The unit was equipped with a split-splitless injector (split ratio, 1:50) and flame ionization detector (FID). OPTIMA-5 fused silica capillary column (30 m x 0.25 mm, 0.25 µm film thickness) was used. The essential oil was analyzed under linear temperature programming applied at 4°C/min from 50 - 340°C. Temperatures of the injector and detector (FID) were maintained at 280°C and 300°C, respectively. Concentrations (% contents) of the essential oil components were calculated using their relative area percentages, obtained by FID, assuming a unity response by all components of GC-MS analysis. The chemical analysis of the essential oil was carried out using gas chromatography-mass spectrometry (GC-MS) coupled to a Saturn 2000 mass spectrometry detector (Varian-Chrompack, model 3800), gas chromatography-mass spectrometry (GC-MS) (Varian chrompack, model 3800, GC/MS/MS-200 (Saturn, Netherlands)). The chromatographic conditions were as follow: column oven program, 60 °C (1 min, isothermal) to 246°C (3 min, isothermal) at 3 °C/min; the injector and detector temperatures were 250 and 300°C, respectively. Helium was used as the carrier gas at a flow rate of 1.2 mL min-1. A HP-5 MS capillary column (30 m × 0.25 mm ID, 0.25 μm film thicknesses) was utilized. The actual temperatures in MS source reached approximately 180°C. The ionization voltage was 70 eV. A hydrocarbon mixture of n- alkanes (C8 - C20) was analyzed separately by GC-MS under same chromatographic conditions using the same HP-5 column.
Identification of Compounds
Essential oils compounds were identified by computer search using their mass spectra either with known components,22 or by comparison of received chemical substances mass-spectrum which are in essential oils composition and according to mass-spectrum library Wiley/NIST database.23
Results and Discussion
Hydrodistillation method is described in the European Pharmacopoeia.24 In this method, the plant material is boiled in water. After that, the volatile material is carried by the steam through tubes and then is cooled. The volatile essential oil is then removed from the top of the hydrosol. As known that hydrodistillation needs a large amount of plant sample and also the time of extraction is quite long (3 – 4 hours) and thus the energy consumption is quite high. But from the literature survey, we can see that hydrodistillation using a clevenger type apparatus is the one that has the best results. Although it is a very common method, the results are still better than the ones obtained using other methods reported.25
Volatile compounds in Jerusalem artichoke leaves and tubers
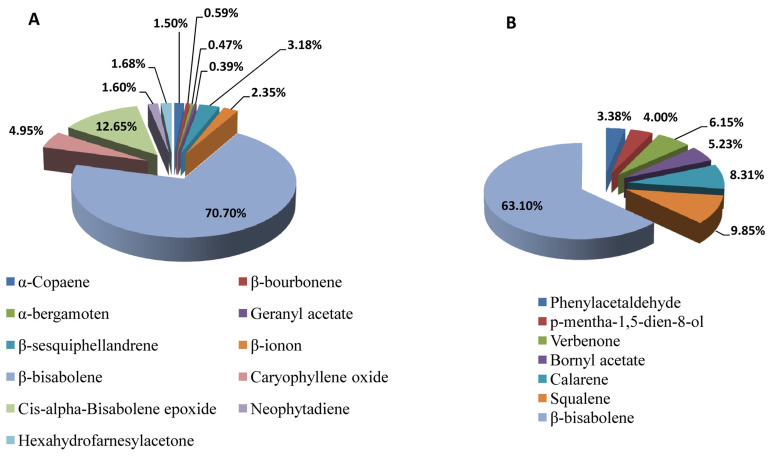
The major component in leaves and tubers was (-)-β-bisabolene with the highest concentration among other volatile compounds concentrations of 70.7% and 63.1%, respectively. Other components in leaves present in significant contents being: α-copaene (1.50%), β-bourbonene (0.59%), (E)-α-bergamoten (0.47%), geranyl acetate (0.39%), β-sesquiphellandrene (3.18%), β-ionon (2.35%), caryophyllene oxide (4.95%), (Z)-α-bisabolene epoxide (12.65%), neophytadiene (1.60%), and hexahydrofarnesylacetone (1.68%) (Figure 1). The retention indices data and chemical composition of leaves essential oil are presented in Table 1, while Figure 2 shows typical chromatogram. On the other hand, other components present in tubers in significant contents as well being: phenylacetaldehyde (3.38%), p-mentha-1,5-dien-8-ol (4.00%), verbenone (6.15%), bornyl acetate (5.23%), calarene (8.31%), and squalene (9.85%) (Figure 1). The retention indices data and chemical composition of tubers essential oil are presented in Table 1, while Figure 3 shows typical chromatogram.
Figure 1.
The composition of the volatile essential oil in leaves and tubers in Jerusalem artichoke, (A): leaves; and (B): tubers
Table 1. Chemical constituents of the essential oil from leaves and tubers of Jerusalem artichoke plant. Data are given as mean (n = 3).
| No. | I | Name of constituent | Chemical group | Leave oil (g/100g) | Tubers oil (g/100g) |
| 1 | 1171 | p-mentha-1,5-dien-8-ol | OM | - | 0.00013 |
| 2 | 1208 | Verbenone | OM | - | 0.00020 |
| 3 | 1296 | Bornyl acetate | OM | - | 0.00017 |
| 4 | 1377 | α-Copaene | SH | 0.00074 | - |
| 5 | 1405 | Phenylacetaldehyde | AL | - | 0.00011 |
| 6 | 1417 | ß-bourbonene | SH | 0.00029 | - |
| 7 | 1431 | (E)-α-bergamoten | SH | 0.00023 | - |
| 8 | 1448 | Geranyl acetone | IP | 0.00019 | - |
| 9 | 1483 | Calarene | SH | - | 0.00027 |
| 10 | 1493 | ß-ionone | IP | 0.00116 | - |
| 11 | 1498 | (-)-ß-bisabolene | SH | 0.03486 | 0.00205 |
| 12 | 1560 | ß-sesquiphellandrene | SH | 0.00157 | - |
| 13 | 1573 | Caryophyllene oxide | OS | 0.00244 | - |
| 14 | 1680 | (Z)-α-bisabolene epoxide | OS | 0.00624 | - |
| 15 | 1836 | Neophytadiene | DT | 0.00079 | - |
| 16 | 1848 | Hexahydrofarnesylacetone | IP | 0.00083 | - |
| 17 | 2834 | Squalene | TT | - | 0.00032 |
I : Retention indices; OM: Oxygenated monoterpenes; SH : Sesquiterpene hydrocarbons; OS : Oxygenated sesquiterpenes; IP : Isoprenoids; TT : Triterpene; DT : Diterpenes hydrocarbons; AL : Aldehyde
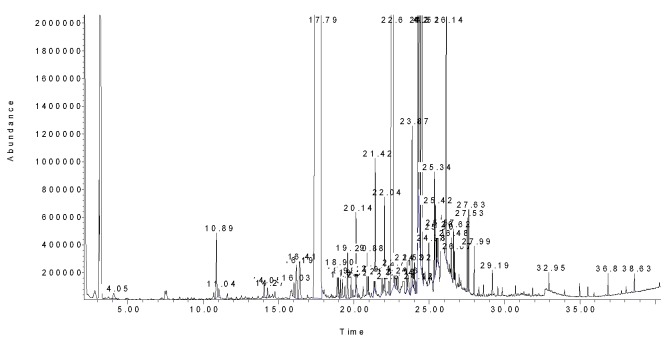
Figure 2.
A typical chromatogram of Jerusalem artichoke leaves essential oil. Please refer to text for GC conditions.
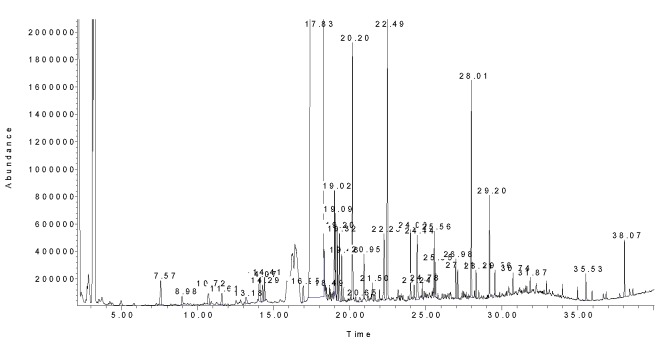
Figure 3.
A typical chromatogram of Jerusalem artichoke tubers essential oil. Please refer to text for GC conditions.
As seen, a total of 17 identified volatile compounds were found in the extract of Jerusalem artichoke leaves and tubers in the range of 0.00019 – 0.03486 and from 0.00011 – 0.00205 g/100g, respectively (Table 1). The results of the qualitative and quantitative essential oils analyses of both leaves and tubers were listed in order of elution based on the column used. All of these compounds were classified based on its chemical group and also identified by comparison of their mass spectral data with the NIST database and retention times of reference standards. The classification of these volatile compounds in leaves and tubers species were as three oxygenated monoterpenes, one diterpenes hydrocarbons, one triterpene, six sesquiterpenes hydrocarbons, two oxygenated sesquiterpenes, three isoprenoids, and one aldehyde. Among the identified compounds only (-)-β-bisabolene has previously been reported as constituent of Jerusalem artichoke (as whole plant without differentiation between leaves and tubers species constituents).19 Alexander et al. (1982) identified 52 volatile compounds (20 were positively identified, 15 were partially characterized and 17 unknown constituents) in raw Jerusalem artichoke tuber using solvent extraction with trichlorofluoromethane. Traditional solvent extraction methods require a large quantity of solvent and are time consuming. Moreover, the large amount of solvent used not only increases operating costs but also causes environmental problems.26 Several extraction techniques have been developed as an alternative to traditional extraction methods, offering better advantages with regard to solvent consumption, extraction time, extraction yields and reproducibility. Hydrodistillation shows to be an excellent method for extracting the essential oil as it results in good yield and recovery, is less labor-intensive, and is considered a simple and fast technique. Additionally, the ability to use either dry or fresh plant tissue, or even to reduce the amount of dried plant material from 75 to 10 g dry weight with no significant changes in content, composition, and results offer great promise in physiological and breeding studies where many samples need to be analyzed and the amount of plant material may be limited issue.26
The concentrations of these compounds are also given in Table 1. Taking into consideration both number and concentration, the major compound of the volatile species in leaves and tubers consisted of (-)-β-bisabolene, with average of 0.03486 and 0.00205 g/100g, respectively. All other identified volatiles in leaves and tubers were quantified and presented in concentrations below 0.00624 and 0.00032 g/100g, respectively.
Alexander et al. (1982) identified all saturated straight chain hydrocarbons between hexadecane and eicosane (C16 – C20) in Jerusalem artichoke plant. In the present study, neophytadiene (C20) was identified; this may be attributed probably due to the difference in the sample preparation used and the region where the plant has been collected and harvested. Only two identified compounds, namely; β-ionone and hexahydrofarnesylacetone were found and quantified in leaves, showing almost close results. Additionally, only one aldehyde (phenylacetaldehyde), which is considered as degradation products of fatty acids, was identified and quantified in tubers.
In general, the essential oils content in leaves and tubers appears somehow influenced by the part of the plant from where it has been taken. Surprisingly, it is interesting to note that the higher content obtained herein for some compounds are due to drying and grinding steps prior hydrodistillation. This observation is of interest from scientifically point of view where the drying steps not only prevents the development of microbial and fungal spoilage, but also it may allow the extension of the shelf-life of the plant and it improves the essential oils content. The improvement of essential oils contents by using dried and grinded raw material has been also previously reported in lime,27 thyme,28 and peppermint.29
Conclusion
The essential oils, obtained from leaves and tubers in Jerusalem artichoke by hydrodistillation, were analysed by gas chromatography (GC-FID) and gas chromatography-mass spectrometry (GC-MS). Essential oil profile of Jerusalem artichoke leaves and tubers was produced; it showed significant differences between leaves and tubers parts. GC-MS analysis indicated that a higher concentration of (-)-β-bisabolene was present in both leaves and tubers. Additionally, the leaves of Jerusalem artichoke are a promising source of natural (-)-β-bisabolene. A total of 17 identified volatile compounds were found in the extract of Jerusalem artichoke leaves and tubers in the range of 0.00019 – 0.03486 and from 0.00011 – 0.00205 g/100g, respectively (Table 1). Moreover, the results obtained showed that GC–MS is a powerful tool that offers a simple and highly sensitive way to evaluate the quality of Jerusalem artichoke species, which may be of significant value to industrial as well as regulatory bodies. To our knowledge, this is the first in-depth and comparative investigation of the essential oils present in leaves and tubers of Jerusalem artichoke. The significant differences in the constituents of the essential oils found in these two parts provide compelling evidence that leaves and tubers must be treated as two different constituents once analysed.
Acknowledgments
This work is supported financially by a National Medical University (O.O.Bogomolets, Ukraine).
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Slimestad R, Seljasen R, Meijer K, Skar SL. Norwegian-grown Jerusalem artichoke (Helianthus tuberosus L.): Morphology and content of sugars and fructo-oligosaccharides in stems and tubers. J Sci Food Agric. 2010;90(6):956–64. doi: 10.1002/jsfa.3903. [DOI] [PubMed] [Google Scholar]
- 2.Ma XY, Zhang LH, Shao HB, Xu G, Zhang F, Ni FT. et al. Jerusalem artichoke (Helianthus tuberosus L.), a medicinal salt-resistant plant has high adaptability and multiple-use values. J Med Plant Res. 2011;5(8):1275–82. [Google Scholar]
- 3.Righetti L, Tassoni A, Bagni N. Polyamines content in plant derived food: A comparison between soybean and Jerusalem artichoke. Food Chem. 2008;111(4):852–6. [Google Scholar]
- 4.Matsuura H, Yoshihara T, Ichihara A. Four New Polyacetylenic Glucosides, Methyl β-D-Glucopyranosyl Helianthenate C-F, from Jerusalem Artichoke (Helianthus tuberosus L.) Biosci Biotechnol Biochem. 1993;57(9):1492–8. [Google Scholar]
- 5.Baba H, Yaoita Y, Kikuchi M. Sesquiterpenoids from the leaves of Helianthus tuberosus L. J Tohoku Pharm Univ. 2005;52:21–5. [Google Scholar]
- 6.Pan L, Sinden MR, Kennedy AH, Chai H, Watson LE, Graham TL. et al. Bioactive constituents of Helianthus tuberosus L. (Jerusalem artichoke) Phytochem Lett. 2009;2(1):15–8. [Google Scholar]
- 7.University of Maryland Medical Center. Traditional Chinese Medicine. [updated 24 August 2009; 19 April 2014]; Available from: www.umm.edu/altmed/articles/traditional-chinese-000363.htm. [Google Scholar]
- 8.Imelouane B, Amhamdi H, Wathelet JP, Ankit M, Kheded K, Elbachiri A. Chemical Composition and Antimicrobial Activity of Essential Oil of Thyme (Thymus vulgaris) from Eastern Morocco. Int J Agric Biol. 2009;11:205–8. [Google Scholar]
- 9.Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antimicrobial activity of some plant essential oils. BMC Complement Altern Med. 2006;6:39–44. doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldini M, Danuso F, Turi M, Vannozzi GP. Evaluation of new clones of Jerusalem artichoke (Helianthus tuberosus L.) for inulin and sugar yield from stalks and tubers. Ind Crop Prod. 2003;19:25–40. [Google Scholar]
- 11.Curt MD, Aguado P, Sanz M, Sánchez G, Fernández J. Clone precocity and the use of Helianthus tuberosus L. stems for bioethanol. Ind Crop Prod. 2006;24:314–20. [Google Scholar]
- 12.Kockis L, Liebhard P, Praznik W. Effect of seasonal changes on content of profile of soluble carbohydrates in tubers of different varieties of Jerusalem artichoke (Helianthus tuberosus L.) J Agric Food Chem. 2007;55(23):9401–8. doi: 10.1021/jf0717485. [DOI] [PubMed] [Google Scholar]
- 13.Javier M, Jerónimo G, Luis R, Rafael AB. Analysis of sugars by liquid chromatography-mass spectrometry in Jerusalem artichoke tubers for bioethanol production optimization. Biomass Bioenergy. 2011;35(5):2006–12. [Google Scholar]
- 14.Junko T, Toshio N. Preparation of dried chips from Jerusalem artichoke (Helianthus tuberosus) tubers and analysis of their functional properties. Food Chem. 2011;126(3):922–6. [Google Scholar]
- 15.Xiaoyan Y, Mingzhe G, Hongbin X, Chengyu T, Yuguang D. Free radical scavenging activities and bioactive substances of Jerusalem artichoke (Helianthus tuberosus L.) leaves. Food Chem. 2012;133:10–4. [Google Scholar]
- 16.Bruggink C, Maurer R, Herrmann H, Cavalli S, Hoefler F. Analysis of carbohydrates by anion exchange chromatography and mass spectrometry. J Chromatogr A. 2005;1085(1):104–9. doi: 10.1016/j.chroma.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 17.Hotchkiss AT, Lecrinier SL, Hicks KB. Isolation of oligogalacturonic acids up to DP 20 by preparative high-performance anion-exchange chromatography and pulsed amperometric detection. Carbohydr Res. 2001;334(2):135–40. doi: 10.1016/s0008-6215(01)00170-7. [DOI] [PubMed] [Google Scholar]
- 18.Di-Ya L, Yan C, Ling L, Zhen-Yu Z, Xin D, Hai Z. et al. Comparative analysis of essential oils found in Rhizomes Curcumae and Radix Curcumae by gas chromatography–mass spectrometry. J Pharm Anal. 2011;1(3):203–7. doi: 10.1016/j.jpha.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLeod AJ, Pieris NM, Gonzalez De Troconis N. Aroma volatiles of Cynara Scolymus and Helianthus tuberosus. Phytochem. 1982;21(7):1647–51. [Google Scholar]
- 20.British pharmacopoeia. London: Her Majesty’s stationary office; 1990. [Google Scholar]
- 21.European Pharmacopoeia. 3rd ed. Strasbourg: Council of Europe; 1997. [Google Scholar]
- 22.Adams RP. Identification of essential oils by ion trap mass spectroscopy. New York: Academic Press, Inc; 1989. [Google Scholar]
- 23.Registry, 8th ed. with NIST 05 MS Spectra, Revision 2005 D.06.00. s.l. Agilent Technologies, Wiley; 2007. [Google Scholar]
- 24.European Pharmacopoeia. 3rd ed. Strasbourg: Council of Europe; 2004. [Google Scholar]
- 25.Okoh OO, Sadimenko AP, Afolayan AJ. Comparative evaluation of the antibacterial activities of the essential oils of Rosmarinus officinalis L. obtained by hydrodistillation and solvent free microwave extraction methods. Food Chem. 2010;120:308–12. [Google Scholar]
- 26.Lijun W, Curtis LW. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol. 2006;17:300–12. [Google Scholar]
- 27.Yadav AR, Chauhan AS, Rekha MN, Rao LJM, Ramteke RS. Flavour quality of dehydrated lime [Citrus aurantifolia (Christm.) Swingle] Food Chem. 2004;85:59–62. [Google Scholar]
- 28.Hanci SS, Sahin S, Yilmaz L. Isolation of volatile oil from thyme (Thymbra spicata) by steam distillation. Nahrung. 2003;47(4):252–5. doi: 10.1002/food.200390059. [DOI] [PubMed] [Google Scholar]
- 29.Rohloff J, Dragland S, Mordal R, Iversen TH. Effect of harvest time and drying method on biomass production, essential oil yield, and quality of peppermint (Mentha x piperita L.) J Agric Food Chem. 2005;53(10):4143–8. doi: 10.1021/jf047998s. [DOI] [PubMed] [Google Scholar]