Abstract
Purpose: This study evaluated the in vitro antioxidant potential of two varieties of Codiaeum variegatum leaves (spiral (CP) and royal like (BP)) extracts.
Methods: The different antioxidant assays, including DPPH free radical scavenging, nitric oxide scavenging, hydrogen peroxide, reducing power, total antioxidant activity, protection of lipid peroxidation and RBC membrane stabilization activity, were studied. Moreover, high-performance liquid chromatography (HPLC) coupled with diode-array detection was used to identify and quantify the phenolic compounds in the royal like (BP) leaves extract.
Results: Codiaeum variegatum extracts showed effective DPPH free radical scavenging, hydrogen peroxide radical scavenging and nitric oxide scavenging activity. However, reducing power of ferric ion was not significant compared to the standard antioxidant activity. In addition, Codiaeum variegatum extracts exhibited protection against lipid peroxidation. The total antioxidant activity was increased dose dependently when compared with standard drug ascorbic acid. (-)-Epicatechin, p-coumaric acid, rutin hydrate and ellagic acid were identified in the extract. Among the phenolic compounds, ellagic acid was abundantly present in the extract.
Conclusion: Our investigation suggests that Codiaeum variegatum leaves contain high amount of phenolic compounds which may responsible for its biological activities in folkloric medicine.
Keywords: Codiaeum variegatum, DPPH, Nitric oxide, Hydrogen peroxide, Ellagic acid, Membrane stabilizing activity
Introduction
Medicinal plants are traditionally used in folk medicine as natural remedies from ancient era and their usages are increasing day by day. Some of them also served as a source for basic lead molecules of modern medicine. More than 70% of the developing world’s population depends on traditional medicinal system.1 Medicinal plants are rich source of active chemical constituents such as polyphenols and flavonoids, glycosides, alkaloids, and tannins.2 Most often, a desired biological response is not due to the presence of one component but the presence of a mixture of bioactive plant components. Medicinal plants are rich source of antioxidants which has the potential to ameliorates oxidative damage in tissues and prevents degenerative diseases such as cardiovascular diseases, cancer, diabetes and aging.3,4 Traditional medical practitioners use a variety of medicinal plants for treatment of different ailments in Bangladesh. Bangladesh is rich in flora and fauna, more than 500 potential medicinal plants are listed including their chemical constituents and traditional uses.5
Garden crotons (Codiaeum variegatum) are a group of beautifully variegated leafy perennial ornamental shrubs found almost everywhere in Bangladesh. It belongs to the family Euphorbiaceae which is an important plant family for diverse biological activities.6 Its native habitats include India, Philippines, Sri Lanka, Thailand, Indonesia, Malaysia and some other Pacific Islands.7 Apart from its ornamental values, Codiaeum variegatum is also used for several medicinal purposes. Root decoction is taken for the treatment of gastric ulcers. Its leaves contain antibacterial and anti-amoebic properties and cures diarrhea.8 A recent report also suggests that Codiaeum variegatum extracts are active against influenza virus and a bioactive cyanoglucoside was isolated as the active component.9 Codiaeum variegatum also possesses alkaloids, anthraquinones, flavanoids, terpenes, steroid, phenol, saponins, tannins, phlobatannin and cardenolide7 and showed potent cytotoxicities in brian shrimp lethality bioassays.10 Phytochemical components, especially polyphenols (such as flavonoids, tannins, phyenyl propanoids, phenolic acids etc) are known to be responsible for the free radical scavenging and antioxidant activities. Phenolic compounds are very well known plant constituents because of their scavenging ability.11 It is demonestrated that polyphenolic compounds possess inhibitory effects on mutagenesis and carcinogenesis. In vitro studies also suggested that polyphenols may exert their inhibitory effects by acting as prooxidants on cancer cells or may inhibit the formation and growth of tumors by induction of cell cycle arrest and apoptosis.11 However, no reports have been found on any antioxidant activities and analysis of phenolic compounds in Codiaeum variegatum extracts. As a part of our ongoing investigation on natural antioxidants and biological activities from local medicinal plants of Bangladesh,10,12,13 in this paper, we evaluated the antioxidant activity of Codiaeum variegatum leaves extracts and analyzed the phenolic compounds present using HPLC-DAD system.
Materials and Methods
Collection and identification of plant materials
In this present investigation, the Codiaeum variegatum leaves were collected from Stamford University campus, Bangladesh and was identified at the Bangladesh National Herbarium, Mirpur, Dhaka where a voucher specimen (no: DACB 31304) has been deposited.
Drying and grinding of plants
The collected plant parts (leaves) were separated from undesirable materials such as other plants or plant parts. The plant parts were ground into a coarse powder with the help of a grinder. The powder was stored in an airtight container and kept in a cool, dark and dry place until further analysis was commenced.
Phytochemical screening
Phytochemical screening of the extract was performed using the following reagents and chemicals: alkaloids were determined using Dragendorffs reagent, flavonoids were determined by using Mg and HCl; tannins were determined using ferric chloride and potassium dichromate solutions and saponins were determined by the ability to produce suds. Gum was tested using Molish reagents and concentrated sulphuric acid.
Chemicals Used
Gallic acid (GA), (+)-catechin hydrate (CH), vanillic acid (VA), caffeic acid (CA), (-)-epicatechin (EC), p-coumaric acid (PCA), rutin hydrate (RH), ellagic acid (EA), myricetin (MC), kaempferol (KF), and quercetin (QU) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Acetonitrile (HPLC), methanol (HPLC), acetic acid (HPLC), and ethanol was obtained from Merck (Darmstadt, Germany). 1,1-diphenyl-2-picrylhydrazyl (DPPH), Butylated hydroxytoluene (BHT), trichloroacetic acid (TCA), Ammonium molybdate, hydrogen peroxide (H2O2), sodium nitroprusside, were also purchased from Merck, Germany and ethylene diamine tetra acetic acid (EDTA), sodium phosphate, sulfanilamide, N-(1-naphthyl) ethylenediamine dihydrochloride from BDH, England. Ferric chloride was obtained from Thomas Baker and Potassium ferricyanide was purchased from Guandong Chemical Reagent, China. All other reagents were of analytical grade.
In vitro antioxidant assays
DPPH radical scavenging activity :
Qualitative assay: Qualitative assay was performed according to the method previously described.14 Test samples were prepared with a suitable solvent system on a TLC plate and sprayed with 0.004% w/v DPPH solution in methanol using a sprayer. The positive activity was detected by the discolored (pale yellow) spots on a reddish purple background.
Quantitative assay: The free radical scavenging capacity of the extracts was determined using DPPH.15 A methanol DPPH solution (0.004% w/v) was mixed with serial dilutions (1 to 500 μg) of Codiaeum variegatum extracts and after 10 min, the absorbance was read at 515 nm using a spectrophotometer (HACH 4000 DU UV – visible spectrophotometer). Ascorbic acid was used as a standard antioxidant.
Nitric oxide radical inhibition assay
Nitric oxide radical inhibition was estimated using Griess reagents.15,16 In this investigation, Griess-Illosvoy reagent was modified by naphthyl ethylene diamine dihydrochloride (0.1% w/v) instead of 1-napthylamine (5%). The reaction mixture (3 ml) containing sodium nitroprusside (10 mM, 2 ml), phosphate buffer saline (0.5 ml) and C. variegatum extract (10 μg to 160 μg) or standard solution (BHT, 0.5 ml) was incubated at 25°C for 150 min. After incubation, 0.5 ml of the reaction mixture was mixed with 1 ml of sulfanilic acid reagent (0.33% in 20% glacial acetic acid) and allowed to stand for 5 min for completing diazotization. Then, 1 ml of naphthyl ethylene diamine dihydrochloride was added, mixed and allowed to stand for 30 min at 25°C. A pink color chromophore formed in diffused light. The absorbances of these solutions were measured at 540 nm against the corresponding blank solutions. Ascorbic acid was used as a standard antioxidant.
Scavenging of hydrogen peroxide
A modified method based on that of Ruch et al.17 was used to determine the ability of the extracts to scavenge hydrogen peroxide.15 Hydrogen peroxide (43 mM) was prepared in phosphate buffered saline (pH 7.4). Standards (ascorbic acid) and extract solutions were prepared at concentrations of 50 to 250 mM. Aliquots of the standard or extract solutions (3.4 mL) were added to 0.6 mL of hydrogen peroxide solution. The reaction mixture was incubated at room temperature for 10 min, and the absorbance was determined at 230 nm. The percentage of scavenging was calculated as follows:
Reducing power
The reducing power of Codiaeum variegatum extracts were determined according to the method previously described.18 Different concentrations of Codiaeum variegatum extract (100 μg – 1000 μg) in 1 ml of distilled water was mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 ml, 1%). The mixture was incubated at 50°C for 20 min. A portion (2.5 ml) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 ml) was mixed with distilled water (2.5 ml) and FeCl3 (0.5 ml. 0.1%) and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. Ascorbic acid was used as a standard antioxidant.
Determination of total antioxidant capacity
The antioxidant activity of the extracts of Codiaeum variegatum were evaluated by the phosphomolybdenum method according to the procedure previously described.15 The assay is based on the reduction of Mo (VI)–Mo (V) by the extract and subsequent formation of a green phosphate/Mo (V) complex at acid pH. 0.3 ml extract was combined with reagent solution (0.6M sulfuric acid, 28mM sodium phosphate and 4mM ammonium molybdate). The tubes containing the reaction solution were incubated at 95°C for 90 min. Then the absorbance of the solution was measured at 695 nm using a spectrophotometer (HACH 4000 DU UV – visible spectrophotometer) against blank after cooling to room temperature. Methanol (0.3 ml) was used as the blank. The antioxidant activity is expressed as the number of equivalents of ascorbic acid.
Lipid peroxidation assay
Thiobarbituric acid reactive substances were determined by using previously described method.18 The reaction mixture contained in a final volume of 1.0 ml, 500 μl of liver microsomal fraction, 300 μl buffer containing the plant extract (50–150 μg), 100 μl of FeCl3 (1 mM) and 100 μl ascorbic acid (1 mM) to start peroxidation. Samples were incubated at 37°C for 1 hour, after that lipid peroxidation was measured using the reaction with thiobarbituric acid (TBA). The absorbance of the organic layer was measured at 532 nm. All reactions were carried out in duplicate. Ascorbic acid was served as a standard antioxidant.
Determination of total phenolic content
The total phenolic content of the extract was determined by the modified Folin-Ciocaltu method.19 Briefly, 1.0 ml of each extract (1 mg/ml) was mixed with 5 ml Folin-Ciocaltu reagent (1:10 v/v distilled water) and 4 ml (75g/l) of sodium carbonate. The mixture was vortexed for 15 second and allowed to stand for 30 min at 40°C for color development. The absorbance was read at 765 nm with a spectrophotometer. Total phenolic content was determined as mg of gallic acid equivalent per gram using the equation obtained from a standard gallic acid calibration curve y = 6.2548x - 0.0925, R2=0.9962.
Membrane stabilizing activity
Preparation of erythrocyte suspension :
Whole blood was obtained with heparinized syringes from rats through cardiac puncture. The blood was washed three times with isotonic buffered solution (154 mM NaCl) in 10 mM sodium phosphate buffer (pH 7.4). The blood was centrifuged each time for 10 minutes at 3000 g.
Hypotonic solution-induced rat erythrocyte haemolysis :
Membrane stabilizing activity of the extracts was assessed using hypotonic solution-induced rat erythrocyte haemolysis.15 The test sample consisted of stock erythrocyte (RBC) suspension (0.50 ml) mixed with 5 ml of hypotonic solution (50 mM NaCl) in 10 mM sodium phosphate buffered saline (pH 7.4) containing the extract (0.25- 2.0 mg/ml) or indomethacin (0.1 mg/ml). The control sample consisted of 0.5 ml of RBC mixed with hypotonic -buffered saline solution alone. The mixtures were incubated for 10 min at room temperature and centrifuged for 10 min at 3000 g and the absorbance of the supernatant was measured at 540 nm. The percentage inhibition of haemolysis or membrane stabilization was calculated according to the below mentioned formula.
Where:
OD1 = Optical density of hypotonic-buffered saline solution alone
OD2 = Optical density of test sample in hypotonic solution
High performance liquid chromatography (HPLC) system
Chromatographic analyses were carried out on a Thermo Scientific Dionex UltiMate 3000 Rapid Separation LC (RSLC) systems (Thermo Fisher Scientific Inc., MA, USA), coupled to a quaternary rapid separation pump (LPG-3400RS), Ultimate 3000RS autosampler (WPS-3000) and rapid separation diode array detector (DAD-3000RS). Phenolic compounds were separated on a Acclaim® C18 (4.6 x 250 mm; 5µm) column (Dionix, USA) which was controlled at 30°C using a temperature controlled column compartment (TCC-3000). Data acquisition, peak integration, and calibrations were performed with Dionix Chromeleon software (Version 6.80 RS 10).
Chromatographic conditions
The phenolic composition of the leaves of C. variegatum ethanol extract was determined by HPLC-DAD, as described previously with some modifications.13,20 The mobile phase consisted of acetonitrile (solvent A), acetic acid solution pH 3.0 (solvent B), and methanol (solvent C). The system was run with the following gradient elution program: 0 min, 5%A/95%B; 10 min, 10%A/80%B/10%C; 20 min, 20%A/60%B/20%C and 30min, 100%A. There was a 5 min post run at initial conditions for equilibration of the column. The flow rate was kept constant throughout the analysis at 1 ml/min and the injection volume was 20 µl. For UV detection, the wavelength program was optimized to monitor phenolic compounds at their respective maximum absorbance wavelengths as follows: λ 280 nm held for 18.0 min, changed to λ 320 nm and held for 6 min, and finally changed to λ 380 nm and held for the rest of the analysis and the diode array detector was set at an acquisition range from 200 nm to 700 nm. The detection and quantification of gallic acid (GA), (+)-catechin hydrate (CH), vanillic acid (VA), caffeic acid (CA), and EC was done at 280 nm, of p-coumaric acid (PCA), Rutin hydrate (RH), and ellagic acid (EA) at 320 nm, and of myricetin (MC), quercetin (QU), and kaempferol (KF) at 380 nm, respectively.
Statistical Analysis
All data are presented as mean ±Standard deviation (SD). IC50 values for scavenging of free radicals by the extracts were calculated from dose-response curve by using default analyzing tab of Graph Pad Prism Software (USA).
Results and Discussion
Plant derived secondary metabolites are receiving great attention in recent years due to their diverse biological activities. It is believed that the use of plants for medicinal purposes have been associated with less side effects. In the present study, we have applied a wide range of established in vitro assays to evaluate the antioxidant and free radical scavenging activities of Codiaeum variegatum extracts. Preliminary phytochemical analysis of Codiaeum variegatum extracts revealed the presence of alkaloids, gums, tannins and saponins in the extracts (Table 1). Phenolic compounds and flavonoids were reported to be associated with antioxidant properties, acting as scavengers of singlet oxygen and free radicals.21,22 Gum, tannin and saponin containing plants are also rich sources of antioxidants.23-25
Table 1. Phytochemical screening of C. variegatum extracts.
| Extract | Alkaloids | Gum | Flavonoids | Tannins | Saponins |
| Extract of C. variegatum (CP) | ++ | +++ | -- | +++ | ++ |
| Extract of C. variegatum (BP) | ++ | ++ | + | +++ | + |
DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity
Phytochemical screening of the extracts indicated the presence of alkaloids, saponin, gum and tannins (Table 1). The DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of Codiaeum variegatum are given in Table 2 and Figure 1. In the TLC-based qualitative antioxidant assay using DPPH spray, the extract of Codiaeum variegatum showed prominent free radical scavenging properties as indicated by the presence of a yellowish spot on a reddish purple background on the TLC plate. The IC50 values of the extracts were found to be 40.93 µg/mL and 73.16 μg/mL for CP and BP respectively whereas IC50 for ascorbic acid was 17.17 μg/mL, which is a well known antioxidant.
Table 2. IC50 values of C. variegatum extracts in different antioxidant assays such as DPPH method, NO- scavenging method, H2O2 scavenging, Scavenging of TBARS method and total phenolic content. Values are expressed as average of duplicate experiments.
| Sample | IC50 (µg/mL) | Total phenolic content (mg of gallic acid equivalent per g of dry extract) | |||
| DPPH scavenging method | NO- scavenging method | H2O2 scavenging method | Scavenging of TBARS | ||
| Extract of C. variegatum (CP) | 40.93 µg/mL | 186.2µg/mL | 148.5 µg/mL | 148.5 µg/mL | 35.73 |
| Extract of C. variegatum (BP) | 73.17 µg/mL | 159.9 µg/mL | 148.3 µg/mL | 169.0 µg/mL | 97.28 |
| Ascorbic acid | 17.17 µg/mL | 91.15 µg/mL | 124.9 µg/mL | 115.8 µg/mL | - |
Figure 1.
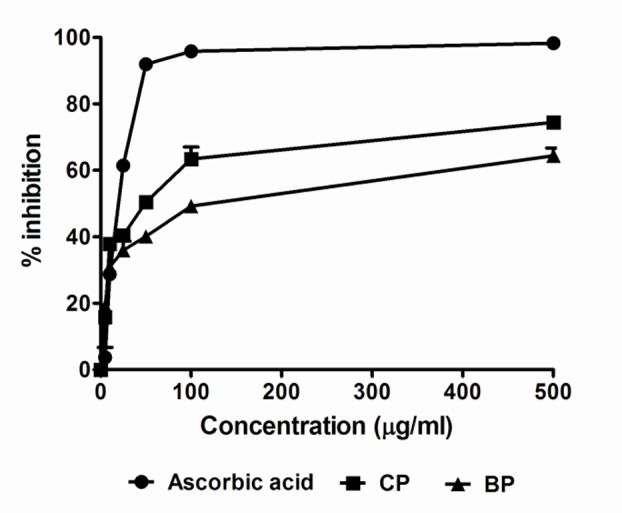
DPPH radical scavenging activity of the ethanol extract of Codiaeum variegatum leaves. DPPH radical scavenging activity was increased by increasing the concentration of the sample extract. Data are represented as Mean ± SD of duplicate experiments.
Euphorbiaceae plant family possess strong antioxidant activities which are greatly associated with the presence of phenolic compounds.26 In this study, Codiaeum variegatum extracts showed significant scavenging of DPPH free radicals compared to the standard antioxidants. DPPH is a stable free radical, pink in solution, which can accept one electron from antioxidant containing plant extracts, thus neutralizes its free radical nature. The degree of decolourization of the DPPH solution can be measure in UV spectrophotometer and indicates the scavenging activity of the plant extracts.27,28
NO· scavenging activity
Suppression of NO· release may partially be attributed to direct NO· scavenging, as all Codiaeum variegatum extracts decreased the amount of nitrite generated from the decomposition of sodium nitroprusside in vitro. The scavenging of nitric oxide by the plant extract was increased in a dose-dependent manner. The IC50 value of the extract was 186.2 μg/mL and 159.9 μg/mL respectively whereas the IC50 value of vitamin C was 95.15 μg/mL (Table 2 and Figure 2).
Figure 2.
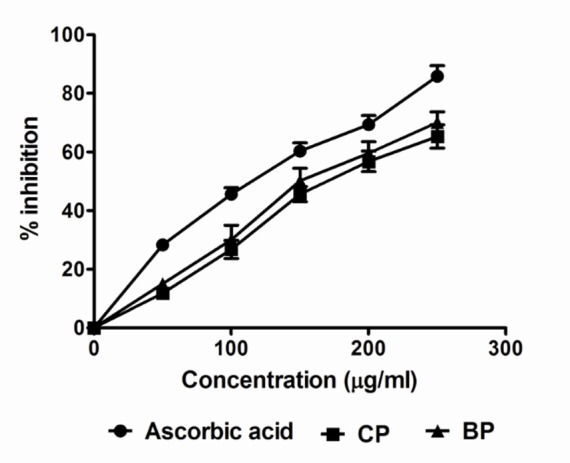
Scavenging of NO radical by Codiaeum variegatum leaf extracts. Data are represented as Mean ± SD of duplicate experiments.
Nitric oxide is a gaseous water soluble molecule, implicated in inflammation, cancer and other pathological conditions.29,30 Both nitric oxide and superoxide anion can cause injury to various tissues. The toxicity and damage caused by NO·and .O-2 is multiplied as they react to produce reactive peroxynitrite (ONOO_), which leads to serious toxic reactions with biomolecules.30,31 The scavenging activity of reactive peroxynitrite helps to arrest the chain of reactions initiated by excess generation of NO that are detrimental to the human health.16 Suppression of NO· release may partially be attributed to direct NO· scavenging, as all Codiaeum variegatum extracts decreased the amount of nitrite generated from the decomposition of sodium nitroprusside in vitro.
Scavenging of H2O2 activity
The scavenging of H2O2 by vitamin C and the extract of Codiaeum variegatum after incubation for 10 min was increased with increased concentration of the sample. The extract exhibited higher H2O2 scavenging activity than vitamin C at similar concentrations. The IC50 values of the extracts CP and BP and ascorbic acid were 148.5 and 148.3 µg/mL and 124.9 µg/mL respectively (Table 2 and Figure 3).
Figure 3.
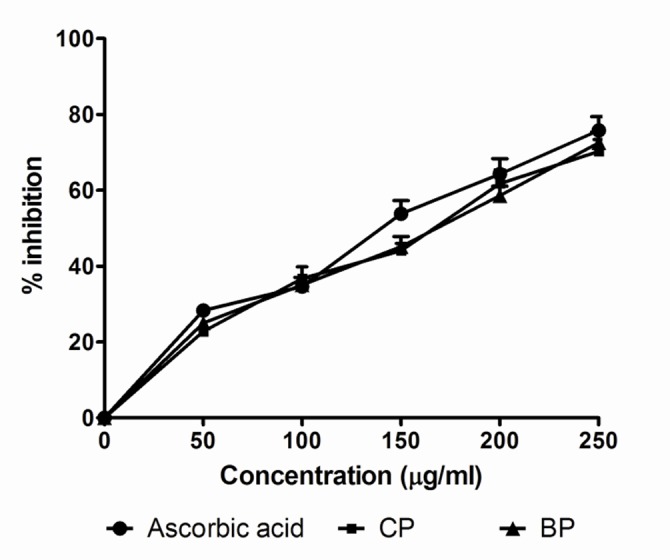
Scavenging of H2O2 by Codiaeum variegatum leaf extracts. Data ara represented as Mean ± SD of duplicate experiments.
Hydrogen peroxide is toxic to cell because it may give rise to hydroxyl radicals due to the presence of iron ions.32 Therefore, removing H2O2 is very important for antioxidant defense in cells. Dietary polyphenols (especially compounds with the orthodihydroxy phenolic structure quercetin, catechin, gallic acid ester, caffeic acid ester) showed protection against hydrogen peroxide induced cytotoxicity in mammalian and bacterial cells.33 The phenolic compounds of the C. variegatum extracts may be involved in removing the H2O2.
Reducing activity, total antioxidant capacity and total phenolic compounds
Our data on the reducing power of the tested extracts suggests low to moderate reducing properties. Like other antioxidant assays, the reducing power of Codiaeum variegatum extracts increased with increasing the amount of samples. Figure 4 shows the reducing ability of Codiaeum variegatum extracts in comparison with ascorbic acid. Total antioxidant capacity of the Codiaeum variegatum extract, expressed as the number of equivalents of ascorbic acid, is shown in Figure 5. Total antioxidant capacity was also increased in a dose-dependent manner.
Figure 4.
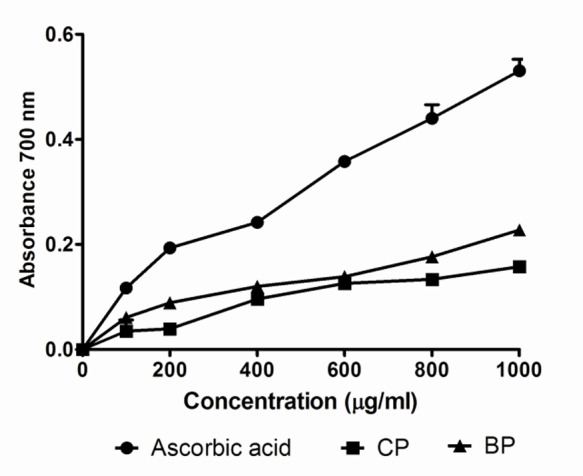
Reducing power of Codiaeum variegatum leaves ethanol extracts. An increase in absorbance in the reducing power method implies that extracts are capable of donating hydrogen atoms in a dose dependent manner. Data was represented as Mean ± SD of duplicate experiments.
Figure 5.
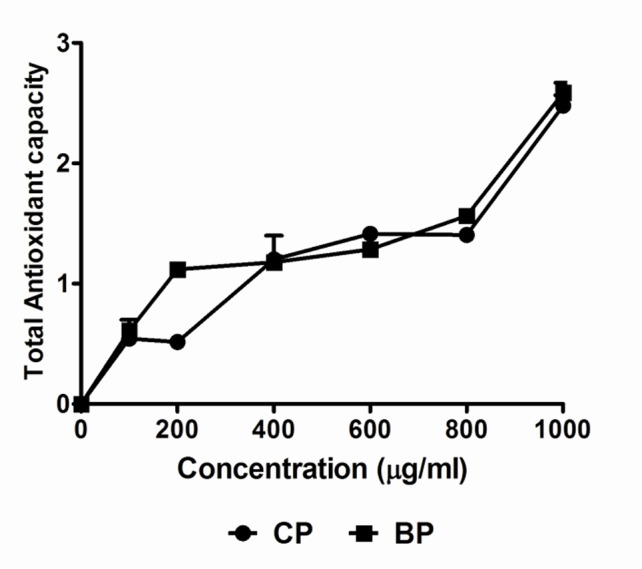
Total antioxidant capacity of Codiaeum variegatum leaf extracts. Data was represented as Mean ± SD of duplicate experiments.
Direct correlation between antioxidant activity and reducing power of certain plant extracts were observed previously.34 The reducing properties are generally associated with the presence of reductones which showed antioxidant activity by breaking the free radical chain donating a hydrogen atom.18 Our data on the reducing power of the tested extracts suggests moderate reducing properties. However, the antioxidant activity of plant extracts are attributed to various antioxidants present followed by various mechanisms such as prevention of chain initiation, binding of transition metal ion catalysts, decomposition of peroxides, prevention of continued hydrogen abstraction, reductive capacity and radical scavenging.18,35 Like the total antioxidant activity, the reducing power of Codiaeum variegatum extracts increased with increasing the amount of samples.
The amount total phenolic compound was calculated as quite high in the methanol extract of Codiaeum variegatum (BP) (97.28 mg of gallic acid equivalent) (Table 2). According to the results of this study, it can be revealed that the high inhibition value in the methanol extract might be due to the high concentration of phenolic compounds present in the extract.
Lipid peroxidation assay and RBC membrane stabilization assay
Activity of plant extract against non-enzymatic lipid peroxidation in rat liver microsomes is shown in Table 2 and Figure 6. Addition of Fe2+/ascorbate to the liver microsomes causes a rise in lipid peroxidation. The extract showed inhibition of peroxidation effect in all concentrations, which showed 50% inhibition at 148.5 and 169.0 μg/mL whereas the vitamin C showed at 115.8 μg/ml. The extract of Codiaeum variegatum at a concentration range of 0.50-2.0 mg/mL also protected the rat erythrocyte membrane against lysis induced by hypotonic solution (Table 3). In contrast, indomethacin (0.10 mg/mL) offered a significant protection of the rat red blood cells (RBC) against the damaging effect of a hypotonic solution.
Figure 6.
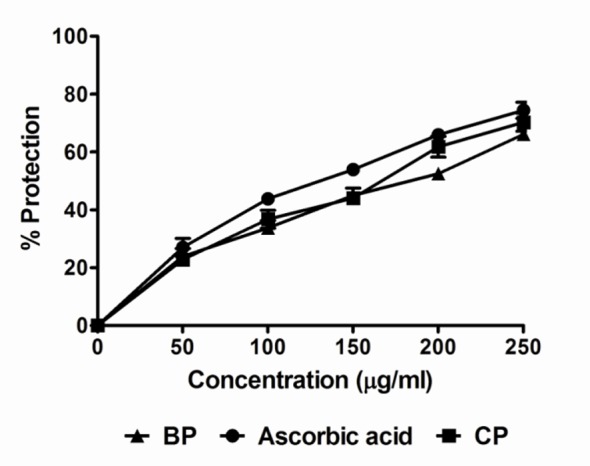
Prevention of lipid peroxidation product TBARS formation by Codiaeum variegatum leaf extracts. Data are represented as Mean ± SD of duplicate experiments.
Table 3. Membrane stabilizing activity by two varieties of C. variegatum extracts. Values are expressed as average of duplicate experiments.
| Concentration |
Absorbance BP |
% Protection |
Absorbance CP |
% Protection |
| Hypotonic medium 50 mM | 0.35± 0.01 | - | 0.35± 0.01 | - |
| C. variegatum 0.25 mg/mL | 0.287 | 17.857 | 0.260 | 25.57 |
| 0.5 mg/mL | 0.280 | 19.857 | 0.223 | 36.285 |
| 1.0 mg/mL | 0.171 | 51 | 0.203 | 41.857 |
| 1.5 mg/mL | 0.106 | 69.571 | 0.116 | 66.714 |
| 2.0 mg/mL | 0.059 | 83.142 | 0.050 | 85.601 |
| Indomethacin ( 0.10 mg/mL) | 0.051±0.003 | 85.26±0.41 | - | - |
Superoxides, ferrous state of iron and H2O2 are able to cause lipid peroxidation via fenton reaction in biological membrane.36 During lipid peroxidation, low molecular-weight end products, probably malondialdehyde, are formed by oxidation of polyunsaturated fatty acids which can react with two molecules of thiobarbituric acid to give a pinkish red chromogen. Exposure to hypotonic medium may cause lysis of red blood cell membrane accompanied by haemolysis and oxidation of haemoglobin.37 The haemolytic effect of hypotonic solution is related to excessive accumulation of fluid within the cell resulting in the rupturing of its membrane. Such injury to RBC membrane will further render the cell more susceptible to secondary damage through free radical induced lipid peroxidation.38 The breakdown of cell membranes leads to enhance further cellular damage.39 Compounds with membrane-stabilizing properties are well known for their ability to interfere with the early phase of inflammatory reactions, namely the prevention of the release of phospholipases that trigger the formation of inflammatory mediators.40 As a rich source of antioxidant phenolic compounds, the extracts of this plant exhibit significant membrane stabilizing property.
HPLC-DAD analysis of phenolic contents in leaves extracts
Identification and quantification of individual phenolic compounds in the large leaves of Codiaeum variegatum were analysed by HPLC-DAD system. In this study, we used eleven different phenolic standards, C18 column with 250 mm length, and rapid separation LC (RSLC) systems while other investigators used six standards,20 C18 column with 150mm length, and HP 1090, series II, liquid chromatography systems to determine the polyphenolic contents. The wavelength between 210 and 380 nm was used for the detection of polyphenolic compounds.41,42 Therefore, 280, 320 and 380 nm wavelength were selected for the detection of all standards in this study. From Figure 7, it can be observed that a good separation can be achieved within 30 min using the above condition described. Symmetrical, sharp and well-resolved peaks were observed for the eleven polyphenolic standards. The elution order and the retention times for GA, CH, VA, CA, EC, PCA, RH, EA, MC, QU, and KF were 6.25, 13.69, 15.95, 16.24, 16.69, 19.89, 21.07, 21.79, 24.54, 26.12, and 27.13 minutes respectively.
Figure 7.
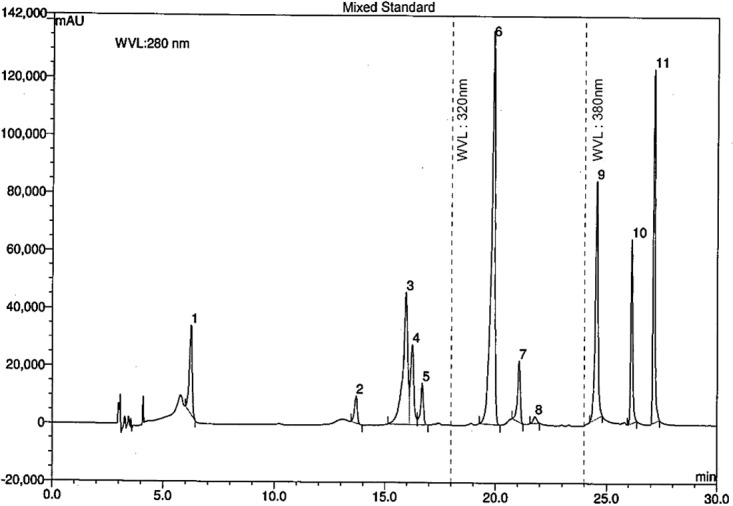
HPLC chromatogram of a standard mixture of polyphenolic compounds. Peaks: 1, gallic acid; 2, (+)-catechin; 3, vanillic acid; 4, caffeic acid; 5, (–)-epicatechin; 6, p-coumaric acid; 7, rutin hydrate; 8, ellagic acid; 9, myricetin; 10, quercetin; 11, kaempferol.
The chromatographic separations of polyphenols in ethanol extract are shown in Figure 8. The experimental results indicated that the extract containes a high concentration of ellagic acid (187.87 mg per 100 g of dry weight, Table 4). It was also found that moderate concentration of (–)-epicatechin, rutin hydrate and p-coumaric acid (26.20 mg, 56.91 mg and 15.82 mg per 100 g of dry weight, Table 4) was present in the large leaf extract. Ellgic acid is a potent antioxidant and anticancer compound. Ellagic acid showed cytotoxicity in HSC-2 oral carcinoma cells by inducing apoptosis but not toxic to normal cells.43 Previously we reported that Codiaeum variegatum extracts showed potent cytotoxicity in brian shrimp lethality bioassays.10 HPLC analysis of the C. variegatum extracts suggested that high ellagic acid content may be responsible for the cytotoxicity of the plant extract. However, other plant phenolics such as (–)-epicatechin, rutin hydrate and p-coumaric acid are also potent antioxidants and inhibitors of tumor cells. Therefore, a synergistic action of those compounds may possible while using this plant extracts.
Figure 8.
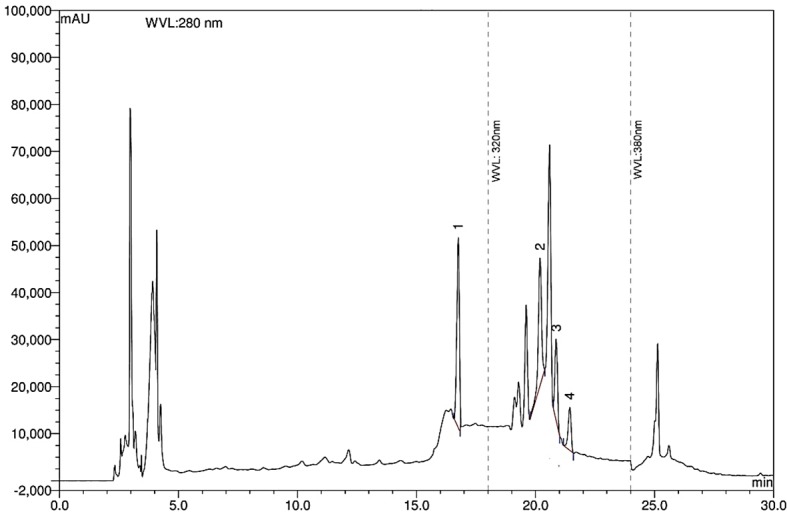
HPLC chromatogram of Codiaeum variegatum extracts. Peaks: 1, (–)-epicatechin; 2, p-coumaric acid; 3, rutin hydrate; 4, ellagic acid.
Table 4. Contents of polyphenolic compounds in the Codiaeum variegatum large leaf extract.
| Polyphenolic compound | Codiaeum variegatum large leaves extract | |
| Content (mg/100 g of dry extract) | % RSD | |
| EC | 26.20 | 1.03 |
| PCA | 15.82 | 0.94 |
| RH | 56.91 | 1.96 |
| EA | 187.87 | 8.52 |
EA, Ellagic acid, PCA, p-Coumaric acid, RH, Rutin hydrate, EC, (-) Epicatechin
Conclusion
On the basis of this study, both Codiaeum variegatum extracts (CP and BP) showed significant antioxidant activities compared to standard compounds in vitro. The various antioxidant mechanisms of Codiaeum variegatum extract may be attributed to its strong abilities as a hydrogen donor or as scavenger of nitric oxide and hydrogen peroxide free radicals. In addition, the antioxidant activities showed by the extracts are mainly due to phenolic compounds present in Codiaeum variegatum extracts. Further HPLC-DAD analysis of the extracts proposed that ellagic acid may be responsible for the antioxidant activity of Codiaeum variegatum extracts. Further work should be carried out using Codiaeum variegatum extracts to determine in-vivo antioxidant and anti-tumor activities. Moreover, the phenolic compounds have been of interest of health benefits, the present HPLC study could be indicative to a potential application to identify and quantify the polyphenolic compounds in any medicinal plant extract.
Acknowledgments
This project was funded by Department of Pharmacy, Stamford University Bangladesh. Authors are grateful to the authority of Stamford University Bangladesh.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Azaizeh H, Saad B, Cooper E, Said O. Traditional Arabic and Islamic Medicine, a Re-Emerging Health Aid. Evid Based Complement Alternat Med. 2010;7(4):419–24. doi: 10.1093/ecam/nen039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Škrovánková S, Mišurcová L, Machů L. Chapter three - antioxidant activity and protecting health effects of common medicinal plants. In: Jeyakumar H, editor. Advances in Food and Nutrition Research. Academic Press; 2012. p. 75-139. [DOI] [PubMed] [Google Scholar]
- 3.Gutteridge JM, Halliwell B. Antioxidants: Molecules, medicines, and myths. Biochem Biophys Res Commun. 2010;393(4):561–4. doi: 10.1016/j.bbrc.2010.02.071. [DOI] [PubMed] [Google Scholar]
- 4.Ndhlala AR, Moyo M, Van Staden J. Natural antioxidants: fascinating or mythical biomolecules? Molecules. 2010;15(10):6905–30. doi: 10.3390/molecules15106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghani A. Medicinal plants of bangladesh with chemical constituents and uses. 2nd ed. Dhaka: Asiatic Society of Bangladesh; 2002. [Google Scholar]
- 6.Mwine JT, Damme PV. Why do Euphorbiaceae tick as medicinal plants?: A review of Euphorbiaceae family and its medicinal features. J Med Plant Res. 2011;5(5):652–62. [Google Scholar]
- 7.Ogunwenmo KO, Idowu OA, Innocent C, Esan EB, Oyelana OA. Cultivars of Codiaeum variegatum (L.) Blume (Euphorbiaceae) show variability in phytochemical and cytological characteristics. Afr J Biotechnol. 2007;6(20):2400–5. [Google Scholar]
- 8.Moundipa P, Kamini G, Charles F, Iris B. Medicinal plants from Cameroon with amoebicidal activity: Codieaum variegatum, a potential source of new products against Amoebiasis. Afr J Tradit Complement Altern Med. 2005;2:113–21. [Google Scholar]
- 9.Forero JE, Avila L, Taborda N, Tabares P, López A, Torres F. et al. In vitro anti-influenza screening of several Euphorbiaceae species: Structure of a bioactive Cyanoglucoside from Codiaeum variegatum. Phytochemistry. 2008;69(16):2815–9. doi: 10.1016/j.phytochem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Saffoon N, Alam M, Uddin M. Phytochemical and cytotoxic investigation of Codiaeum variegatum Linn, leaf. S J Pharm Sci. 2010;3(2):51–3. [Google Scholar]
- 11.Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–52. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alam M, Nyeem M, Awal M, Mostofa M, Alam M, Subhan N. et al. Antioxidant and hepatoprotective action of the crude methanolic extract of the flowering top of Rosa damascena. Orient Pharm Exp Med. 2008;8:164–70. [Google Scholar]
- 13.Uddin R, Saha MR, Subhan N, Hossain H, Jahan IA, Akter R. et al. HPLC-analysis of polyphenolic compounds in Gardenia jasminoides and determination of antioxidant activity by using free radical scavenging assays. Adv Pharm Bull. 2014;4(3):273–81. doi: 10.5681/apb.2014.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadhu SK, Okuyama E, Fujimoto H, Ishibashi M. Separation of Leucas aspera, a medicinal plant of Bangladesh, guided by prostaglandin inhibitory and antioxidant activities. Chem Pharm Bull (Tokyo) 2003;51(5):595–8. doi: 10.1248/cpb.51.595. [DOI] [PubMed] [Google Scholar]
- 15.Alam M, Ghani A, Subhan N, Rahman M, Haque M, Majumder M. et al. Antioxidant and membrane stabilizing properties of the flowering tops of Anthocephalus cadamba. Nat Prod Commun. 2008;3:65–7. [Google Scholar]
- 16.Das S, Bala A, Bhowmik M, Ghosh LK. Attenuation of reactive nitrogen species by different flavonoids enriched fractions of Schima Wallichii. Asian Pac J Trop Biomed. 2012;2:S632–6. [Google Scholar]
- 17.Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003–8. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 18.Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Sendra E, Sayas-Barberá E, Pérez-Álvarez JA. Antioxidant properties of pomegranate (Punica granatum L.) bagasses obtained as co-product in the juice extraction. Food Res Int. 2011;44(5):1217–23. [Google Scholar]
- 19.Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51(3):609–14. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- 20.Chuanphongpanich S, Phanichphant S. Method development and determination of phenolic compounds in broccoli seeds samples. Chiang Mai J Sci. 2006;33(1):103–7. [Google Scholar]
- 21.Mustafa RA, Abdul Hamid A, Mohamed S, Bakar FA. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. J Food Sci. 2010;75(1):C28–35. doi: 10.1111/j.1750-3841.2009.01401.x. [DOI] [PubMed] [Google Scholar]
- 22.Caillet S, Côté J, Doyon G, Sylvain JF, Lacroix M. Antioxidant and antiradical properties of cranberry juice and extracts. Food Res Int. 2011;44(5):1408–13. [Google Scholar]
- 23.Okamura H, Mimura A, Yakou Y, Niwano M, Takahara Y. Antioxidant activity of tannins and flavonoids in Eucalyptus rostrata. Phytochemistry. 1993;33:557–61. [Google Scholar]
- 24.Surveswaran S, Cai YZ, Corke H, Sun M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007;102:938–53. [Google Scholar]
- 25.Behera S, Babu S, Ramani Y, Choudhury P, Panigrahi R. Phytochemical investigation and study on antioxidant properties of Ocimum canum hydro-alcoholic leaf extracts. J Drug Deliv Ther. 2012;2(4):122–8. [Google Scholar]
- 26.Shahwar D, Rehman S, Ahmad N, Ullah S, Raza M. Antioxidant activities of the selected plants from the family Euphorbiaceae, Lauraceae, Malvaceae and Balsaminaceae. Afr J Biotechnol. 2010;9(7):1086–96. [Google Scholar]
- 27.Jayaprakasha GK, Girennavar B, Patil BS. Radical scavenging activities of Rio Red grapefruits and Sour orange fruit extracts in different in vitro model systems. Bioresource Technol. 2008;99(10):4484–94. doi: 10.1016/j.biortech.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 28.Thambiraj J, Paulsamy S, Sevukaperumal R. Evaluation of in vitro antioxidant activity in the traditional medicinal shrub of western districts of Tamilnadu, India, Acalypha fruticosa Forssk. (Euphorbiaceae) Asian Pac J Trop Biomed. 2012;2:S127–30. [Google Scholar]
- 29.Tennyson AG, Lippard SJ. Generation, translocation, and action of nitric oxide in living systems. Chem Biol. 2011;18(10):1211–20. doi: 10.1016/j.chembiol.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, Wu PF, Wang F, Chen JG. Targeting gaseous molecules to protect against cerebral ischaemic injury: Mechanisms and prospects. Clin Exp Pharmacol Physiol. 2012;39(6):566–76. doi: 10.1111/j.1440-1681.2011.05654.x. [DOI] [PubMed] [Google Scholar]
- 31.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142(2):231–55. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jagetia GC, Reddy TK. Alleviation of iron induced oxidative stress by the grape fruit flavanone naringin in vitro. Chem Biol Interact. 2011;190(2–3):121–8. doi: 10.1016/j.cbi.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama T, Yamada M, Osawa T, Kawakishi S. Suppression of active oxygen-induced cytotoxicity by flavonoids. Biochem Pharmacol. 1993;45(1):265–7. doi: 10.1016/0006-2952(93)90402-i. [DOI] [PubMed] [Google Scholar]
- 34.Tung YT, Wu JH, Hsieh CY, Chen PS, Chang ST. Free radical-scavenging phytochemicals of hot water extracts of Acacia confusa leaves detected by an on-line screening method. Food Chem. 2009;115(3):1019–24. [Google Scholar]
- 35.Ashokkumar D, Thamilselvan V, Senthikumar GP, Mazumder UK, Gupta M. Antioxidant and Free Radical Scavenging Effects of Lippia nodiflora. Pharm Biol. 2008;46(10-11):762–71. [Google Scholar]
- 36.Gulcin I, Huyut Z, Elmastas M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem. 2010;3(1):43–53. [Google Scholar]
- 37.Sam GPG, Suresh AJ, Aruna A, Niraimathi V. In-vitro studies of antioxidant and membrane stabilization activity of 2-substituted 4, 5-diphenyl imidazole derivatives. Am J PharmTech Res. 2012;2(4):643–8. [Google Scholar]
- 38.Kar B, Kumar RBS, Karmakar I, Dolai N, Bala A, Mazumder UK. et al. Antioxidant and in vitro anti-inflammatory activities of Mimusops elengi leaves. Asian Pac J Trop Biomed. 2012:S976–80. [Google Scholar]
- 39.Maxwell SR. Prospects for the use of anti-oxidant therapies. Drugs. 1995;49(3):345–61. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- 40.Aitadafoun M, Mounieri C, Heyman SF, Binistic C, Bon C, Godhold J. 4-Alkoxybenzamides as new potent phosholipase A2 inhibitors. Biochem Pharmacol. 1996;51:737–42. doi: 10.1016/0006-2952(95)02172-8. [DOI] [PubMed] [Google Scholar]
- 41.Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–84. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakakibara H, Honda Y, Nakagawa S, Ashida H, Kanazawa K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J Agric Food Chem. 2003;51:571–81. doi: 10.1021/jf020926l. [DOI] [PubMed] [Google Scholar]
- 43.Weisburg JH, Schuck AG, Reiss SE, Wolf BJ, Fertel SR, Zuckerbraun HL. et al. Ellagic Acid, a Dietary Polyphenol, Selectively Cytotoxic to HSC-2 Oral Carcinoma Cells. Anticancer Res. 2013;33(5):1829–36. [PubMed] [Google Scholar]


