Abstract
Purpose: Vitamin D, a liposoluble vitamin has many benefits on health. Encapsulation of bioactives in lipid-based carrier systems like nanoliposomes preserves their native properties against oxidation over time along with providing its stable aqueous dispersion.
Methods: In the current study, vitamin D3 nanoliposomes were prepared using thin-film hydration-sonication method and fully characterized by different instrumental techniques.
Results: According to FTIR and DSC results, no interaction was observed between encapsulated nutraceutical and liposome constituents. The particle size and size distribution (Span value) were calculated 82–90 nm and 0.70–0.85, respectively. TEM analysis showed nano sized globular and bilayer vesicles. In all formations, the encapsulation efficiency of vitamin D3 was calculated more than 93%. Addition of cholesterol to lecithin bilayer increased the negative zeta potential from -29 to -43mV.
Conclusion: The results of this study concluded that the liposomal nanoparticles may be introduced as a suitable carrier for fortification of beverages with vitamin D3.
Keywords: Nutraceutical, Nanoliposomes, Vitamin D3, Fortification
Introduction
Vitamin D as a sterol compound is the main part of the cartilage and bone matrix.1 Various studies have shown that vitamin D deficiencies are seen often in regions with short periods of sunshine, which result in lower UVB radiation delivery that mostly occurs in winter or at evenings in other seasons. Vitamin D deficiency can occur even in countries with sufficient sunshine due to traditional costumes that prevent people to expose themselves to sunlight.2 Therefore, use of supplements or diets containing vitamin D such as milk is critical in these countries.3 Because of low polarity, vitamin D3 cannot be dissolved completely in aqueous solutions, also liposoluble vitamins like vitamin D3 sensitive to oxidation. In this circumstance, encapsulation can protect vitamin D3 during storage and improve their physiological effectiveness.1 Encapsulation provides the possibility of controlled release of various materials in small (nano or micro) capsules.4 Nanocarriers supply further surface area and capable to improve solubility, bioavailability, controlled release, and targeting of the incorporated ingredients to a greater amount than micron-sized carriers. Indeed, use of nanocarriers, especially lipid based carriers such as nanoemulsions, nanoliposomes, nanonoisome, solid lipid nanoparticles (SLNs) and nanostructure lipid carriers (NLCs), for encapsulation can help stabilizing reactive and sensitive materials.5,6 In aqueous solvents such as water, polar lipids (e.g. phosphatidylcholine or phosphatidylethanolamine) alone or in combination with cholesterol or ergosterol have a tendency to self-assemble in the form of bilayer membranes. These bilayer membranes are relatively flexible and under shear (homogenization or sonication) can be forced to assume a curvature that leads to the creation of round shaped particles called liposomes that can be used to encapsulation, delivery, and release materials with various solubility properties, Because of the possession of both lipid and aqueous phases.7 Liposomes and nanoliposomes have a wide range of uses in food industries by protecting sensitive materials or food additives.8 A liposomal nano colloids containing vitamins E and C has been reported previously.9 It was suggested that liposomes could preserve these oxidation-sensitive vitamins in aqueous foods. Ko and Lee,10 found that nanoliposomes had a good effect on the stabilization of incorporated retinol in various temperatures, UV light statuses and times. Moreover, rapid degradation of retinol occurred in nanoliposomes by increasing the temperature and the highest protective effect of nanoliposome obtained under dark condition at 4°C. According to our literature review results, there is no report on the preparation of vitamin D-loaded nanoliposomes intended for beverage fortification. Therefore, the objective of the present study was to prepare vitamin D3 nanoliposomes using thin film hydration-sonication method followed by fully in-vitro characterization of the prepared formulations.
Materials and Methods
Materials
Soybean phosphatidylcholine (PC) isolated from soybeans, vitamin D3 (cholecalciferol) standard, cholesterol and other chemicals including methanol, dichloromethane, acetonitrile and ethanol were purchased from Acros Organics (USA), DSM (France) and Merck (Germany) with analytical grade, respectively.
Experimental design
Preparation of liposomes containing vitamin D3 :
Liposomes were produced using thin film hydration-sonication technique with a slight modification. lecithin and cholesterol with various quantities (60:0, 50:10, 40:20, 30:30 w/w) were mixed in 15ml ethanol/methanol as solvent (2:1 v/v). Then Vitamin D3 was added to a mixture in round flask. Solvents were evaporated in a rotary evaporator at 30°C and dry lipid sediment remained on the wall of the flask. The residual solvent removed by nitrogen stream at 25°C. Hydration of the lipids was carried out by adding 10 ml of distilled water and 0.5 g of glass beads to the flask and then a rotary evaporator (without vacuum) was used to form MLVs.11 In order to decrease the size, first the sample was subjected to homogenization (15 min/60°C) (Silent Crusher M homogenizer, Germany). Then the formulations were exposed to a probing sonication (Vibra Cell - Sonics & Material, 130 W, 20 kHz, USA) at 70% sonication strength in ice bath for 5 min (5 cycles of 1 min sonication and 3 min rest intermittently to allow cooling of the sample).12
Formulation characterization
Measurement of the liposome size :
The size of vitamin D3 nanoliposomes was assessed by measuring the severity of laser beam scattered by the samples at the angle of 90° with the dynamic light scattering technique at room temperature (Particle Size Analyzer, Wing SALD 2101, Shimadzo, Japan). Four milliliters of distilled water were added to 0.1 ml of liposomal suspension to avoid multiple laser dispersion induced by accumulation of particles. The average particle size was calculated according to the volume mean diameter (VMD) or DeBroukere mean (D4,3) in the equation below:
The particle size distribution was measured using the Span formula as follows:13
Higher Span values indicate greater varieties in size distribution or polydispersity.
Transmission electron microscopy (TEM) :
One drop of each sample was set on a carbon grid and allowed to dry. Then the dried samples studied by a Zeiss-Leo 906 TEM (Germany) at 150 kV.
Measurement of zeta potential :
Almost all particles gain electric charges on their surface when contact with a liquid. This is called zeta potential. Zeta potential is used to show the stability of colloidal suspensions or emulsions; therefore, higher zeta potential values mean more stable suspensions.14 To assess the zeta potential of nanoliposomes, a Zeta Sizer 2000 (Malvern Instruments Ltd., UK) was used at 25 ±0.1ºC. All samples were repeated triples.
Fourier transforms infrared spectroscopy (FTIR) :
Lyophilized empty liposome and liposome containing vitamin D3 were embedded in KBr pellets with the sample: KBr ratio of 1:10 and Fourier transforms infrared (FTIR) spectra of samples recorded using FTIR spectrophotometer (Shimadzo, Japan). The scans were performed over a wave number range of 4000–400 cm−1.
Differential scanning colorimetry (DSC) :
Thermal analysis was performed using a differential scanning calorimeter (model 200 F 3 Maia, Germany). Thermograms were obtained at a scanning rate of 30°C/min. The analyses were performed using 5 mg of each sample in standard aluminum pans. Empty liposome and vitamin D3 liposomes were scanned between 25 to 300°C, and -20 to 300°C, respectively.
Encapsulation efficiency :
To determine the amount of vitamin D3 loaded in liposomes, HPLC (Knauer, Germany) was used with Knauer C18 column (10 µm, 250 × 4.6 mm), mobile phase of methanol-acetonitrile (80:20), flow rate of 2 ml/min and UV detector wavelength at 265 nm. Briefly, the aqueous solution was centrifuged and the free vitamins and lipids were removed. Then, the vitamin D3 inside the liposomes was extracted by adding chloroform to the sample. HPLC linearity was calculated using four working standard solutions (5–20 µg/ml) of vitamin D3 methanol/acetonitrile (80:20 v/v). The injection volume was 20 µl and the relative retention time was 8.147 min. Then, the correlation (r2), intercept and slope of the standard curve were calculated. The vitamin concentration in nanoliposomes was calculated using the following equation:10
Liposome stability :
The stability of liposomes was assessed by determining the particle mean diameter and studying the leak out of the vitamin from the liposome after one month of storage at refrigerated temperature by the following equation:15
Statistical analysis
Statistical analysis was carried out on a completely randomized design using One-way ANOVA and Duncken’s mean comparison tests at the significant level of 5% with SPSS version 16.0.
Results and Discussion
Particle size analysis
Particle size analysis showed that the size and size distribution of nanoliposome formulations were in the range of 82 to 89 nm and 0.70 to 0.85, respectively (Table 1). In colloid systems, the particle diameter distribution is often determined by polydispersity. The smaller Span values show that the particle size distribution is narrow and thus almost particles have the same diameter.16 Figure 1B displays the size pattern of formulation F2 obtained from particle sizer device in both volume (VMD) and number (NMD) mean diameters. The small difference among VMD and NMD indicates the homogeneity of the prepared nanoliposomes. However, cholesterol plays a role in lipid carrier structure and orientation of the head groups within the membrane interface regions. Results showed no significant changes in size and size distribution of the vesicles using various amounts of cholesterol in vitamin loaded liposomes. Cholesterol occupies cavities formed by the surfactant monomers assembled into vesicles within liposomal bilayer. Furthermore, cholesterol-phospholipid complex can reduce bilayer permeability to small hydrophilic solutes and ions.17 Mohammad et al,18 reported no significant differences in vesicle size following changing the cholesterol content of the blank and drug loaded liposomes (0–50% mol/mol); similar to reports from the current study. In contrast, Frisken et al,19 showed that cholesterol increased particle size of the Dipalmitoylphosphatidylcholine (DPPC) bilayer during the extrusion method of the liposome preparation. However, no significant changes are seen in the size distribution of liposomes in all DPPC to cholesterol proportions. Another research showed that addition of cholesterol to liposome bilayer resulted in the reduction of particle size.20 Therefore, it can be suggested that the effect of cholesterol on particle size can vary based on the preparation method of liposome and type of phospholipids. Alexander et al,21 added plant sterols into soy phospholipid vesicles using high pressure homogenization to assess the effect of sterol on size, stability and encapsulation efficiency. Sterol made liposomes showed a higher vesicle size and encapsulation efficiency and lower size stability than sterol- free liposomes. These observations were suggested to be attributed to differences in packing of phospholipids with plant sterols.
Table 1. Size stability of vitamin D3-loaded nanoliposomes prepared with various quantities of lecithin: cholesterol.
| Formulation Code | PC:Chola (W:W) | Size (nm)b | ||
| 1 day | 15 days | 30 days | ||
| F1 | 60:0 | 86 ± 8.72 | 593±41.30 | 989±60.26 |
| F2 | 50:10 | 89±11.53 | 80±12.58 | 87±16.17 |
| F3 | 40:20 | 86±10.41 | 180±26.31 | 263±48.95 |
| F4 | 30:30 | 82±12.58 | 210±24.61 | 400±35.92 |
a phosphatidylcholine:Cholestrol
b Size based on volume mean diameter
Figure 1.
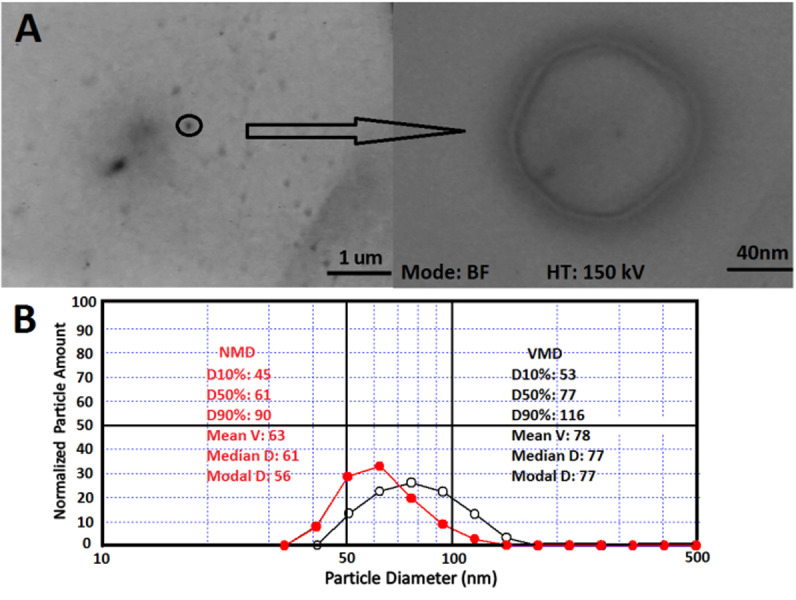
A) Transmission electron microscopy, and B) Particle size pattern of formulation F2 based on volume mean diameter (VMD) (black line) and number mean diameter (NMD) (red line).
Transmission electron microscopy study
TEM analysis showed round, nano-sized bilayer liposomes with a mean diameter of 120 nm with no agglomeration; as shown by particle size analyzer as well (Figure 1A).
Zeta potential
Effect of cholesterol on electrostatic stability of empty liposomes :
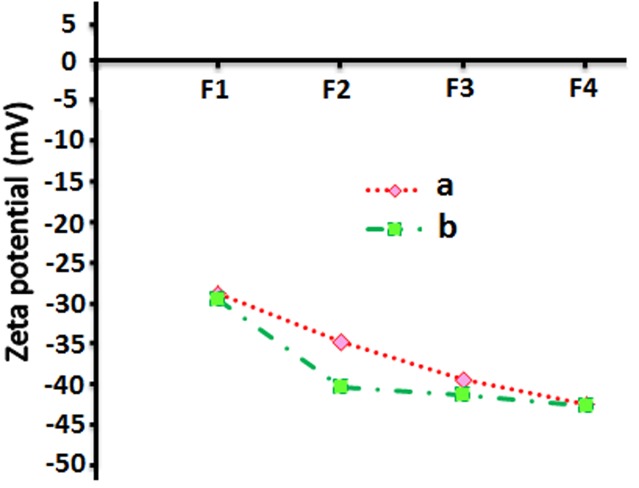
The fact that cholesterol is able to make liposomal membrane stable in biological fluids (e.g. blood) and enhance viscosity and decline permeability has been proven previously.11 In the present study, this stability was assessed by measuring zeta potential of the pure lecithin liposomes by incorporating of various quantities of cholesterol into the phosphatidylcholine bilayer (Figure 2a). However all liposomes consist of zwitterionic phospholipids and neutral cholesterol at the operating pH (7.0), had negative charges. This can be explained by configurational effects of lipids in the assembly the PC dipole head group generates sheltering of the positive net that might be reinforced by the existence of cholesterol, where more of the negative side of the dipole sticks-out of the exterior of the liposome and the positive side pulled more to the interior of the membrane because of hydrogen-bonding interaction with hydroxyl group of cholesterol. These results are similar to results of the study by Tseng et al,22 who observed that zeta potential and electrostatic repulsion among PC liposome membranes increased when cholesterol was added to the suspension. Furthermore, liposomes composed of PC-Cholesterol were more stable and could tolerate severe shear stress. According to previous studies,12 zeta potential of the liposomes containing ferrous sulfate increased as the concentration of cholesterol increased from 0 to 10 mol%.
Figure 2.
Zeta potential of empty nanoliposome (a), vitamin D3 nanoliposome (b) with various quantities of lecithin: Cholesterol
Zeta potential of vitamin D3 nanoliposomes :
Results of zeta potential of nanoliposomes containing vitamin D3 have been shown in Figure 2b. The results indicated that no significant change occurred on zeta potential value by encapsulation of vitamin D3 into liposomes, as it can be predicted due to the low amounts of vitamin D3. In contrast to this study, Fatouros and Antimisiaris14 showed that encapsulation of hydrophobic steroid drugs could influence surface tension of PC liposomes considerably because of change in direction of phosphatidylcholine head groups at the outside of liposomes. Also, Padamwar and Pokharkar23 reported a higher zeta potential value by adding vitamin E to lecithin-cholesterol liposomes.
Encapsulation efficiency
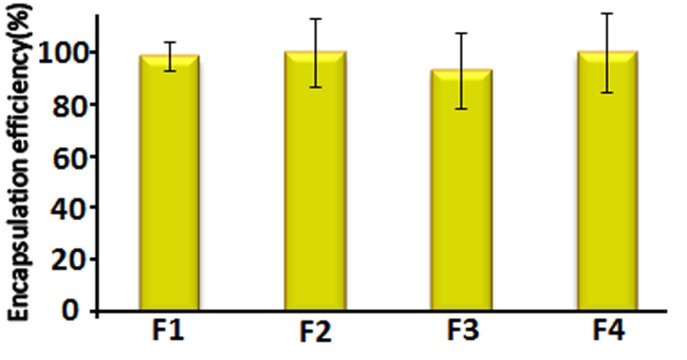
Vitamin D3 is entirely encapsulated in liposomes; nearly 93% for all formulations to be analyzed by HPLC (Figure 3). These results are associated with the fact that vitamin D3 is liposoluble compound and it is encapsulated nearly completely in the bilayer. Compared to hydrophilic compounds, lipophilic compounds exhibit higher encapsulation efficiency and they are more stable against hydrolytic degradation, oxidation and show slower release rate of ingredients. Data from the present study are similar to data from the study by Marsanasco et al,9 who showed a high encapsulation efficiency of vitamin E entrapped into PC liposomes using centrifugation. Montenegro et al24 assessed a high encapsulation efficiency of hydrophobic ingredient in liposomes composed of dipalmitoylphosphatidylcholine. Furthermore, Ko and Lee10 reported the incorporation efficiency of retinol in nanoliposomes over 99%. They demonstrated that the preparation method of liposome is important in the incorporation efficiency of active materials with the highest effectiveness. It has been reported that the thin layer hydration method used in this study, displays maximum encapsulation efficiency of bioactive.
Figure 3.
Encapsulation efficiency of nanoliposome containing vitamin D3 with various quantities of lecithin: cholesterol
Bioactive-carrier interaction
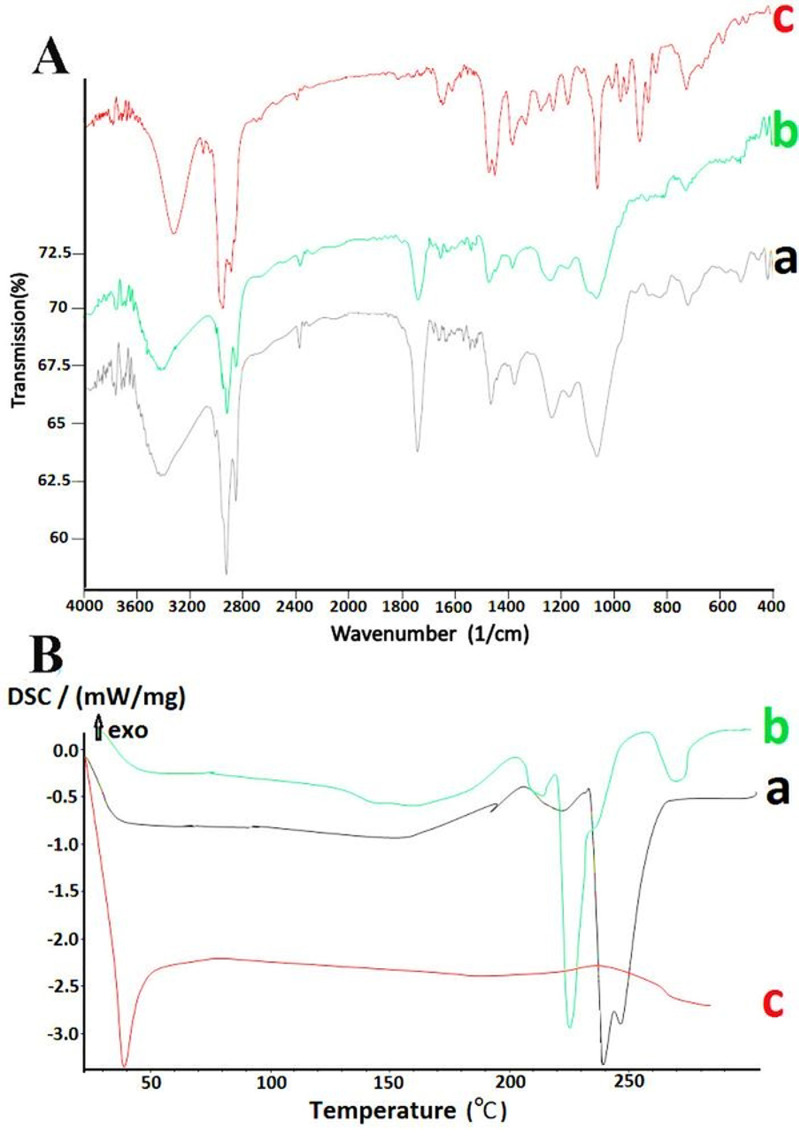
In this study, we have used FTIR spectroscopy and DSC to determine any possible chemical interaction between the bioactive and carrier. Figure 4A, shows the infrared spectrum of empty liposome (a), vitamin D3 liposome (b) and vitamin D3 (c). At the wavenumbers of 3400, 2925, 2850, 1740, 1631, 1446, 1375, 1225, 1056, 800, 720, 520 cm-1, the peak values of liposome containing vitamin D3 and empty liposome were identical. This suggests that there was no chemical interaction between bioactive and carrier compounds. According to another study, vitamin D3 is able to interchange among membranes at a greater speed than cholesterol is, suggesting a mild link between vitamin D3 and phospholipid bilayer than that between D3 and cholesterol.25
Figure 4.
A) Infrared spectrum and B) Differential scanning colorimetry curves of a) lyophilized empty nanoliposome, b) vitamin D3 nanoliposome, and c) vitamin D3.
However, this has been further substantiated by DSC analysis of vitamin D3 and the empty and vitamin D3 nanoliposome. As shown in Figure 4B, liposomes containing vitamin interestingly showed the endotherm at 227°C (lower temperature compered to empty liposome) and the melting endotherm of vitamin D3 has been disappeared. Therefore, changes in thermal properties can be explained by the insertion of vitamin D3 into the lipid bilayer of nanoliposomes and interchange with phospholipid membrane. Indeed, it can be suggested that the weak interaction of vitamin D3 molecules with bilayer of nanoliposomes results in disordered structures and lower melting temperatures, compared to that of empty liposome. Many researchs have been carried out on the effect of active substances on thermal properties of nanocarriers. Chanda et al15 showed that insertion of fluconazole into the lipid bilayer could dissemble the melting endotherm of fluconazole. Pople and Singh26 showed that tacrolimus endotherm was entirely eliminated, which means that tacrolimus was completely inserted into the lipid matrix.
Liposome stability
Particle mean diameter :
The colloidal systems are defined based on their size of particles and with the narrow size distribution in the submicron range. Moreover, particles larger than 1 µm can be a pointer of physical instability.27 In the current study, liposomes were prepared under different reaction conditions such as various ratios of lecithin to cholesterol. The prepared formulation with a ratio of 50 to 10 w/w lecithin and cholesterol (F2) exhibited small particle size (89 nm) with suitable size distribution (span value 0.77) indicating homogenous nature of dispersion. Furthermore this formulation remained stable for one month (1, 15 and 30 days) at refrigeration condition, which suggests a good stability. The mean particle sizes of other formulations (F1, F3 and F4) increased to 989, 262, and 399nm at the end of one month, respectively (Table 1).
Stability of entrapped active material :
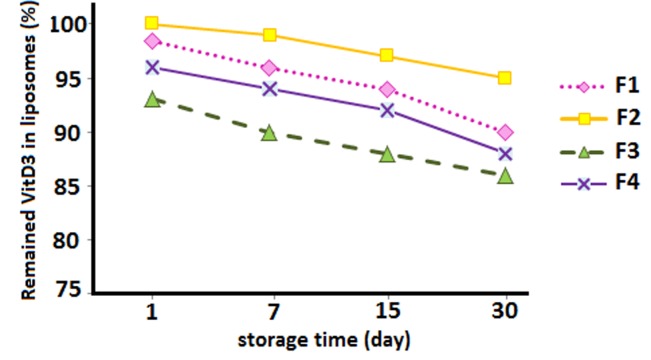
Determination of vitamin D3 stability entrapped in nanoliposome at refrigeration temperature has been measured by HPLC (Figure 5). Results of the present study showed that entrapped substances leaked out slightly at 2–8°C and no significant differences were found in one month, compared to that assessed immediately after the preparation. This can be explained by the following reasons: 1) Presence of cholesterol in liposome structure results in an increases zeta potential and electrostatic repulsion between phosphatidylcholine liposomes. Furthermore, addition of cholesterol to liposome formulation was also shown to reduces the permeability of liposome membrane; therefore, entrapped materials cannot leak out easily; The effect of cholesterol on shear stress tolerance of phosphatidylcholine vesicles had been demonstrated by other researches.28
Figure 5.
Stability studies- percentage of vitamin D3 remaining in nanoliposomes prepared with various quantities of lecithin: cholesterol at refrigerated temperature (2- 8°C) for a period of one month.
2) based on the lipophilic nature of entrapped materials, degradation does not occur; and 3) The best condition to keep vitamin D3 stable in nanoliposomes was darkness and cold temperature (4°C) during the storage period. Zeta potential tends to decline by increasing temperature then the crystalline structure of lipids changes; therefore, the active materials leak out more quickly and the stability reduces. These results are similar to results of a study by Caddeo et al,29 who found that the amount of resveratrol in liposomes did not change during two months at 4°C.
Chanda et al15 assessed the stability and potency of liposomes to reserve inclusions during one month under refrigeration (2-9°C), room (25 ±2°C) and high (45°C) temperatures. Results showed that liposomes were more stable and potent to keep inclusions (leakage < 5%) at refrigeration temperatures and higher temperatures resulted in a higher fluidity of lipid bilayer and higher leakage of inclusions.
Conclusion
In this study, vitamin D3 nanoliposomes were produced successfully by thin film hydration-sonication method. The electrostatic stability of empty liposomes improved by the addition of certain amounts of cholesterol but zeta potential was not considerably changed when the vitamin was incorporated in liposomes. The greatest protective effect of nanoliposome was shown under dark condition at 4°C. These effects of temperature and light are proven by the report that stable gel-phase lipids provide the greatest protection against vitamin D3 degradation. These results also indicate that the nanoliposomes containing bioactive materials have potential applications in improvement of the shelf life of nutraceuticals, stability of cosmetic materials and drug delivery systems.
Acknowledgments
This paper was financially supported by grant no. 92/125 from the Drug Applied Research Center of Tabriz University of Medical Sciences.
Conflict of Interest
There is no conflict of interest to be reported.
References
- 1.Gonnet M, Lethuaut L, Boury F. New trends in encapsulation of liposoluble vitamins. J Control Release. 2010;146(3):276–90. doi: 10.1016/j.jconrel.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 2.Ovesen L, Brot C, Jakobsen J. Food contents and biological activity of 25-hydroxyvitamin D: a vitamin D metabolite to be reckoned with? Ann Nutr Metab. 2003;47(3-4):107–13. doi: 10.1159/000070031. [DOI] [PubMed] [Google Scholar]
- 3.Lamberg-Allardt C. Vitamin D in foods and as supplements. Prog Biophys Mol Biol. 2006;92(1):33–8. doi: 10.1016/j.pbiomolbio.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Mozafari MR, Khosravi-Darani K, Borazan GG, Cui J, Pardakhty A, Yurdugul S. Encapsulation of Food Ingredients Using Nanoliposome Technology. Int J Food Prop. 2008;11(4):833–44. [Google Scholar]
- 5.Fathi M, Mozafari MR, Mohebbi M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci Tech. 2012;23(1):13–27. [Google Scholar]
- 6.Weiss J, Gaysinsky S, Davidson PM, McClements DJ. Nanostructured Encapsulation Systems: Food Antimicrobials In: Barbosa-Canovas G, Mortimer A, Lineback D, Spiess W, Buckle K, Colonna P, editors. Global Issues in Food Science and Technology. New York: Academic Press; 2009. [Google Scholar]
- 7.Taylor TM, Davidson PM, Bruce BD, Weiss J. Liposomal nanocapsules in food science and agriculture. Crit Rev Food Sci Nutr. 2005;45(7-8):587–605. doi: 10.1080/10408390591001135. [DOI] [PubMed] [Google Scholar]
- 8.Gibis M, Zeeb B, Weiss J. Formation, characterization, and stability of encapsulated hibiscus extract in multilayered liposomes. Food Hydrocolloid. 2014;38(0):28–39. [Google Scholar]
- 9.Marsanasco M, Márquez AL, Wagner JR, Alonso SDV, Chiaramoni NS. Liposomes as vehicles for vitamins E and C: An alternative to fortify orange juice and offer vitamin C protection after heat treatment. Food Res Int. 2011;44(9):3039–46. [Google Scholar]
- 10.Ko S, Lee SC. Effect of nanoliposomes on the stabilization of incorporated retinol. Afr J Biotechnol. 2013;9(37):6158–61. [Google Scholar]
- 11.Kirby C, Brooker B, Law B. Accelerated ripening of cheese using liposome‐encapsulated enzyme. Int J Food Sci Tech. 1987;22(4):355–75. [Google Scholar]
- 12.Xia S, Xu S. Ferrous sulfate liposomes: preparation, stability and application in fluid milk. Food Res Int. 2005;38(3):289–96. [Google Scholar]
- 13.Hamishehkar H, Emami J, Najafabadi AR, Gilani K, Minaiyan M, Mahdavi H. et al. The effect of formulation variables on the characteristics of insulin-loaded poly(lactic-co-glycolic acid) microspheres prepared by a single phase oil in oil solvent evaporation method. Colloids Surf B Biointerfaces. 2009;74(1):340–9. doi: 10.1016/j.colsurfb.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Fatouros DG, Antimisiaris SG. Effect of amphiphilic drugs on the stability and zeta-potential of their liposome formulations: a study with prednisolone, diazepam, and griseofulvin. J Colloid Interface Sci. 2002;251(2):271–7. doi: 10.1006/jcis.2002.8432. [DOI] [PubMed] [Google Scholar]
- 15.Chanda H, Das P, Chakraborty R, Ghosh A. Development and evaluation of liposomes of fluconazole. J Pharm Biomed Sci. 2011;5(27):1–9. [Google Scholar]
- 16.Ruozi B, Tosi G, Forni F, Fresta M, Vandelli MA. Atomic force microscopy and photon correlation spectroscopy: two techniques for rapid characterization of liposomes. Eur J Pharm Sci. 2005;25(1):81–9. doi: 10.1016/j.ejps.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Devaraj GN, Parakh SR, Devraj R, Apte SS, Rao BR, Rambhau D. Release studies on niosomes containing fatty alcohols as bilayer stabilizers instead of cholesterol. J Colloid Interface Sci. 2002;251(2):360–5. doi: 10.1006/jcis.2002.8399. [DOI] [PubMed] [Google Scholar]
- 18.Mohammed AR, Weston N, Coombes AG, Fitzgerald M, Perrie Y. Liposome formulation of poorly water soluble drugs: optimisation of drug loading and ESEM analysis of stability. Int J Pharm. 2004;285(1-2):23–34. doi: 10.1016/j.ijpharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Frisken B, Asman C, Patty P. Studies of vesicle extrusion. Langmuir. 2000;16(3):928–33. [Google Scholar]
- 20.Viriyaroj A, Ngawhirunpat T, Sukma M, Akkaramongkolporn P, Ruktanonchai U, Opanasopit P. Physicochemical properties and antioxidant activity of gamma-oryzanol-loaded liposome formulations for topical use. Pharm Dev Technol. 2009;14(6):665–71. doi: 10.3109/10837450902911937. [DOI] [PubMed] [Google Scholar]
- 21.Alexander M, Acero Lopez A, Fang Y, Corredig M. Incorporation of phytosterols in soy phospholipids nanoliposomes: Encapsulation efficiency and stability. Lwt-Food Sci Technol. 2012;47(2):427–36. [Google Scholar]
- 22.Tseng L, Liang H, Chung T, Huang Y, Liu D. Liposomes incorporated with cholesterol for drug release triggered by magnetic field. J Med Biol Eng. 2007;27(1):29. [Google Scholar]
- 23.Padamwar MN, Pokharkar VB. Development of vitamin loaded topical liposomal formulation using factorial design approach: drug deposition and stability. Int J Pharm. 2006;320(1-2):37–44. doi: 10.1016/j.ijpharm.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Montenegro L, Panico AM, Bonina F. Quantitative determination of hydrophobic compound entrapment in dipalmitoylphosphatidylcholine liposomes by differential scanning calorimetry. Int J Pharm. 1996;138(2):191–7. [Google Scholar]
- 25.Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta. 2007;1771(6):663–76. doi: 10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Pople PV, Singh KK. Development and evaluation of colloidal modified nanolipid carrier: application to topical delivery of tacrolimus. Eur J Pharm Biopharm. 2011;79(1):82–94. doi: 10.1016/j.ejpb.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Heurtault B, Saulnier P, Pech B, Proust JE, Benoit JP. Physico-chemical stability of colloidal lipid particles. Biomaterials. 2003;24(23):4283–300. doi: 10.1016/s0142-9612(03)00331-4. [DOI] [PubMed] [Google Scholar]
- 28.Lu Q, Li DC, Jiang JG. Preparation of a tea polyphenol nanoliposome system and its physicochemical properties. J Agric Food Chem. 2011;59(24):13004–11. doi: 10.1021/jf203194w. [DOI] [PubMed] [Google Scholar]
- 29.Caddeo C, Teskac K, Sinico C, Kristl J. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int J Pharm. 2008;363(1-2):183–91. doi: 10.1016/j.ijpharm.2008.07.024. [DOI] [PubMed] [Google Scholar]