Abstract
Purpose: Exploration of plant combinations could be an alternative approach for diabetes treatment. The aim of this study is to evaluate the hypoglycemic effect of combination of A. indica and G. procumbens ethanolic extracts in alloxan-induced diabetic rats.
Methods: Powder of A. indica and G. procumbens leaves were macerated with ethanol 70%. Determination of rutin in A. indica and quercetin in G. procumbens were performed by TLC-densitometry. Hyperglycemia in rats was induced by an intraperitoneal injection of alloxan monohydrate at a single dose of 150 mg/kgBW. The rats were treated with 3 dosage variation of combinations for 15 days. Hypoglycemic effect was evaluated by estimating the blood glucose levels and the rats pancreas histological study.
Results: A. indica contained 2.90±0.15% of rutin and G. procumbens contained 18.86±0.86% of quercetin. Combination at the ratio of 50mg/kgBW A. indica:112.5mg/kgBW G. procumbens showed the highest hypoglycemic effect: 68.74±4.83% (preprandial) and 73.91±3.18% (postprandial). Histological studies indicated that this combination improved the morphology of the islets of Langerhans and β cells. It also increased insulin expression and decreased the elevated-glucose concentrations.
Conclusion: This study showed that combination of both extracts has better hypoglycemic effect than the single treatment of A. indica or G. procumbens. Combination of both extracts was potential to develop as a blood glucose-lowering agent for diabetic patients.
Keywords: Diabetes mellitus, Alloxan, A. indica, G. procumbens
Introduction
Diabetes mellitus (DM) remains a global health problem that continues to increase rapidly.1 However, the treatment of DM that is available at this time are relatively expensive and can cause side effects in patients, which causes the need of alternative treatments. Azadirachta indica A. Juss. and Gynura procumbens (Lour.) Merr. are 2 types of plants that have been traditionally used by the people of Indonesia to treat various diseases including DM. Several researches has reported that A.indica significantly lowered blood glucose in alloxan and streptozotocin induced diabetic rats.2-5 Quercetin, rutin, and nimbidin contained in A. indica are reported to be active components that contributes to its hypoglycemic effect.6 Previous studies reported that the ethanolic extract of G. procumbens leaves significantly lowered blood glucose in streptozotocin-induced rats.7 G. procumbens contains several compounds such as glycosides, flavonoids, tannins and alkaloids that were known to have hypoglycemic activity.8,9 Both plants has been widely investigated and reported for its potential as antidiabetic agent, but the hypoglycemic effect of the combination of both plants has not been reported. Both of these plants were potential to be developed as polyherbal to treat DM. The aim of this research is to study the hypoglycemic effect of combination of A. indica and G. procumbens in alloxan-induced hyperglycemic rats.
Materials and Methods
Materials
A. indica and G. procumbens leaves were collected from Moyudan, Sleman, Yogyakarta. TLC mobile phases, TLC spray reagents, TLC plates (silica gel F254), ethanol 70%, sodium carboxymethyl cellulose, glucose, materials for Hematoxylin-eosin staining, antibodies antiinsulin were obtained from E. Merck, Darmstadt, Germany. Glibenclamide (PT. Kalbe Farma, Tbk.) and GOD-PAP kit with glucose oxidase and 4-aminoantioyrine (DiaSys, Diagnostic Systems GmbH, Holzheim, Germany).
Animals
Male wistar rats (2-3 months) weighing 150-200 g were used in the study. The rats were obtained from the Laboratory of Pharmacology and Toxicology, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, Indonesia. The animals were conditioned for one week before used and were fed with standard laboratory food and water at libitum. During the treatment, they were maintained on constant temperature (22±2°C) and constant relative humidity (55±10%). Ethical clearance for the animal study was obtained from Research Ethics Committee, Integrated Research and Testing Laboratory Universitas Gadjah Mada, Indonesia (Ethical clearance certificate number: 140/KEC-LPPT/III/2014).
Preperation of ethanolic extracts of A. indica and G. procumbens
A. indica and G. procumbens leaves were authentized at Department of Pharmaceutical Biology, Faculty of Pharmacy, Universitas Gadjah Mada, Indonesia. The leaves were washed with water and dried with oven. The powder was macerated with ethanol 70% for 24 hours, respectively, with two times remaceration. Filtrate obtained was then evaporated to get a viscous extract.
Determination of A. indica and G. procumbens marker compounds
Determination of both A. indica and G. procumbens marker compounds were performed by Thin Layer Chromatography (TLC) using silica gel F254 plate as a stationary phase but with different mobile phase. Rutin in A. indica was determined by using n-butanol:glacial acetic acid:water (4:1:5) as mobile phase. Meanwhile, quercetin in G. procumbens was determined by using chloroform:ethyl acetate:methanol:glacial acetic acid (7:2:0.5:0.5). Both rutin and quercetin were detected under UV 254 and UV 366 nm before and after sprayed. AlCl3 spray reagent was used to detect flavonoid and the determination of the compounds amount was perform by densitometer.
Experimental design
Thirty two male Wistar rats aged 1.5-2 months were aclimatized for 1 week and were divided into 8 groups. Diabetic condition was induced by intraperitoneal injection of alloxan monohydrate solution (150 mg/kgBB) in rats that had fasted for 8 hours. Blood glucose levels were measured 72 hours after injection of alloxan. Rats with serum glucose levels ≥ 150 mg/dL is stated to be diabetes and used for further research. Treatments were conducted for 15 days, 2 times a day, with treatment groups as follows: normal group (no treatment), negative control (CMC-Na 0.5%), glibenclamide 0.45 mg/kgBW, ethanolic extract of A. indica 200 mg/kgBW, ethanolic extract of G. procumbens 150 mg/kgBW, combination of A. indica 150 mg/kgBW and G. procumbens 37.5 mg/kgBW (combination 1), combination of A. indica 100 mg/kgBW and G. procumbens 75 mg/kgBW (comb. 2), and combination of A. indica 50 mg/kgBW and G. procumbens 112.5 mg/kgBW (comb. 3). Preprandial and postprandial blood samples were collected on day 0, 5, 10, and 15. Blood glucose levels were determined by Glucose Oxidase Phenol Aminoantipyrine (GOD-PAP) method.
Pancreas histological observation
Pancreas histological observation was conducted on day 15, the end of the experimental period. Rats were euthanized by cervical dislocation and the pancreas were collected and fixed with 4% paraformaldehyde in phosphate buffer saline for 24 hours. The pancreas was then stained with Hematoxylin eosin (HE) for observation of islet Langerhans morphology. Pancreatic insulin expression was conducted by Imunohistochemistry (IHC) analysis.
Statistical analysis
Statistically significant difference of hypoglycemic effect by ANOVA continued with LSD. Significance was set at p<0.05 for all tests.
Results
The extract yeild obtained from the maceration process was 7.87% for A. indica and 7.91% for G. procumbens. Qualitative analysis of A. indica and G. procumbens ethanol extract was done by TLC method and showed the presence of rutin in A. indica (Figure 1) and quercetin in G. procumbens (Figure 2). The amount of the marker compounds was determined by TLC-densitometry. The result was A. indica contained 2.90% rutin and G. procumbens contained 1.89% quercetin.
Figure 1.

TLC profile of A. indica ethanol extract. (a) visible light, (b) under UV 254nm. Spots= (1): Rutin standard (2): A. indica ethanol extract
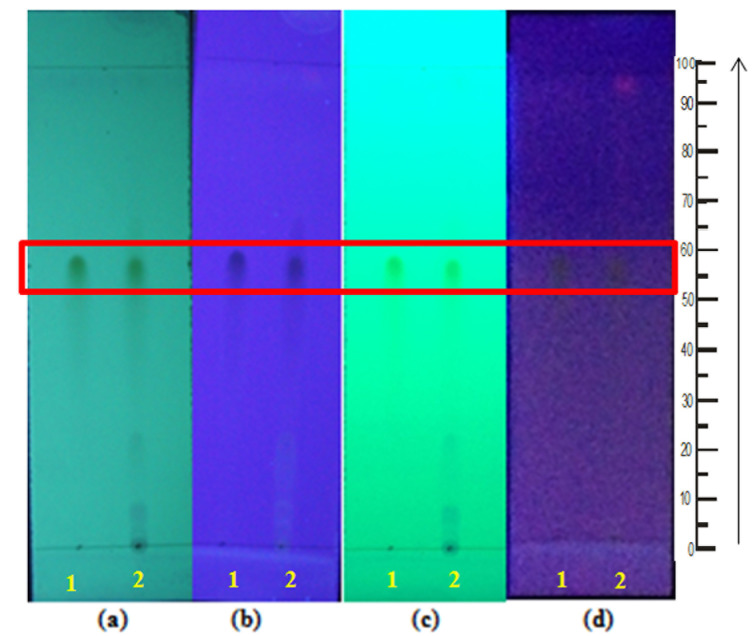
Figure 2.
TLC profile of G. procumbens ethanol extract. (a) under UV 254nm, (b) under UV 366 nm, (c) under UV 254 nm after sprayed with AlCl3, (d) under UV 366 nm after sprayed with AlCl3. Spots = (1) Quercetin standard; (2) G. procumbens ethanol extract
Alloxan induction was conducted before treatment. The result was preprandial and postprandial blood glucose levels of normal and alloxan-induced rats that were significantly higher than normal rats (p<0.05). It means that induction with alloxan 150 mg/kgBW succesfully caused diabetic condition in rats. Determination of blood glucose level was performed after 72 hours of alloxan induction.
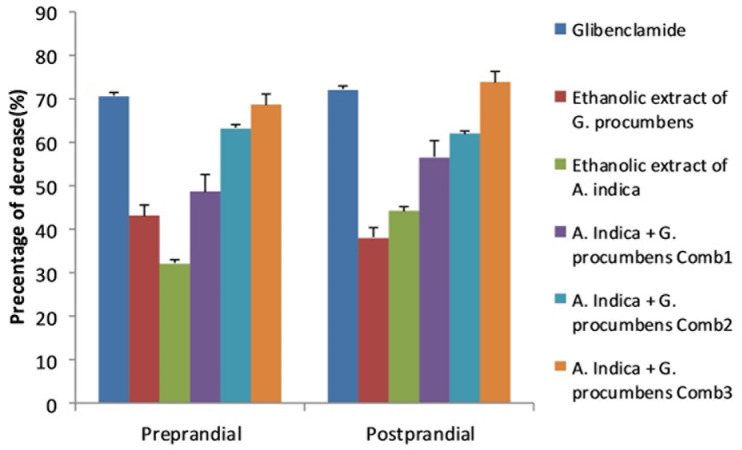
This study showed that single administration of A. indica and G. procumbens and its combination reduced blood glucose level in alloxan-induced rats (Figure 3). Glibenclamide showed the highest preprandial hypoglycemic effect, followed by combination 3, combination 2, combination 1, G. procumbens ethanol extract, A. indica ethanol extract, and CMC-Na 0.5%. Combination 3 had the highest postprandial glycemic effect, followed by glibenclamide, combination 2, combination 1, A. indica ethanol extract, G. procumbens ethanol extract, and CMC-Na 0.5%. Combination 3 showed the highest hypoglycemic effect 68.74±4.83% (preprandial) and 73.91±3.18% (postprandial).
Figure 3.
Profile of hypoglycemic activities (%) of all treatments. Data represent mean±SEM, and are four to five independet experiments. The treatments were glibenclamide 0.45 mg/kgBW; G. procumbens 150 mg/kgBW; A. indica 200 mg/kgBW; A. indica 150 mg/kgBW+ G.procumbens 37.5 mg/kgBB (combination 1); A. indica 100 mg/kgBW + G.procumbens 75 mg/kgBW (combination 2); A. indica 50 mg/kgBW + G.procumbens 112.5 mg/kgBW (combination 3). *P,0.05 compared to the control value.
Histological observation using HE staining showed that A. indica, G. procumbens and its combination improved the morphology of Langerhans islets and β cells in comparison to the negative control group. Combination 3 showed the best improvement based on the number of cell and its shape (Figure 4). IHC analysis also showed that this combination also increased insulin productions. Insulin expression could be seen in the IHC observation showed by brown area in the islets (Figure 5).
Figure 4.
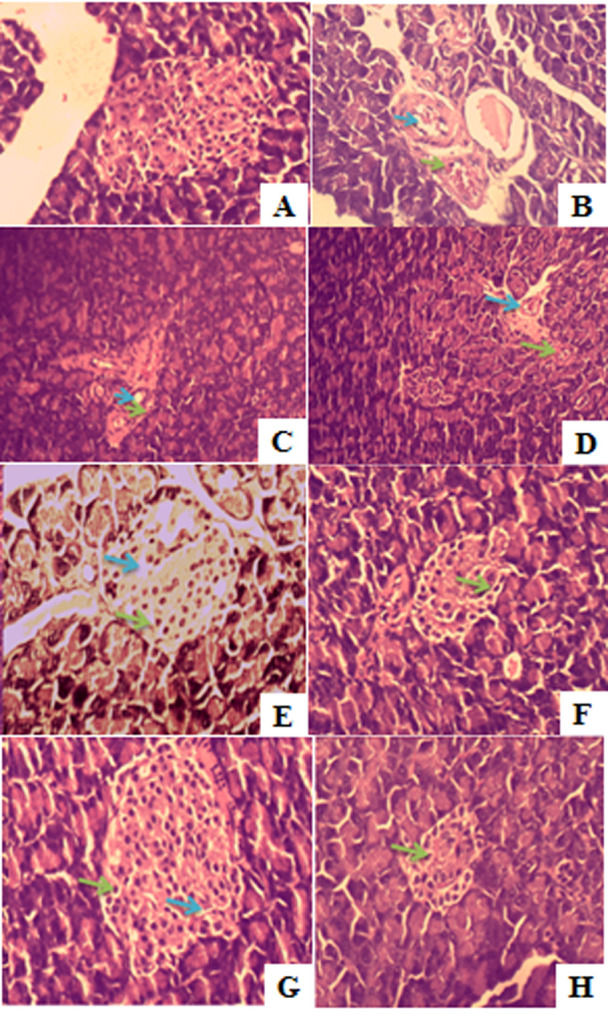
Rat pancreas histology observation with HE staining. (A) Normal group (B) Negative control group (C) Positive control group (D) G. procumbens ethanol extract (E) A. indica ethanol extract (F) Combination 1 (G) Combination 2 (H) Combination 3.
Figure 5.
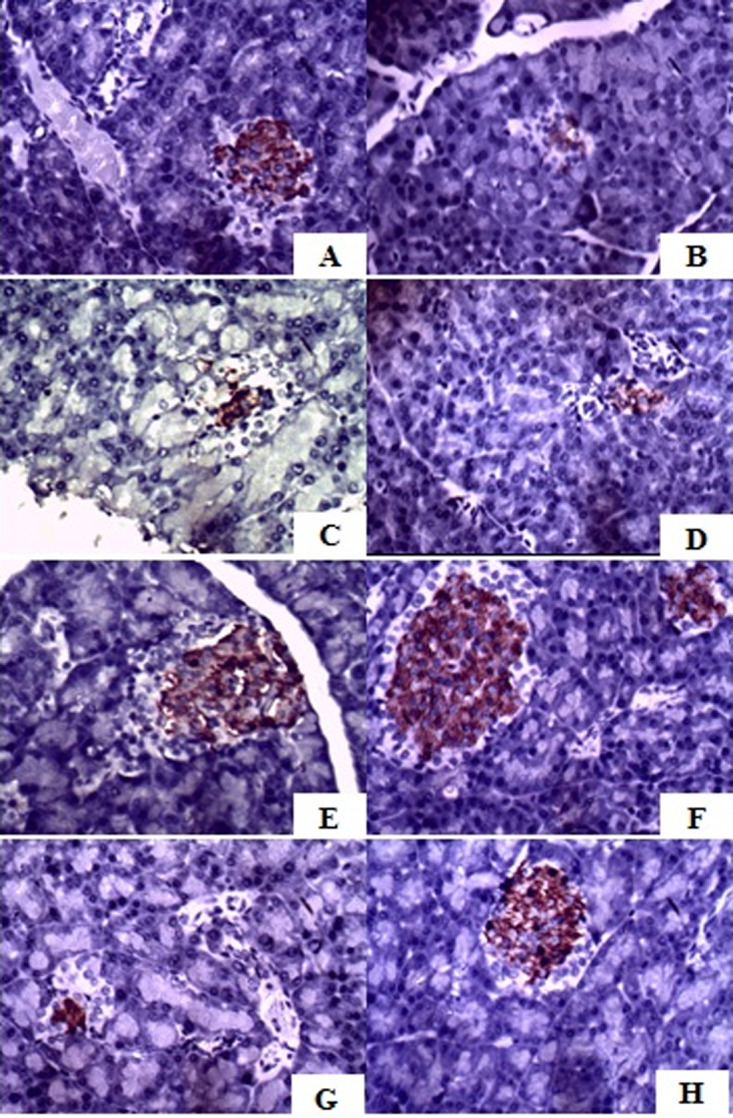
Immunohistochemical-stained sections of insulin rat pancreas observation. A) Normal group (B) Negative control group (C) Glibencamide group (D) Ethanolic extract of G. procumbens (E) Ethanolic extract of A. indica (F) Combination 1 (G) Combination 2 (H) Combination 3.
Discussion
A. indica and G. procumbens are two plants that are known for their hypoglycemic activities. A. indica, also known as neem tree, was reported to have various biological and pharmacological activities, including antiplasmodial, antitrypanosomal, antioxidant, anticancer, antibacterial, antiviral, antiulcer, spermicidal, anthelminthic, larvicidal and fungicidal activities.10 Previous researches has reported that most of the active compound of A. indica belong to the group of tetratriterpenoids and a small number of nonterpenoidal ingredients. About 300 compounds were isolated from various parts of the tree, such as azadirachtin, nimbin, nimbidin, flavonoids and alkaloids.11,12 Meanwhile, G. procumbens, also known as Sambung Nyawa, has also been reported to possess several pharmacological activities such as antiinflammatory, antiherpes simplex virus, antihypertensive, antihyperlipidemic, antisterility and antioxidative capabilities.13 G. procumbens is reported to contain various compounds such as flavonoids, tannins and terpenoids.14,15 A.indica significantly lowered blood glucose in alloxan and streptozotocin-induced diabetic rats.2-5 Quercetin, rutin, and nimbidin contained in A. indica is reported to be the active component that contributes to DM.6 Other studies reported that the ethanolic leaf extract of G. procumbens significantly lowered glycemia in streptozotocin-induced rats.7 Several compounds such as glycosides, flavonoids, tannins and alkaloids in G. procumbens is known to have hypoglycemic activity.8,9
From this study, A. indica contained 2.86% rutin and G. procumbens contained 1.89% quercetin. Rutin and quercetin are both flavonoids which possess high antioxidant activity. A. indica and G. procumbens potential in lowering blood glucose and improving the morphology of the islets of Langerhans and β cells is related to the antioxidant activity of the plants compounds. It is known that oxidative stress plays a role in the pathogenesis of diabetes mellitus. In hyperglycemia state, there is an increase in oxidative stress, which causes defect in insulin action and insulin secretion.16 Rutin has the ability to scavenge free radicals, inhibit lipid proxidation and protect the β cells of the pancreas. It significantly decreases elevated reactive oxygen species while increasing endogenous antioxidant enzymes in kidney of diabetic rats.17 This causes the increase of insulin production and decreased of blood glucose levels. Quercetin also protects againts oxidative damage and preserves pancreatic β cell integrity.18 Several studies reported quercetin mechanism of action in diabetes such as decreases lipid peroxidation; increases antioxidant enzymes activity like superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase18 inhibition of insulin-dependent activation of phosphoinositol-3-kinase (PI-3K);19 and reduces intestinal glucose absorption by inhibiting GLUT 2.20,21
Pharmacology and histology studies in this research indicated that the hypoglycemic effect of the combination of both plants was better than that of single treatment of A. indica or G. procumbens. The various active compounds in the extract of both plants may synergistically increase hypoglycemic effect. However the mechanism action of the active compounds in both extracts needs further investigation.
Conclusion
This study showed that combination of ethanolic extracts of A. indica and G. procumbens has a synergistic effect in lowering blood glucose and improving the morphology of Langerhans islet and β cells. Both plants contained antioxidative compounds. Combination of both extracts was potential to develop as a blood glucose-lowering agent for diabetic patients.
Acknowledgments
We thank the Faculty of Pharmacy Universitas Gadjah Mada for facilitating some part of the study. We also thank the DP2M DIKTI (Directorate of Higher Education) Ministry of Education, Indonesia through “Hibah Unggulan Perguruan Tinggi” Research Grant 2014 for financial support in the study.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization Obesity. Preventing and Manging the Global Epidemic. Geneva: WHO Technical Report Series; 2000. [PubMed] [Google Scholar]
- 2.Dixit VP, Sinha R, Tank R. Effect of Neem seed oil on the blood glucose concentration of normal and alloxan diabetic rats. J Ethnopharmacol. 1986;17(1):95–8. doi: 10.1016/0378-8741(86)90076-0. [DOI] [PubMed] [Google Scholar]
- 3.Murty KS, Rao DN, Rao DK, Murty LBG. A Preliminary Study on the Hypoglycemic and Antihyperglycemic Effect of Azadirachta indica. Indian J Pharmacol. 1978;10:247–50. [Google Scholar]
- 4.Pillai NR, Santkumari G. Hypoglycemic Activity of Melia Azadirachta Linn. (Neem) Indian J Med Res. 1984;14:931–3. [Google Scholar]
- 5.Sukla R, Sunil S, Bhandari CR. Preliminary Clinical Trials on Antidiabetic Effects of Azadirachta indica. Med Surg. 1973;13:11–2. [Google Scholar]
- 6.Chattopadhyay RR. Possible mechanism of antihyperglycemic effect of Azadirachta indica leaf extract: part V. J Ethnopharmacol. 1999;67(3):373–6. doi: 10.1016/s0378-8741(99)00094-x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang XF, Tan BK. Effects of an ethanolic extract of Gynura procumbens on serum glucose, cholesterol and triglyceride levels in normal and streptozotocin-induced diabetic rats. Singapore Med J. 2000;41(1):9–13. [PubMed] [Google Scholar]
- 8.Orhan I, Kupeli E, Sener B, Yesilada E. Appraisal of anti-inflammatory potential of the clubmoss, Lycopodium clavatum L. J Ethnopharmacol. 2007;109(1):146–50. doi: 10.1016/j.jep.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Sharma B, Balomajumder C, Roy P. Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food Chem Toxicol. 2008;46(7):2376–83. doi: 10.1016/j.fct.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Atowadi SE, Atowadi JC. Azadirachta indica (neem): A Plant of Multiple Biological and Pharmacological Activities. Phytochem Rev. 2009;8:601–20. [Google Scholar]
- 11.Satdive RK, Eapen S, Fulzele DP. Bioactive Constituents and Antimicrobial Activity of Cell Cultures of Azadirachta indica. Int J Pharma Bio Sci. 2011;2(4):617–28. [Google Scholar]
- 12.Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological Activities and Medicinal Properties of Neem (Azadirachta indica) Curr Sci. 2002;82(11):1336–45. [Google Scholar]
- 13.Lee H, Hakim P, Rabu A, Sani HA. Antidiabetic Effect of Gynura procumbens Leaves Extracts Involve Modulation of Hepatic Carbohydrate Metabolism in Streptozotocin-induced Diabetic Rats. J Med Plant Res. 2012;6(5):796–812. [Google Scholar]
- 14.Akowuah GA, Amirin S, Mariam A, Aminah I. Blood Sugar Lowering Activity of Gynura procumbens Leaf Extracts. J Trop Med Plant. 2001;2:5–10. [Google Scholar]
- 15.Akowuah GA, Sadikun A, Mariam A. Flavonoid Identification and Hypoglycemic Studies of Butanol Fraction from Gynura procumbens. Pharm Biol. 2002;40(6):405–10. [Google Scholar]
- 16.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 17.Alsaif MA. Beneficial Effects of Rutin and Vitamin C Coadministration in a Streptozotocin-Induced Diabetes Rat Model of Kidney Nephrotoxicity. Pak J Nutr. 2009;8(6):745–54. [Google Scholar]
- 18.Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res. 2005;51(2):117–23. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Stewart LK, Wang Z, Ribnicky D, Soileau JL, Cefalu WT, Gettys TW. Failure of dietary quercetin to alter the temporal progression of insulin resistance among tissues of C57BL/6J mice during the development of diet-induced obesity. Diabetologia. 2009;52(3):514–23. doi: 10.1007/s00125-008-1252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon O, Eck P, Chen S, Corpe CP, Lee JH, Kruhlak M. et al. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007;21(2):366–77. doi: 10.1096/fj.06-6620com. [DOI] [PubMed] [Google Scholar]
- 21.Manzano S, Williamson G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol Nutr Food Res. 2010;54(12):1773–80. doi: 10.1002/mnfr.201000019. [DOI] [PubMed] [Google Scholar]