Abstract
BACKGROUND
A puzzling feature of the long QT syndrome (LQTS) is that family members carrying the same mutation often have divergent symptoms and clinical outcomes.
OBJECTIVES
We tested the hypothesis that vagal and sympathetic control, as assessed from spectral analysis of spontaneous beat-to-beat variability of RR and QT intervals from standard 24-hour electrocardiogram Holter recordings, can modulate the severity of LQTS type 1 (LQT1) in 46 members of a South-African LQT1 founder population carrying the clinically severe KCNQ1-A341V mutation.
METHODS
Nonmutation carriers (NMCs, n = 14) were compared with mutation carriers (MCs, n = 32), 22 with and 10 without major symptoms. We assessed the effect of circadian rhythm and of beta-blocker therapy over traditional time and frequency domain RR and QT variability indices.
RESULTS
The asymptomatic MCs differed significantly from the symptomatic MCs and from NMCs in less vagal control of heart rate and more reactive sympathetic modulation of the QT interval, particularly during daytime when arrhythmia risk for LQT1 patients is greatest.
CONCLUSIONS
The present data identify an additional factor contributing to the differential arrhythmic risk among LQT1 patients carrying the same mutation. A “normal” autonomic control confers a high risk, whereas patients with higher sympathetic control of the QT interval and reduced vagal control of heart rate are at lower risk. This differential “autonomic make-up,” likely under genetic control, will allow refinement of risk stratification within LQTS families, leading to more targeted management.
Keywords: autonomic nervous system, beta-blocker therapy, cardiovascular control, heart rate variability, QT variability
Despite major progress in the understanding (1,2) and management (1,3,4) of the congenital long QT syndrome (LQTS), several unsolved questions of high clinical relevance remain. One of the most puzzling, and most emotionally disquieting for the affected families, is represented by the unequal arrhythmic risk present among family members who carry the same disease-causing mutation. Difficulty foreseeing a benign or life-threatening outcome, even among genetically affected siblings, proves problematic for physicians caring for these patients.
Over the last decade, this challenge prompted numerous attempts to identify “modifier genes,” genetic variants associated with a higher or lower arrhythmic risk (2,5). The current view holds that these modifiers include factors that either modify the underlying arrhythmogenic myocardial substrate or affect the probability and magnitude of arrhythmia-triggering events (2). The former include genes encoding proteins that likely contribute to the balance of inward and outward currents operating during the cardiac action potential, and several of these have already been identified (6–10), while the latter include genes modulating differences in sympathetic and vagal responses (11–13).
The present study aims precisely at expanding the understanding of the relationship between neural control and arrhythmic risk in LQTS. Two concepts, preliminary for the design of our study, were: 1) the focus should have been on the LQTS type 1 (LQT1) patients because they are at risk specifically during sympathetic activation (14); and 2) these LQT1 patients should all be from a single founder population and, thus, have the same mutation, to avoid the phenotypic variability attributed to mutation heterogeneity. Founder populations represent the ideal human model to study modifier genes (6).
We have previously shown that LQT1 asymptomatic mutation carriers (AMCs) were more likely to have a lower heart rate (11), lower baroreflex sensitivity (11), and a smaller heart rate decrease at the end of an exercise stress test than symptomatic mutation carriers (SMCs) (12). The last two findings point to a protective effect of reduced vagal reflexes. A limitation of our studies: although providing novel data about vagal control in LQTS patients, we had no specific information on the sympathetic control at the ventricular level.
The analysis of the spontaneous changes of the heart period and QT interval provides indices that allow noninvasive inferences on the autonomic modulation directed to the sinus node and to the ventricles (15–17). The power of the respiratory-related heart period changes in the high-frequency band (HF; 0.15–0.5 Hz) decreases with the vagal withdrawal progressively induced by graded head-up tilt (18–20). By contrast, the magnitude of fluctuations of the QT interval in the low-frequency band (LF; 0.04–0.15 Hz) positively correlates with the inclination of the tilt table (21,22), suggesting that QT variability and sympathetic control are directly linked (23,24). The combination of these 2 indices (i.e., the HF power of heart period variability and the LF power of QT variability) provides a unique possibility to dissect vagal and sympathetic influences on the heart and to test whether different autonomic patterns might help distinguish AMCs and SMCs.
We tested this hypothesis in a well-characterized LQT1 South African founder population in which all the affected members carry KCNQ1-A341V, one of the LQTS mutations with the most severe phenotype (25–27). This relatively common mutation (26) produces a 50% reduction in basal IKs current and, in vitro, severe reduction in cAMP responsiveness due to failure to phosphorylate KCNQ1 at terminal N-position S27 (28).
METHODS
STUDY POPULATION AND PROTOCOL
The study population included only members of the 25 families constituting the South African LQT1 founder population carrying the KCNQ1-A341V mutation (25–27). Holter recordings were performed in 46 of them, 32 MCs and 14 non-MCs (NMCs) who served as controls. The MCs were further subdivided in SMCs or AMCs according to having suffered or not, regardless of therapy, either syncope (fainting spells with transient but complete loss of consciousness) or aborted cardiac arrest requiring resuscitation. We defined as AMC an individual who had reached age 20 without cardiac events while not being treated with beta-blocker therapy (βB). The 3 groups were of similar age with the median ranging between 35 and 39 years. All patients were studied off βB (βBoff), while 28 out of the 32 MCs (87.5%) were studied also on βB (βBon), which was almost always propranolol. βBoff corresponded to a plasma concentration of propranolol <20 ng·ml−1. Patient selection depended mostly on the physical proximity of their residence to the Stellenbosch area in the Western Cape and on their willingness to come a few times for the Holter recordings.
The study protocol consisted in the acquisition of 74 Holter recordings (12-lead 24-hour; Mortara Instrument Inc., Milwaukee, Wisconsin). The sampling rate was 180 Hz. The analyses were carried out on lead II and were performed during daytime (DAY, from 2:00 PM to 6:00 PM) or nighttime (NIGHT, from midnight to 4:00 AM). The protocol adhered to the principles of the Declaration of Helsinki for medical research involving human subjects. All probands and family members provided written informed consent for clinical and genetic evaluations, as approved by the ethical review boards of the Universities of Stellenbosch, Vanderbilt, and Pavia.
VARIABILITY SERIES EXTRACTION AND DATA ANALYSIS
Electrocardiogram (ECG) recordings were preprocessed to limit broadband noise and cancel baseline wandering (29). Heart period was approximated as the temporal distance between two consecutive R-wave peaks (RR) on the ECG. The R-wave peak was detected using a derivative-threshold algorithm and its occurrence was fixed using parabolic interpolation. The T-wave end was located according to a threshold on the absolute first derivative set as a fraction (i.e., 30%) of the absolute maximal first derivative value computed on the T-wave downslope (29). The temporal distance between R-wave peak and T-wave end (RTe) was taken as an approximation of QT interval automatically measured from the ECG recording (30). All R-wave peak detections were carefully checked. Erroneous identifications were corrected and missed beats manually inserted. Cubic spline interpolation technique was applied over those RR and RTe values that were directly influenced by the occurrence of nonsinus beats. RR and RTe beat-to-beat series were extracted from 24-hour Holter monitoring during DAY and NIGHT. We considered frames of 250 cardiac beats. After calculating the RR and RTe mean (μRR and μRTe), the RR and RTe series were linearly detrended. RR and RTe variance (σ2RR and σ2RTe) were calculated from detrended series.
Spectral analysis was performed via a parametric approach exploiting the autoregressive model (31). Briefly, the autoregressive model describes the beat-to-beat series in the time domain as a linear combination of p past samples weighted by constant coefficients plus a zero mean random white noise. The Levinson-Durbin recursion algorithm was utilized to estimate, directly from the data, the coefficients of the autoregressive model and the white noise variance. The number of coefficients, p, was chosen according to the Akaike’s figure of merit. Power spectral density was computed from the model coefficients and from the white noise variance. The power spectral density was factorized into spectral components, the sum of which provides the entire power spectral density. A spectral component was labeled as LF if its central frequency was in the LF band, whereas it was classified as HF if its central frequency was in the HF band (15). The LF and HF powers were defined as the sum of the powers of all LF and HF spectral components respectively. We assessed the HF power over RR series (HFaRR) as an index of vagal modulation (32,33) and the LF power of RTe series (LFaRTe) as an index of sympathetic modulation (21,22). HFaRR and LFaRTe were expressed in absolute units (ms2). All the considered indices (i.e. μRR, μRTe, σ2RR, σ2RTe, HFaRR and LFaRTe) were calculated for each frame. Analysis was iterated with 50% overlap over the entire period, thus resulting in a distribution of parameters. The median of the distribution was extracted for successive statistical analyses (34).
STATISTICAL ANALYSIS
One-way analysis of variance (Dunnett’s test for multiple comparisons), or Kruskal-Wallis one-way analysis of variance on ranks (Dunn’s test for multiple comparisons) when appropriate, was applied to check whether NMCs, AMCs, and SMCs could be distinguished based on the considered parameters. Two-way repeated measures analysis of variance (one factor repetition, Holm-Sidak test for multiple comparisons) was performed to evaluate the significance of circadian rhythm of the time and frequency domain parameters in MCs (i.e. AMCs and SMCs were assessed during DAY and NIGHT). Two-way repeated measures analysis of variance (Holm-Sidak test for multiple comparisons) was performed to evaluate the significance of changes of time and frequency domain parameters induced by B in MCs (i.e. AMCs and SMCs were assessed βBoff and βBon). If heterogeneity of variance was detected according to either the Bartlett’s test or, when appropriate, the Levene’s test, the data were log transformed before the application of one- or two-way analysis of variance. Values are reported as mean plus standard deviation in figures. The statistical analysis was carried out using a commercial statistical program (Sigmastat, ver.3.0.1, Systat Software, San Jose, California). A p value < 0.05 was always considered as significant.
RESULTS
COMPARISON BETWEEN NMCs AND MCs
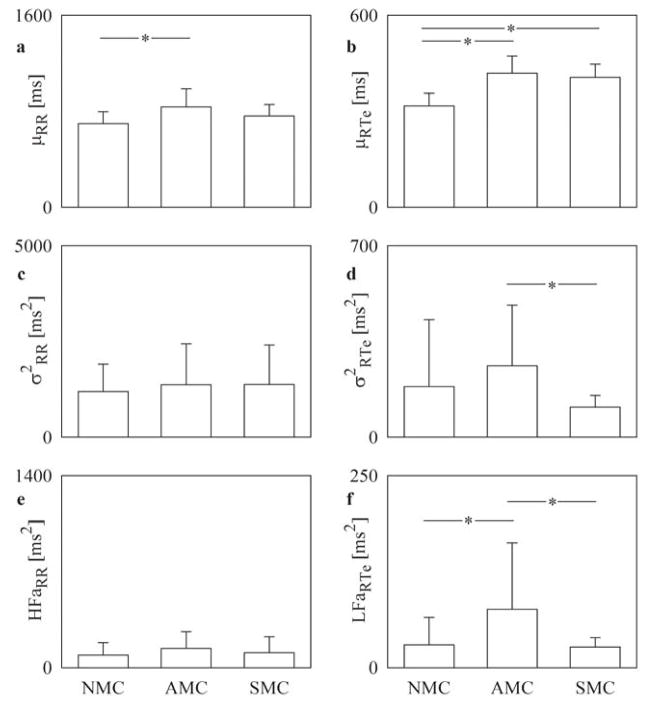
Figure 1 shows that while μRR was similar among SMCs and NMCs, it was longer in AMCs (Figure 1A). As expected, both AMCs and SMCs had longer μRTe than NMCs (Figure 1B). Analysis of RR variability revealed no significant differences among the 3 groups (Figure 1C and 1E). By contrast, analysis of RTe variability differentiated AMCs from SMCs: indeed, σ2RTe (Figure 1D) and LFaRTe (Figure 1F) were greater in AMCs compared to SMCs. Importantly, LFaRTe of AMCs was significantly greater also than that of NMCs (Figure 1F). Thus, AMCs appear to have a sympathetically-mediated greater variability of the QT interval.
FIGURE 1. βBoff in NMCs, AMCs, and SMCs during DAY.
Bar graphs report the RR mean, μRR (A); RTe mean, μRTe (B); RR variance, σ2RR (C); RTe variance, σ2RTe (D); high-frequency (HF) power of the RR series expressed in absolute units, HFaRR (E); and low-frequency (LF) power of the RTe series expressed in absolute units, LFaRTe (F) assessed βBoff in NMCs, AMCs, and SMCs during DAY (from 2:00 PM to 6:00 PM). Values are mean + SD. *p < 0.05. AMC =asymptomatic mutation carrier; βBoff = off β-blocker therapy; NMC = nonmutation carrier; SMC = symptomatic mutation carrier.
CIRCADIAN RHYTHM OF THE AUTONOMIC ACTIVITY IN MCs
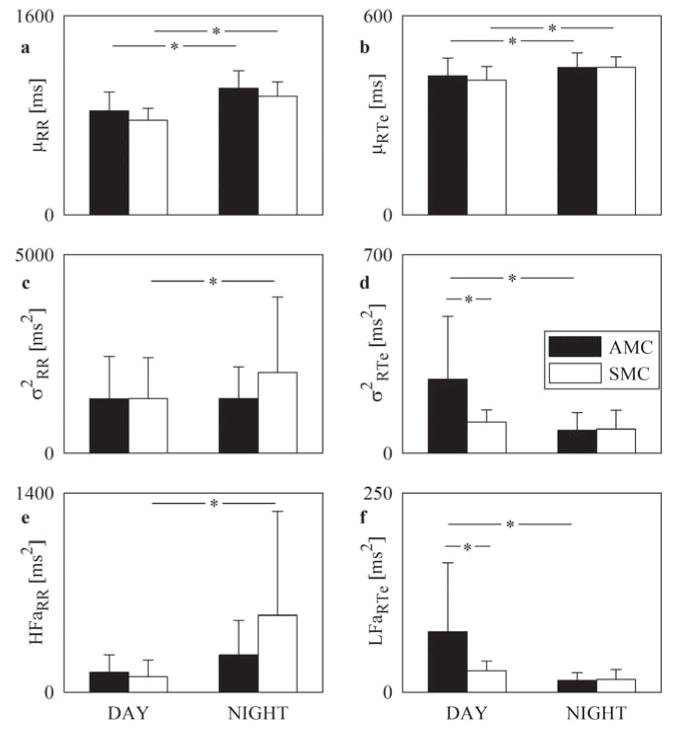
Figure 2A shows that μRR lengthened during NIGHT in both AMCs and SMCs. During both DAY and NIGHT, AMCs tended to have longer μRR than SMCs (Figure 2A). Also μRTe exhibited a circadian rhythmicity in MCs (Figure 2B). While σ2RR increased during NIGHT in SMCs, this value remained unchanged in AMCs (Figure 2C). Conversely, σ2RTe decreased during NIGHT in AMCs, while it did not change in SMCs (Figure 2D). During DAY, σ2RTe was larger in AMCs than in SMCs, while no difference was observed during NIGHT (Figure 2D). HFaRR (Figure 2E) and LFaRTe (Figure 2F) confirmed the differences observed in Figure 2C and Figure 2D respectively and suggested a greater reactivity of the vagal control of heart rate in SMCs and of the sympathetic control of the QT interval in AMCs.
FIGURE 2. βBoff in NMCs, AMCs, and SMCs during DAY and NIGHT.
Grouped bar graphs report μRR (A), μRTe (B), σ2RR (C), σ2RTe (D), HFaRR (E), and LFaRTe (F) assessed βBoff in AMCs (dark bars) and SMCs (white bars) during DAY and NIGHT (midnight to 4:00 AM). Values are mean + SD. *p < 0.05. Abbreviations as in Figure 1.
EFFECT OF β-BLOCKERS
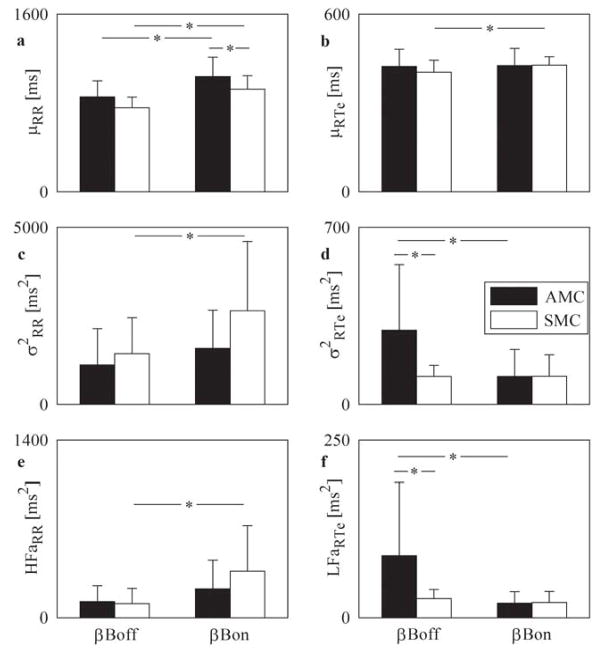
Figure 3A shows that βB lengthened μRR in both groups, but elongated μRTe only in SMCs (Figure 3B). Further, bradycardia induced by βB was significantly greater in AMCs than in SMCs (Figure 3A). Beta-blocker therapy significantly increased σ2RR in SMCs, while treatment did not affect this value in AMCs (Figure 3C). Conversely, βB significantly decreased σ2RTe in AMCs, while treatment did not influence this parameter in SMCs (Figure 3D). The difference between σ2RTe in AMCs and SMCs observed in βBoff, disappeared in βBon (Figure 3D). HFaRR (Figure 3E) and LFaRTe (Figure 3F) confirmed the differences observed in Figure 3C and Figure 3D respectively and corroborated that, in response to βB, there was a larger reactivity of the vagal control of heart rate in SMCs and of the sympathetic control of QT interval in AMCs as observed with the circadian changes.
FIGURE 3. βBoff and βBon in AMCs and SMCs during DAY.
Grouped bar graphs report μRR (A), μRTe (B), σ2RR (C), σ2RTe (D), HFaRR (E), LFaRTe (F) assessed βBoff and βBon in AMCs (dark bars) and SMCs (white bars) during DAY. Values are mean + SD.*p < 0.05. βBon = on β-blocker; other abbreviations as in Figure 1.
DISCUSSION
The present study significantly extends our previous work, which identified differences in autonomic responses, especially in vagal reflexes, between LQT1 patients with and without cardiac events (11,12). The main novel finding here is the previously unsuspected fact that AMCs have a greater degree of sympathetic modulation directed to the ventricles than SMCs and this is especially evident in daytime, when the arrhythmic risk for LQT1 patients is higher (14). Contrary to common wisdom, this finding – which is directly associated with a higher variability of the QT interval – suggests that a greater sympathetic drive to the ventricles is a protective factor in LQT1. These new observations make an important contribution to our understanding of the physiologic mechanisms underlying the otherwise puzzling phenomenon of patients carrying the same disease-causing mutation but with much lower arrhythmic risk (Central Illustration).
CENTRAL ILLUSTRATION. Differences in Autonomic Control of Long QT Type 1 Patients Account for Divergent Arrhythmic Risk.
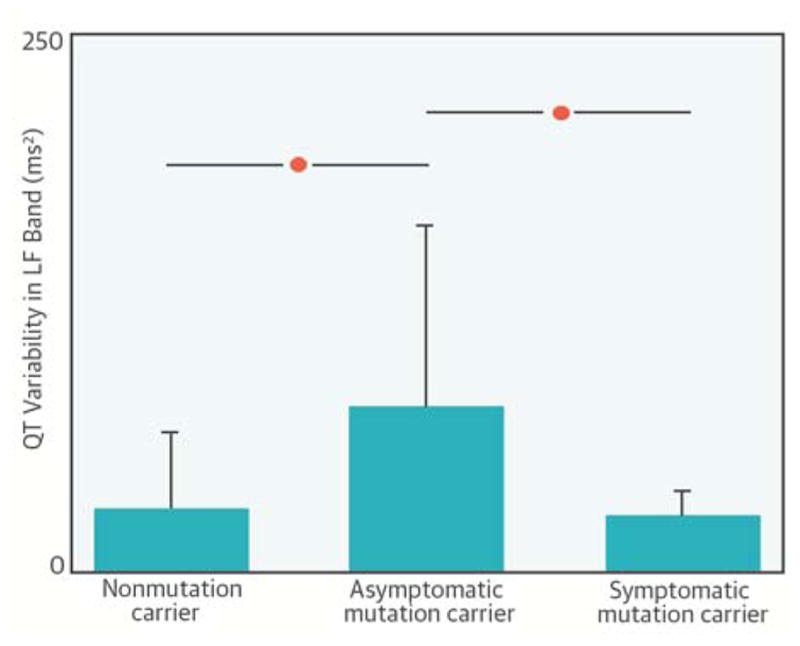
Asymptomatic long QT syndrome type 1 (LQT1) patients have higher-than-normal sympathetic control of the QT interval as assessed from the power of QT variability in the low-frequency (LF) band, whereas the autonomic responses of the symptomatic LQT1 individuals and of normal controls are similar.
Additionally, we observed greater reactivity of the sympathetic control of the QT interval and lesser reactivity of the vagal regulation of heart rate in AMCs compared to SMCs. Indeed, markers of sympathetic modulation derived from QT interval variability in AMCs decreased during nighttime or on βB therapy, while they were unmodified in SMCs. Conversely, indices of vagal modulation derived from RR variability in SMCs increased during nighttime or on βB therapy, while remaining unchanged in AMCs. The present results are relevant to a better understanding of LQT1, offering a clue to a more sophisticated approach to risk stratification, and help dissect out the mechanisms underlying the efficacy of therapeutic interventions.
AUTONOMIC CONTROL OF HEART RATE
AMCs tended to have a lower baseline heart rate, as reported previously (25), but the difference was significant only compared to NMCs. During daytime, time and frequency domain indices derived from heart rate variability did not distinguish SMCs from AMCs. Circadian changes in heart rate were present in both the AMCs and SMCs but nighttime RR variability was significantly greater only in the SMCs. In addition, the RR interval variability was greater in the SMCs than in the AMCs in response to β-blockade. Overall, the data pointed to greater vagally-mediated RR control in SMCs, thus increasing the likelihood of abrupt changes in the RR interval that would produce a proarrhythmic effect in LQT1 patients. These data fit with and extend our previous observations (11,12). The extension is mainly the consequence of the evaluation of frequency domain heart rate variability parameters computed from 24-hour Holter recordings in individuals both on and off βB therapy. We observed larger increases in heart rate variability power in the high-frequency band, a widely recognized index of vagal modulation directed to the sinus node (15,18–20), during nighttime and under βB therapy in SMCs compared to AMCs. This supports the conclusion that SMCs show greater reactivity in the vagal control of heart rate compared to AMCs and that a sluggish vagal responsiveness to challenges is a protective factor in LQT1.
AUTONOMIC CONTROL OF THE QT INTERVAL
The data on the RTe interval, an accurate proxy for the QT interval, provide unexpected and novel insights regarding the autonomic control of ventricular repolarization in LQT1 patients. Indeed, the analysis of RTe variability demonstrated significant differences between AMCs and SMCs. Both the overall magnitude of RTe variability and its portion in the LF band were greater in AMCs. Since these indices can be utilized as markers of sympathetic modulation directed to the ventricles (16,17,21–24), our finding supports the conclusion that AMCs have a greater degree of sympathetic control. It is most intriguing that this greater sympathetic control of ventricular repolarization differentiates the AMCs not only from the SMCs but also from the NMCs. This observation indicates that the group with ‘abnormal’ autonomic responses is not, as one might have thought, the one with cardiac events but rather the asymptomatic group. We had reached an almost identical conclusion when examining baroreflex sensitivity, a very different but equally important autonomic parameter (11).
A greater degree of sympathetic control of ventricular repolarization implies a greater ability to adapt QT duration to rapid changes of the RR interval. This factor proves key to survival when heart rate increases rapidly and the QT interval must shorten appropriately to avoid the R-on-T phenomenon because, when depolarization encroaches the vulnerable period of the T wave, ventricular fibrillation likely occurs. This general concept is especially important for survival in LQT1 patients, given their impairment of the IKs current that is essential for the control of repolarization during heart rate increases.
This finding and the related analysis permit the following interpretation of what underlies the individual and hitherto mysterious propensity to be or not be a symptomatic LQT1 patient. We have already demonstrated that the KCNQ1-A341V mutation is a highly malignant one, with 80% of the MCs suffering major cardiac events (26). If a carrier of this mutation has a “normal” autonomic control, he/she will very likely develop life-threatening arrhythmias. The possibility of reducing this risk depends on either “external” protection, such as that afforded by the effective antiadrenergic therapies available (3), or on “internal” protection, such as a spontaneous or genetically-mediated autonomic modulation characterized by a reduced vagal control of heart rate (11,12) associated with an enhanced sympathetic control of ventricular repolarization, as we demonstrate here. This is why the AMCs are different, in terms of autonomic control, not only from the SMCs but also from the NMCs.
The effect of β-blockade, assessed by internal control analysis, also provided interesting data. As expected, the RR interval increased significantly in both groups but, once again, the more interesting finding came from the analysis of RTe variability. The magnitude of the RTe changes was significantly reduced in the AMCs whereas it was unmodified in the SMCs, largely because it was already very low. The reduction of RTe variability in the AMCs could raise concerns at first glance, but this is just one more case in medicine of an apparently negative side effect of therapy overshadowed by a more powerful protective effect; a classic example of this is the coronary vasoconstrictor effect of β-blockers being overcome by the reduction in oxygen consumption secondary to the heart rate reduction. For these AMCs, the loss in RTe variability is more than compensated by the RR increase and, above all, by the prevention of the arrhythmogenic effects of norepinephrine release. Analogously, the RR lengthening can compensate for the decrease of the RTe variability observed nighttime in AMCs in the absence of beta-blocker therapy.
Furthermore, the observed decrease of RTe variability during the nighttime and in response to βB therapy in AMCs suggests a more important reactivity of the sympathetic control to challenges in AMCs compared to SMCs that again can be taken as an indication of the more flexible regulation of the QT interval in AMCs than in SMCs.
Finally, an important question for the understanding of the concepts underlying these observations and for their clinical translation: Is the propensity for different autonomic responses an independent, genetically-controlled variable in respect to the presence/absence of the disease-causing mutation? For this to be true, it would be necessary that both patterns of autonomic responsiveness be present in both MCs and NMCs. This has indeed already been demonstrated by the fact that while baroreflex sensitivity differs significantly between SMCs and AMCs, the distribution of baroreflex responses is the same between MCs and NMCs (11). This proves that the coexistence between disease-causing mutations with autonomic responses that are divergent and carrying opposite influences on outcome is the play of chance.
CLINICAL IMPLICATIONS
Even though good clinical management requires that all carriers of an LQTS-causing mutation who have a prolonged QT interval be treated with β-blockers (3), the ability to stratify patients based on arrhythmic risk would allow a more targeted therapeutic strategy. When the findings from the present study are integrated with those from our 2 previous analyses of autonomic parameters (11,12), a single coherent picture emerges.
LQT1 patients can, rather confidently, be stratified as at low arrhythmic risk if they have a relatively low heart rate at rest, a relatively low baroreflex sensitivity, a sluggish heart rate reduction at the end of an exercise stress test, and if, as shown here, they have an active sympathetic control of ventricular repolarization and a reduced vagal control of heart rate (Table 1). The converse is true for those with the opposite pattern, who can justifiably be regarded as at high risk.
TABLE 1.
Distinguishing Features of LQT1 AMCs
| Reference | Distinguishing Features |
|---|---|
| Previous studies | Lower heart rate than SMCs (11) |
| Smaller post-exercise heart rate reduction than SMCs (12) | |
| Lower baroreflex sensitivity than SMCs (11) | |
| Present study | Lower heart rate than SMCs |
| Lower increase in heart rate variability during NIGHT than SMCs | |
| Lower increase in heart rate variability in response to βB than SMCs | |
| Greater QT variability than both SMCs and NMCs | |
| Greater decrease in QT variability during NIGHT than SMCs | |
| Greater decrease in QT variability in response to βB than SMCs |
AMCs = asymptomatic mutation carriers; βB = beta-blocker therapy; NIGHT = nighttime; SMCs = symptomatic mutation carriers.
CONCLUSIONS
We have learned a lot about the differential arrhythmic risk related to the characteristics of the specific mutations and their interaction with more common genetic variants. In this study, the data identify an additional factor contributing to the differential arrhythmic risk among LQT1 patients carrying the same mutation. The combination of the individual genetic make-up (with the knowledge of the electrophysiological actions of the LQTS-causing mutations through their impact on ionic currents) and with clinically quantifiable parameters describing autonomic function (as derived from routine 24-hour Holter recordings) will significantly refine our clinical decision making for the benefit of our patients.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Members of families with long QT syndrome type 1 with the same mutation have disparate symptoms and clinical outcomes. Asymptomatic patients often have greater than normal sympathetically-mediated variations in QT interval but less reactive vagal control of heart rate, whereas the autonomic responses of high-risk individuals are similar to normal controls.
TRANSLATIONAL OUTLOOK
Variations in autonomic control characteristics may help explain why patients with the same mutation may face divergent arrhythmic risk, but further studies are needed to translate these observations into targeted strategies for clinical management.
Acknowledgments
The authors are grateful to Pinuccia De Tomasi, BS for her expert editorial support
Financial support: This study was supported by the Telethon grant GGP09247 to P.J.S., L.C. and A.P., by the National Institutes of Health grant HL068880 to A.L.G., P.J.S. and L.C, and by Italian Ministry of Health Malattie Rare RF-IAI_2008-1216776 to P.J.S. and L.C.
LIST OF ABBREVIATIONS
- AMC
asymptomatic mutation carrier
- HFaRR
high-frequency power computed over the RR series and expressed in absolute units
- LFaRTe
low-frequency power computed over the RTe series and expressed in absolute units
- LQTS
long QT syndrome
- MC
mutations carrier
- NMC
nonmutation carrier
- RR
time interval between two consecutive R-wave peaks on the electrocardiogram
- RTe
time interval between R-wave peak and T-wave end on the electrocardiogram
- SMC
symptomatic mutation carrier
Footnotes
Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwartz PJ, Crotti L, Insolia R. Long QT syndrome: from genetics to management. Circ Arrhythm Electrophysiol. 2012;5:868–77. doi: 10.1161/CIRCEP.111.962019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz PJ, Ackerman MJ, George AL, Jr, Wilde AM. Impact of genetics on the clinical management of channelopathies. J Am Coll Cardiol. 2013;62:169–80. doi: 10.1016/j.jacc.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz PJ, Ackerman MJ. The long QT syndrome. A transatlantic clinical approach to diagnosis and therapy. Eur Heart J. 2013;34:3109–16. doi: 10.1093/eurheartj/eht089. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz PJ, Crotti L. Long QT and short QT syndromes. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: from Cell to Bedside. 6. Philadelphia, PA: Elsevier/Saunders; 2014. pp. 935–46. [Google Scholar]
- 5.Schwartz PJ. Sudden cardiac death, founder populations and mushrooms. What is the link with gold mines and modifier genes? Heart Rhythm. 2011;8:548–50. doi: 10.1016/j.hrthm.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Crotti L, Lundquist AL, Insolia R, et al. KCNH2-K897T is a genetic modifier of latent congenital long QT syndrome. Circulation. 2005;112:1251–58. doi: 10.1161/CIRCULATIONAHA.105.549071. [DOI] [PubMed] [Google Scholar]
- 7.Crotti L, Monti MC, Insolia R, et al. NOS1AP is a genetic modifier of the long-QT syndrome. Circulation. 2009;120:1657–63. doi: 10.1161/CIRCULATIONAHA.109.879643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin AS, Giudicessi JR, Tijsen AJ, et al. Variants in the 3′ untranslated region of the KCNQ1-encoded Kv7. 1 potassium channel modify disease severity in patients with type 1 long QT syndrome in an allele-specific manner. Eur Heart J. 2012;33:714–23. doi: 10.1093/eurheartj/ehr473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duchatelet S, Crotti L, Peat R, et al. Identification of a KCNQ1 polymorphism acting as a protective modifier against arrhythmic risk in the long QT syndrome. Circ Cardiovasc Genet. 2013;6:354–61. doi: 10.1161/CIRCGENETICS.113.000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Villiers CP, van der Merwe L, Crotti L, et al. AKAP9 is a genetic modifier of congenital long-QT syndrome type 1. Circulation Cardiovasc Genet. 2014 Aug 2; doi: 10.1161/CIRCGENETICS.113.000580. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz PJ, Vanoli E, Crotti L, et al. Neural control of heart rate is an arrhythmia risk modifier in long QT syndrome. J Am Coll Cardiol. 2008;51:920–9. doi: 10.1016/j.jacc.2007.09.069. [DOI] [PubMed] [Google Scholar]
- 12.Crotti L, Spazzolini C, Porretta AP, et al. Vagal reflexes following an exercise stress test: a simple clinical tool for gene-specific risk stratification in the Long QT Syndrome. J Am Coll Cardiol. 2012;60:2515–24. doi: 10.1016/j.jacc.2012.08.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tank J, Jordan J, Diedrich A, et al. Genetic influences on baroreflex function in normal twins. Hypertension. 2001;37:907–10. doi: 10.1161/01.hyp.37.3.907. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 15.Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability Standard of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. No authors listed. [PubMed] [Google Scholar]
- 16.Berger RD. QT Interval variability. Is it a measure of autonomic activity? J Am Coll Cardiol. 2009;54:821–52. doi: 10.1016/j.jacc.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Malik M. Beat-to-beat QT variability and cardiac autonomic regulation. Am J Physiol. 2009;295:H923–H925. doi: 10.1152/ajpheart.00709.2008. [DOI] [PubMed] [Google Scholar]
- 18.Montano N, Gnecchi-Ruscone T, Porta A, Lombardi F, Pagani M, Malliani A. Power spectrum analysis of heart rate variability to assess changes in sympatho-vagal balance during graded orthostatic tilt. Circulation. 1994;90:1826–31. doi: 10.1161/01.cir.90.4.1826. [DOI] [PubMed] [Google Scholar]
- 19.Porta A, Tobaldini E, Guzzetti S, Furlan R, Montano N, Gnecchi-Ruscone T. Assessment of cardiac autonomic modulation during graded head-up tilt by symbolic analysis of heart rate variability. Am J Physiol. 2007;293:H702–8. doi: 10.1152/ajpheart.00006.2007. [DOI] [PubMed] [Google Scholar]
- 20.Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KUO, Eckberg DL. Human responses to upright tilt: a window on central autonomic integration. J Physiol. 1999;517:617–28. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porta A, Bari V, Badilini F, Tobaldini E, Gnecchi-Ruscone T, Montano N. Frequency domain assessment of the coupling strength between ventricular repolarization duration and heart period during graded head-up tilt. J Electrocardiol. 2011;44:662–8. doi: 10.1016/j.jelectrocard.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porta A, Tobaldini E, Gnecchi-Ruscone T, Montano N. RT variability unrelated to heart period and respiration progressively increases during graded head-up tilt. Am J Physiol. 2010;298:H1406–14. doi: 10.1152/ajpheart.01206.2009. [DOI] [PubMed] [Google Scholar]
- 23.Baumert M, Lambert GW, Dawood T, et al. QT interval variability and cardiac norepinephrine spillover in patients with depression and panic disorder. Am J Physiol. 2008;295:H962–8. doi: 10.1152/ajpheart.00301.2008. [DOI] [PubMed] [Google Scholar]
- 24.Baumert M, Schlaich MP, Nalivaiko E, et al. Relation between QT interval variability and cardiac sympathetic activity in hypertension. Am J Physiol. 2011;300:H1412–7. doi: 10.1152/ajpheart.01184.2010. [DOI] [PubMed] [Google Scholar]
- 25.Brink PA, Crotti L, Corfield V, et al. Phenotypic variability and unusual clinical severity of congenital Long QT Syndrome in a founder population. Circulation. 2005;112:2602–10. doi: 10.1161/CIRCULATIONAHA.105.572453. [DOI] [PubMed] [Google Scholar]
- 26.Crotti L, Spazzolini C, Schwartz PJ, et al. The common long QT syndrome mutation KCNQ1/A341V causes unusually severe clinical manifestations in patients with different ethnic backgrounds: toward a mutation-specific risk stratification. Circulation. 2007;116:2366–75. doi: 10.1161/CIRCULATIONAHA.107.726950. [DOI] [PubMed] [Google Scholar]
- 27.Brink PA, Schwartz PJ. Of founder populations, long QT syndrome, and destiny. Heart Rhythm. 2009;6(Suppl 11S):S25–S33. doi: 10.1016/j.hrthm.2009.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heijman J, Spätjens RL, Seyen SR, et al. Dominant-negative control of cAMP-dependent IKs upregulation in human long-QT syndrome type 1. Circ Res. 2012;110:211–9. doi: 10.1161/CIRCRESAHA.111.249482. [DOI] [PubMed] [Google Scholar]
- 29.Porta A, Baselli G, Lombardi F, et al. Performance assessment of standard algorithms for dynamic R-T interval measurement: comparison between R-Tapex and R-Tend approach. Med Biol Eng Comput. 1998;36:35–42. doi: 10.1007/BF02522855. [DOI] [PubMed] [Google Scholar]
- 30.Baumert M, Starc V, Porta A. Conventional QT variability measurement vs. template matching techniques: comparison of performance using simulated and real ECG. PLoS ONE. 2012;7:e41920. doi: 10.1371/journal.pone.0041920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 32.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger RD, Cohen RJ. Power spectrum analysis of heart rate fluctuations: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–3. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 33.Pomeranz B, Macaulay RJB, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:H151–3. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 34.Porta A, Faes L, Masé M, et al. An integrated approach based on uniform quantization for the evaluation of complexity of short-term heart period variability: application to 24h Holter recordings in healthy and heart failure humans. Chaos. 2007;17:015117. doi: 10.1063/1.2404630. [DOI] [PubMed] [Google Scholar]