SUMMARY
Optimally orchestrating complex behavioral states such as the pursuit and consumption of food is critical for an organism’s survival. The lateral hypothalamus (LH) is a neuroanatomical region essential for appetitive and consummatory behaviors, but whether individual neurons within the LH differentially contribute to these interconnected processes is unknown. Here we show that selective optogenetic stimulation of a molecularly defined subset of LH GABAergic (Vgat-expressing) neurons enhances both appetitive and consummatory behaviors, while genetic ablation of these neurons reduced these phenotypes. Furthermore, this targeted LH subpopulation is distinct from cells containing the feeding-related neuropeptides, melanin-concentrating hormone (MCH) and orexin (Orx). Employing in vivo calcium imaging in freely behaving mice, to record activity dynamics from hundreds of cells, we identified individual LH GABAergic neurons that preferentially encode aspects of either appetitive or consummatory behaviors, but rarely both. These tightly regulated, yet highly intertwined, behavioral processes are thus dissociable at the cellular level.
INTRODUCTION
Complex, but evolutionary well-conserved, behavioral states such as those related to feeding, require both precurrent and consummatory responses (Sherrington, 1906). These oftentimes sequential reactions, which are historically conceptualized as appetitive and consummatory behaviors (Ball and Balthazart, 2008; Craig, 1917; Lorenz, 1950; Tinbergen, 1951), represent highly intertwined processes that are not fully distinguished at the neural level. The lateral hypothalamus (LH), a critical modulator of both appetitive and consummatory processes, is a heterogeneous brain area containing numerous genetically distinct cell populations that utilize various signaling modalities (Berthoud and Münzberg, 2011). Gene expression patterns within the LH suggest that individual neurons likely release either inhibitory or excitatory neurotransmitters, γ-aminobutyric acid (GABA) and glutamate, as well as a host of neuropeptides (Hökfelt et al., 2000; Lein et al., 2007), implying that identifiable subdivisions within the global LH neuronal network can be genetically targeted. Electrical stimulation of the LH, which non-specifically activates various cell types and processes, evokes voracious consummatory feeding as well as appetitive reward-related behaviors (Hoebel and Teitelbaum, 1962; Margules and Olds, 1962; Olds and Milner, 1954; Wise, 1971), while ablation of the region results in emaciation and aphagia (Anand and Brobeck, 1951; Hoebel, 1965). Moreover, the activity of LH neurons changes in response to food and associated stimuli (Ono et al., 1981, 1986). These electrically evoked behaviors and feeding-specific activity patterns were discovered in numerous species of the Kingdom Animalia, from lizards (Molina-Borja and Gómez-Soutullo, 1989) to humans (Quaade et al., 1974). These past findings emphasize the importance and evolutionary conservation of the LH for regulating these survival-oriented behaviors. However, given the heterogeneous cellular composition of the LH (Adamantidis and de Lecea, 2009; Karnani et al., 2013; Knight et al., 2012), and the fact that multiple fibers of passage traverse this region (Hahn and Swanson, 2012), classical electrical stimulation, lesion, or electrophysiological recording studies are unable to determine whether genetically defined cell types, such as LH GABAergic neurons, encode and regulate precise aspects of appetitive food-seeking and/or consummatory behaviors.
Neural circuit tracing and manipulation experiments revealed that optogenetic modulation of LH glutamatergic neurons influences feeding and motivated behavioral responding (Jennings et al., 2013a). In the current study, we examined if molecularly defined LH neurons that express the gene for the vesicular GABA transporter (Vgat), and thus synthesize and release GABA, selectively promote and encode appetitive and consummatory behaviors.
RESULTS
Optogenetic Stimulation and Inhibition of LH GABAergic Neurons Bidirectionally Modulate Feeding and Reward-Seeking Behavior
Given that electrical stimulation of the LH produces multiple behavioral responses, including feeding and positive reinforcement (Hoebel and Teitelbaum, 1962; Margules and Olds, 1962), and that the highest concentration of GABA in the hypothalamus is located within the anterior portions of the LH (Kimura and Kuriyama, 1975), we first explored the functional role of LH GABAergic neurons in modulating feeding and reward-related behaviors by using optogenetic techniques. Applying established viral procedures (Jennings et al., 2013b), we first expressed channelrhodopsin-2 conjugated to enhanced yellow fluorescent protein (AAV5-EF1α-DIO-hChR2(H134R)-eYFP) selectively in GABAergic neurons located within the anterior and dorsolateral subdivisions of the LH (Figures 1A and 1B) in Vgat-IRES-Cre mice (Vong et al., 2011), and implanted optical fibers directly above the LH for somata photostimulation (Figures 1C and S1A). Approximately 4 weeks after surgery, we tested whether direct photostimulation of LH GABAergic neurons influenced feeding and reward-related behavioral phenotypes in ad libitum fed mice. Photoactivation of these neurons at 20 Hz significantly increased the time spent in a designated food area (Figures 1D and 1E), food consumption (Figure 1F), time spent in a location paired with photostimulation (Figure 1G), and optical self-stimulation behavior (Figure 1H). Next, we tested whether photoinhibition of LH GABAergic neurons disrupted feeding and reward-related behaviors in food-restricted mice. Utilizing similar procedures as described above, we targeted a modified variant (Mattis et al., 2012) of the inhibitory opsin, archaerhodopsin-3 (AAV5-EF1α-DIO-eArch3.0-eYFP; Chow et al., 2010), to LH GABAergic neurons in Vgat-IRES-Cre mice (Figures 1I-1K and Figure S1B). Photoinhibition of LH GABAergic neurons led to significant reductions in time spent in a designated food area (Figures 1L and 1M), food consumption (Figure 1N), and time spent in a location paired with photoinhibition (Figure 1O). These data indicate that selective optogenetic modulation of neurochemically distinct LH GABAergic neurons (Figures S1C-S1Q) influences both feeding and reward-related phenotypes, supporting the idea that both appetitive and consummatory behavioral processes are represented in the LH by GABAergic neurons.
Figure 1. Optogenetic Modulation of LH GABAergic Neurons Bidirectionally Modulates Feeding and Reward-Related Behaviors.
(A) Scheme for viral targeting of AAV5-EF1α-DIO-ChR2-eYFP to the LH of Vgat-IRES-Cre animals.
(B) 20x confocal image depicting ChR2-eYFP expression in LH GABAergic neurons. EP: entopeduncular nucleus, LH: lateral hypothalamus, Fx: fornix, DMH: dorsomedial hypothalamic nucleus, VMH: ventromedial hypothalamic nucleus, Arc: arcuate nucleus, 3v: third ventricle, D: dorsal, L: lateral, M: medial, V: ventral. Scale bar, 200 µm.
(C) Diagram for photostimulation of LH GABAergic neurons.
(D) Color map encoding spatial location from an ad lib fed LHGABA::ChR2 mouse during the free-access feeding paradigm.
(E) Photostimulation of LH GABAergic neurons significantly increased time spent in the food zone compared to controls and time epochs without photostimulation (n = 5 mice per group, F2,27 = 86.24, p < 0.0001).
(F) 20-Hz photostimulation significantly increased food intake in LHGABA::ChR2 mice compared to controls and time epochs without photostimulation (n = 5 mice per group, F2,27 = 17.05, p < 0.0001).
(G) LHGABA::ChR2 mice spent significantly more time in the chamber paired with photostimulation compared to controls (n = 5 mice per group, t8 = 6.796, p < 0.0001).
(H) LHGABA::ChR2 mice nose poked significantly more for 20-Hz photostimulation compared to controls (n = 4 mice per group, t5 = 5.744, p = 0.0012).
(I) Viral targeting of AAV5-EF1α-DIO-eArch3.0-eYFP into the LH of Vgat-IRES-Cre mice.
(J) 20x confocal image showing eArch3.0-eYFP expression in the LH of a Vgat-IRES-Cre mouse. Scale bar, 200 µm.
(K) Illustration for somata LH GABAergic photoinhibition.
(L) Color map encoding spatial location of an example food-deprived LHGABA::eArch3.0 animal during the free-access feeding paradigm.
(M) Upon photoinhibition exposure, LHGABA::eArch3.0 animals spent significantly less time in the food zone compared to controls (n = 5 mice per group, F1,16 = 9.39, p = 0.007).
(N) Photoinhibition of LH GABAergic neurons significantly suppressed food intake in food-restricted mice when compared to controls and time epochs without photoinhibition (n = 5 mice per group, F1,16 = 5.43, p = 0.033).
(O) LHGABA::eArch3.0 mice spent significantly less time in the photoinhibition-paired chamber compared to controls (n = 5 mice per group, t8 = 4.512, p = 0.002).
All values are mean ± SEM. Student’s t-test or Two-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001. See also Figure S1.
Chemogenetic Activation of LH GABAergic Neurons Enhances Consummatory Behaviors
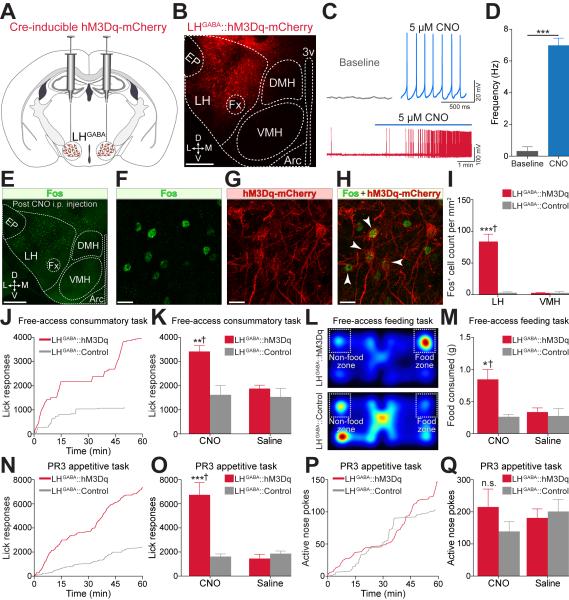
To expand upon the acute behavioral effects observed from optogenetic manipulations (Figure 1), we investigated if sustained activation of LH GABAergic neurons, over a longer timescale, influenced consumption and work effort to earn caloric rewards. Thus, we virally targeted the Gq-coupled excitatory designer receptor exclusively activated by designer drugs (DREADD), hM3Dq, to LH GABAergic neurons by injecting the Cre-inducible viral construct, AAV8-hSyn-DIO-hM3D(Gq)-mCherry (Krashes et al., 2011), into similar target zones within the LH of Vgat-IRES-Cre mice (LHGABA::hM3Dq; Figures 2A and 2B and Figures S2A-S2G). The inert molecule, clozapine-N-oxide (CNO), selectively binds to hM3Dq and activates neurons through Gq signaling pathways (Alexander et al., 2009). Therefore, to verify CNO-mediated activation in LHGABA::hM3Dq neurons, we performed whole-cell recordings in brain slices and found that the spontaneous firing rate of a subset of LHGABA::hM3Dq neurons significantly increased upon CNO (5 μM) bath application (Figures 2C and 2D). Additionally, we examined whether in vivo stimulation of LH GABAergic neurons, via systemic CNO administration, enhanced the expression of Fos, a marker for neuronal activity, within the LH. Intraperitoneal (i.p.) injections of CNO (1 mg/kg) in LHGABA::hM3Dq mice significantly increased Fos expression in the LH compared to LHGABA::Control mice injected with CNO (Figures 2E-2I). To determine whether DREADD-mediated activation of LH GABAergic neurons affected consummatory behavior, mice were trained in a free-access caloric consumption task (1 hr duration) that permitted the quantification of lick responses at the delivery spout for a palatable caloric liquid reward. Chemogenetic activation of LH GABAergic neurons in LHGABA::hM3Dq mice via CNO (1 mg/kg, i.p.) injection led to a significant increase in the number of lick (consummatory) responses when compared to controls (Figures 2J and 2K). In addition, CNO administration 45 min prior to a 1 hr free-access feeding task (Figure 2L) enhanced food intake in ad libitum fed LHGABA::hM3Dq mice (Figure 2M; see Extended Experimental Procedures).
Figure 2. Bulk Chemogenetic Activation of LH GABAergic Neurons Enhances Consummatory Behaviors.
(A) Viral targeting of AAV8-hSyn-DIO-hM3D(Gq)-mCherry into the LH of Vgat-IRES-Cre mice.
(B) 20x confocal image showing hM3Dq-mCherry expression in the LH of a Vgat-IRES-Cre animal. Scale bar, 200 µm.
(C) Example current-clamp traces from a LHGABA::hM3Dq brain slice before (baseline) and after 5 μM CNO demonstrating DREADD-mediated action potentials (bottom).
(D) CNO significantly increases spontaneous firing of LHGABA::hM3Dq neurons (n = 3 cells, n = 3 mice, t4 = 12.370, p = 0.0002).
(E) 20x confocal image from an example LHGABA::hM3Dq animal, where Fos-induction was assessed 2 hr after CNO (1 mg/kg; i.p.) injection. Scale bar, 500 µm.
(F-H) 63x confocal images displaying Fos immunoreactivity (F) and hM3Dq-mCherry expression (G) in a LHGABA::hM3Dq animal injected with CNO. (H) Merged image of (F) and (G) showing colocalization of Fos and hM3Dq-mCherry expression as indicated by white arrows. Scale bars, 20 µm.
(I) CNO administration in LHGABA::hM3Dq mice significantly increases Fos expression in the LH compared to controls and neighboring regions (n = 3 LH sections, n = 3 mice per group, F1,8 = 46.00, p < 0.0001).
(J) Cumulative lick responses from individual LHGABA::hM3Dq and LHGABA::Control mice during a single 1 hr free-access caloric consumption task.
(K) Systemic CNO application significantly increases lick responses in LHGABA::hM3Dq mice compared to LHGABA::Control mice and saline injections during a free-access caloric consumption task (n = 6 mice per group, F1,20 = 8.12, p = 0.01).
(L) Example color maps from LHGABA::hM3Dq (top) and LHGABA::Control mice (bottom) during the free-access feeding task.
(M) Systemic application of CNO in LHGABA::hM3Dq mice significantly increased food consumption during the free-access feeding task when compared to controls and saline injections (n = 6 mice per group, F1,20 = 6.37, p = 0.02).
(N) Cumulative lick responses from example LHGABA::hM3Dq and LHGABA::Control animals during a single 1 hr progressive ratio 3 (PR3) session.
(O) Systemic CNO application significantly increased lick responses in LHGABA::hM3Dq mice during the PR3 paradigm when compared to controls and saline injections (n = 6 mice per group, F1,20 = 24.37, p < 0.0001).
(P) Nose poke responses from example LHGABA::hM3Dq and LHGABA::Control mice during a single PR3 session.
(Q) CNO administration did not significantly affect nose poke responses during the PR3 session (n = 6 mice per group, F1,20 = 1.47, p = 0.24).
All values are mean ± SEM. Student’s t-test or Two-way ANOVA. See also Figure S2.
To examine whether chemogenetic activation of LH GABAergic neurons alters work effort exerted to earn caloric rewards, we initially trained mice on a fixed-ratio of 1 (FR1) schedule, where each active nose poke resulted in the delivery of a palatable and calorie-dense liquid. Following stable behavioral responding, on subsequent sessions, LHGABA::hM3Dq and LHGABA::Control mice were either administered saline or CNO (counterbalanced) 45 min prior to progressive ratio 3 (PR3) test sessions, which is an established behavioral assay for measuring an animal’s motivation to obtain caloric rewards (Krashes et al., 2011). Intriguingly, activation of these cells significantly increased lick responses (metrics of consumption; Figures 2N and 2O), but did not alter the number of active nose pokes nor the break point (metrics of motivation; Figures 2P and 2Q). Anecdotally, we observed that LHGABA::hM3Dq mice treated with CNO spent majority of the time licking at the reward receptacle (despite reward delivery being contingent upon nose poke responding), suggesting that bulk chemogenetic activation of these neurons may over-ride optimal behavioral performance by biasing behavior towards aspects of consumption. Taken together, these data indicate that chemogenetic activation of LH GABAergic neurons enhances consummatory behaviors.
Vgat-targeted LH GABAergic Neurons are Molecularly Distinct from MCH and Orx Cells
The LH exclusively houses two separate molecularly defined cell types, melanin-concentrating hormone (MCH) and orexin (Orx) neurons (Elias et al., 1998), which are known to have diverse roles in regulating food intake, energy balance, reward, and sleep-wakefulness (Jego et al., 2013; Karnani et al., 2011; Sakurai et al., 1998; Whiddon and Palmiter, 2013). Since MCH and Orx are thought to promote aspects of feeding and reward-related behaviors we next assessed whether Vgat-targeted neurons within the LH coexpress either of these orexigenic neuropeptides. To achieve this, we immunolabeled both peptide-producing neuronal populations in Vgat-IRES-Cre mice that endogenously express eYFP in Vgat + cells (Vgat-eYFP) and determined whether Orx and/or MCH neurons colocalize with Vgat + cells in the LH. Strikingly, immunostaining for MCH and Orx in Vgat-eYFP (Vgat-IRES-Cre mice crossed to a Ai3 reporter line; Madisen et al., 2010) brain slices revealed that Vgat + LH neurons do not coexpress either of these neuropeptides, signifying that these Vgat-targeted neurons in the LH represent a neurochemically distinct GABAergic subpopulation that is separate from MCH and Orx cells (Figures 3A-3F and Figure S3). Although these neuropeptide-producing neurons have been implicated as crucial modulators of feeding, selective activation of Vgat-targeted neurons within the LH does not directly stimulate MCH and Orx cells to produce feeding and reward-related behaviors (Figures S1F-S1Q and Figures S2B-S2G).
Figure 3. Genetic Ablation of LH Vgat Neurons that are Separate from MCH and Orx Cells Attenuates Weight Gain, Food-seeking, and Consummatory Behaviors.
(A-C) 20x confocal images of the dorsolateral LH demonstrating the absence of colocalization between Vgat-eYFP and MCH immunostaining (n = 223 +/− 13.77 Vgat-eYFP cells per mm2, n = 76 +/− 11.37 MCH cells per mm2, and 0% overlap, n = 3 LH sections, n = 3 Vgat-eYFP mice). Scale bars, 100 µm.
(D-F) Representative 20x confocal images from a Vgat-eYFP brain slice (D) immunostained for Orx in red (E) displaying a lack of eYFP and Orx-immunoreactivity coexpression in Vgat + LH cells (F; n = 266 +/− 24.2 Vgat-eYFP cells per mm2, n = 200 +/− 4.41 Orx cells per mm2, and 0% overlap, n = 3 LH sections, n = 3 Vgat-eYFP mice). Scale bars, 100 µm.
(G) Viral injection of AAV2-flex-taCasp3-TEVp into the LH of Vgat-IRES-Cre mice.
(H and I) 20x confocal images demonstrating decreased GAD67 expression in LHGABA::taCasp3 mice (H) compared to LHGABA::Control animals (I). Scale bars, 200 µm.
(J) GAD67 expression is significantly decreased in the LH of LHGABA::taCasp3 animals compared to LHGABA::Controls. Ablation of LH GABAergic neurons does not significantly alter GAD67-expression levels within the VMH and EP of LHGABA::taCasp3 and LHGABA::Control mice (n = 3 LH sections, n = 3 mice per group, F2,15 = 5.58, p = 0.01).
(K) Ablation of LH GABAergic neurons significantly blunted weight gain induced from a calorie-dense diet (n = 7 mice per group, F1,720 = 377.01, p = 0.0174).
(L) Four weeks after taCasp3-TEVp viral injection, LH GABAergic cell death significantly reduced daily consumption of a calorie-dense diet (n = 7 mice per group, t12 = 2.597, p = 0.0234).
(M) Color map locations from example LHGABA::taCasp3 (top) and LHGABA::Control (bottom) mice during the free-access feeding paradigm.
(N) LHGABA::taCasp3 mice display significant decreases in food consumption when compared to controls during the free-access feeding paradigm (n = 7 mice per group, t12 = 3.239, p = 0.0071).
(O) LH GABAergic neuron ablation significantly decreases lick responses in LHGABA::taCasp3 animals compared to controls during a free-access caloric consumption paradigm (n = 7 mice per group, t12 = 5.3320, p = 0.0002).
(P) Lick responses from example LHGABA::taCasp3 and LHGABA::Control animals during a single (1 hr) PR3 session.
(Q) Ablation of LH GABAergic neurons significantly decreases lick responses in LHGABA::taCasp3 animals compared to LHGABA::Controls during the PR3 task (n = 6 mice per group, t10 = 3.024, p = 0.012).
(R) Nose poke responses from example LHGABA::taCasp3 and LHGABA::Control animals during a single PR3 session.
(S) Ablation of LH GABAergic neurons significantly decreases nose poke responding in LHGABA::taCasp3 animals compared to LHGABA::Controls during the PR3 task (n = 6 mice per group, t10 = 2.773, p = 0.019).
(T) LHGABA::taCasp3 mice display significantly lower breakpoints when compared to LHGABA::Controls during the PR3 session (n = 6 mice per group, t10 = 2.692, p = 0.022).
All values are mean ± SEM. Student’s t-test or Two-way ANOVA. See also Figures S3 and S4.
Genetic Ablation of LH GABAergic Neurons Reduces Consummatory Behaviors and Motivation to Obtain Caloric Rewards
We next assessed the necessity of LH GABAergic neurons for regulating these feeding-related processes with cell-type specific ablation methods. To selectively ablate LH GABAergic neurons, we injected a Cre-inducible viral construct coding for taCasp3-2A-TEVp (AAV2-FLEX-taCasp3-TEVp) into the LH of Vgat-IRES-Cre mice (LHGABA::taCasp3; Figure 3G)(Yang et al., 2013). In situ hybridization for glutamic acid decarboxylase (GAD67), a marker for GABAergic neurons (Erlander et al., 1991), revealed a significant reduction in the number of LH GABAergic neurons following bilateral LH injections of AAV-flex-taCasp3-TEVp, and no change in the number of GABAergic neurons within neighboring regions, including the entopeduncular nucleus (EP) and ventromedial hypothalamus (VMH; Figures 3H-3J). Previous studies demonstrated that chemical or electrolytic lesions of the LH can result in significant aphagia and weight loss (Harrell et al., 1975; Schallert and Whishaw, 1978). Therefore, we monitored the daily body weight of LHGABA::taCasp3 and LHGABA::Control mice while both groups were maintained on a calorie-dense diet for 60 days. Ablation of LH GABAergic neurons significantly blunted weight gain in LHGABA::taCasp3 mice compared to controls (Figure 3K). Additionally, LHGABA::taCasp3 mice displayed a significant reduction in daily food intake measured at one month post virus injection (Figure 3L).
We next tested the effects of LH GABAergic neuron ablation on acute feeding and motivation to obtain caloric rewards. Food-restricted LHGABA::taCasp3 mice showed reduced food intake in the acute free-access feeding assay (Figures 3M and 3N) and diminished lick responding in the free-access caloric consumption task compared to controls (Figure 3O). When tested on the PR3 task, LHGABA::taCasp3 mice exhibited reduced lick responding compared to controls (Figures 3P and 3Q). Congruently, LHGABA::taCasp3 mice showed a significant reduction in metrics of appetitive responding (active nose pokes and breakpoints) compared to controls (Figures 3R-3T). Furthermore, genetic ablation of LH GABAergic neurons did not significantly alter the number of MCH or Orx cells in the LH (Figures S4A-S4F), corroborating our findings that Vgat targeted LH neurons are a separate population from MCH and Orx cells (Figures 3A-3F and Figure S3). Lastly, LHGABA::taCasp3 mice did not display locomotor deficits or anxiety-related phenotypes when tested in an open-field test (Figures S4G and S4H). Together, these data highlight a causal and specific role for LH GABAergic neurons in regulating appetitive responding, consumption, and energy balance.
In Vivo Ca2+ Imaging in Large Populations of LH GABAergic Neurons
The optogenetic, chemogenetic, and cell ablation experiments outlined above demonstrate that bulk modulation of LH GABAergic neurons causally regulates appetitive and consummatory behavioral output. Though these approaches provide important causal information for linking cell-type specific function to behavior, bulk modulation of even genetically defined neurons fails to account for the high degree of functional diversity within a targeted cell population, nor do they accurately reflect endogenous cellular activity dynamics that underlie complex behaviors. Therefore, it is unclear whether appetitive and consummatory processes are orchestrated by functionally discrete LH GABAergic subpopulations or whether individual neurons are participant in aspects of both appetitive and consummatory behaviors. To examine this in detail, we applied in vivo microendoscopic imaging strategies (Barretto et al., 2011; Flusberg et al., 2008; Ghosh et al., 2011; Jennings and Stuber, 2014) to resolve somatic Ca2+ activity dynamics from hundreds of LH GABAergic neurons (n = 743 cells) in freely behaving mice (n = 6 mice; Movie S1). First, we expressed the green fluorescent sensor of neuronal activity, GCaMP6m, in LH GABAergic neurons by injecting a Cre-inducible AAV viral construct (AAVDJ-EF1α-DIO-GCaMP6m) into the LH of Vgat-IRES-Cre mice (LHGABA::GCaMP6m; Figures 4A-4C and Figures S5A-S5L). To circumvent the optical aberrations and the turbidity of tissue that typically preclude imaging in deep brain structures like the LH (~ 5 mm deep), we implanted 8 mm long microendoscopes (0.5 mm diameter; consisting of a relay lens fused to doublet gradient refractive index microlenses) directly above the LH for optical detection of GCaMP6m fluorescence emission (Figures 4D-4F and Figure S5M). Next, we interfaced the implanted microendoscope with a detachable miniaturized fluorescence microscope (Ghosh et al., 2011; Ziv et al., 2013) to visualize Ca2+ signals from large populations of LH GABAergic neurons in freely moving LHGABA::GCaMP6m mice (Figure 4G). Furthermore, applying this technique in conjunction with established computational algorithms (Mukamel et al., 2009), we were able to identify and track the Ca2+ activity from individual LH GABAergic neurons within single recording sessions and across multiple days and behavioral tasks (Figures 4H and 4I; See Extended Experimental Procedures).
Figure 4. In Vivo Ca2+ Imaging of LH GABAergic Neurons in Freely Moving Mice.
(A) Diagram showing unilateral viral injection of AAVDJ-EF1α-DIO-GCaMP6m into the LH of Vgat-IRES-Cre mice.
(B) 10x confocal image of GCaMP6m expression in LH GABAergic neurons. Scale bar, 500 µm.
(C) 63x confocal image demonstrating stable and healthy GCaMP6m expression in LH GABAergic neurons several months after viral delivery. Scale bar, 20 µm.
(D) Integration of the mini-epifluorescence microscope with the microendoscope for deep-brain imaging of LH GABAergic neurons expressing GCaMP6m.
(E) 20x confocal image showing lens (microendoscope) placement and GCaMP6m-expressing neurons within the LH. Focal plane in tissue is 300 µm from the bottom of the lens as indicated by the dashed red box. Scale bar, 500 µm.
(F) In vivo mini-epifluorescence image of GCaMP6m expression in the LH. Green arrow directs to a LH GABAergic neuron expressing GCaMP6m. Red arrow highlights a blood vessel. Scale bar, 100 µm.
(G) Illustration of the in vivo Ca2+ imaging setup.
(H) Schematized cell map of an example animal’s LH GABAergic neurons visualized during free-access feeding and PR3 tasks. The same neurons can be tracked between sessions (colored cells).
(I) Ca2+ traces of individual neurons tracked in (H).
See also Figure S5.
Food-Location Coding Profiles of LH GABAergic Neurons
Because the activity of unclassified LH neurons can be modulated in response to nutrient-related information (Ono et al., 1981, 1986), we first determined whether LH GABAergic ensembles are engaged when food is readily available. Thus, we characterized the response profiles of individual LH GABAergic neurons during the same free-access feeding task used above and recorded Ca2+ signals as food-restricted LHGABA::GCaMP6m mice freely explored an arena that possessed discrete food-containing zones (FZ) in two of the outer corners (Figure 5A and Movie S2). This approach allowed for a visual representation of discrete spatial Ca2+ responses (Figures 5B and 5C) with some neurons exhibiting increased Ca2+ spiking in the presence of food (Figures 5B and 5D) and others showing decreased Ca2+ transients while the animal was in the FZ (Figures 5C and 5E). Further, we calculated a response ratio for each LH GABAergic neuron based on the frequency of Ca2+ responses in the FZ over the frequency in non-food zones (FZ to NFZ; Figure 5F). We categorized response profiles from individual neurons as food zone excited (FZe) or food zone inhibited (FZi) if their log FZ/NFZ ratios were > ±1 standard deviation of the mean (0.0 ± 0.3) of the entire population (n = 612 cells). Total Ca2+ activity from both groups decreased over time (Figure S6A); however, average responses to FZ and NFZ areas within FZe and FZi neurons revealed significant differences in their respective directions (Figures 5G and 5H), supporting the design of our classification model. To spatially represent the response profiles of each neuron, we pseudocolored individual cells from an example animal’s cell map by its log-transformed response ratio and observed that cells of differing response profiles intermingled with each other rather than segregating into separate clusters (Figure 5I). These data demonstrate that the endogenous activity dynamics of subsets of LH GABAergic neurons are preferentially modulated in environmental locations paired with food.
Figure 5. Subsets of LH GABAergic Neurons Display Enhanced or Reduced Activity to Environmental Locations Containing Food.
(A) Example trace of animal’s location during the free-access feeding task.
(B and C) Spatial Ca2+ activity maps of a single FZe (B) and FZi (C) cell. Behavioral arena is divided into 0.33 × 0.33 cm bins, where number of Ca2+ events per unit time is represented in color.
(D and E) Example Ca2+ traces from one FZe (orange; D) and one FZi (blue; E) cell during the free-access feeding task in relation to animal’s location and state of interaction with food.
(F) Distribution of food zone (FZ) responses from all detected cells (mean = 0.0, sd = 0.3). FZe cells are classified as above one standard deviation (sd) from the mean. FZi cells fall below one standard deviation from the mean (n = 612 total cells imaged, n = 87 FZe cells, n = 73 FZi cells, n = 6 mice).
(G and H) Average Ca2+ transients per min for FZe (G) and FZi (H) cells in each zone (n = 87 FZe cells, t86 = 14.92, p < 0.0001, n = 73 FZi cells, t72 = 15.08, p < 0.0001).
(I) Example cell map with cells’ color encoding response to FZ. Scale bar, 100 µm. Error bars represent SEM. Student’s t-test. See also Figure S6.
Individual LH GABAergic Neurons Selectively Encode Aspects of Appetitive or Consummatory Behaviors
Next, we sought to determine whether individual LH GABAergic neurons selectively compute aspects of appetitive and/or consummatory behaviors. Therefore, we tracked Ca2+ dynamics in LH GABAergic neurons during the same PR3 task described above, where LHGABA::GCaMP6m mice worked to obtain a caloric liquid reward. Numerous individual LH GABAergic neurons showed time-locked Ca2+ transients in response to either the first lick after reward delivery (consummatory responses) or unreinforced active nose pokes (appetitive responses; Figures 6A and 6B and Movie S3). Non-consummatory licks, those following unreinforced nose pokes, also evoked Ca2+ changes but to a much lesser degree (Figure S7A), signifying that these consummatory Ca2+ responses depend on the presence of the caloric reward. We profiled all imaged neurons based on their differences between the average Ca2+ activity 1.5 s before and after each behavioral event to quantify their responses to aspects of feeding. LH GABAergic neurons displayed a variety of classifiable response profiles, such as cells that were modulated during reward consumption or immediately following active nose pokes (Figures 6C and 6D and Figures S7B-S7E). Thus, we categorized these neurons as responsive to a particular behavioral event (Consumption or Nose poke) if their Ca2+ activity (averaged from 0 to 1.5 s after the event) was statistically enhanced compared to the activity −1.5 to 0 s before the event. Averaging responses from excited neurons to consummatory licks revealed separate subpopulations of neurons with distinct Ca2+ responses to consummatory licks (Figure 6E). A much larger population of LH GABAergic neurons displayed significant Ca2+ signals time-locked to appetitive nose pokes (Figure 6F), although the amplitudes of these responses were lower compared to consummatory responses. LH GABAergic neurons displayed a diverse range of responses to consummatory licks and nose pokes (Figures S7F and S7G). However, we found that individual neurons that significantly responded to reward consumption were largely separate from those that respond to appetitive nose pokes (Figures 6G and 6H). Taken together, these data demonstrate that functionally segregated subpopulations of LH GABAergic neurons encode aspects of appetitive (nose poke responsive cells) or consummatory (lick responsive cells) behaviors, but rarely both.
Figure 6. Separate LH GABAergic Neurons Selectively Encode Aspects of Appetitive or Consummatory Behaviors.
(A) Ca2+ response to consummatory licks. (Top) Ca2+ response to individual consummatory licks from an example cell. (Bottom) Average Ca2+ response to all consummatory licks from the example cell.
(B) Ca2+ response to nose pokes. (Top) Ca2+ response to individual nose pokes from an example cell. (Bottom) Average Ca2+ response to all nose pokes from the example cell.
(C and D) Cell activity maps from an example animal. Color codes consummatory lick or appetitive nosepoke responses (average difference between Ca2+ signals from 1.5 s before and after the respective event) for each cell. Scale bars, 100 µm.
(E) Average Ca2+ response to consummatory licks following reward delivery from all excited cells from all animals. (Top) Average Ca2+ response to consummatory licks following reward delivery from all cells. (Bottom) Ca2+ response to consummatory licks averaged across cells excited by consummatory licks (n = 75 lick excited, n = 743 total cells).
(F) Average Ca2+ response to nose pokes from all excited cells of all animals. (Top) Average Ca2+ response to nose pokes from all cells. (Bottom) Ca2+ response to nose pokes averaged across cells excited by nose pokes (n = 168 nose poke excited, n = 743 total cells).
(G) Cell map from example animal. Cells excited by consummatory licks (cyan), nose pokes (yellow), or both (green). Scale bar, 100 µm.
(H) Venn diagram representing distribution and overlap of classified responsive cells in the PR3 task. Green shaded regions represent SEM. See also Figure S7.
Lastly, we explored the Ca2+ dynamics of the same neurons between both behavioral paradigms (free-access feeding and the PR3 task) to determine if the same neurons that respond to the presence of food during the free-access feeding task also responded to consummatory licks and/or nose pokes during the PR3 task. To accomplish this, we registered IC unit cell maps from the same animal between sessions (Figures 7A-7C) and applied a 5-µm cutoff between center points of paired cells based on the distribution of cells from the same imaging session (Figure 7D) and the distance between cells from different sessions (Figure 7E). Using these criteria, only a subset of neurons imaged during the free-access feeding behavioral task were also detected in the PR3 task (n = 40 PR excited cells out of 125 FZ responsive cells from paired sessions. Total paired cells between recording sessions: 472, Figure 7F). Neurons tracked in both sessions were not localized to any portion of the field of view and showed no distinct anatomical pattern or layout (Figures 7G–7I). However, paired cells displayed a high degree of functional overlap in the schematized IC unit cell maps between distinct imaging sessions. A large proportion of FZe neurons that also respond to PR3 behavioral events (28/40 cells) displayed increased activity to either nose pokes or consummatory licks in the PR3 task, while a smaller portion of FZi neurons responded to PR3 behavioral events (Figure 7J). Taken together, these data reveal that subsets of LH GABAergic neurons functionally overlap between tasks that require different behavioral processes to obtain food, signifying the flexibility and complexity of these neurons for integrating and regulating components of feeding.
Figure 7. Tracking the Activity Dynamics of Individual LH GABAergic Neurons Across Separate Behavioral Tasks.
(A) Cell map from example animal during free-access feeding task.
(B) Cell map from same example animal during PR3 appetitive task.
(C) Merge of free-access feeding and PR3 appetitive task cell maps from same example animal.
(D) Distribution of nearest neighbor distances between cells of different behavioral tasks but within the same animal for all subjects.
(E) Distribution of nearest-neighbor distances between all cells within the same behavioral task and imaging session.
(F) Distribution of cell responses in free-access feeding task of only paired cells (n = 472 cells from 6 mice, n = 73 FZe cells, n = 52 FZi cells).
(G-I) Maps of only paired cells from an example animal across the free-access feeding and PR3 tasks. Scale bars, 100 µm.
(J) Bar graph showing cells that respond in both the free-access feeding and PR3 tasks.
DISCUSSION
Historically, the LH has been viewed as a critical governor of both appetitive and consummatory behaviors (Hoebel and Teitelbaum, 1962; Margules and Olds, 1962). However, given that the LH encompasses a plethora of genetically, anatomically, and functionally distinct neurons that utilize various neurotransmitters and neuropeptides, the precise mechanisms by which these cell populations orchestrate behavior have remained a mystery. The results described here demonstrate that Vgat-expressing neurons in the LH function to promote both appetitive and consummatory behaviors. Importantly, we show that these Vgat-targeted cells in the LH do not co-localize with either of the neuropeptides, MCH or Orx, in agreement with previous findings that certain LH GABAergic subpopulations (GAD65-expressing neurons) are neurochemically and electrophysiologically distinct from MCH and Orx cells (Karnani et al., 2013). However, it has also been reported that some MCH neurons do express GABAergic markers such as GAD67 and release GABA from distal synaptic terminals (Jego et al., 2013). Thus, transgene penetrance in Vgat-IRES-Cre mice may be reduced compared to endogenous LH Vgat expression and/or MCH neurons may express extremely low levels of Vgat which can be dynamically regulated as observed in other GABAergic neuronal populations (Jarvie and Hentges, 2012; Lamas et al., 2001; Sperk et al., 2003). In addition, the present results do not rule out the possibility that these unique Vgat-targeted GABAergic neurons contain other neuropeptides, such as Neurotensin or Galanin (Allen and Cechetto, 1995; Goforth et al., 2014; Laque et al., 2013). Thus, parceling out precise neuronal subtypes from these multidimensional populations in the LH to then elucidate their function still remains a major challenge.
Divergent circuit connectivity between LH neurons and upstream and downstream circuit nodes could also account for the diverse appetitive and consummatory response profiles of the LH GABAergic neuronal population. Previous retrograde and anterograde tracing studies demonstrate strong connectivity between the LH and other feeding- and reward-associated brain regions that are also important for motivated behaviors, including the hypothalamus, midbrain, hindbrain, and striatal structures (Adamantidis et al., 2011; Betley et al., 2013; Gutierrez et al., 2011; Hahn and Swanson, 2012; Kempadoo et al., 2013; Leinninger et al., 2011), implying that the selective-coding properties within separate LH GABAergic subpopulations might be input- and/or projection-dependent.
While the LH controls both appetitive and consummatory behaviors, these processes are encoded by distinct cellular LH GABAergic subpopulations. Our data suggests that separate subsets of appetitive-coding and consumption-coding ensembles exist within the LH GABAergic network. Thus, the LH GABAergic network can be viewed as a mosaic of functionally and computationally distinct cell types, requiring further definition. Nevertheless, these important computational differences among individual LH GABAergic neurons would have gone unnoticed if only bulk neuromodulatory approaches were employed, further underscoring the necessity of identifying the natural activity dynamics within a network to better understand the precise neural underpinnings of complex behavioral states.
EXPERIMENTAL PROCEDURES
Experimental Subjects
All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted by the NIH, and with approval of the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill (UNC). Adult (25 – 30 g) male Vgat-IRES-Cre (Vong et al., 2011) or wild type littermate mice were used.
Behavioral Assays
All food-deprived mice were restricted to 85 to 90% of their initial body weight by administering one daily feeding of ~ 2.5 to 3.0 g of standard grain-based chow immediately following each behavioral experiment, if performed. Animals were run on free-access feeding, real-time place preference, optical self-stimulation, and operant feeding (FR1 and PR3) assays. For optogenetic manipulations, light from solid-state lasers (473 nm or 532 nm) was delivered via custom-made patch cables to implanted chronic fibers on animals’ head as described previously (Jennings et al., 2013b). For each chemogenetic behavioral manipulation, mice received i.p. injections of either vehicle or CNO (1 mg/kg) 45 min prior to testing with at least 3 days in between each session (counterbalanced). For further details, refer to Extended Experimental Procedures.
Freely Moving Ca2+ Imaging
A miniature microscope with an integrated LED (475 nm) was used to image GCaMP6m fluorescence in LH GABAergic neurons through an implanted microendoscope. Using nVista HD Acquisition Software (version 2; Inscopix, Palo Alto, CA), images were acquired at 15 frames per second with the LED transmitting 0.1 to 0.2 mW of light on average. Ca2+ imaging was synchronized with time-stamped behavioral data at the start of each session. All images were processed using the Mosaic software (version 1.0.0b; Inscopix, Palo Alto, CA) and then analyzed with custom MATLAB scripts. For further details, refer to Extended Experimental Procedures.
Statistical Analysis
Mean values are accompanied by SEM values. Comparisons were tested using paired or unpaired t-tests. Two-way ANOVA tests followed by Bonferroni post-hoc comparisons were applied for comparisons with more than two groups.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Spencer Smith, Ilana Witten, and members of the Stuber lab for helpful discussion. We thank the UNC vector core for viral packaging, Bradford Lowell and Linh Vong for the Vgat-IRES-Cre mice, and the UNC Neuroscience Center Microscopy Core (P30 NS045892). A tribute to Johnathon Gregory Taylor (1996 – 2014), whose contributions were greatly appreciated and valued for this project. This study was supported by The Klarman Family Foundation, The Brain and Behavior Research Foundation, the Foundation for Prader-Willi Research, the Foundation of Hope, and the National Institute on Drug Abuse (DA032750 and DA038168) and the National Institute on Alcohol Abuse and Alcoholism (P60 AA011605). J.H.J. was supported by (MH104013) and A.M.S. was supported by (NS007431 and DA034472).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adamantidis A, de Lecea L. The hypocretins as sensors for metabolism and arousal. J. Physiol. 2009;587:33–40. doi: 10.1113/jphysiol.2008.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis AR, Tsai H-C, Boutrel B, Zhang F, Stuber GD, Budygin EA, Touriño C, Bonci A, Deisseroth K, de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GV, Cechetto DF. Neurotensin in the lateral hypothalamic area: origin and function. Neuroscience. 1995;69:533–544. doi: 10.1016/0306-4522(95)00261-g. [DOI] [PubMed] [Google Scholar]
- Anand BK, Brobeck JR. Hypothalamic Control of Food Intake in Rats and Cats. Yale J. Biol. Med. 1951;24:123–140. [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. How useful is the appetitive and consummatory distinction for our understanding of the neuroendocrine control of sexual behavior? Horm. Behav. 2008;53:307–311. doi: 10.1016/j.yhbeh.2007.09.023. author reply 315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretto RPJ, Ko TH, Jung JC, Wang TJ, Capps G, Waters AC, Ziv Y, Attardo A, Recht L, Schnitzer MJ. Time-lapse imaging of disease progression in deep brain areas using fluorescence microendoscopy. Nat. Med. 2011;17:223–228. doi: 10.1038/nm.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H-R, Münzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol. Behav. 2011;104:29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Cao ZFH, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. Appetites and Aversions as Constituents of Instincts. Proc. Natl. Acad. Sci. U. S. A. 1917;3:685–688. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J. Comp. Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Flusberg BA, Nimmerjahn A, Cocker ED, Mukamel EA, Barretto RPJ, Ko TH, Burns LD, Jung JC, Schnitzer MJ. High-speed, miniaturized fluorescence microscopy in freely moving mice. Nat. Methods. 2008;5:935–938. doi: 10.1038/nmeth.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, Schnitzer MJ. Miniaturized integration of a fluorescence microscope. Nat. Methods. 2011;8:871–878. doi: 10.1038/nmeth.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goforth PB, Leinninger GM, Patterson CM, Satin LS, Myers MG. Leptin Acts via Lateral Hypothalamic Area Neurotensin Neurons to Inhibit Orexin Neurons by Multiple GABA-Independent Mechanisms. J. Neurosci. 2014;34:11405–11415. doi: 10.1523/JNEUROSCI.5167-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R, Lobo MK, Zhang F, de Lecea L. Neural integration of reward, arousal, and feeding: Recruitment of VTA, lateral hypothalamus, and ventral striatal neurons. IUBMB Life. 2011;63:824–830. doi: 10.1002/iub.539. [DOI] [PubMed] [Google Scholar]
- Hahn JD, Swanson LW. Connections of the lateral hypothalamic area juxtadorsomedial region in the male rat. J. Comp. Neurol. 2012;520:1831–1890. doi: 10.1002/cne.23064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell LE, Decastro JM, Balagura S. A critical evaluation of body weight loss following lateral hypothalamic lesions. Physiol. Behav. 1975;15:133–136. doi: 10.1016/0031-9384(75)90292-9. [DOI] [PubMed] [Google Scholar]
- Hoebel BG. Hypothalamic Lesions by Electrocauterization: Disinhibition of Feeding and Self-Stimulation. Science. 1965;149:452–453. doi: 10.1126/science.149.3682.452. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science. 1962;135:375–377. doi: 10.1126/science.135.3501.375. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Broberger C, Xu ZQ, Sergeyev V, Ubink R, Diez M. Neuropeptides--an overview. Neuropharmacology. 2000;39:1337–1356. doi: 10.1016/s0028-3908(00)00010-1. [DOI] [PubMed] [Google Scholar]
- Jarvie BC, Hentges ST. Expression of GABAergic and glutamatergic phenotypic markers in hypothalamic proopiomelanocortin neurons. J. Comp. Neurol. 2012;520:3863–3876. doi: 10.1002/cne.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R, Friedman J, Burdakov D, Adamantidis AR. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat. Neurosci. 2013a;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R, Friedman J, Burdakov D, Adamantidis AR. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat. Neurosci. 2013b;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Stuber GD. Tools for Resolving Functional Activity and Connectivity within Intact Neural Circuits. Curr. Biol. CB. 2014;24:R41–R50. doi: 10.1016/j.cub.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The Inhibitory Circuit Architecture of the Lateral Hypothalamus Orchestrates Feeding. Science. 2013a;341:1517–1521. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013b;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnani MM, Apergis-Schoute J, Adamantidis A, Jensen LT, de Lecea L, Fugger L, Burdakov D. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron. 2011;72:616–629. doi: 10.1016/j.neuron.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Karnani MM, Szabó G, Erdélyi F, Burdakov D. Lateral hypothalamic GAD65 neurons are spontaneously firing and distinct from orexin- and melanin-concentrating hormone neurons. J. Physiol. 2013;591:933–953. doi: 10.1113/jphysiol.2012.243493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger G-M, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, et al. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:7618–7626. doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Kuriyama K. Distribution of Gamma-Aminobutyric Acid (gaba) in the Rat Hypothalamus: Functional Correlates of Gaba with Activities of Appetite Controlling Mechanisms. J. Neurochem. 1975;24:903–907. doi: 10.1111/j.1471-4159.1975.tb03654.x. [DOI] [PubMed] [Google Scholar]
- Knight ZA, Tan K, Birsoy K, Schmidt S, Garrison JL, Wysocki RW, Emiliano A, Ekstrand MI, Friedman JM. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell. 2012;151:1126–1137. doi: 10.1016/j.cell.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas M, Gómez-Lira G, Gutiérrez R. Vesicular GABA transporter mRNA expression in the dentate gyrus and in mossy fiber synaptosomes. Mol. Brain Res. 2001;93:209–214. doi: 10.1016/s0169-328x(01)00202-9. [DOI] [PubMed] [Google Scholar]
- Laque A, Zhang Y, Gettys S, Nguyen T-A, Bui K, Morrison CD, Muenzberg-Gruening H. Leptin receptor neurons in the mouse hypothalamus are co-localized with the neuropeptide galanin and mediate anorexigenic leptin action. Am. J. Physiol. Endocrinol. Metab. 2013 doi: 10.1152/ajpendo.00643.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Opland DM, Jo Y-H, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz KZ. Physiological Mechanisms in Animal Behavior. Academic Press; Oxford, England: 1950. The comparative method in studying innate behavior patterns; pp. 221–268. Society’s Symposium IV. [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margules DL, Olds J. Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science. 1962;135:374–375. doi: 10.1126/science.135.3501.374. [DOI] [PubMed] [Google Scholar]
- Mattis J, Tye KM, Ferenczi EA, Ramakrishnan C, O’Shea DJ, Prakash R, Gunaydin LA, Hyun M, Fenno LE, Gradinaru V, et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods. 2012;9:159–172. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Borja M, Gómez-Soutullo T. Electrical stimulation of lateral hypothalamic area and behavioural sequences in a lacertid lizard. Behav. Brain Res. 1989;32:197–201. doi: 10.1016/s0166-4328(89)80085-3. [DOI] [PubMed] [Google Scholar]
- Mukamel EA, Nimmerjahn A, Schnitzer MJ. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron. 2009;63:747–760. doi: 10.1016/j.neuron.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J. Comp. Physiol. Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Ono T, Nishino H, Sasaki K, Fukuda M, Muramoto K. Long-term lateral hypothalamic single unit analysis and feeding behavior in freely moving rats. Neurosci. Lett. 1981;26:79–83. doi: 10.1016/0304-3940(81)90429-8. [DOI] [PubMed] [Google Scholar]
- Ono T, Sasaki K, Nishino H, Fukuda M, Shibata R. Feeding and diurnal related activity of lateral hypothalamic neurons in freely behaving rats. Brain Res. 1986;373:92–102. doi: 10.1016/0006-8993(86)90319-7. [DOI] [PubMed] [Google Scholar]
- Quaade F, Vaernet K, Larsson S. Stereotaxic stimulation and electrocoagulation of the lateral hypothalamus in obese humans. Acta Neurochir. (Wien) 1974;30:111–117. doi: 10.1007/BF01405759. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schallert T, Whishaw IQ. Two types of aphagia and two types of sensorimotor impairment after lateral hypothalamic lesions: observations in normal weight, dieted, and fattened rats. J. Comp. Physiol. Psychol. 1978;92:720–741. doi: 10.1037/h0077504. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System (CUP Archive) 1906.
- Sperk G, Schwarzer C, Heilman J, Furtinger S, Reimer RJ, Edwards RH, Nelson N. Expression of plasma membrane GABA transporters but not of the vesicular GABA transporter in dentate granule cells after kainic acid seizures. Hippocampus. 2003;13:806–815. doi: 10.1002/hipo.10133. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. The study of instinct. Clarendon Press/Oxford University Press; New York, NY, US: 1951. [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiddon BB, Palmiter RD. Ablation of neurons expressing melanin-concentrating hormone (MCH) in adult mice improves glucose tolerance independent of MCH signaling. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:2009–2016. doi: 10.1523/JNEUROSCI.3921-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Individual differences in effects of hypothalamic stimulation: the role of stimulation locus. Physiol. Behav. 1971;6:569–572. doi: 10.1016/0031-9384(71)90207-1. [DOI] [PubMed] [Google Scholar]
- Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, Shah NM. Sexually Dimorphic Neurons in the Ventromedial Hypothalamus Govern Mating in Both Sexes and Aggression in Males. Cell. 2013;153:896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nat. Neurosci. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.