Abstract
The progression of many neurodegenerative diseases is thought to be driven by the template-directed misfolding, seeded aggregation and cell–cell transmission of characteristic disease-related proteins, leading to the sequential dissemination of pathological protein aggregates. Recent evidence strongly suggests that the anatomical connections made by neurons — in addition to the intrinsic characteristics of neurons, such as morphology and gene expression profile — determine whether they are vulnerable to degeneration in these disorders. Notably, this common pathogenic principle opens up opportunities for pursuing novel targets for therapeutic interventions for these neurodegenerative disorders. We review recent evidence that supports the notion of neuron–neuron protein propagation, with a focus on neuropathological and positron emission tomography imaging studies in humans.
Neurodegenerative diseases are a major cause of disability and premature death among older people worldwide1–3. Although these diseases, for which there are currently no disease-modifying therapies, show a great diversity of clinical phenotypes, they share a common pathological hallmark — the accumulation of characteristic proteins into insoluble aggregates in or among selectively vulnerable neurons and glial cells.
Aggregates of the phosphorylated microtubule-associated protein tau in neurofibrillary tangles and neuropil threads, together with deposits of amyloid-β (Aβ), are characteristic of sporadic Alzheimer disease (AD). Tau pathology alone also characterizes a subgroup of cases of frontotemporal lobar degeneration (FTLD), which is designated as FTLD-tau, as well as other rare tauopathies4–7. Moreover, neuronal accumulations of α-synuclein in Lewy bodies and Lewy neurites are the pathological signatures of sporadic Parkinson disease (PD) and PD with dementia, as well as of dementia with Lewy bodies8. Furthermore, almost all cases of amyotrophic lateral sclerosis (ALS) and a further subgroup of cases of FTLD (FTLD-TDP) are characterized by aggregates of TAR DNA-binding protein 43 (TDP43)9.
The disease-related proteins are transformed from their normal conformation into fibrillar or multimeric species that function as seeds and templates to drive non-pathological protein counterparts to adopt a similar structural alteration (BOX 1). In a self-perpetuating process that has been likened to that observed in prion diseases10, progressive seeded aggregation of conformationally altered proteins spreads to interconnected neurons and adjacent glial cells. Based on converging lines of evidence from human tissue, cell culture and animal model studies11–13, the paradigm of pathological protein propagation in neurodegenerative diseases is now firmly established.
This Review links experimental evidence of the molecular mechanisms of protein propagation in AD (involving tau and Aβ), FTLD-tau (involving tau), PD (involving α-synuclein), and ALS and FTLD-TDP (both of which involve TDP43) to what is known from studies in humans regarding vulnerable types of neurons and glial cells and the pathways by which disease-protein pathology might spread through the CNS. We focus on studies of neuropathology but also underline the importance of novel protein-specific neuroimaging markers that offer the opportunity to validate the possibility of sequential spreading that has been implicated by human pathology studies. Finally, we discuss how the unifying pathological principle of protein propagation may offer new disease-modifying therapeutic approaches to treating neurodegenerative diseases.
Molecular mechanisms
In prion disease, an infectious protein replicates by recruiting and inducing pathological conformational changes in its normal counterpart, resulting in the aggregation of pathological prions14–16. Prions thus act as corruptive templates that induce a chain-reaction-like process of protein misfolding and progressive aggregation. Although such propagation mechanisms were long thought to be exclusively associated with prion diseases, recent studies have provided convincing evidence that a ‘prion-like’ self-propagating mechanism may apply to a wider range of proteins that are associated with neurodegenerative diseases, including misfolded Aβ, tau and α-synuclein, mutant huntingtin with polyglutamine repeats (which is characteristic of Huntington disease), mutant superoxide dismutase 1 (SOD1) and phosphorylated TDP43 (BOX 2; TABLE 1).
Table 1.
Summary of evidence supporting the propagation and transmission of non-prion neurodegenerative disease proteins
| Pathogenic protein | Associated diseases in humans | Main localization of protein | Studies in human tissue supporting sequential spread | References supporting neuron-neuron transmission | ||
|---|---|---|---|---|---|---|
| Wild-type | Pathological | Cell culture | Animal models | |||
| α-synuclein | PD, DLB and MSA | Presynaptic | Cytoplasmic | PD80 and MSA105 | 39,40,62,177 178 | 41–44 |
| Amyloid-β | AD | Transmembrane (APP) | Extracellular | AD66,71 | 179 | 23,31,180–182 |
| Mutant huntingtin | HD | Nuclear | Nuclear | – | 60 | – |
| Mutant superoxide dismutase 1 | ALS | Cytoplasmic | Cytoplasmic | – | 56 | – |
| RNA-binding protein FUS | ALS and FTLD-FUS | Nuclear | Cytoplasmic | – | – | – |
| Tau | AD, FTLD-tau (including PiD, PSP and CBD) and CTE | Cytoplasmic | Cytoplasmic | AD66 and CTE7 | 50,55 | 25,183 |
| TDP43 | ALS and FTLD-TDP | Nuclear | Cytoplasmic | ALS96, FTLD-TDP104 and AD103* | 48,49 | – |
AD, Alzheimer disease; ALS, amyotrophic lateral sclerosis; APP, amyloid precursor protein (a transmembrane precursor protein whose proteolysis generates amyloid-P); CBD, corticobasal degeneration; CTE, chronic traumatic encephalopathy; DLB, dementia with Lewy bodies; FTLD, frontotemporal lobar degeneration; HD, Huntington disease; MSA, multiple system atrophy; PD, Parkinson disease; PiD, Pick disease; PSP, progressive supranuclear palsy; TDP43, TAR DNA-binding protein 43.
Although AD is primarily characterized by tau and amyloid-P pathology, TDP43 pathology is observed in up to 57% of cases.
Initiation of protein pathology
To date, the mechanisms that trigger the initial conversion of normally soluble proteins into filamentous polymers remain unresolved (FIG. 1). In template-directed misfolding, a misfolded version of a protein interacts directly with the native protein and converts the latter into a misfolded replicate. For example, mutant SOD1 can induce the misfolding of native, wild-type dimeric SOD1 in human mesenchymal and neural cell lines17, and aggregates that contain both mutant and wild-type SOD1 have been found in individuals with familial ALS18 and in mice that express both wild-type and mutant human SOD1 (REF. 19).
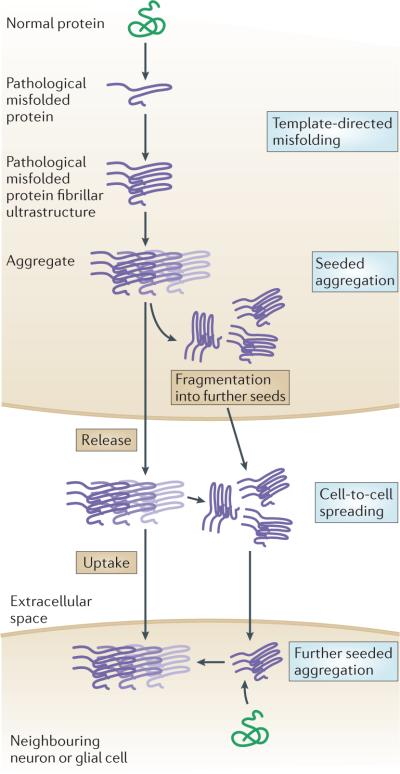
Figure 1. Hypothetical molecular mechanisms of prion-like disease protein transmission in neurodegenerative diseases.
In template-directed misfolding, the deposited pathological disease proteins are transformed from their normal conformation, via intermediates, into fibrillar species. These species have the properties of amyloid (for instance, a fibrillar ultrastructure that consists of sheets of β-strands) and serve as templates to drive normal physiological versions of the protein to adopt similar structural alterations13. In a self-perpetuating process, the progressive seeded aggregation of conformationally changed proteins results in intracellular aggregates that fragment into ‘daughter seeds’. Finally, in cell–cell transmission, pathological proteins spread to anatomically interconnected neurons and adjacent glial cells via an autocatalytic chain-reaction-like process11.
Seeded aggregation is the process whereby mis-folded proteins recruit and initiate template-directed misfolding of the native protein to form new aggregates. The first non-prion neurodegenerative disease protein that was shown to disseminate in this way was misfolded Aβ in marmosets; subsequently, seeded aggregation was also observed in mice overexpressing a mutant variant of the amyloid precursor protein, from which Aβ is derived20–24. Similarly, when brain extracts from mice expressing mutant human tau were injected into the hippocampus and the over-lying cerebral cortex of transgenic animals expressing wild-type human tau, tau pathology was seeded that went on to spread progressively25. Tauopathy was even observed in wild-type mice after seeding with exogenously prepared aggregates of tau, although there was a lower density of aggregates than in transgenic mice that expressed human mutant tau26. Furthermore, synthetic preformed tau fibrils induced tau aggregation in cultured cells and in transgenic mice that expressed human mutant tau27,28. Remarkably, recent studies have also demonstrated that tau, Aβ and α-synuclein exist in different conformational variants, or ‘strains’, that show different seeding and/or cross-seeding properties and different levels of neurotoxicity, and could thereby contribute to the tremendous heterogeneity of neurodegenerative diseases29–31.
Early in vivo evidence to show that α-synuclein pathology can propagate originated from studies on individuals with PD who had received transplants of fetal mesencephalic dopaminergic neurons, which developed α-synuclein-positive Lewy bodies and showed signs of neuronal degeneration32–36. Such studies were followed by in vitro studies37–39 and animal model experiments37,38,40–47 that demonstrated seeded aggregation and transmission of α-synuclein. In contrast to the evidence for tau and α-synuclein transmission, evidence for the transmission of TDP43 is only beginning to emerge and to date is limited to in vitro studies48,49.
Propagation
The mechanisms by which misfolded proteins can propagate from one neuron to the next (or, in the case of extracellular Aβ aggregates, from one region of the CNS to another) remain to be fully determined. Generally, for intracellular aggregates to propagate in this way, these pathogenic proteins need to be released from the originating neuron or glial cell and taken up by a neighbouring neuron or glial cell. Aβ (similar to prion protein) is naturally present in the extracellular space; however, tau, α-synuclein and TDP43 need to cross cell membrane barriers to propagate among cells. Although tau aggregates could be released into the extracellular space as fibrils50, neuronal cell culture studies have shown that non-fibrillar (that is, monomeric or oligomeric) α-synuclein and tau could be secreted via exosomes51–54. Fluid-phase and receptor-mediated endocytosis have been implicated in the cellular uptake of mutant tau, mutant SOD1, and fibrils, oligomers and monomers of α-synuclein39,55–59, and there is evidence that mutant huntingtin aggregates are capable of directly penetrating cell membranes without passage through endocytic compartments60. Neurodegenerative disease proteins might be transferred among the bodies and processes of cells via several of these mechanisms simultaneously. Notably, this transfer may occur across synapses and thus correspond to intrinsic connectivities between cells11.
Importantly, there is evidence to indicate that tau and α-synuclein can spread via neuronal connections (BOX 3). In vitro studies indicate that mutant and wild-type tau, fibrillar α-synuclein and wild-type TDP43 can be transported through the axon anterogradely as well as retrogradely39,61–63 (and the transport of tau has recently been summarized in detail64). In transgenic mice expressing mutant human tau or mutant human α-synuclein, injection of tau or α-synuclein preformed fibrils, respectively, into the brain caused aggregates in anatomical structures that were relatively distant from the original injection sites, whereas no aggregates formed at the injection site28,42, thus implying that neuronal connections are probably involved in disease protein propagation and disease spread. In addition, intramuscular injection of mutant α-synuclein in two mouse strains — one of which expressed mutant human α-synuclein and the other of which expressed wild-type human α-synuclein — caused intraneuronal α-synuclein pathology in the brain and the spinal cord65.
Evidence from studies in humans
In this section, we review evidence from post-mortem and positron emission tomography (PET) studies of human tissue, and explain how these studies support the concept that neurodegenerative disease progression (in the disorders discussed here) probably reflects the cell–cell propagation of non-prion disease proteins.
Stereotypical patterns of pathology progression
Neuropathological studies have identified that stereotypical patterns of pathology occur in various neurodegenerative diseases over time, and that progression of these patterns is associated with increasing severity of the clinical phenotype, thus enabling the development of staging systems for these diseases. These studies noted that pathological lesions develop at different CNS sites sequentially, and that disease progression is associated with an increase in the size of aggregates and an increase in the number of cells showing pathological inclusions at these sites66,67. Such a stereotypical pathology pattern was first established for AD, in which tau aggregates are found first in the locus coeruleus of the pontine tegmentum68 and then in the transentorhinal cortex of the anteromedial temporal lobe (FIG. 2a,b). Subsequently, lesions are found in other parts of the temporal lobe, including the entorhinal and hippocampal areas, with lesions next becoming detectable in the basal temporal lobe and the insular cortex, before finally developing in the neocortex66,69. Stereotypical patterns of tau pathology are not restricted to AD; tau lesions in chronic traumatic encephalopathy can originate close to perivascular spaces within the depths of cortical sulci70 and become subsequently detectable in large regions of the neocortex and allocortex, diencephalon, basal ganglia, brainstem and spinal cord7. Thus, even though tau pathology characterizes both AD and chronic traumatic encephalopathy, the overall directions in which this pathology progresses in these diseases are different.
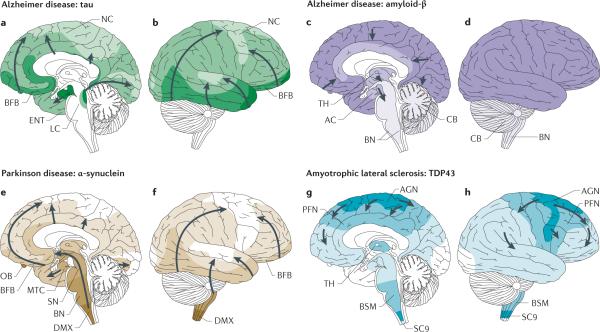
Figure 2. Sequential topographical dissemination of non-prion proteins in neurodegenerative diseases.
In all panels, the pathology is first detected in areas delineated by darker colours and subsequently in regions shown in lighter colours. a, b | In Alzheimer disease, tau aggregates develop in the locus coeruleus (LC), then in the transentorhinal and entorhinal regions and subsequently in the hippocampal formation and in broad areas of the neocortex (NC)69. c, d | In contrast to tau pathology, amyloid-β deposits in Alzheimer disease are first observed in the NC and are then detected in allocortical, diencephalic and basal ganglia structures (in a caudal direction) and in the brainstem, and occasionally in the cerebellum (CB)71. e, f | The progression of α-synuclein-immunoreactive Lewy body and Lewy body and neurite pathology in Parkinson disease follows an ascending pattern from the brainstem to the telencephalon80. The earliest lesions can be detected in the olfactory bulb (OB), as well as in the dorsal motor nucleus of the vagus nerve (DMX) in the medulla oblongata. At later stages, the α-synuclein aggregate pathology is found more rostrally through the brainstem via the pons and midbrain, in the basal forebrain and, ultimately, in the NC. g, h | In amyotrophic lateral sclerosis cases with a low burden of TAR DNA-binding protein 43 (TDP43) pathology, TDP43 inclusions are seen in the agranular motor cortex (AGN), in the brainstem motor nuclei of cranial nerves XII–X, VII and V, and in α-motor neurons in the spinal cord. Later stages of disease are characterized by the presence of TDP43 pathology in the prefrontal neocortex (PFN), brainstem reticular formation, precerebellar nuclei, pontine grey and the red nucleus. Subsequently, prefrontal and postcentral neocortices, as well as striatal neurons, are affected by pathological TDP43, before the pathology is found in anteromedial portions of the temporal lobe, including the hippocampus96. AC, allocortex; BFB, basal forebrain; BN, brainstem nuclei; BSM, brainstem somatomotor nuclei; ENT, entorhinal cortex; MTC, mesiotemporal cortex; SC9, spinal cord grey-matter lamina IX; SN, substantia nigra; TH, thalamus. Part f reprinted from Neurobiology of Aging, 24, Braak, H. et al. Staging of brain pathology related to sporadic Parkinson's disease, 197–211, © 2003, with permission from Elsevier.
In contrast to the dissemination of tau, patterns of Aβ plaques in AD follow essentially the opposite direction: plaques are initially found in the cortex and then, at later disease stages, spread to the brainstem71 (FIG. 2c,d). Indeed, studies have shown that Aβ plaques are observed first in the neocortex and then in allocortical, diencephalic and basal ganglial structures before being found — in cases with the highest burden of pathology — in the brainstem and cerebellum66,71,72. Why these two major disease proteins of AD show such fundamentally different patterns is incompletely understood. Although tau and Aβ are likely to exert toxicity via separate mechanisms73, observations from in vitro and in vivo models indicate that Aβ may drive the formation of tau pathology74 and that tau may mediate Aβ toxicity75–77. Furthermore, these proteins could have synergistic deleterious effects on, for instance, mitochondrial function78. Future studies of possible interactions between these key players of AD pathology are needed to explain their divergent spreading patterns in AD79.
Although tau pathology in AD is first found in caudal areas and then later in rostral regions, and Aβ plaque pathology is detected in increasingly caudal areas with disease progression, α-synuclein-containing Lewy bodies and Lewy neurites in PD, PD with dementia, and dementia with Lewy bodies are first detected in ventral areas and subsequently in more dorsal brain regions69,71,80. The earliest lesions in PD and in dementia with Lewy bodies can be detected in the olfactory bulb and anterior olfactory nucleus, as well as in the dorsal motor nucleus of the vagus nerve in the medulla oblongata. In PD and in dementia with Lewy bodies, with increasing burden of pathology, α-synuclein aggregate pathology is found in the pons and midbrain before being found in the basal forebrain and, ultimately, in the neocortex80–85 (FIG. 2e,f). Thus, only in more advanced stages of PD does α-synuclein aggregation cause the loss of midbrain dopaminergic neurons in the pars compacta of the substantia nigra80. It is this specific cell loss that has been linked to the classic motor symptoms of PD, including rigidity, resting tremor and bradykinesia. The accumulation of α-synuclein aggregates in the anterior olfactory nucleus86 and olfactory bulb is clinically reflected by hyposmia, which is frequently observed before the onset of motor symptoms in PD87.
Intriguingly, in individuals with early PD, α-synuclein pathology has also been detected outside the CNS, in neurons of Auerbach's and Meissner's plex-uses of the enteric nervous system (ENS)88,89, as well as in autonomic nerves of the submandibular gland90. This distribution of α-synuclein pathology could explain other early symptoms of PD, such as dysphagia and constipation91, and has prompted speculation about the possible spread of early α-synuclein pathology between the closely connected ENS and the dorsal motor nucleus of the vagus nerve. However, it currently remains unclear whether it is the olfactory bulb, the ENS or the lower brainstem that is the earliest focus of α-synuclein pathology92–94. Indeed, the appearance of the initial pathology in these separate locations gave rise to the ‘dual-hit theory’ of PD95.
Our group recently proposed four sequential stages of TDP43 pathology in ALS, whereby the protein pathology initially seems to spread rostrally and caudally from the motor neocortex and towards the spinal cord and brainstem, and then to frontal, parietal and, ultimately, anteromedial temporal lobes at later stages96 (FIG. 2g,h). Moreover, other studies show stereotypical patterns of TDP43 pathology that are suggestive of sequential spreading in other neurodegenerative diseases. Indeed, TDP43 aggregates have frequently been observed in addition to tau pathology and Aβ plaques in AD97–102. In such cases, TDP43 aggregates have been reported to first appear in the amygdala and, with increasing burden of pathology, to be subsequently detected in the entorhinal and hippocampal areas, the occipitotemporal and inferior temporal cortical areas and, finally, the frontal cortex and basal ganglia103. Similarly, in FTLD-TDP presenting as behavioural variant FTD, a stereotypical distribution pattern suggestive of a sequential spreading of TDP43 aggregates along a fronto-occipital gradient was observed, with the first lesions developing in orbitofrontal areas and the amygdala, and other lesions appearing later in premotor, primary motor, parietal and, finally, occipital areas of the cortex104. In chronic traumatic encephalopathy, TDP43 pathology has been observed among widespread cortical regions (as in FTLD-TDP), as well as in the spinal cord, although no clear regional spreading pattern has been described7. It is therefore possible that TDP43 pathology spreads along different pathways in different neurodegenerative diseases.
Vulnerable types of neurons
Human tissue studies and animal model experiments indicate that the spatiotemporal patterns of neurodegenerative disease protein pathology are highly selective. Only a small number of the many neuronal types are prone to developing the abnormal aggregations, with the remainder — often directly in the vicinity of the affected neurons — remaining functionally and morphologically normal. For instance, α-synuclein pathology in early multiple system atrophy is observed in neurons of the inferior olive in the medulla oblongata105, whereas the dorsal motor nuclei of the vagus nerve and the hypoglossal nerves remain virtually free of α-synuclein aggregates (except in patients with severe overall pathology). The reasons underlying this selective vulnerability of distinct neuronal populations are unknown. Neuronal vulnerability could be determined by inherent biochemical and structural characteristics that would render neurons more prone to developing aggregates, and/or by the location and connectivity of these cells. Glial cells, particularly oligodendrocytes, exhibit α-synuclein inclusions early in multiple system atrophy105; however, the mechanisms of possible spread between glial cells and neurons remain largely unknown (BOX 4).
Several studies have noted that neurons affected by the aggregation of α-synuclein, tau or TDP43 have specific anatomical characteristics. In PD, the neurons found to be particularly vulnerable to α-synuclein aggregation belong to the class of projection neurons80. Specifically, projection neurons with axons that were very long exhibited a pronounced tendency to develop α-synuclein inclusions. By contrast, projection neurons with short axons (such as the pyramidal cells of neo cortical layers II and IV, the neurons of the presubicular parvocellular layer of the hippocampus and local-circuit neurons with short axons) were generally resistant to α-synuclein aggregation80. Furthermore, projection neurons with long and thin axons that were only sparsely myelinated or unmyelinated were vulnerable to α-synuclein aggregates, whereas neurons with long, but thickly myelinated axons with large diameters were resistant to the formation of such aggregates80,106.
It has been suggested that projection neurons with sparsely myelinated axons would require prodigious energy expenditure to maintain axonal function and transport107, and that such high energy demands would result in continuously high levels of oxidative stress that could increase neuron vulnerability to α-synuclein aggregation in PD108–111. In the final stages of PD (when α-synuclein aggregates can be found in the neocortex), the cortical areas that myelinate ontogenetically last and therefore have thinly myelinated axons develop severe α-synuclein pathology, whereas thickly myelinated axons in primary cortices are comparatively impervious to α-synuclein aggregation112. Intriguingly, a highly similar pattern can be noted in AD: the first tau aggregates appear in the sparsely myelinated temporal mesocortex, whereas heavily myelinated primary cortical fields are the last to exhibit such pathology113.
Moreover, motor neurons are very large with very long axons that require high levels of mitochondrial activity compared with other neurons, and this could make them more vulnerable in ALS114. Importantly, however, neuronal susceptibility to TDP43 aggregation in ALS is not confined to motor neurons but also includes non-motor neurons such as the pyramidal layer II or III cells of large (non-motor) areas of the neocortex, especially in frontal areas96. By contrast, some motor neurons — for example, the motor neurons of the cranial nerves III, IV and VI that innervate the extrinsic eye muscles — are not (or are only at very late stages of disease) affected by TDP43 lesions96.
Routes and pathways
Imaging studies of patients with FTD or AD suggest that the differential vulnerability of brain regions to neurodegenerative changes is correlated with the strength of neuronal connections among the involved areas115,116. For example, brain regions with intense neuronal connectivity — as determined by functional MRI — correlated with foci of brain atrophy in patients with behavioural variant FTD or other neurodegenerative diseases115. Neuropathology studies of ALS, PD and AD also provide evidence to suggest that neurodegenerative protein pathologies may spread via neuronal connections.
In ALS, the vulnerability of subcortical neurons to developing TDP43 aggregates seems to be determined by the presence of direct cortical connections from already affected areas. Post-mortem analysis of ALS brain tissue revealed that areas that receive direct projections from the cortex develop TDP43 inclusions once the corresponding area of cortex is affected96. By contrast, neurons that receive no relevant cortical input — such as the motor nuclei of the extrinsic eye muscles — remain resistant to TDP43 lesions until late in the disease96.
In PD, the detection of α-synuclein pathology in the vagal dorsal motor nucleus in cases with early disease is intriguing because this nucleus is a synaptic relay station of the parasympathetic nervous system, which includes the ENS, and Lewy body and Lewy neurite pathologies have been repeatedly observed in neurons of the ENS89,117–119. The mechanisms that trigger misfolding of α-synuclein in the ENS remain unresolved, although local inflammation, oxidative stress and the crossing of external pathogens through the mucosal barrier to the ENS are all proposed triggers112,119.
In individuals with AD, or PD and concomitant AD, glial tau pathology is rare, but all of the brain regions and neuronal types that do exhibit tau pathology are anatomically interconnected. This pattern indicates that physical contacts, axonal transport and/or transynaptic transmission between involved regions could play a key role in the pathogenesis of AD120–122. The locus coeruleus — the initial site of tau pathology in AD — is anatomically distant from the cortical transentorhinal region, the second site in which pretangles (and subsequently, neurofibrillary tangles) develop in AD. Tau aggregates are at first confined to isolated cortical pyramidal cells that are located chiefly in the transentorhinal region11,25,120,123,124; however, axonal projections from the coeruleus project to this region, suggesting that these projections may mediate the propagation of tau pathology.
Some nuclei within the brainstem (including the locus coeruleus), midbrain, basal forebrain and hypothalamus send long and extensively ramifying projections to the olfactory bulb, many subcortical nuclei, the entire cerebral cortex, the cerebellum and the spinal cord. Such nuclei, in contrast to specific thalamic nuclei (the neurons of which project and relay data to specific cortical regions), belong to a functionally unified group called the non-thalamic nuclei with diffuse cortical projections, and these projections are assumed to have a more generalized effect on cortical regions125. Even at early stages of AD, all of these diffusely projecting non-thalamic nuclei display some degree of tau pathology126–128.
Phylogenetic influences may also be partially responsible for the spread of tau pathology from the locus coeruleus to cortical neurons in the transentorhinal region in AD. The transentorhinal region is an interface between the basal temporal neocortex (which develops late, both phylogenetically and ontogenetically speaking) and the portions of the entorhinal region that are close to the neocortex. Among primates, humans have the largest transentorhinal region and the transentorhinal region that shows the highest degree of laminar differentiation129. The most evolutionarily recent portions of the locus coeruleus project to and synapse in the transentorhinal region. The fact that this connection is evolutionarily recent may partially explain why neurons from these two areas develop the earliest AD-associated tau lesions within the CNS68,122,130.
PET studies
Human tissue pathology-staging studies are limited by the relative lack of early (that is, prodromal) cases, because most patients die as a consequence of the neurodegenerative disease. Furthermore, these studies are limited by the fact that the resulting neuropathological data are by definition cross-sectional, and can propose a sequential order of involvement only by correlating findings with clinical phenotype and disease duration. Although experimental evidence from animal studies suggests a similar spreading in humans is most likely, this cannot be proven in any given individual autopsy case.
To validate the sequential involvement of different CNS regions proposed by human autopsy studies, imaging biomarkers specific to the different disease proteins are necessary because they should enable the in vivo detection and monitoring of any spreading of protein pathology in longitudinal studies. Currently, the most promising markers are PET ligands. Such markers have been developed to visualize Aβ pathology in individuals with AD, and PET ligands for pathological tau are also emerging131,132.
The PET tracer that is most widely used for imaging Aβ is 11C-6-OH-BTA-1, which is also known as 11C-Pittsburgh compound B (11C-PiB), which was developed by Klunk and colleagues133. 11C-PiB PET-imaging trials in humans have shown that tracer uptake in the neocortical brain regions of patients with AD is substantially greater than in those of individuals without dementia133. Moreover, trial data have shown that tracer uptake is predictive of conversion of mild cognitive impairment to AD134 and correlates with Aβ deposition (as determined post-mortem)133,135. Individuals carrying rare autosomal-dominant AD mutations showed elevated 11C-PiB levels in nearly every cortical region and also in subcortical grey-matter structures 15 years before the estimated age of dementia onset136. In addition to 11C-PiB, several studies have used the 18F-labelled Aβ-targeting tracers flutemetamol137, florbetapir138 and florbetaben139. Flutemetamol showed good discrimination between AD and controls137, and florbetapir showed close correlation of 18F uptake in PET with post-mortem Aβ pathology138. Furthermore, florbetaben showed a higher tracer uptake in individuals with AD than in age-matched normal controls, especially in neocortical regions (particularly the posterior cingulate gyrus)139. Moreover, there was a lower uptake of an Aβ-specific PET ligand within the hippocampus than in neocortical brain areas, and this may closely reflect the more prominent Aβ pathology in neocortical areas than in allocortical areas, as reported in post-mortem neuropathology studies71. A caveat, however, is that Aβ-specific PET tracers may bind to fibrillar rather than to early-stage diffuse Aβ plaques; therefore, the earliest deposits of Aβ may not be visualized by these imaging ligands. In addition, 11C-PiB and the 18F-labelled PET tracers bind with greater affinity to β-sheet (fibrillar) Aβ than to diffuse plaques140.
Several PET markers that are highly specific to neurofibrillary tangles and other types of tau pathology (including glial tau pathology) have recently been developed, such as the 18F-labelled tracers 18F-THK5105 (REF. 184), 18F-THK523 (REF. 132), 18F-T807 (REF. 141), 18F-T808 (REF. 142) and the 11C-labelled tracer 11C-PBB3 (REF. 143). In vitro studies in AD brain homogenates have shown that THK5105 and T808 have an affinity for tau fibrils that is 25–27-fold higher than that for Aβ fibrils144. In a group of eight individuals with late-stage AD, the 18F-THK5105 PET tracer was substantially enriched in the medial and inferior temporal lobe (as compared with cognitively normal age-matched controls)145, thus reflecting the early effects of tau neurofibrillary tangle pathology on these areas in AD69. In addition, 18F-THK5105 retention was strongly associated with decreases in cognitive parameters and with reduced hippocampal and whole-brain grey-matter volumes. All of these associations are consistent with findings from previous post-mortem studies that showed marked correlations between neurofibrillary tangle density and dementia severity or neuronal loss69.
A notable feature of the tau tracers is that they have different sensitivities to different types of tau lesions depending on the underlying disease. Tau exists in six isoforms: three three-repeat (3R) isoforms and three four-repeat (4R) isoforms. Thus, tau deposits can contain 3R tau, 4R tau or a mixture of 3R and 4R tau iso-forms, depending on the specific disease6. The 18F-tracer THK523 exclusively labels neurofibrillary tangles (which contain a mixture of 3R and 4R isoforms) that are present in AD, but not the 3R tau lesions that occur in Pick disease or the 4R tau lesions that occur in corticobasal degeneration and progressive supra-nuclear palsy, whereas 11C-PBB3 seems to detect all of these tau lesions143. Thus, these tracers possess different clinical applications, either as general markers of AD and related tauopathies (for instance, in the case of 11C-PBB3) or for the differential diagnosis to distinguish between tauopathies (for example, 18F-THK523).
A current limitation of PET studies is their relatively low spatial resolution, which makes it difficult to adequately detect neurofibrillary tangles within subcortical regions and small anatomical structures such as the locus coeruleus (which is where, according to neuro-pathological staging models, tau pathology first occurs in AD69). Thus, compared with autopsy staging studies that can focus on individual lesions and involved cells, PET studies can only provide an assessment of regional effects. In addition, the majority of PET studies have been cross-sectional; to show a sequential spread of pathology, additional longitudinal studies are necessary that should also compare their findings to those from post-mortem pathology analyses. Finally, there are still no specific neuroimaging markers for synucleinopathies or TDP43 proteinopathies, although the development of such ligands is the focus of intense ongoing research. Indeed, for these latter diseases, we are currently limited to indirect approaches that monitor the neuronal and axonal loss that is associated with potentially spreading pathology. Given the close relationship between the presence of protein deposits and neuronal loss (as seen in pathology studies of tau in AD or TDP43 in ALS and in FTLD-TDP69,96,104) in certain regions, serial analyses of changes in brain connectivity115 and of progressive damage to fibre tracts could help to delineate spreading pathways in proteinopathies for which no specific in vivo marker yet exists. For example, we demonstrated that ALS-induced axonal loss in white-matter tracts — as measured by diffusion tensor imaging — affected the same regions as seen in our staging study of TDP43 pathology146, and we are currently implementing this diffusion tensor imaging-based approach in a prospective study. However, prospective studies that implement serial fibre-tract and connectivity analyses are still rare and have been limited to AD147. Moreover, these prospective studies have mostly included small numbers of individuals and have not compared their findings with data from post-mortem analysis of protein pathology.
Therapeutic implications
To date, no disease-modifying therapy exists for the neurodegenerative diseases discussed here, and neuroprotective and anti-inflammatory therapies have largely proved unsatisfactory. Template-directed replication and subsequent cell–cell transmission of pathology-associated proteins provides a common molecular pathway that could be targeted by novel therapeutic strategies with the aim of disrupting or delaying propagation.
As template-directed misfolding is likely to be a very early event in the pathological cascade of the non-prion neurodegenerative diseases discussed here, one therapeutic approach could be to stabilize the wild-type proteins in their normal conformation, ideally rendering them resistant to template-directed conformational change while not interfering with their normal cellular function13. This approach has already been applied with some success to the treatment of transthyretin amyloidosis148. In this disease, the transthyretin tetramer must first dissociate into monomers to form amyloid. Ligands that stabilize the tetramer can prevent amyloidosis, and a drug with that effect (tafamidis) has been approved by the European Medicines Agency for the treatment of a familial form of transthyretin amyloidosis148.
Moreover, agents that interfere with the release or uptake of neurodegenerative disease proteins could prevent transmission of pathology to neighbouring neurons. For example, specific antibodies could capture protein seeds in the extracellular space as they are ‘in transit’ between neurons149, or target receptors or other cellular proteins needed for the uptake or release of pathogenic proteins10,13,17,40,50,150,151. However, such immunological interventions in humans would face the formidable obstacles of the blood–brain barrier and the blood–cerebrospinal fluid barrier, both of which limit the passage of extrathecally administered antibodies into the CNS152. Similarly, interfering with protein transmission by nonspecifically enhancing cell membrane stability, inhibiting endocytosis or inhibiting exosomal protein secretion would probably interfere with the homeostasis of other cellular proteins and thus entail unacceptable adverse effects. However, these therapeutic strategies are worthy of further exploration.
Open questions and future directions
Although the propagation of disease proteins offers a unifying pathophysiological concept of neurodegenerative diseases, several key issues need to be resolved. The specific molecular mechanisms that trigger the initial conversion of normally soluble proteins into filamentous or aggregated polymers remain unknown. Understanding the initial event or events in the protein-misfolding cascade would be valuable for identifying therapeutic targets that could be modulated before potentially irreversible spreading of protein pathology and neuron loss.
Furthermore, it will be crucial to determine the specific biochemical characteristics of protein species that are transmissible. For instance, it remains to be determined whether pre-fibrillar protein species, such as oligomers, are more detrimental to cells than are fibrillar forms153. Many mechanistic aspects of cell–cell transmission require clarification. Although the uptake and release of pathological proteins via different types of endocytosis and exocytosis processes can explain pathological protein transmission in cell culture models, they can only partially account for the spreading of disease proteins over considerable anatomical distances as observed in animal models and human tissue studies62,154. An understanding of the pathways and directionality of spreading would be facilitated by the definition of primary (early) foci of protein pathology in the human disease. However, for some diseases, such as PD, the initial focus is still being discussed95, and for others, such as ALS, it has not been determined96. This is attributable partly to the limitations of neuropatho-logical studies, which analyse selected and often only small blocks of tissue (obviously, with the aim of preserving invaluable human material) that do not allow detailed analysis of pathology in large regions or across different regions of the CNS. Thus, comprehensive pathology studies performed on entire cortical regions or entire hemispheres, the brainstem and the full length of the spinal cord may be necessary to more reliably define the early foci of protein pathology.
Finally, many neurodegenerative diseases are likely to be non-cell autonomous, with an important part played by astroglia, oligodendroglia and microglia. For example, in multiple system atrophy, α-synuclein pathology chiefly presents as cytoplasmic inclusions in oligodendrocytes (although it is also observed to a much lesser extent in neurons)105. The relevance and extent of glial contributions to the transmission of protein pathology in neurodegenerative diseases remain unclear.
Considerable effort will be needed to resolve each of these problems and to make progress towards effective therapies for neurodegenerative diseases. Indeed, any progress in resolving the key issues outlined in this Review is likely to advance our understanding of each of these diseases substantially, and future strategies that target the transmission of any of these pathological proteins will most probably constitute a fundamental step forward in treating neurodegenerative diseases.
Box 1 | Structural characteristics of neurodegenerative disease proteins.
The misfolded conformations of amyloid-β, tau, α-synuclein, huntingtin and superoxide dismutase 1 are characterized by bundles of twisted, non-branching filaments that are composed of β-sheets that in turn consist of β-strands13.
These filaments, known as amyloids, are defined by specific X-ray diffraction patterns, as well as by chemical stains such as Congo red, Thioflavin-S and Thioflavin-T155. TAR DNA-binding protein 43 (TDP43) aggregates are less amyloid-like than the pathological proteins mentioned above: TDP43 inclusions mainly contain TDP43-immunoreactive, amorphous disordered aggregates, in which occasional 10–20 nm-diameter straight filaments are found156. However, a recent study in human tissue examining a small set of inclusions also observed skein-like inclusions composed of amyloid-like filamentous TDP43, indicating that a certain proportion of TDP43 inclusions are amyloid-like157. Furthermore, human-derived carboxy-terminal fragments of both wild-type and mutant TDP43 formed Thioflavin-T-reactive fibrils in culture158. In addition, the C-terminal region of human TDP43 has a prion-like domain159, and this was suggested to be required for amyloid-like fibril formation. Importantly, several studies suggest that pre-fibrillar species of neurodegenerative disease proteins may be more detrimental than fibrillar species153. For example, amyloid-β oligomers were suggested to induce synaptic dysfunction by interfering with signalling pathways downstream of certain NMDA or AMPA receptors at synaptic plasma membranes153.
Sequential spreading
The staged spatial dissemination of intracellular or extracellular protein aggregate pathology in neurodegenerative diseases.
Skein-like inclusions
Elongated filamentous intraneuronal inclusions of neurodegenerative disease proteins.
Cross-seeding
A process whereby seeds of one pathological protein cause the aggregation of another protein.
Exosomes
Cell-derived vesicles that are released from the plasma membrane and could thus constitute a mechanism of disease-protein release in neurodegenerative diseases.
Box 2 | Neurodegenerative disease proteins: prions or not?
Prions arise from an abnormal version of the cell membrane prion protein PrPC, called PrPSc, which misfolds and may aggregate into plaques. The name was coined by Prusiner in 1982 when he suggested that the scrapie agent be designated a “proteinacious infectious particle” or “prion” (REF. 14). Prions isolated from different sources (such as sheep, deer and cattle) target different brain areas, induce unique symptoms and have varied incubation times, indicating that prions exist in different conformational variants — so-called strains160,161. Prions are by definition infectious, and have caused epidemics162. Accordingly, infectivity is one of the essential components of the definition of prions. The infectious transmission of prions between species is not trivial and thus justifies categorizing infectious prion diseases as zoonoses. This was dramatically evidenced by the spread of bovine spongiform encephalopathy (which killed almost 200,000 cattle) to humans in the form of Creutzfeldt–Jakob disease, which caused more than 200 human deaths162.
Prionoids (also called ‘prion-like proteins’ to emphasize their similarities to prions, or ‘non-prion proteins’ to underline differences) is a term coined by Aguzzi10 to refer to disease proteins that seem to spread from cell to cell, possibly underlying the progression of non-prion neurodegenerative diseases such as Alzheimer disease and Parkinson disease11,13. Evidence from animal model studies and cell culture experiments indicates that misfolding, cell–cell propagation and spreading of prionoids within regions probably occurs in non-prion neurodegenerative diseases, and there is increasing evidence to indicate that these disease proteins can also exist as different strains29–31. However, although misfolded variants of these proteins from one diseased animal can, when injected at high concentrations into the brains of unaffected animals, spread in the recipient, they cannot be termed prions because they are neither infectious nor zoonoses in the way prion diseases are161,163. There are no epidemiological data to support an infectious spread of tauopathies and synucleinopathies through blood transfusions, organ transplants or other means. Indeed, we found no evidence of inter-individual transmission of any of these diseases in a cohort of more than 7,000 middle-aged and older individuals who, as children, had been treated with daily systemic injections of human growth hormone extracted from post-mortem human pituitaries, whereas 24 cases of prion PrPSc disease occurred163.
An even more notable difference between prions and other transmissible neurodegenerative disease proteins, again with respect to infectivity, comes from data on the very high prevalence of readily transmissible or infectious prion diseases in sheep, cattle, moose, elk and other animals for which there is just no counterpart for tauopathies or synucleinopathies. Nothing comparable to the epidemic of, for instance, bovine spongiform encephalopathy has occurred for tauopathies or synucleinopathies in livestock, and nor is anything likely to occur, because there is no reservoir of infectious human proteinopathies in livestock or other mammals. These clinical and epidemiological differences are important for human medicine, and suggest that prion diseases and neurodegenerative diseases caused by non-prion neurodegenerative disease proteins or prionoids should not be grouped together. In agreement with Aguzzi161, and to avoid unfounded public health concerns about whether Alzheimer disease, Parkinson disease and other non-prion neurodegenerative diseases may be infectious, we do not recommend referring to these other disease proteins as prions. Instead, we think it is preferable to refer to these non-prion neurodegenerative disease proteins by their long-accepted traditional names — pathological amyloid-β, tau and α-synuclein — rather than as amyloid-β, tau and α-synuclein prionoids.
Chronic traumatic
encephalopathy
A form of neurodegeneration that occurs in individuals who have sustained multiple concussions or injuries to the brain.
Rigidity
Stiffness and resistance to limb movement due to increased muscle tone. It is a key clinical symptom of Parkinson disease and can be uniform (known as lead-pipe rigidity) or ratchety (known as cogwheel rigidity).
Hyposmia
A reduced ability to smell and detect odours. This deficit can be due to any of a wide range of causes, including neurodegenerative diseases such as Parkinson disease or Alzheimer disease.
Dual-hit theory
The proposal that α-synuclein pathology in Parkinson disease starts in two different locations: the lower brainstem (dorsal motor nucleus of the vagus nerve) and the olfactory bulb.
Box 3 | Evidence from animal model studies of spreading via neuronal connections.
If the vulnerability of neurons to protein pathology were determined solely by inherent neuronal characteristics, one would expect the same types of neurons to be affected, independently of the location of the initial focus. However, animal models in which the initial focus of pathology is varied have shown that depending on where preformed fibrils (PFFs) are injected, different neuronal populations are sequentially affected25,28,42,44,124, implying that neuronal connectivity could be an important clue to understanding neuronal vulnerability to protein aggregation in neurodegenerative diseases. For example, injections of human tau PFFs into the striatum of PS19 human mutant tau transgenic mice induced tau aggregation in regions interconnected with the striatum, including the substantia nigra and thalamus, whereas after PFFs were injected into the hippocampus, aggregates first spread to the entorhinal cortex and contralateral hippocampus28.
Furthermore, as inclusion pathology can spread to anatomically distant structures while neurons closer to the injection site are spared, the transmission of protein pathology in these diseases might not simply occur by diffusion among neighbouring cells but rather occur via axonal connections25,28,42,124. A recent study in mice expressing human mutant tau indicated that tau seeded aggregates, similar to prions and amyloid-β aggregates, can reach the CNS from the periphery164. Recently, the hypothesis that intestinal α-synuclein spreads to the CNS via postganglionic enteric neurons and the vagus nerve was strengthened by the observation that human Parkinson disease brain lysates injected into the intestinal wall of wild-type Sprague–Dawley rats cause α-synuclein pathology in the dorsal motor nucleus of the vagus after ~144 hours165. Although mechanisms such as glial cell–glial cell or glial cell–neuron spreading, migration through interstitial fluids and/or cerebrospinal fluid, or blood-cell-mediated transport of tau amyloid pathology166 cannot be eliminated, the patterns of pathology in animal models and human tissue studies make the idea of neuronal connection-mediated propagation particularly attractive, at least for pathological tau and α-synuclein154.
Pretangles
Early intracellular aggregates of hyperphosphorylated tau protein that develop into filamentous neurofibrillary tangles.
Box 4 | Do glia contribute to protein propagation?
There is now abundant evidence to support a prominent role of astrocytes, microglia and oligodendrocytes in the initiation and progression of different neurodegenerative diseases (recently summarized in REFS 167–170). By contrast, little is known about how glia could specifically contribute to protein propagation.
Some initial studies on the role of glia in protein propagation exist for synucleinopathies. Here, both monomeric and aggregated α-synuclein released from neuronal cells were shown to be endocytosed by astrocytes in a co-culture of primary astrocytes with a neuronal cell line overexpressing α-synuclein171. This neuron-to-astroglia transfer of α-synuclein led to changes in the expression of genes related to neuroinflammatory response171. The glial accumulation of misfolded α-synuclein through transmission of the neuronal protein was also demonstrated in transgenic mice expressing human α-synuclein171. These results indicate that astroglial α-synuclein pathology observed in Parkinson disease and dementia with Lewy bodies could be caused by a direct transmission of neuronal α-synuclein to astroglia, and could result in inflammatory responses, including microglial activation172. Suppression of this subsequent microglial activation extended mouse survival.
To replicate the prominent pathological effects on oligodendroglia in multiple system atrophy, several transgenic mouse models that express human wild-type α-synuclein under the control of oligodendroglia-specific promoters have been developed. These models reproduce the accumulation of α-synuclein in glial cytoplasmic inclusions and these mice exhibit various levels of motor impairment173,174. Indeed, Yazawa et al.174 described α-synuclein pathology in axons of transgenic mice overexpressing wild-type human α-synuclein specifically in oligodendrocytes that at the time was difficult to explain, but that in retrospect may have signified the transfer of α-synuclein pathology from oligodendrocytes to axons.
In addition, oligodendrocytes in cell culture were shown to internalize α-synuclein monomers, oligomers and fibrils. Furthermore, in the brains of rats that overexpressed human α-synuclein, a transfer of human α-synuclein to rat oligodendroglia that had been grafted into the striatum was observed175. These findings support the notion of neuron-to-oligodendrocyte transfer of α-synuclein, a mechanism that could have a crucial role in the propagation of α-synuclein pathology in multiple system atrophy.
For tauopathies, Clavaguera et al.26 showed the induction of tau pathology in oligodendrocytes and astrocytes following injection of progressive supranuclear palsy, corticobasal degeneration and argyrophilic grain disease brain lysates into the brains of mice expressing wild-type tau. This demonstrates that tau pathology can spread to these cells, but does not explain the potential role of glia in tau propagation.
Finally, with regard to amyloid-β, both in cases of Alzheimer disease and transgenic mice, the expression of β-secretase 1 and amyloid-β were not restricted to neurons but to also occur in astroglia176, indicating that astrocytes may contribute to the generation of amyloid-β aggregates in vivo.
Thus, although the current evidence from cell culture studies and animal models is limited, it is plausible that pathological aggregates may be induced not only in neurons, but also (to a variable extent) in glial cells, and that pathological disease proteins may be transferred between neurons and glia. Therefore, future studies that more specifically examine the role of glial cells in protein propagation are necessary.
Acknowledgements
The authors thank H. Braak for reviewing the manuscript and M. Leonhard for help with designing the figures.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Bird T, et al. Epidemiology and genetics of frontotemporal dementia/Pick’s disease. Ann. Neurol. 2003;54:S29–S31. doi: 10.1002/ana.10572. [DOI] [PubMed] [Google Scholar]
- 2.Huisman MH, et al. Population based epidemiology of amyotrophic lateral sclerosis using capture-recapture methodology. J. Neurol. Neurosurg. Psychiatry. 2011;82:1165–1170. doi: 10.1136/jnnp.2011.244939. [DOI] [PubMed] [Google Scholar]
- 3.Savica R, et al. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol. 2013;70:1396–1402. doi: 10.1001/jamaneurol.2013.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 5.Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl Acad. Sci. USA. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 7.McKee AC, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. α-synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc. Natl Acad. Sci. USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 10.Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:783–790. doi: 10.1016/j.neuron.2009.12.016. [This article defines neurodegenerative disease proteins as prionoids.] [DOI] [PubMed] [Google Scholar]
- 11.Guo JL, Lee VM. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nature Med. 2014;20:130–138. doi: 10.1038/nm.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polymenidou M, Cleveland DW. Prion-like spread of protein aggregates in neurodegeneration. J. Exp. Med. 2012;209:889–893. doi: 10.1084/jem.20120741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [An excellent summary of amyloid-like characteristics of neurodegenerative disease proteins.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [A landmark publication that defines prions as infectious proteinaceous particles.] [DOI] [PubMed] [Google Scholar]
- 15.Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu. Rev. Pathol. 2008;3:11–40. doi: 10.1146/annurev.pathmechdis.3.121806.154326. [DOI] [PubMed] [Google Scholar]
- 16.Aguzzi A. Cell biology: beyond the prion principle. Nature. 2009;459:924–925. doi: 10.1038/459924a. [DOI] [PubMed] [Google Scholar]
- 17.Grad LI, et al. Intermolecular transmission of superoxide dismutase 1 misfolding in living cells. Proc. Natl Acad. Sci. USA. 2011;108:16398–16403. doi: 10.1073/pnas.1102645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruijn LI, et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 19.Deng HX, et al. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc. Natl Acad. Sci. USA. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Evidence for the experimental transmission of cerebral β-amyloidosis to primates. Int. J. Exp. Pathol. 1993;74:441–454. [This paper provides evidence that senile plaques in the brains of nonhuman primates can be induced by the intracerebral injection of human AD brain homogenates.] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Induction of β(A4)-amyloid in primates by injection of Alzheimer's disease brain homogenate. Comparison with transmission of spongiform encephalopathy. Mol. Neurobiol. 1994;8:25–39. doi: 10.1007/BF02778005. [DOI] [PubMed] [Google Scholar]
- 22.Kane MD, et al. Evidence for seeding of β-amyloid by intracerebral infusion of Alzheimer brain extracts in β-amyloid precursor protein-transgenic mice. J. Neurosci. 2000;20:3606–3611. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer-Luehmann M, et al. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 24.Rosen RF, et al. Exogenous seeding of cerebral β-amyloid deposition in βAPP-transgenic rats. J. Neurochem. 2012;120:660–666. doi: 10.1111/j.1471-4159.2011.07551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clavaguera F, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nature Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [A pioneering study reporting that tau pathology can be induced in tau-transgenic mice by the intracerebral injection of brain extracts containing aggregated tau.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clavaguera F, et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl Acad. Sci. USA. 2013;110:9535–9540. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo JL, Lee VM. Neurofibrillary tangle-like tau pathology induced by synthetic tau fibrils in primary neurons over-expressing mutant tau. FEBS Lett. 2013;587:717–723. doi: 10.1016/j.febslet.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iba M, et al. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer's-like tauopathy. J. Neurosci. 2013;33:1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo JL, et al. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell. 2013;154:103–117. doi: 10.1016/j.cell.2013.05.057. [This study demonstrates the existence of strains in non-prion neurodegenerative disease proteins.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders DW, et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. 2014;82:1271–1288. doi: 10.1016/j.neuron.2014.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heilbronner G, et al. Seeded strain-like transmission of β-amyloid morphotypes in APP transgenic mice. EMBO Rep. 2013;14:1017–1022. doi: 10.1038/embor.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li JY, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nature Med. 2008;14:501–503. doi: 10.1038/nm1746. [This study suggests that endogenous α-synuclein seeds in the brains of humans with PD can induce the aggregation of α-synuclein in grafted neurons.] [DOI] [PubMed] [Google Scholar]
- 33.Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov. Disord. 2008;23:2303–2306. doi: 10.1002/mds.22369. [DOI] [PubMed] [Google Scholar]
- 34.Li JY, et al. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson's disease. Mov. Disord. 2010;25:1091–1096. doi: 10.1002/mds.23012. [DOI] [PubMed] [Google Scholar]
- 35.Chu Y, Kordower JH. Lewy body pathology in fetal grafts. Ann. NY Acad. Sci. 2010;1184:55–67. doi: 10.1111/j.1749-6632.2009.05229.x. [DOI] [PubMed] [Google Scholar]
- 36.Kurowska Z, et al. Signs of degeneration in 12–22-year old grafts of mesencephalic dopamine neurons in patients with Parkinson's disease. J. Parkinsons Dis. 2011;1:83–92. doi: 10.3233/JPD-2011-11004. [DOI] [PubMed] [Google Scholar]
- 37.Hansen C, et al. α-synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kordower JH, et al. Transfer of host-derived α synuclein to grafted dopaminergic neurons in rat. Neurobiol. Dis. 2011;43:552–557. doi: 10.1016/j.nbd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volpicelli-Daley LA, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc. Natl Acad. Sci. USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mougenot AL, et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol. Aging. 2012;33:2225–2228. doi: 10.1016/j.neurobiolaging.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Luk KC, et al. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 2012;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masuda-Suzukake M, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luk KC, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [This report describes the induction of α-synuclein pathology by the intracerebral injection of synthetic α-synuclein fibrils into non-transgenic mice.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watts JC, et al. Transmission of multiple system atrophy prions to transgenic mice. Proc. Natl Acad. Sci. USA. 2013;110:19555–19560. doi: 10.1073/pnas.1318268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Recasens A, et al. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann. Neurol. 2014;75:351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- 47.Angot E, et al. α-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in vivo. PLoS ONE. 2012;7:e39465. doi: 10.1371/journal.pone.0039465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furukawa Y, Kaneko K, Watanabe S, Yamanaka K, Nukina N. A seeding reaction recapitulates intracellular formation of Sarkosyl-insoluble transactivation response element (TAR) DNA-binding protein-43 inclusions. J. Biol. Chem. 2011;286:18664–18672. doi: 10.1074/jbc.M111.231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nonaka T, et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 2013;4:124–134. doi: 10.1016/j.celrep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of Tau aggregation by fibrillar species. J. Biol. Chem. 2012;287:19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emmanouilidou E, et al. Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Danzer KM, et al. Exosomal cell-to-cell transmission of α synuclein oligomers. Mol. Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saman S, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grad LI, et al. Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosome-dependent and -independent mechanisms. Proc. Natl Acad. Sci. USA. 2014;111:3620–3625. doi: 10.1073/pnas.1312245111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munch C, O'Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc. Natl Acad. Sci. USA. 2011;108:3548–3553. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holmes BB, et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl Acad. Sci. USA. 2013;110:E3138–E3147. doi: 10.1073/pnas.1301440110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu JW, et al. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J. Biol. Chem. 2013;288:1856–1870. doi: 10.1074/jbc.M112.394528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee HJ, et al. Assembly-dependent endocytosis and clearance of extracellular α-synuclein. Int. J. Biochem. Cell Biol. 2008;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 60.Ren PH, et al. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nature Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fallini C, Bassell GJ, Rossoll W. The ALS disease protein TDP-43 is actively transported in motor neuron axons and regulates axon outgrowth. Hum. Mol. Genet. 2012;21:3703–3718. doi: 10.1093/hmg/dds205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freundt EC, et al. Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Ann. Neurol. 2012;72:517–524. doi: 10.1002/ana.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Utton MA, et al. The slow axonal transport of the microtubule-associated protein tau and the transport rates of different isoforms and mutants in cultured neurons. J. Neurosci. 2002;22:6394–6400. doi: 10.1523/JNEUROSCI.22-15-06394.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scholz T, Mandelkow E. Transport and diffusion of tau protein in neurons. Cell. Mol. Life Sci. 2014;71:3139–3150. doi: 10.1007/s00018-014-1610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sacino AN, et al. Intramuscular injection of α-synuclein induces CNS α-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc. Natl Acad. Sci. USA. 2014;111:10732–10737. doi: 10.1073/pnas.1321785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [A pioneering study that defines stages of tau pathology in AD and establishes the first sequential staging scheme in neurodegenerative diseases.] [DOI] [PubMed] [Google Scholar]
- 67.Mori F, et al. Maturation process of TDP-43-positive neuronal cytoplasmic inclusions in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol. 2008;116:193–203. doi: 10.1007/s00401-008-0396-9. [DOI] [PubMed] [Google Scholar]
- 68.Braak H, Del Tredici K. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 69.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geddes JF, Vowles GH, Nicoll JA, Revesz T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. 1999;98:171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- 71.Thal DR, Rub U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 72.Thal DR, et al. Sequence of Aβ-protein deposition in the human medial temporal lobe. J. Neuropathol. Exp. Neurol. 2000;59:733–748. doi: 10.1093/jnen/59.8.733. [A description of the sequential dissemination of Aβ pathology in AD.] [DOI] [PubMed] [Google Scholar]
- 73.Small DH, Mok SS, Bornstein JC. Alzheimer's disease and Aβ toxicity: from top to bottom. Nature Rev. Neurosci. 2001;2:595–598. doi: 10.1038/35086072. [DOI] [PubMed] [Google Scholar]
- 74.De Felice FG, et al. Alzheimer's disease-type neuronal tau hyperphosphorylation induced by Aβ oligomers. Neurobiol. Aging. 2008;29:1334–1347. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roberson ED, et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 76.Ittner LM, et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 77.Chetelat G. Alzheimer disease: Aβ-independent processes-rethinking preclinical AD. Nature Rev. Neurol. 2013;9:123–124. doi: 10.1038/nrneurol.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rhein V, et al. Amyloid-β and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc. Natl Acad. Sci. USA. 2009;106:20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jellinger KA. Interaction between pathogenic proteins in neurodegenerative disorders. J. Cell. Mol. Med. 2012;16:1166–1183. doi: 10.1111/j.1582-4934.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Braak H, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 81.Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 82.Del Tredici K, Braak H. Spinal cord lesions in sporadic Parkinson's disease. Acta Neuropathol. (Berl.) 2012;124:643–664. doi: 10.1007/s00401-012-1028-y. [DOI] [PubMed] [Google Scholar]
- 83.Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson's disease. Adv. Anat. Embryol. Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- 84.van de Berg WD, et al. Patterns of α-synuclein pathology in incidental cases and clinical subtypes of Parkinson's disease. Parkinsonism Relat. Disord. 2012;18:S28–S30. doi: 10.1016/S1353-8020(11)70011-6. [DOI] [PubMed] [Google Scholar]
- 85.Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nature Rev. Neurol. 2013;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 86.Pearce RK, Hawkes CH, Daniel SE. The anterior olfactory nucleus in Parkinson's disease. Mov. Disord. 1995;10:283–287. doi: 10.1002/mds.870100309. [DOI] [PubMed] [Google Scholar]
- 87.Hawkes CH, Shephard BC, Daniel SE. Is Parkinson's disease a primary olfactory disorder? QJM. 1999;92:473–480. doi: 10.1093/qjmed/92.8.473. [DOI] [PubMed] [Google Scholar]
- 88.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson's disease: the presence of Lewy bodies in Auerbach's and Meissner's plexuses. Acta Neuropathol. 1988;76:217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- 89.Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson's disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990;79:581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- 90.Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson's disease. Acta Neuropathol. 2010;119:703–713. doi: 10.1007/s00401-010-0665-2. [DOI] [PubMed] [Google Scholar]
- 91.Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease: frequency and pathophysiology. Neurology. 1992;42:726–732. doi: 10.1212/wnl.42.4.726. [DOI] [PubMed] [Google Scholar]
- 92.Doty RL. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann. Neurol. 2008;63:7–15. doi: 10.1002/ana.21327. [DOI] [PubMed] [Google Scholar]
- 93.Beach TG, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Del Tredici K, Braak H. Lewy pathology and neurodegeneration in premotor Parkinson's disease. Mov. Disord. 2012;27:597–607. doi: 10.1002/mds.24921. [DOI] [PubMed] [Google Scholar]
- 95.Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: the dual hit theory revisited. Ann. NY Acad. Sci. 2009;1170:615–622. doi: 10.1111/j.1749-6632.2009.04365.x. [DOI] [PubMed] [Google Scholar]
- 96.Brettschneider J, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013;74:20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amador-Ortiz C, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann. Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arai T, et al. Phosphorylated TDP-43 in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–136. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 99.Bigio EH, et al. TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta Neuropathol. 2010;120:43–54. doi: 10.1007/s00401-010-0681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davidson YS, et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer's disease, late onset Alzheimer's disease and Down's syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. (Berl.) 2011;122:703–713. doi: 10.1007/s00401-011-0879-y. [DOI] [PubMed] [Google Scholar]
- 101.Hu WT, et al. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol. 2008;116:215–220. doi: 10.1007/s00401-008-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Josephs KA, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–1857. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Josephs KA, et al. Staging TDP-43 pathology in Alzheimer's disease. Acta Neuropathol. 2014;127:441–450. doi: 10.1007/s00401-013-1211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brettschneider J, et al. Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD). Acta Neuropathol. 2014;127:423–439. doi: 10.1007/s00401-013-1238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jellinger KA, Seppi K, Wenning GK. Grading of neuropathology in multiple system atrophy: proposal for a novel scale. Mov. Disord. 2005;20:S29–S36. doi: 10.1002/mds.20537. [DOI] [PubMed] [Google Scholar]
- 106.Orimo S, et al. Unmyelinated axons are more vulnerable to degeneration than myelinated axons of the cardiac nerve in Parkinson's disease. Neuropathol. Appl. Neurobiol. 2011;37:791–802. doi: 10.1111/j.1365-2990.2011.01194.x. [DOI] [PubMed] [Google Scholar]
- 107.Aiello GL, Bach-y-Rita P. The cost of an action potential. J. Neurosci. Methods. 2000;103:145–149. doi: 10.1016/s0165-0270(00)00308-3. [DOI] [PubMed] [Google Scholar]
- 108.Giasson BI, et al. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 109.Chung CY, et al. Identification and rescue of α-synuclein toxicity in Parkinson patient-derived neurons. Science. 2013;342:983–987. doi: 10.1126/science.1245296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ryan SD, et al. Isogenic human iPSC Parkinson's model shows nitrosative stress-induced dysfunction in MEF2-PGC1α transcription. Cell. 2013;155:1351–1364. doi: 10.1016/j.cell.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zuo L, Motherwell MS. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson's disease. Gene. 2013;532:18–23. doi: 10.1016/j.gene.2013.07.085. [DOI] [PubMed] [Google Scholar]
- 112.Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 113.Braak H, Braak E. Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol. 1996;92:197–201. doi: 10.1007/s004010050508. [DOI] [PubMed] [Google Scholar]
- 114.Shaw PJ, Eggett CJ. Molecular factors underlying selective vulnerability of motor neurons to neurodegeneration in amyotrophic lateral sclerosis. J. Neurol. 2000;247(Suppl. 1):17–27. doi: 10.1007/BF03161151. [DOI] [PubMed] [Google Scholar]
- 115.Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73:1216–1227. doi: 10.1016/j.neuron.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raj A, Kuceyeski A, Weiner M. A network diffusion model of disease progression in dementia. Neuron. 2012;73:1204–1215. doi: 10.1016/j.neuron.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric α-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci. Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 118.Beach TG, et al. Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shannon KM, et al. α-synuclein in colonic submucosa in early untreated Parkinson's disease. Mov. Disord. 2012;27:709–715. doi: 10.1002/mds.23838. [DOI] [PubMed] [Google Scholar]
- 120.Dujardin S, et al. Neuron-to-neuron wild-type Tau protein transfer through a trans-synaptic mechanism: relevance to sporadic tauopathies. Acta Neuropathol. Commun. 2014;2:14. doi: 10.1186/2051-5960-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc. Natl Acad. Sci. USA. 1985;82:4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saper CB, Wainer BH, German DC. Axonal and transneuronal transport in the transmission of neurological disease: potential role in system degenerations, including Alzheimer's disease. Neuroscience. 1987;23:389–398. doi: 10.1016/0306-4522(87)90063-7. [DOI] [PubMed] [Google Scholar]
- 123.Duyckaerts C. Neurodegenerative lesions: seeding and spreading. Rev. Neurol. 2013;169:825–833. doi: 10.1016/j.neurol.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 124.Liu L, et al. Trans-synaptic spread of tau pathology in vivo. PLoS ONE. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morrison JH, Foote SL, O'Connor D, Bloom FE. Laminar, tangential and regional organization of the noradrenergic innervation of monkey cortex: dopamine-β-hydroxylase immunohistochemistry. Brain Res. Bull. 1982;9:309–319. doi: 10.1016/0361-9230(82)90144-7. [DOI] [PubMed] [Google Scholar]
- 126.Agnati LF, Bjelke B, Fuxe K. Volume versus wiring transmission in the brain: a new theoretical frame for neuropsychopharmacology. Med. Res. Rev. 1995;15:33–45. doi: 10.1002/med.2610150104. [DOI] [PubMed] [Google Scholar]
- 127.Nieuwenhuys R. Comparative aspects of volume transmission, with sidelight on other forms of intercellular communication. Prog. Brain Res. 2000;125:49–126. doi: 10.1016/S0079-6123(00)25006-1. [DOI] [PubMed] [Google Scholar]
- 128.O'Donnell J, Zeppenfeld D, McConnell E, Pena S, Nedergaard M. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem. Res. 2012;37:2496–2512. doi: 10.1007/s11064-012-0818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Braak H, Braak E. The human entorhinal cortex: normal morphology and lamina-specific pathology in various diseases. Neurosci. Res. 1992;15:6–31. doi: 10.1016/0168-0102(92)90014-4. [DOI] [PubMed] [Google Scholar]
- 130.Braak H, Del Tredici K. Evolutional aspects of Alzheimer's disease pathogenesis. J. Alzheimers Dis. 2013;33(Suppl. 1):S155–S161. doi: 10.3233/JAD-2012-129029. [DOI] [PubMed] [Google Scholar]
- 131.Klunk WE, et al. The binding of 2-(4′-methylaminophenyl)benzothiazole to postmortem brain homogenates is dominated by the amyloid component. J. Neurosci. 2003;23:2086–2092. doi: 10.1523/JNEUROSCI.23-06-02086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fodero-Tavoletti MT, et al. 18F-THK523: a novel in vivo tau imaging ligand for Alzheimer's disease. Brain. 2011;134:1089–1100. doi: 10.1093/brain/awr038. [DOI] [PubMed] [Google Scholar]
- 133.Klunk WE, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann. Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [The study describes the development of 1 1C-PiB, which is currently the most widely used PET tracer for Aβ.] [DOI] [PubMed] [Google Scholar]
- 134.Rowe CC, et al. Predicting Alzheimer disease with β-amyloid imaging: results from the Australian imaging, biomarkers, and lifestyle study of ageing. Ann. Neurol. 2013;74:905–913. doi: 10.1002/ana.24040. [DOI] [PubMed] [Google Scholar]
- 135.Bacskai BJ, et al. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch. Neurol. 2007;64:431–434. doi: 10.1001/archneur.64.3.431. [DOI] [PubMed] [Google Scholar]
- 136.Benzinger TL, et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer's disease. Proc. Natl Acad. Sci. USA. 2013;110:E4502–E4509. doi: 10.1073/pnas.1317918110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vandenberghe R, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann. Neurol. 2010;68:319–329. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- 138.Clark CM, et al. Use of florbetapir-PET for imaging β-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Barthel H, et al. Cerebral amyloid-β PET with florbetaben (18F) in patients with Alzheimer's disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol. 2011;10:424–435. doi: 10.1016/S1474-4422(11)70077-1. [DOI] [PubMed] [Google Scholar]
- 140.Ikonomovic MD, et al. Early AD pathology in a [C-11] PiB-negative case: a PiB-amyloid imaging, biochemical, and immunohistochemical study. Acta Neuropathol. 2012;123:433–447. doi: 10.1007/s00401-012-0943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chien DT, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J. Alzheimers Dis. 2013;34:457–468. doi: 10.3233/JAD-122059. [DOI] [PubMed] [Google Scholar]
- 142.Chien DT, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F18]-T808. J. Alzheimers Dis. 2014;38:171–184. doi: 10.3233/JAD-130098. [DOI] [PubMed] [Google Scholar]
- 143.Maruyama M, et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79:1094–1108. doi: 10.1016/j.neuron.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang W, et al. A highly selective and specific PET tracer for imaging of tau pathologies. J. Alzheimers Dis. 2012;31:601–612. doi: 10.3233/JAD-2012-120712. [DOI] [PubMed] [Google Scholar]
- 145.Okamura N, et al. Non-invasive assessment of Alzheimer's disease neurofibrillary pathology using 18F-THK5105 PET. Brain. 2014;137:1762–1771. doi: 10.1093/brain/awu064. [DOI] [PubMed] [Google Scholar]
- 146.Kassubek J, et al. Diffusion tensor imaging analysis of sequential spreading of disease in amyotrophic lateral sclerosis confirms patterns of TDP-43 pathology. Brain. 2014;137:1733–1740. doi: 10.1093/brain/awu090. [DOI] [PubMed] [Google Scholar]
- 147.Amlien IK, Fjell AM. Diffusion tensor imaging of white matter degeneration in Alzheimer's disease and mild cognitive impairment. Neuroscience. 2014;276:206–215. doi: 10.1016/j.neuroscience.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 148.Johnson SM, Connelly S, Fearns C, Powers ET, Kelly JW. The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug. J. Mol. Biol. 2012;421:185–203. doi: 10.1016/j.jmb.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bae EJ, et al. Antibody-aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J. Neurosci. 2012;32:13454–13469. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Holmes BB, Diamond MI. Cellular mechanisms of protein aggregate propagation. Curr. Opin. Neurol. 2012;25:721–726. doi: 10.1097/WCO.0b013e32835a3ee0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tran HT, et al. α-synuclein immunotherapy blocks uptake and templated propagation of misfolded α-synuclein and neurodegeneration. Cell Rep. 2014;7:2054–2065. doi: 10.1016/j.celrep.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Reiber H, Felgenhauer K. Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin. Chim. Acta. 1987;163:319–328. doi: 10.1016/0009-8981(87)90250-6. [DOI] [PubMed] [Google Scholar]
- 153.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nature Rev. Mol. Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 154.Braak H, et al. Amyotrophic lateral sclerosis — a model of corticofugal axonal spread. Nature Rev. Neurol. 2013;9:708–714. doi: 10.1038/nrneurol.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schmidt ML, et al. The fluorescent Congo red derivative, (trans, trans)-1-bromo-2,5-bis-(3-hydroxycarbonyl-4-hydroxy)styrylbenzene (BSB), labels diverse β-pleated sheet structures in postmortem human neurodegenerative disease brains. Am. J. Pathol. 2001;159:937–943. doi: 10.1016/s0002-9440(10)61769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cairns NJ, et al. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am. J. Pathol. 2007;171:227–240. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Robinson JL, et al. TDP-43 skeins show properties of amyloid in a subset of ALS cases. Acta Neuropathol. 2013;125:121–131. doi: 10.1007/s00401-012-1055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen AK, et al. Induction of amyloid fibrils by the C-terminal fragments of TDP-43 in amyotrophic lateral sclerosis. J. Am. Chem. Soc. 2010;132:1186–1187. doi: 10.1021/ja9066207. [DOI] [PubMed] [Google Scholar]
- 159.Goldschmidt L, Teng PK, Riek R, Eisenberg D. Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proc. Natl Acad. Sci. USA. 2010;107:3487–3492. doi: 10.1073/pnas.0915166107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aguzzi A, Heikenwalder M, Polymenidou M. Insights into prion strains and neurotoxicity. Nature Rev. Mol. Cell Biol. 2007;8:552–561. doi: 10.1038/nrm2204. [DOI] [PubMed] [Google Scholar]
- 161.Aguzzi A. Neurodegeneration: Alzheimer's disease under strain. Nature. 2014;512:32–34. doi: 10.1038/512032a. [DOI] [PubMed] [Google Scholar]
- 162.Sikorska B, Liberski PP. Human prion diseases: from Kuru to variant Creutzfeldt-Jakob disease. Subcell. Biochem. 2012;65:457–496. doi: 10.1007/978-94-007-5416-4_17. [DOI] [PubMed] [Google Scholar]
- 163.Irwin DJ, et al. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol. 2013;70:462–468. doi: 10.1001/jamaneurol.2013.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Clavaguera F, et al. Peripheral administration of tau aggregates triggers intracerebral tauopathy in transgenic mice. Acta Neuropathol. 2014;127:299–301. doi: 10.1007/s00401-013-1231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Holmqvist S, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [This study provides experimental evidence that supports the notion that α-synuclein can spread from the ENS to the lower brainstem in PD.] [DOI] [PubMed] [Google Scholar]
- 166.Sponarova J, Nystrom SN, Westermark GT. AA-amyloidosis can be transferred by peripheral blood monocytes. PLoS ONE. 2008;3:e3308. doi: 10.1371/journal.pone.0003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Philips T, Rothstein JD. Glial cells in amyotrophic lateral sclerosis. Exp. Neurol. 2014;262:111–120. doi: 10.1016/j.expneurol.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fellner L, Jellinger KA, Wenning GK, Stefanova N. Glial dysfunction in the pathogenesis of α-synucleinopathies: emerging concepts. Acta Neuropathol. 2011;121:675–693. doi: 10.1007/s00401-011-0833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brambilla L, Martorana F, Rossi D. Astrocyte signaling and neurodegeneration: new insights into CNS disorders. Prion. 2013;7:28–36. doi: 10.4161/pri.22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Farfara D, Lifshitz V, Frenkel D. Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer's disease. J. Cell. Mol. Med. 2008;12:762–780. doi: 10.1111/j.1582-4934.2008.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee HJ, et al. Direct transfer of α-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gu XL, et al. Astrocytic expression of Parkinson's disease-related A53T α-synuclein causes neurodegeneration in mice. Mol. Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fernagut PO, Tison F. Animal models of multiple system atrophy. Neuroscience. 2012;211:77–82. doi: 10.1016/j.neuroscience.2011.09.044. [DOI] [PubMed] [Google Scholar]
- 174.Yazawa I, et al. Mouse model of multiple system atrophy α-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 2005;45:847–859. doi: 10.1016/j.neuron.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 175.Reyes JF, et al. α-synuclein transfers from neurons to oligodendrocytes. Glia. 2014;62:387–398. doi: 10.1002/glia.22611. [DOI] [PubMed] [Google Scholar]
- 176.Rossner S, Lange-Dohna C, Zeitschel U, Perez-Polo JR. Alzheimer's disease β-secretase BACE1 is not a neuron-specific enzyme. J. Neurochem. 2005;92:226–234. doi: 10.1111/j.1471-4159.2004.02857.x. [DOI] [PubMed] [Google Scholar]
- 177.Nonaka T, Watanabe ST, Iwatsubo T, Hasegawa M. Seeded aggregation and toxicity of α-synuclein and tau: cellular models of neurodegenerative diseases. J. Biol. Chem. 2010;285:34885–34898. doi: 10.1074/jbc.M110.148460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of α-synuclein and its aggregates. J. Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nath S, et al. Spreading of neurodegenerative pathology via neuron-to-neuron transmission of β-amyloid. J. Neurosci. 2012;32:8767–8777. doi: 10.1523/JNEUROSCI.0615-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Langer F, et al. Soluble Aβ seeds are potent inducers of cerebral β-amyloid deposition. J. Neurosci. 2011;31:14488–14495. doi: 10.1523/JNEUROSCI.3088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Eisele YS, et al. Peripherally applied Aβ-containing inoculates induce cerebral β-amyloidosis. Science. 2010;330:980–982. doi: 10.1126/science.1194516. [This study shows that cerebral Aβ pathology can be caused by the introduction of Aβ seeds into the peritoneum.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Harris JA, et al. Transsynaptic progression of amyloid-β-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron. 2010;68:428–441. doi: 10.1016/j.neuron.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.de Calignon A, et al. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Okamura N, et al. Novel 18F-labeled arylquinoline derivatives for noninvasive imaging of tau pathology in Alzheimer disease. J. Nucl. Med. 2013;54:1420–1427. doi: 10.2967/jnumed.112.117341. [DOI] [PubMed] [Google Scholar]