Abstract
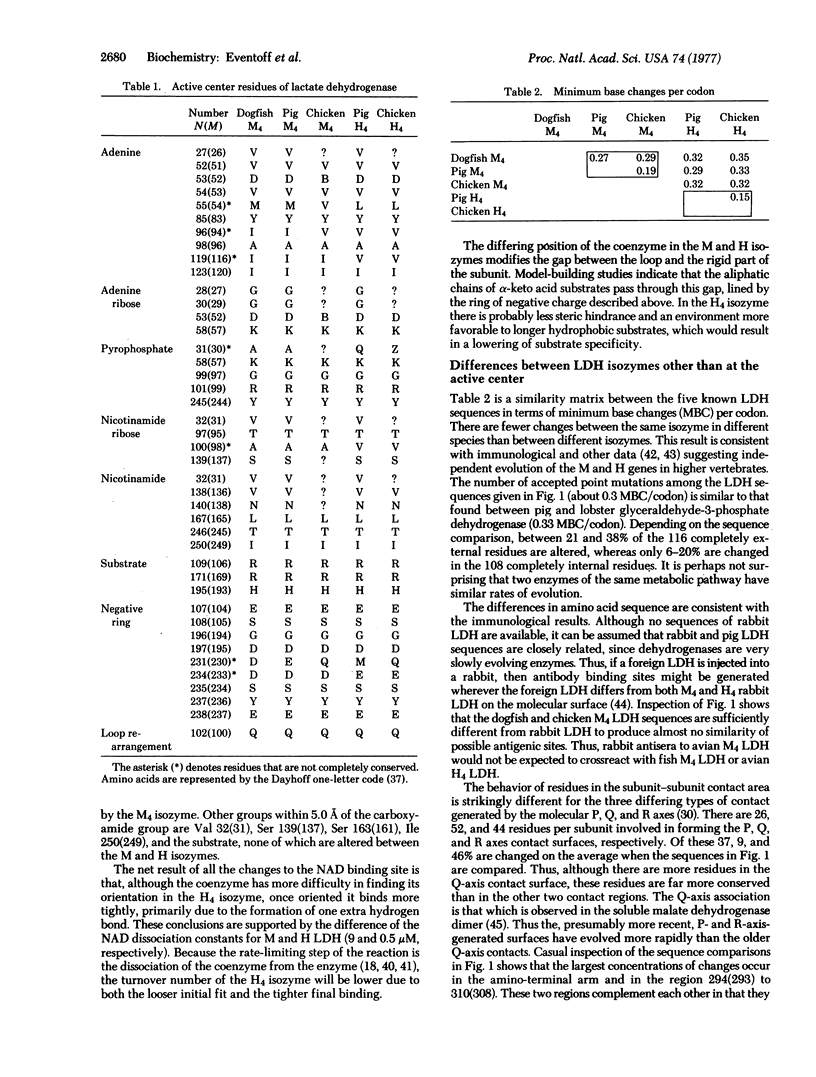
The three-dimensional structures of dogfish M4 (muscle) and pig H4 (heart) lactate dehydrogenase (L-lactate:NAD+ oxidoreductase, EC 1.1.1.27) have been determined and correlated with the amino acid sequences of the dogfish M4, pig M4, pig H4, chicken M4, and chicken H4 lactate dehydrogenase isozymes. These results have been related to the known differences of physicochemical properties between the M and H lactate dehydrogenase isozymes. By far the largest structural alterations occur in the transition between the "apo" and "ternary complex" conformational states of the enzyme rather than between species or isozymes. The major catalytic difference can be explained by a replacement of alanine (in the M chain) with a glutamine (in the H chain) in the vicinity of the binding site of the coenzyme phosphates. The known immunological differentiation of the M and H isozymes is consistent with the differences in their amino acid sequences.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON B. M., KAPLAN N. O. Enzymatic studies with analogues of diphosphopyridine nucleotide. J Biol Chem. 1959 May;234(5):1226–1232. [PubMed] [Google Scholar]
- Adams M. J., Buehner M., Chandrasekhar K., Ford G. C., Hackert M. L., Liljas A., Rossmann M. G., Smiley I. E., Allison W. S., Everse J. Structure-function relationships in lactate dehydrogenase. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1968–1972. doi: 10.1073/pnas.70.7.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battellino L. J., Blanco A. Catalytic properties of the lactate dehydrogenase isozyme "X" from mouse testis. J Exp Zool. 1970 Jun;174(2):173–186. doi: 10.1002/jez.1401740207. [DOI] [PubMed] [Google Scholar]
- Cahn R. D., Zwilling E., Kaplan N. O., Levine L. Nature and Development of Lactic Dehydrogenases: The two major types of this enzyme form molecular hybrids which change in makeup during development. Science. 1962 Jun 15;136(3520):962–969. doi: 10.1126/science.136.3520.962. [DOI] [PubMed] [Google Scholar]
- Eventoff W., Hackert M. L., Rossmann M. G. A low-resolution crystallographic study of porcine heart lactate dehydrogenase. J Mol Biol. 1975 Oct 15;98(1):249–258. doi: 10.1016/s0022-2836(75)80113-6. [DOI] [PubMed] [Google Scholar]
- FONDY T. P., PESCE A., FREEDBERG I., STOLZENBACH F., KAPLAN N. O. THE COMPARATIVE ENZYMOLOGY OF LACTIC DEHYDROGENASES. II. PROPERTIES OF THE CRYSTALLINE HM3 HYBRID FROM CHICKEN MUSCLE AND OF H2M2 HYBRID AND H4 ENZYME FROM CHICKEN LIVER. Biochemistry. 1964 Apr;3:522–530. doi: 10.1021/bi00892a010. [DOI] [PubMed] [Google Scholar]
- FROMM H. DETERMINATION OF DISSOCIATION CONSTANTS OF COENZYMES AND ABORTIVE TERNARY COMPLEXES WITH RABBIT MUSCLE LACTATE DEHYDROGENASE FROM FLUORESCENCE MEASUREMENTS. J Biol Chem. 1963 Sep;238:2938–2944. [PubMed] [Google Scholar]
- Heck H. D., McMurray C. H., Gutfreund H. The resolution of some steps of the reactions of lactate dehydrogenase with its substrates. Biochem J. 1968 Aug;108(5):793–796. doi: 10.1042/bj1080793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E., Tsernoglou D., Webb L., Banaszak L. J. Polypeptide conformation of cytoplasmic malate dehydrogenase from an electron density map at 3.0 angstrom resolution. J Mol Biol. 1972 Dec 30;72(3):577–589. doi: 10.1016/0022-2836(72)90176-3. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Jaenicke R. Thermodynamics of complex formation between nicotinamide adenine dinucleotide and pig skeletal muscle lactate dehydrogenase. Biochemistry. 1975 Jan 14;14(1):24–27. doi: 10.1021/bi00672a005. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Stinson R. A. The use of ternary complexes to study ionizations and isomerizations during catalysis by lactate dehydrogenase. Biochem J. 1973 Apr;131(4):739–748. doi: 10.1042/bj1310739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. S. Evolution of lactate dehydrogenase genes. FEBS Lett. 1972 Nov 15;28(1):51–55. doi: 10.1016/0014-5793(72)80675-6. [DOI] [PubMed] [Google Scholar]
- Jeckel D., Anders R., Pfleiderer G. Zum Wirkungsmechanismus der Lactat-Dehydrogenase. VI. Anderung der biochemischen Eigenschaften von Lactat-Dehydrogenase aus Schweineherzmuskel nach Nitrierung mit Tetranitromethan. Hoppe Seylers Z Physiol Chem. 1971 Jun;352(6):769–779. [PubMed] [Google Scholar]
- KAPLAN N. O., CIOTTI M. M. Evolution and differentiation of dehvdrogenases. Ann N Y Acad Sci. 1961 Nov 2;94:701–722. doi: 10.1111/j.1749-6632.1961.tb35567.x. [DOI] [PubMed] [Google Scholar]
- Kaplan N. O., Everse J., Admiraal J. Significance of substrate inhibition of dehydrogenases. Ann N Y Acad Sci. 1968 Jun 14;151(1):400–412. doi: 10.1111/j.1749-6632.1968.tb11903.x. [DOI] [PubMed] [Google Scholar]
- Kiltz H. H., Keil W., Griesbach M., Petry K., Meyer H. The primary structure of porcine lactate dehydrogenase: isoenzymes M4 and H4. Hoppe Seylers Z Physiol Chem. 1977 Jan;358(1):123–127. doi: 10.1515/bchm2.1977.358.1.123. [DOI] [PubMed] [Google Scholar]
- Lane R. S., Dekker E. E. 2-keto-4-hydroxybutyrate. Synthesis, chemical properties, and as a substrate for lactate dehydrogenase of rabbit muscle. Biochemistry. 1969 Jul;8(7):2958–2966. doi: 10.1021/bi00835a041. [DOI] [PubMed] [Google Scholar]
- Markert C. L., Møller F. MULTIPLE FORMS OF ENZYMES: TISSUE, ONTOGENETIC, AND SPECIES SPECIFIC PATTERNS. Proc Natl Acad Sci U S A. 1959 May;45(5):753–763. doi: 10.1073/pnas.45.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert C. L., Shaklee J. B., Whitt G. S. Evolution of a gene. Multiple genes for LDH isozymes provide a model of the evolution of gene structure, function and regulation. Science. 1975 Jul 11;189(4197):102–114. doi: 10.1126/science.1138367. [DOI] [PubMed] [Google Scholar]
- McPherson A., Jr Interaction of lactate dehydrogenase with its coenzyme, nicotinamide-adenine dinucleotide. J Mol Biol. 1970 Jul 14;51(1):39–46. doi: 10.1016/0022-2836(70)90268-8. [DOI] [PubMed] [Google Scholar]
- Musick W. D., Adams A. D., Rossmann M. G., Wheat T. E., Goldberg E. A low-resolution study of testicular lactate dehydrogenase using the molecular replacement technique. J Mol Biol. 1976 Jul 5;104(3):659–668. doi: 10.1016/0022-2836(76)90127-3. [DOI] [PubMed] [Google Scholar]
- NISSELBAUM J. S., PACKER D. E., BODANSKY O. COMPARISON OF THE ACTIONS OF HUMAN BRAIN, LIVER, AND HEART LACTIC DEHYDROGENASE VARIANTS ON NUCLEOTIDE ANALOGUES AND ON SUBSTRATE ANALOGUES IN THE ABSENCE AND IN THE PRESENCE OF OXALATE AND OXAMATE. J Biol Chem. 1964 Sep;239:2830–2834. [PubMed] [Google Scholar]
- PLAGEMANN P. G., GREGORY K. F., WROBLEWSKI F. [The electrophoretically separable lactic dehydrogenases in mammals. III. Influence of temperature on the lactic dehydrogenases in rabbits]. Biochem Z. 1961;334:37–48. [PubMed] [Google Scholar]
- Pesce A., Fondy T. P., Stolzenbach F., Castillo F., Kaplan N. O. The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of vertebrates. J Biol Chem. 1967 May 10;242(9):2151–2167. [PubMed] [Google Scholar]
- Pfleiderer G., Jeckel D. Kupplungsreaktion an Lactatdehydrogenase nach reversibler Maskierung der SH-Gruppen. Umsetzung mit p-Diazobenzolsulfonsäure zur Markierung essentieller Histidin- oder Tyrosinreste. Eur J Biochem. 1967 Sep;2(2):171–175. doi: 10.1111/j.1432-1033.1967.tb00122.x. [DOI] [PubMed] [Google Scholar]
- RAJEWSKY K., AVRAMEAS S., GRABAR P., PFLEIDERER G., WACHSMUTH E. D. IMMUNOLOGISCHE SPEZIFITAET VON LACTATDEHYDROGENASE ISOZYMEN DREIER SAEUGETIER-ORGANISMEN. Biochim Biophys Acta. 1964 Nov 22;92:248–259. [PubMed] [Google Scholar]
- Schwert G. W., Miller B. R., Peanasky R. J. Lactic dehydrogenase. X. A re-evaluation of the effects of pH upon the kinetics of the reaction. J Biol Chem. 1967 Jul 25;242(14):3245–3252. [PubMed] [Google Scholar]
- Schwert G. W. The estimation of kinetic constants for the lactate dehydrogenase system by the use of integrated rate equations. J Biol Chem. 1969 Mar 10;244(5):1285–1290. [PubMed] [Google Scholar]
- Sugrobova N. P., Kurganov B. I., Iakovlev V. A. Kinetika Obrazovaniia Neproduktiznogo Troinogo Kompleksa Laktatdegidrogenaza (Izoferment N4) Nad-Piruvat. Biokhimiia. 1975 Mar-Apr;40(2):281–289. [PubMed] [Google Scholar]
- Taylor S. S., Allison W. S., Kaplan N. O. The amino acid sequence of the tryptic peptides isolated from dogfish M4 lactate dehydrogenase. J Biol Chem. 1975 Nov 25;250(22):8740–8747. [PubMed] [Google Scholar]
- Taylor S. S. Amino acid sequence of dogfish muscle lactate dehydrogenase. J Biol Chem. 1977 Mar 10;252(5):1799–1806. [PubMed] [Google Scholar]
- Taylor S. S., Oxley S. S., Allison W. S., Kaplan N. O. Aminoacid sequence of dogfish M4 lactate dehydrogenase. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1790–1793. doi: 10.1073/pnas.70.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERLICK S. F. Fluorescence spectra and polarization of glyceraldehyde-3-phosphate and lactic dehydrogenase coenzyme complexes. J Biol Chem. 1958 Dec;233(6):1455–1467. [PubMed] [Google Scholar]
- WIELAND T., PFLEIDERER G. Nachweis der Heterogenität von Milchsäure-dehydrogenasen verschiedenen Ursprungs durch Trägerelektrophorese. Biochem Z. 1957;329(2):112–116. [PubMed] [Google Scholar]
- WILSON A. C., KAPLAN N. O., LEVINE L., PESCE A., REICHLIN M., ALLISON W. S. EVOLUTION OF LACTIC DEHYDROGENASES. Fed Proc. 1964 Nov-Dec;23:1258–1266. [PubMed] [Google Scholar]


