Abstract
A fuller understanding of the social epidemiology of disease requires an extended description of the relationships between social factors and health indicators in a systematic manner. In the present study, we investigated the correlations between income and 330 indicators of physiological, biochemical, and environmental health in participants in the US National Health and Nutrition Examination Survey (NHANES) (1999–2006). We combined data from 3 survey waves (n = 249–23,649 for various indicators) to search for linear and nonlinear (quadratic) correlates of income, and we validated significant (P < 0.00015) correlations in an independent testing data set (n = 255–7,855). We validated 66 out of 330 factors, including infectious (e.g., hepatitis A), biochemical (e.g., carotenoids, high-density lipoprotein cholesterol), physiological (e.g., upper leg length), and environmental (e.g., lead, cotinine) measures. We found only a modest amount of association modification by age, race/ethnicity, and gender, and there was no association modification for blacks. The present study is descriptive, not causal. We have shown in our systematic investigation the crucial place income has in relation to health risk factors. Future research can use these correlations to better inform theory and studies of pathways to disease, as well as utilize these findings to understand when confounding by income is most likely to introduce bias.
Keywords: biomarkers, environment, income, nutrition, socioeconomics
For the past century, results from studies in the United States have indicated that income and poverty are correlated with mortality (1–4), and this information has guided specific actions to improve health (2). Over the past several decades, this work has been extended to examine correlations of income with other markers or indicators of health (5–8). Longitudinal studies have demonstrated that the correlation between income and health is bidirectional: There are ways in which health could impact income, and conversely income could impact health (9, 10).
Although in the majority of these studies, investigators have reported analyses of 1 correlate at a time, there have been a few studies with a broader approach in which investigators examined how multiple health-related factors were associated with income (11, 12). Associations with income varied substantially, even for factors with similar implications for health (12). Because the literature is likely biased to present associations found to be statistically significant and to conform to prior beliefs of low income being associated with increased risk of worse health, the true extent of associations of infections, biochemical, physiological, and environmental factors with household income is unknown.
Another issue arises as to whether these correlations with income are modified by age, gender, or race/ethnicity. Associations between income and mortality have been shown to be stronger among persons aged 45–64 years than among those who are either younger or older (3). In several studies, investigators have reported that income is more strongly associated with mortality and health outcomes among women than among men (13–18), particularly for measures of adiposity (19–21). Many correlational studies of income and health present results stratified by race/ethnicity, and generally the associations are stronger among whites (6, 7, 22). However, the extent to which these reflect true differences in correlation or instead are the result of multiple comparisons and selective publication of results remains unclear.
Social epidemiologic understanding of disease might benefit from systematic evaluation of correlations using environment-wide association studies (23). This approach borrows from the genome-wide association study, which scans all common variants in the genome for association with a phenotype. Prior environment-wide association studies examined the associations of environmental factors with disease outcomes of interest, such as type 2 diabetes mellitus (23), elevated blood pressure (24), metabolic syndrome (25), elevated cholesterol levels (26), all-cause mortality (27), and preterm birth (28).
In the present analysis, we extended the methodology to examine whether income, a measure of socioeconomic position, was associated with a wide array of infectious, biochemical, physiological, and environmental factors. We tested whether there was an association between each factor and income. After controlling for multiple hypotheses, we validated significant findings in an independent sample to reduce the likelihood of spurious associations. We next investigated whether income had a nonlinear relationship with infectious, biochemical, physiological, and environmental exposure factors. Finally, we considered interactions between income and age, gender, and race/ethnicity.
METHODS
Study sample
In our analysis, we used data on participants from 4 waves of the continuous National Health and Nutrition Examination Survey (NHANES): 1999–2000, 2001–2002, 2003–2004, and 2005–2006. The survey samples were representative of the noninstitutionalized population of the United States. The total sample sizes of the 1999–2000, 2001–2002, 2003–2004, and 2005–2006 surveys were 9,965, 11,032, 10,122, and 10,348, respectively.
Our analysis focused on the participants aged 1–65 years. We excluded persons older than 65 years of age because they are usually not working, and the income of those who are no longer working is less appropriate for capturing socioeconomic position (29). The sample sizes were 7,433, 9,003, 8,242, and 8,785 for the 1999–2000, 2001–2002, 2003–2004, and 2005–2006 surveys, respectively. They were smaller than the overall sample sizes primarily because of the restricted age range of analysis. Sample sizes for each factor are presented in Web Table 1 (available at http://aje.oxfordjournals.org/).
Income measure
Total combined household income for the 12 months before survey included wages, salaries, income from self-employment, veteran's benefits, interest dividends, rental income, and public assistance. We used a poverty income ratio to account for household size and defined it as the ratio of household income to the official US poverty line. The poverty income ratio was standardized (mean subtracted and divided by the standard deviation) and treated as a continuous linear variable. Because the top category of household income in NHANES is open-ended, households with a poverty income ratio greater than or equal to 5 (13% of the sample) were assigned a level of 5 in the survey. The distribution of poverty income ratio by year, gender, age, and race/ethnicity is shown in Web Figure 1.
Infectious, biochemical, physiological, and environmental factor measures
We assessed 330 infectious, biochemical, physiological, and environmental factors (Web Table 1). We described the type of factors by category; however, we modeled each factor separately in analyses.
We assessed indicators of 31 infectious agents, 24 of which were bacterial and 7 of which were viral. Results for 26 of these assays were either positive or negative, and those for the other 5 were continuous (as indicated in Web Table 1). The 90 biochemical and physiological factors included 19 body measures, 2 blood pressure measures, 2 pulse/heart rate measures, 20 blood cell parameters, and 47 serum- or urine-based biochemical measures. This last group included indicators of metabolic or cardiovascular-related phenotypes (n = 12), liver function (n = 6), kidney function (n = 6), iron levels (n = 6), bone health (n = 2), acid/base status of blood (n = 5), prostate health (n = 3), and hormone levels (n = 2) (see Web Table 1 for all factors).
The remaining factors included 209 environmental exposure markers, such as environmental chemicals and nutrients assayed from serum and urine. These included a serum marker of nicotine metabolism, 7 types of dioxins, 10 furans, 27 heavy metals, 21 hydrocarbons, 18 nutrients, 35 polychlorinated biphenyls, 24 pesticides, 13 phthalates, 6 phytoestrogens, and 25 volatile compounds.
Statistical analysis
A graphical overview of the statistical approach is shown in Web Figure 2. We first separately modeled each of the 330 factors as a dependent variable in a regression model in which income was an independent variable for each of the training surveys (1999–2000, 2001–2002, and 2005–2006). To model continuous factors, we used survey-weighted linear regression. Continuous biomarker factors that had a right-skewed distribution were log-transformed and z-standardized as previously described (23, 26). To model the 26 infectious agent factors that were binary variables, we applied survey-weighted logistic regression. We adjusted all models for age, gender, and race/ethnicity (black, white, Mexican-American, or other).
For each factor, we obtained a single association size and P value for the training data using a random-effects synthesis. We also calculated the I2 estimate of heterogeneity for each of the validated factors.
We used the Bonferroni correction to adjust for multiple hypotheses in the training data, a conservative approach to mitigating false-positive associations with a significance threshold of P = 1.5 × 10−4 (= 0.05/330 total tests). We attempted to validate the significant associations found in the training surveys in the data from the 2003–2004 survey wave (using a validation P value threshold of 0.05 and association sizes in the training and validation sets of either greater than 0 or less than 0). For validated associations, we computed an overall random-effects synthesis estimate and P value. We also conducted a sensitivity analysis by repeating the analyses using Holm's method of adjustment for multiple comparisons (30).
Second, we determined whether there was a nonlinear (quadratic) association with income by adding an income-squared term to each of the 330 models. We observed the P values and association sizes for the coefficient that represented the income-squared term to infer the presence of possible quadratic associations between income and the 330 infectious, biochemical, physiological, and environmental variables. Quadratic associations were deemed significant after multiplicity control (P < 0.05/330 = 1.5 × 10−4) and were tentatively validated in the 2003–2004 survey data (P < 0.05).
Third, we determined whether the associations between income and the 330 variables interacted with gender, Mexican-American or black race/ethnicity, and age group. We modeled the interaction with a multiplicative term between the income variable and an indicator variable for gender, Mexican-American race/ethnicity, or black race/ethnicity. To examine interactions for age, we categorized age into the following groups: 1–18, 19–44, and 45–65 years, and we tested for interactions with the youngest and oldest age categories. When examining interactions between race/ethnicity or gender and income, we retained continuous age, gender, and other race/ethnicity categories as the main covariates in the model. For models in which we examined the interactions between age groups and income, we retained the other age groups, gender, and race/ethnicity in the models. We observed the P values and association sizes for the coefficient that represented the interaction to infer the presence of statistical interactions of gender, race/ethnicity, and age group with income. Interactions that were deemed significant after multiplicity control (P < 0.05/330 = 1.5 × 10−4) were validated in the 2003–2004 survey data (P < 0.05). We also examined factors that had opposite associations in the compared groups (e.g., positive in males and negative in females). We used the survey package in R for all analyses and accounted for cluster pseudostrata, pseudosampling units, and participant weights to accommodate the complex sampling of the data (31).
Finally, we assessed the nonparametric correlations among validated factors. Specifically, we computed biserial correlations between binary factors and Spearman correlation coefficients when considering quantitative factors. We visualized these pairwise correlations in a heat map and arranged the factors using a hierarchical clustering algorithm (32) as previously described (24).
RESULTS
Systematic scan of linear correlations with income
We found income to be significantly correlated (P < 1.5 × 10−4) with 83 (25%) of the 330 environmental or clinical variables in the training surveys (Web Figures 3 and 4). The distribution of observed training set P values deviated from the expected P value distribution of no correlation (Web Figure 4). Of these 83 variables, 66 had a P value less than 0.05 in the independent verification survey (2003–2004). Of these 66 variables, 23 were positively correlated with income (e.g., higher levels of income were correlated with higher levels of the variable), whereas 43 were negatively correlated with income (Web Figure 3). Web Table 2 lists the associations of all variables with income.
Table 1 shows the overall combined meta-analytic association sizes and P values for the 23 variables that were positively associated with income. Nine of the 23 positive associations were for nutrition components, including trans- and cis-β-carotene, α-carotene, folate, and trans-lycopene. Income was also positively associated with several physical environmental factors, including heavy metal (mercury), 4 types of fluorinated compounds (including perfluorohexane sulfonic acid), and 2 body measures (upper leg length and bone mineral density).
Table 1.
Overall Meta-Analytic Association Size for Validated Variables With Positive Correlations With Income, National Health and Nutrition Examination Survey, 1999–2006
| Variable | Category | Association Size | Standard Error | P Value |
|---|---|---|---|---|
| α-Tocopherol | Nutrients | 0.11 | 0.01 | 6.00E−41 |
| trans-β-carotene | Nutrients | 0.20 | 0.02 | 1.00E−40 |
| Lutein/zeaxanthin | Nutrients | 0.17 | 0.01 | 4.00E−33 |
| cis-β-carotene | Nutrients | 0.18 | 0.02 | 1.00E−32 |
| Total mercury | Heavy metals | 0.17 | 0.02 | 4.00E−26 |
| trans-lycopene | Nutrients | 0.10 | 0.01 | 5.00E−26 |
| β-Cryptoxanthin | Nutrients | 0.16 | 0.02 | 2.00E−24 |
| Serum folate | Nutrients | 0.12 | 0.01 | 4.00E−23 |
| Red blood cell folate | Nutrients | 0.09 | 0.01 | 8.00E−19 |
| α-Carotene | Nutrients | 0.20 | 0.02 | 2.00E−18 |
| Perfluorooctanoic acid | Perfluorochemicals | 0.14 | 0.02 | 4.00E−13 |
| HDL-cholesterol | Biochemicals | 0.11 | 0.02 | 5.00E−13 |
| Perfluorooctane sulfonic acid | Perfluorochemicals | 0.13 | 0.02 | 9.00E−13 |
| Urine mercury | Heavy metals | 0.10 | 0.02 | 2.00E−10 |
| Bone mineral density | Body measures | 0.05 | 0.01 | 2.00E−09 |
| Blood urea nitrogen | Biochemicals | 0.07 | 0.01 | 3.00E−09 |
| Perfluorononanoic acid | Perfluorochemicals | 0.15 | 0.03 | 5.00E−09 |
| Total bilirubin | Biochemicals | 0.07 | 0.01 | 6.00E−09 |
| Albumin | Biochemicals | 0.05 | 0.01 | 1.00E−08 |
| Upper leg length | Body measures | 0.06 | 0.01 | 9.00E−08 |
| Bicarbonate | Biochemicals | 0.05 | 0.01 | 2.00E−07 |
| Monocyte percent | Blood measures | 0.05 | 0.01 | 2.00E−07 |
| Perfluorohexane sulfonic acid | Perfluorochemicals | 0.09 | 0.02 | 3.00E−06 |
Income was negatively associated with 43 of the 66 validated factors (Table 2, Web Table 2). Eleven of these are known clinical risk factors for morbidity and mortality, including the following indicators of cardiovascular and metabolic health: blood glucose level, glycohemoglobin level, pulse rate, C-reactive protein concentration, triglyceride level, and white blood cell count. Another validated association included that of income with cotinine, a metabolite of nicotine. Income was also negatively correlated with presence or amount of 5 viral agents, including indicators of hepatitis A and herpes 1 and 2. Income was negatively correlated with exposure to physical chemical agents, including blood benzene, cadmium, lead, blood toluene, fluorene, and styrene. The sensitivity analysis using Holm's method resulted in the identification of 1 additional factor that was negatively correlated with income—urinary cobalt.
Table 2.
Overall Meta-Analytic Association Size for Validated Variables With Negative Correlations With Income, National Health and Nutrition Examination Survey, 1999–2006
| Variable | Category | Association Size | Standard Error | P Value |
|---|---|---|---|---|
| Cadmium, blood | Heavy metals | −0.20 | 0.01 | 5.00E−101 |
| Lead | Heavy metals | −0.16 | 0.01 | 3.00E−81 |
| Cotinine | Smoking | −0.27 | 0.02 | 3.00E−67 |
| Segmented neutrophil number | Blood measures | −0.10 | 0.01 | 9.00E−37 |
| Blood benzene | Volatile compounds | −0.24 | 0.02 | 3.00E−34 |
| White blood cell count | Blood measures | −0.11 | 0.01 | 3.00E−31 |
| C-reactive protein | Biochemical | −0.08 | 0.01 | 1.00E−22 |
| Hepatitis C antibody | Viral infection | −0.73 | 0.08 | 2.00E−21 |
| 2-Fluorene | Hydrocarbons | −0.22 | 0.02 | 4.00E−21 |
| Blood 2,5-Dimethylfuran | Volatile compounds | −0.27 | 0.03 | 3.00E−19 |
| Blood toluene | Volatile compounds | −0.19 | 0.02 | 4.00E−18 |
| Hepatitis B core antibody | Viral infection | −0.41 | 0.05 | 5.00E−18 |
| Blood ethylbenzene | Volatile compounds | −0.15 | 0.02 | 1.00E−17 |
| Monobenzyl phthalate | Phthalates | −0.15 | 0.02 | 1.00E−17 |
| 3-Fluorene | Hydrocarbons | −0.22 | 0.03 | 8.00E−17 |
| Hepatitis A antibody | Viral infection | −0.23 | 0.03 | 1.00E−15 |
| Red cell distribution width | Blood measures | −0.10 | 0.01 | 8.00E−15 |
| γ-tocopherol | Nutrients | −0.16 | 0.02 | 1.00E−14 |
| Homocysteine | Biochemical | −0.07 | 0.01 | 4.00E−14 |
| Urine albumin | Biochemical | −0.07 | 0.01 | 2.00E−13 |
| Herpes 1 | Viral infection | −0.24 | 0.03 | 3.00E−12 |
| Urine lead | Heavy metals | −0.12 | 0.02 | 4.00E−11 |
| Serum glucose | Biochemical | −0.07 | 0.01 | 5.00E−11 |
| 1-Pyrene | Hydrocarbons | −0.17 | 0.03 | 5.00E−11 |
| Lymphocyte number | Blood measures | −0.05 | 0.01 | 1.00E−10 |
| Γ-glutamyl transferase | Biochemical | −0.06 | 0.01 | 3.00E−10 |
| Globulins | Biochemical | −0.09 | 0.01 | 7.00E−10 |
| Blood m-/p-xylene | Volatile compounds | −0.11 | 0.02 | 1.00E−09 |
| Eosinophils number | Blood measures | −0.05 | 0.01 | 1.00E−09 |
| Plasma glucose | Biochemical | −0.07 | 0.01 | 2.00E−09 |
| Herpes 2 | Viral infection | −0.27 | 0.05 | 2.00E−09 |
| Pulse rate | Heart rate/blood pressure | −0.06 | 0.01 | 1.00E−08 |
| Urine cadmium | Heavy metals | −0.13 | 0.02 | 3.00E−08 |
| 2-Phenanthrene | Hydrocarbons | −0.13 | 0.02 | 5.00E−08 |
| Floor lead dusta | Heavy metals | −0.18 | 0.03 | 1.00E−07 |
| 3-Phenanthrene | Hydrocarbons | −0.12 | 0.02 | 2.00E−07 |
| Triglycerides | Biochemical | −0.09 | 0.02 | 2.00E−07 |
| Alkaline phosphatase | Biochemical | −0.05 | 0.01 | 3.00E−07 |
| Blood styrene | Volatile compounds | −0.17 | 0.03 | 3.00E−07 |
| Blood 1,4-dichlorobenzene | Volatile compounds | −0.06 | 0.01 | 7.00E−07 |
| Monocyte number | Blood measures | −0.06 | 0.01 | 9.00E−07 |
| Protoporphyrin | Biochemical | −0.05 | 0.01 | 9.00E−07 |
| Glycohemoglobin | Biochemical | −0.07 | 0.02 | 3.00E−05 |
Measured using graphite furnace atomic absorption spectrometry.
We discovered evidence of heterogeneity in correlations (measured using I2) across waves of data. For 1 of the validated factors (blood styrene), the estimate was between 75% and 100% (very large heterogeneity); for 5 factors (triglycerides, glycohemoglobin, red cell distribution width, γ-tocopherol, urine cadmium), it was between 50% and 75% (large heterogeneity); and for another 6 factors, it was between 25% and 50% (moderate heterogeneity) (Web Table 3). For the 5 factors for which P < 0.05 using the I2 statistic, we present forest plots of the associations by wave of data with the random-effect synthesis estimate (Web Figures 5–9). Given the limited number of data sets, we cannot exclude the possibility that some heterogeneity might exist even for factors that have low or even 0% estimates of I2.
Quadratic income associations with environmental and clinical factors
We estimated correlations between income-squared and the 330 infectious, biochemical, physiological, and environmental factors. Income had positive quadratic relationships with serum cis-β-carotene, trans-β-carotene, and total mercury (Web Table 4). The coefficients for the quadratic and linear terms were similar for all 3 factors, indicating that the levels of serum cis- and trans-β-carotene and total mercury have a convex relationship with income that deviates from linearity at higher income levels (1 to 2 standard deviations away from the mean) more so than at lower incomes (−1 to 2 standard deviations from the mean).
Interactions between income and age, gender, and race/ethnicity
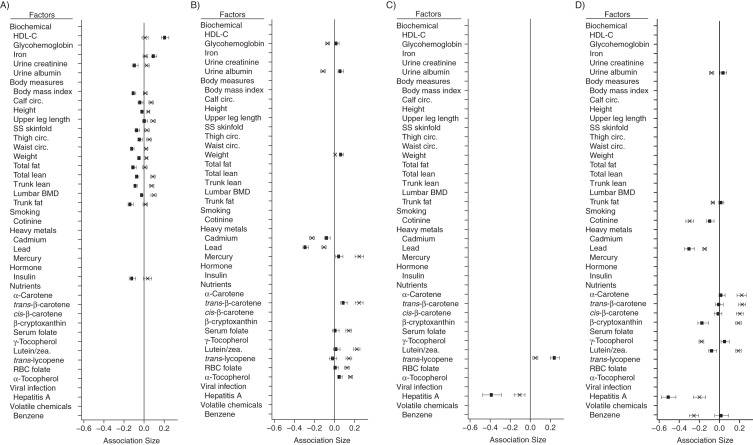
We found 17 interactions for gender, 13 of which were with body measures (Figure 1A), including weight, body mass index (weight (kg)/height (m)2), waist circumference, thigh circumference, calf circumference, lumbar bone mineral density, total fat mass, total lean fat minus bone mineral content, upper leg length, height, and skinfold thickness. In women, there were more negative associations between income and adiposity-related measures.
Figure 1.
Overall sizes of the associations between income and factors with a validated interaction, National Health and Nutrition Examination Survey, 1999–2006. The magnitude of association for each variable is shown for A) interactions with gender (rectangle indicates women; X indicates men); B) interactions with age of 1–18 years (rectangle indicates 1–18; X indicates 19–65); C) interactions with age of 45–65 years (rectangle indicates 45–65; X indicates 1–44); and D) interactions with Mexican-American ethnicity (rectangle indicates Mexican American ethnicity; X indicates non–Mexican-American ethnicity). BMD, bone mineral density; Circ., circumference; HDL-C, high-density lipoprotein cholesterol; RBC, red blood cell; SS skinfold, subscapular skinfold thickness; zea., zeaxanthene. Bars, 95% confidence intervals.
A total of 13 factors had different correlations by age group (Figure 1B and 1C). For example, income had more positive correlations with trans-β-carotene, lutein/zeaxanthin, and total mercury in participants older than 18 years than in those younger than 18 years (Figure 1B).
We found 12 factors that had stronger associations in Mexican-American participants (Figure 1D) than in non–Mexican-American participants. For example, hepatitis A antibody was more strongly negatively correlated with income in Mexican Americans than in non–Mexican Americans (Figure 1D). There was also a much greater negative association between income and cotinine for non–Mexican Americans. Of the 330 factors we examined, there were none for which associations were found in black subjects but not non-black subjects.
Correlation pattern among validated infectious, biochemical, physiological, and environmental factors
We assessed the correlations among each of the variables that were correlated in the training surveys (n = 83) and the adjustment variables (n = 6) and observed that there were many modest correlations among the 3,916 correlations computed (Web Figure 10). Approximately two thirds of the correlations (2,468; 63%) were significant at the Bonferroni-adjusted α = 0.05 level. The 5th to 95th percentile range of the absolute value of ρ was 0.006–0.42 (interquartile range, 0.03–0.15; median, 0.08; Web Figure 10). The 5th and 95th percentile of correlations that were significant had absolute values of 0.04–0.51 (interquartile range, 0.07–0.20; median, 0.11). As documented in prior environment-wide association studies (26, 33), there were significant correlations between environmental chemical or nutrient factors belonging to the same group, such as fluorinated compounds, volatile organic compounds, hydrocarbons, and carotenoid nutrients.
DISCUSSION
After accounting for multiple hypotheses and replicating the results in a separate sample, we found a substantial number of correlations between income and infectious, biochemical, physiological, and environmental factors in the United States from 1999 to 2006. These correlations were calculated in models that took into account age, gender, and race/ethnicity and thus were unlikely to be driven by these factors. In tests for linear correlations, 83 out of 330 factors were significant at the Bonferroni-level of significance, and 66 of these factors were validated in our test sample. Although not universal, our results demonstrate the dense correlation pattern that ties income to infectious, biochemical, physiological, and environmental factors. Of particular note were associations with dietary micronutrients and contaminants from physical environmental factors, as well as with infectious agents. We also found a small number of interactions of income with gender, age, and Mexican-American race/ethnicity; many of the associations were with anthropometric factors. We found no association modification by black race/ethnicity.
Our approach is a systematic alternative to analyses of income in which 1 or a few factors are reported at a time. Our findings are useful in providing direction for future studies of the causes of income-based socioeconomic differentials in health. Although causal studies of income effects are a challenge, there are some opportunities for the examination of policies that change income (34–36), as well as studies that provide randomized income supplementation (37, 38). Given the dense correlation pattern of income with many factors, randomized or quasirandomized studies are likely to be a more reasonable approach to isolating associations of household income with health. Our findings also address the fact that prior literature on income and health is subject to publication bias, which can give the impression that income is associated with all risk factors for disease rather than a particular subset of factors as we have demonstrated.
Our analyses have limitations. The estimated coefficients should not be interpreted as estimations of causal effects. Although we adjusted for age, race/ethnicity, and gender, a large number of other potential confounders are likely, such as parental educational level, wealth, and measures of the physical environment (39). Income is not randomly assigned to households, and some of the correlates examined might have effects on income. We cannot identify the directionality of correlations using cross-sectional data, and there have been a number of prior studies that demonstrated how health-related factors might influence income level (10). Spurious associations with false-positives due to confounding and other reasons are a concern even for associations that have been validated across several time periods and that pass significance thresholds regardless of the stringency of adjustment. Our analyses allow these associations to be seen in context by examining how strong they are (in the distribution of effect sizes noted in this field) (33), how much heterogeneity exists across time periods, and how they correlate with other variables. A further limitation is that income was assessed only at a single time point (40). Unfortunately, previous studies that had higher-quality measures of income did not have comprehensive measures of health-related risks. Income was capped at 5 times the poverty line. However, given current evidence that suggests that the strongest associations of income with biomarkers and mortality are across the lower half of the income distribution (3, 15), it is unlikely that this truncation has a meaningful effect on our findings. It is also important to acknowledge that the income associations are but a snapshot of associations in 1 country over a relatively short period of time (41, 42). Finally, we used Bonferroni adjustment to account for the influence of testing for multiple associations, although there are other potentially less conservative approaches, including false discovery rate (43) and Holm's method (44). Using Holm's method sensitivity analysis resulted in the identification of only 1 additional factor—urinary cobalt.
We present these analyses as a useful first step toward comprehensively understanding the ways in which a specific social variable, household income, is correlated with factors that might be related to disease. There are several ways in which the present analysis can advance studies of social determinants of health and epidemiologic practice more generally. First, we confirmed previous correlational findings on the associations of income with environmental measures, biomarkers, and anthropometric measures, such as blood lead (45), cadmium (46), high-density lipoprotein cholesterol (15), and C-reactive protein (47). Within the factors examined, a number of domains stand out, including dietary factors and environmental factors. Second, some of these findings are new contributions to the literature or reinforce findings from small convenience samples. We found strong and consistently negative associations between income and polycyclic aromatic hydrocarbons (e.g., fluorene), which support findings from a prior 2-home pilot study (48). Nutritional biomarkers, such as carotenoids, have previously been correlated with socioeconomic differences (49).
Our analysis of potential interactions between income and gender, age, and race/ethnicity was done based on prior work that suggested that income associations were greater among persons aged 45–64 years (3) and women (13–18) and that associations were stronger among whites (6, 7, 22). We found specific support for the hypothesis that associations of income with anthropometric measures were generally more negative and stronger in women. In contrast, we found little support for generally different associations between the ages of 1–64. Perhaps in strongest contrast to prior work, we found no differences in income associations between blacks and non-blacks, which suggest that household income correlates with health risk factors in a similar way in each group.
For more general epidemiologic practice, our findings suggest that these might be factors for which associations with health outcomes (e.g., mortality) might be particularly susceptible to confounding by income. Thus, a further application of our findings is to inform when controlling specifically for income as a potential confounder might be particularly important. The strongest positive association with income that we observed in our data was with β-carotene. It is perhaps not coincidental that there are large differences between the positive findings in observational studies of β-carotene, cardiovascular disease, and cancer and subsequent null and reversed findings in randomized trials (50). The ubiquity of associations between environmental contaminants and income also raises cautions for researchers interested in studies of particular environmental factors because our findings suggest that confounding by level of household income might be substantial. As noted previously, many studies control for only a single measure of socioeconomic position (e.g., educational level), and additional control for income might reduce bias (42).
Our findings provide comprehensive evidence for the long-recognized critical importance of the association between income and health. Compared with prior environment-wide association studies, income appears to be more crucial in our study than other factors that have been examined, such as lipid levels, diabetes, and mortality (23, 26, 27). Our correlations suggest that traditional methods of covariate-controlled statistical analysis of observational data might make it difficult to obtain causal estimates of the association of income with health. These determinations support the need to emphasize alternative approaches in social epidemiology (51, 52).
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Center for Biomedical Informatics, Harvard Medical School, Boston, Massachusetts (Chirag J. Patel); Department of Medicine, Stanford Prevention Research Center, School of Medicine, Stanford University, Stanford, California (John P. A. Ioannidis); Department of Health Research and Policy, School of Medicine, Stanford University, Stanford, California (John P. A. Ioannidis); Department of Statistics, School of Humanities and Sciences, Stanford University, Stanford, California (John P. A. Ioannidis); Meta-Research Innovation Center at Stanford, Stanford, California (John P. A. Ioannidis); and Department of Medicine, Division of General Medical Disciplines, School of Medicine, Stanford University, Stanford, California (Mark R. Cullen, David H. Rehkopf).
C.J.P. was supported by grant K99ES023504 from the National Institute of Environmental Health Sciences and the Pharmaceutical Researchers and Manufacturers of America Foundation. D.H.R. was supported by grant K01AG047280 from the National Institute of Aging. The Meta-Research Innovation Center at Stanford is supported by a grant by the Laura and John Arnold Foundation.
The content of this article is solely the responsibility of the authors.
Conflict of interest: none declared.
REFERENCES
- 1.Kitagawa E, Hauser P. Differential Mortality in the United States. Cambridge, MA: Harvard University Press; 1973. [Google Scholar]
- 2.Goldberger J, Wheeler GA, Sydenstricker E. A study of the relation of family income and other economic factors to Pellagra incidence in seven cotton-mill villages of South Carolina in 1916. Public Health Rep. 1920;35(46):2673–2714. [Google Scholar]
- 3.Backlund E, Sorlie PD, Johnson NJ. The shape of the relationship between income and mortality in the United States. Evidence from the National Longitudinal Mortality Study. Ann Epidemiol. 1996;6(1):12–20. doi: 10.1016/1047-2797(95)00090-9. [DOI] [PubMed] [Google Scholar]
- 4.Dowd JB, Albright J, Raghunathan TE, et al. Deeper and wider: income and mortality in the USA over three decades. Int J Epidemiol. 2011;40(1):183–188. doi: 10.1093/ije/dyq189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiscock R, Bauld L, Amos A, et al. Socioeconomic status and smoking: a review. Ann N Y Acad Sci. 2012;1248:107–123. doi: 10.1111/j.1749-6632.2011.06202.x. [DOI] [PubMed] [Google Scholar]
- 6.Crespo CJ, Smit E, Andersen RE, et al. Race/ethnicity, social class and their relation to physical inactivity during leisure time: results from the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Prev Med. 2000;18(1):46–53. doi: 10.1016/s0749-3797(99)00105-1. [DOI] [PubMed] [Google Scholar]
- 7.Miller JE. The effects of race/ethnicity and income on early childhood asthma prevalence and health care use. Am J Public Health. 2000;90(3):428–430. doi: 10.2105/ajph.90.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loucks EB, Magnusson KT, Cook S, et al. Socioeconomic position and the metabolic syndrome in early, middle, and late life: evidence from NHANES 1999–2002. Ann Epidemiol. 2007;17(10):782–790. doi: 10.1016/j.annepidem.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Kawachi I, Adler NE, Dow WH. Money, schooling, and health: mechanisms and causal evidence. Ann N Y Acad Sci. 2010;1186:56–68. doi: 10.1111/j.1749-6632.2009.05340.x. [DOI] [PubMed] [Google Scholar]
- 10.Benzeval M, Judge K. Income and health: the time dimension. Soc Sci Med. 2001;52(9):1371–1390. doi: 10.1016/s0277-9536(00)00244-6. [DOI] [PubMed] [Google Scholar]
- 11.Kant AK, Graubard BI. Secular trends in the association of socio-economic position with self-reported dietary attributes and biomarkers in the US population: National Health and Nutrition Examination Survey (NHANES) 1971–1975 to NHANES 1999–2002. Public Health Nutr. 2007;10(2):158–167. doi: 10.1017/S1368980007246749. [DOI] [PubMed] [Google Scholar]
- 12.Rehkopf DH, Krieger N, Coull B, et al. Biologic risk markers for coronary heart disease: nonlinear associations with income. Epidemiology. 2010;21(1):38–46. doi: 10.1097/EDE.0b013e3181c30b89. [DOI] [PubMed] [Google Scholar]
- 13.Denton M, Walters V. Gender differences in structural and behavioral determinants of health: an analysis of the social production of health. Soc Sci Med. 1999;48(9):1221–1235. doi: 10.1016/s0277-9536(98)00421-3. [DOI] [PubMed] [Google Scholar]
- 14.Prus SG, Gee E. Gender differences in the influence of economic, lifestyle, and psychosocial factors on later-life health. Can J Public Health. 2003;94(4):306–309. doi: 10.1007/BF03403611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rehkopf DH, Berkman LF, Coull B, et al. The non-linear risk of mortality by income level in a healthy population: US National Health and Nutrition Examination Survey mortality follow-up cohort, 1988–2001. BMC Public Health. 2008;8:383. doi: 10.1186/1471-2458-8-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang M, Chen Y, Krewski D. Gender-related differences in the association between socioeconomic status and self-reported diabetes. Int J Epidemiol. 2003;32(3):381–385. doi: 10.1093/ije/dyg075. [DOI] [PubMed] [Google Scholar]
- 17.Zick CD, Smith KR. Patterns of economic change surrounding the death of a spouse. J Gerontol. 1991;46(6):S310–S320. doi: 10.1093/geronj/46.6.s310. [DOI] [PubMed] [Google Scholar]
- 18.Loucks EB, Rehkopf DH, Thurston RC, et al. Socioeconomic disparities in metabolic syndrome differ by gender: evidence from NHANES III. Ann Epidemiol. 2007;17(1):19–26. doi: 10.1016/j.annepidem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Rehkopf D, Dow WH, Gruenewald T, et al. Cardiovascular consequences of income change. In: Wolfe B, Evans WN, Seeman T, editors. The Biological Consequences of Socioeconomic Inequalities. New York, NY: Russell Sage Foundation; 2012. pp. 126–157. [Google Scholar]
- 20.McLaren L. Socioeconomic status and obesity. Epidemiol Rev. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- 21.Schmeiser MD. Expanding wallets and waistlines: the impact of family income on the BMI of women and men eligible for the earned income tax credit. Health Econ. 2009;18(11):1277–1294. doi: 10.1002/hec.1430. [DOI] [PubMed] [Google Scholar]
- 22.Miller JE, Korenman S. Poverty and children's nutritional status in the United States. Am J Epidemiol. 1994;140(3):233–243. doi: 10.1093/oxfordjournals.aje.a117242. [DOI] [PubMed] [Google Scholar]
- 23.Patel CJ, Bhattacharya J, Butte AJ. An environment-wide association study (EWAS) on type 2 diabetes mellitus. PLoS One. 2010;5(5):e10746. doi: 10.1371/journal.pone.0010746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzoulaki I, Patel CJ, Okamura T, et al. A nutrient-wide association study on blood pressure. Circulation. 2012;126(21):2456–2464. doi: 10.1161/CIRCULATIONAHA.112.114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lind PM, Risérus U, Salihovic S, et al. An environmental wide association study (EWAS) approach to the metabolic syndrome. Environ Int. 2013;55:1–8. doi: 10.1016/j.envint.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Patel CJ, Cullen MR, Ioannidis JP, et al. Systematic evaluation of environmental factors: persistent pollutants and nutrients correlated with serum lipid levels. Int J Epidemiol. 2012;41(3):828–843. doi: 10.1093/ije/dys003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel CJ, Rehkopf DH, Leppert JT, et al. Systematic evaluation of environmental and behavioural factors associated with all-cause mortality in the United States National Health and Nutrition Examination Survey. Int J Epidemiol. 2013;42(6):1795–1810. doi: 10.1093/ije/dyt208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel CJ, Yang T, Hu Z, et al. Investigation of maternal environmental exposures in association with self-reported preterm birth. Reprod Toxicol. 2014;45:1–7. doi: 10.1016/j.reprotox.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galobardes B, Lynch J, Smith GD. Measuring socioeconomic position in health research. Br Med Bull. 2007;81-82:21–37. doi: 10.1093/bmb/ldm001. [DOI] [PubMed] [Google Scholar]
- 30.Entringer S, Epel ES, Kumsta R, et al. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A. 2011;108(33):E513–E518. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lumley T. Vienna, Austria: R Foundation for Statistical Computing; 2012. Survey: analysis of complex survey samples. R package version 3.28-2. [Google Scholar]
- 32.Gordon AD. Classification. 2nd ed. Boca Raton, FL: Chapman & Hall/CRC; 1999. [Google Scholar]
- 33.Patel CJ, Ioannidis JP. Placing epidemiological results in the context of multiplicity and typical correlations of exposures. J Epidemiol Community Health. 2014;68(11):1096–1100. doi: 10.1136/jech-2014-204195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans WN, Garthwaite CL. Giving Mom a Break: The Impact of Higher EITC Payments on Maternal Health. Cambridge, MA: National Bureau of Economic Research; 2010. [Google Scholar]
- 35.Strully KW, Rehkopf DH, Xuan Z. Effects of prenatal poverty on infant health: state earned income tax credits and birth weight. Am Sociol Rev. 2010;75(4):534–562. doi: 10.1177/0003122410374086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruckner TA, Rehkopf DH, Catalano RA. Income gains and very low-weight birth among low-income black mothers in California. Biodemography Soc Biol. 2013;59(2):141–156. doi: 10.1080/19485565.2013.833802. [DOI] [PubMed] [Google Scholar]
- 37.Baird SJ, Garfein RS, McIntosh CT, et al. Effect of a cash transfer programme for schooling on prevalence of HIV and herpes simplex type 2 in Malawi: a cluster randomised trial. Lancet. 2012;379(9823):1320–1329. doi: 10.1016/S0140-6736(11)61709-1. [DOI] [PubMed] [Google Scholar]
- 38.Fernald LC, Hamad R, Karlan D, et al. Small individual loans and mental health: a randomized controlled trial among South African adults. BMC Public Health. 2008;8:409. doi: 10.1186/1471-2458-8-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 40.Duncan GJ, Peterson E. The long and short of asking questions about income, wealth, and labor supply. Soc Sci Res. 2001;30(2):248–263. [Google Scholar]
- 41.Kunitz SJ. The Health of Populations: General Theories and Particular Realities. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 42.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294(22):2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 43.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 44.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 45.Brody DJ, Pirkle JL, Kramer RA, et al. Blood lead levels in the US population. Phase 1 of the Third National Health and Nutrition Examination Survey (NHANES III, 1988 to 1991) JAMA. 1994;272(4):277–283. doi: 10.1001/jama.272.4.277. [DOI] [PubMed] [Google Scholar]
- 46.McElroy JA, Shafer MM, Hampton JM, et al. Predictors of urinary cadmium levels in adult females. Sci Total Environ. 2007;382(2-3):214–223. doi: 10.1016/j.scitotenv.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 47.Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health. 2007;7:212. doi: 10.1186/1471-2458-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chuang JC, Callahan PJ, Lyu CW, et al. Polycyclic aromatic hydrocarbon exposures of children in low-income families. J Expo Anal Environ Epidemiol. 1999;9(2):85–98. doi: 10.1038/sj.jea.7500003. [DOI] [PubMed] [Google Scholar]
- 49.Kant AK, Graubard BI. Ethnic and socioeconomic differences in variability in nutritional biomarkers. Am J Clin Nutr. 2008;87(5):1464–1471. doi: 10.1093/ajcn/87.5.1464. [DOI] [PubMed] [Google Scholar]
- 50.van Poppel G. Epidemiological evidence for beta-carotene in prevention of cancer and cardiovascular disease. Eur J Clin Nutr. 1996;50((Suppl 3):S57–S61. [PubMed] [Google Scholar]
- 51.Oakes JM, Andrade KN. Methodologic innovations and advances in social epidemiology. Curr Epidemiol Rep. 2014;1(1):1–7. [Google Scholar]
- 52.Galea S, Link BG. Six paths for the future of social epidemiology. Am J Epidemiol. 2013;178(6):843–849. doi: 10.1093/aje/kwt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.