Abstract
Photic cues influence daily patterns of activity via two complementary mechanisms: (1) entraining the internal circadian clock and (2) directly increasing or decreasing activity, a phenomenon referred to as “masking”. The direction of this masking response is dependent on the temporal niche an organism occupies, as nocturnal animals often decrease activity when exposed to light, while the opposite response is more likely to be seen in diurnal animals. Little is known about the neural mechanisms underlying these differences. Here, we examined the masking effects of light on behavior and the activation of several brain regions by that light, in diurnal Arvicanthis niloticus (Nile grass rats) and nocturnal Mus musculus (mice). Each species displayed the expected behavioral response to a 1 h pulse of light presented 2 h after lights-off, with the diurnal grass rats and nocturnal mice increasing and decreasing their activity, respectively. In grass rats light induced an increase in cFOS in all retinorecipient areas examined, which included the suprachiasmatic nucleus (SCN), the ventral subparaventricular zone (vSPZ), intergeniculate leaflet (IGL), lateral habenula (LH), olivary pretectal nucleus (OPT) and the dorsal lateral geniculate (DLG). In mice, light led to an increase in cFOS in one of these regions (SCN), no change in others (vSPZ, IGL and LH) and a decrease in two (OPT and DLG). In addition, light increased cFOS expression in three arousal-related brain regions (the lateral hypothalamus, dorsal raphe, and locus coeruleus) and in one sleep-promoting region (the ventrolateral preoptic area) in grass rats. In mice, light had no effect on cFOS in these four regions. Taken together, these results highlight several brain regions whose responses to light suggest that they may play a role in masking, and that the possibility that they contribute to species-specific patterns of behavioral responses to light should be explored in future.
Keywords: masking, diurnality, nocturnality, cFOS, temporal niche
1. Introduction
Light is a powerful environmental cue that can have a major impact on daily patterns of behavior and physiology by entraining the endogenous circadian pacemaker and through more acute mechanisms that lead to a process referred to sometimes as “masking” [1]. Disentangling the influences of these two processes can be difficult in natural conditions because in environments with rhythmic alteration of light and darkness masking and circadian mechanisms complement each other to coordinate an animal’s patterns of adaptation to the day/night cycle [2]. Early circadian biologists devised experimental protocols to measure the influences of these two systems on behavior, but in doing so they generally dismissed masking as an important biological process in its own right [3]. This is evident in the original definition of the term, “…certain (sometimes overlooked) experimental conditions can obscure the real Zeitgeber-mechanism. We may call them masking conditions” [4]. Masking, however, can reflect adaptive mechanisms that contribute to regulation of the daily patterning of activity, rather than processes that simply obscure the influences of the circadian system.
Masking is a complex process [e.g. 5–8] and is typically quite different in day- and night-active animals, as light is more likely to increase activity in the former (a process referred to as positive masking) and decrease it in the latter (a process referred to as negative masking [9]). The patterns of response to photic cues can also change across the day in different ways [e.g. 1,10]. Many experiments have documented the suppression of activity by light in nocturnal mice [11–13], rats [14], and hamsters [15]. In recent years, several studies have described masking in diurnal rodents, such as Nile grass rats [10, 16], degus [6–8, 17], Mongolian gerbils [18] and golden spiny mice [19]. In a recent study we directly compared the behavior of nocturnal mice and diurnal Nile grass rats exposed to the same light stimuli presented at the same times of day and found that the animals responded in opposite ways: the light that suppressed activity of mice increased it in grass rats [10].
Although several experimental approaches have been used to study the neural substrates of masking responses, relatively little is known about them. One approach has been to examine the effects of lesions of retinorecipient regions of the brain on acute behavioral responses to photic stimuli. The suprachiasmatic nucleus (SCN) has been implicated in masking through such studies, though its role has been somewhat controversial [5, 20–21] and effects that have been seen could be due to damage to cells in the surrounding region (the ventral subparaventricular zone, vSPZ) [21] or damage to retinal fibers that go through the region of the lesions but do not terminate in the SCN [22]. Other areas that have been implicated in masking through lesion studies include the intergeniculate leaflet (IGL) [23–24], the dorsal lateral geniculate nucleus (DLG) [25], visual cortex [26], and the olivary pretectal nucleus (OPN) [27–28]. Consideration of the effects of lesions of different retinorecipient areas of the brain led Redlin [2] to propose that multiple areas mediate masking of activity by light in nocturnal species.
A second approach to exploration of neural substrates of masking has focused on the recently discovered melanopsin-containing intrinsically photo-responsive retinal ganglion cells (ipRGCs) and the brain regions to which they project. In mice, the masking response is absent when these cells are absent or reduced [29–31]. ipRGCs project to many areas of the brain [32], one or more of which is likely to be functionally linked to masking.
Finally, several studies of nocturnal rodents have used the immediate early gene cFOS to characterize responsiveness to light of cells in regions to which the ipRGCs project. Results from these studies have revealed considerable differences across regions, species, and strains, as summarized in Table 1. For example, in two strains of mice exposure to 1 h of light of the same intensity in the same lab elicited different cFOS responses within the IGL [36, 38]. There are very few studies that have examined light-induced cFOS activation in diurnal species and most of these have focused exclusively on the SCN [57–60]. Only two have looked outside the SCN in a diurnal species, and these have revealed light-induced increases in cFOS in the peri-SCN region of Nile grass rats [59] and the IGL of degus [58]. In diurnal animals, nothing is known about patterns of responsiveness to photic stimuli in other brain regions that receive input from ipRGCs.
TABLE 1.
cFOS activation in response to light pulses in nocturnal mice, rats, and hamsters
| Author | Strain | Time | Duration | Areas | Trend |
|---|---|---|---|---|---|
| (a) Mice | |||||
| Brooks et al., 2011 [33] | C57BL/6J | CT16 | 30min | SCN SPZ |
↑ ↑ |
| LeGate et al., 2012 [34] | B6/129 F1 hybrid | ZT14 | 10min | SCN SPZ LHb |
↑ ↑ ↑ |
| Lima et al., 2003 [35] | C57BL/6J & C3H/HeJ | Not Reported | 1hr | OPN | ↑ |
| Lupi et al., 1999 [36] | C57BL/6J | CT16 LP and Perfused at CT17.5 | 15min | SCN IGL |
↑ (=) |
| Lupi et al., 2008 [37] | Control: C3H Mutant: C57BL/6 & 129/SvJ hybrid |
ZT16 | 1hr | SCN VLPO |
↑ ↑ |
| Lupi et al., 2012 [38] | C3H/He | ZT16 | 15min | SCN IGL |
↑ ↑ |
| Mendoza et al., 2010 [39] | C57BL/6J | CT12 | 30min | LH (ORX) Raphe (5-HT) |
(=) N/A |
| Tsai et al., 2009 [40] | C57BL/6 & 129/SvJ hybrid | ZT15 | 1hr | SCN VLPO |
(=) (=), ↑ GAL+ cell |
| Huerta et al., 1999 [41]† | C57BL/6J | CT16 &22 | 1hr | SCN | ↑* |
| Masana et al., 1996 [42] | C3H/HeN | CT2,6,10,14,18, and 22 | 15min | SCN | ↑ |
| Delogu et al., 2012 [43]† | Not Reported | ZT19 | 60min | SCN | ↑* |
| (b) Rats | |||||
| Rusak et al., 1990 [44] | Not Reported | D and L phases in DD, ZT4.5 | 30 or 60min | SCN IGL |
↑* ↑* |
| Peters et al., 1996 [45] | Sprague-Dawley | Subjective Day & Subjective Night | 2hr (FOS) & 30min (fos) | IGL | ↑ |
| Aronin et al., 1990 [46] | Sprague-Dawley | ZT4 | 4hr | SCN IGL |
↑ ↑ |
| Aronin et al., 1990 [46] | Sprague-Dawley | ZT4 | 4hr | SCN IGL |
↑ ↑ |
| Park et al., 1993 [47]† | Sprague-Dawley | CT16 | 1, 1.5, 2, 3hr | SCN IGL |
(=) 30–90min, ↑ 2+ |
| Janik et al., 1992 [48] | Sprague-Dawley | CT14 | 2hr | SCN IGL |
↑* |
| Cha et al., 2011 [49] | Sprague-Dawley | Not Reported | 1hr | DLG | ↑* |
| Juhl et al., 2007 [50] | Wistar | ZT6, ZT14, ZT19 | 90min | IGL | ↑ ZT6, (=) ZT14 and 19 |
| Prichard et al., 2002 [51] | Fischer F344 | ZT6 & 18 Control; ZT7 & 19 pulsed | 60min | OPN IGL DLG |
(=) ZT7, ↑ ZT19 ↑ ZT7 and (=) ZT19 (=) |
| (c) Hamsters | |||||
| Rusak et al., 1990 [44] | Not Reported | D and L phases in DD, ZT4.5 | 30 or 60min | SCN IGL |
↑ night, (=) day ↑ night, (=) day |
| Janik et al., 1995 [52] | Golden | CT18 | 30min | SCN IGL |
↑ ↑ |
| Marchant et al., 1999 [53] | Golden | CT18.5 | 15 min | SCN IGL DR |
↑ ↑ ↑ |
| Muscat et al., 2006 [54] | Golden | CT19 | 5 min | SCN IGL |
↑ ↑ |
| Zhang et al., 1993 [55] | Golden | CT19 | 5 min | SCN IGL SPZ |
↑* ↑* ↑* |
| Zhane et al., 1996 [56] | Golden | CT19 | 5 min | SCN | ↑ |
Indicates that the study did not quanitatively analyze the data
Indicates that the study used both sexes
In the current study we examined cFOS in several brain regions of animals exposed to light that triggered an increase in activity of grass rats and a decrease in mice. First, we looked at areas that receive direct input from the retina, including the SCN, vSPZ, IGL, lateral hypothalamus (LH), olivary pretectal area (OPT) and the DLG. These areas might produce acute effects on general activity via pathways extending to structures that regulate sleep/wake state [5], as light can rapidly trigger sleep in nocturnal mammals (e.g. mice [61–62]) and heighten arousal/alertness in diurnal ones (e.g. humans [63]). We therefore also examined responses to light in the ventrolateral preoptic area (VLPO), a sleep-promoting region that receives retinal input (32), as well as in three brain regions that stimulate arousal, the lateral hypothalamus (LH), dorsal raphe (DR), and locus coeruleus (LC). Although the latter areas receive little or no direct visual input they could respond to light via indirect projections from the retina.
2. Methods
2.1. Animals
Adult female grass rats (n=8) were obtained from the breeding colony at Michigan State University and adult male CD1 mice (n=10) were obtained from Charles River Laboratory (Wilmington, MA, USA). Female grass rats are anestrous in our standard laboratory conditions [64], and masking responses to light are the same in male and female grass rats [10]. All animals were maintained on a 12:12 light-dark (LD) cycle with 300 lux of white light during the light phase and <1 lux of red light during the dark phase. All animals were singly housed in Plexiglas cages (34×28×17 cm) equipped with an enrichment device (PVC, length: 8 cm, diameter: 6 cm); food (PMI Nutrition Prolab RMH 2000, Brentwood, MO) and water were available ad libitum. The Institutional Animal Care and Use Committee of Michigan State University approved all experimental procedures.
2.2. Experimental Procedures
All animals were kept on a 12:12 LD cycle unless otherwise noted; zeitgeber time (ZT) 0 refers to the time of lights-on. Activity levels were monitored via infrared motion detectors (IRs, Visonic Tel Aviv, Israel), and all counts from them were recorded with the VitalView Program (Minimitter, Bend, OR, USA). After two weeks animals were assigned to either a control group (grass rat: n=4, mice: n=5) or a group that was exposed to a one-hour light pulse (LP; grass rats: n=4, mice: n=5) beginning at ZT 14. All of these animals were perfused (see above) at the end of the light pulse (i.e. ZT 15).
2.3. Immunocytochemistry (ICC)
One series of sections from each animal (i.e. every third section) was processed with immunohistochemistry to visualize the distribution of cells containing the protein cFOS. The protocol for grass rats followed the same steps outlined for the CTβ reaction. In brief, sections were incubated in (i) 5% normal donkey serum (Jackson ImmunoResearch, West Grow, PA, USA), (ii) a primary rabbit anti-cFOS antibody (1:50,000, Santa Cruz Biochemistry, Santa Cruz, CA, USA), (iii) biotinylated donkey anti-rabbit antibody (1:200, Jackson ImmunoResearch) and (iv) the ABC complex (Vector Laboratory). From this point the procedure differs from that used for the CTβ. Sections were rinsed in a 0.14M acetate buffer (pH 7.2), and then reacted in a mixture of DAB (0.5mg/mL) and nickel sulfate in 0.14M acetate buffer (pH 7.2) with 3% hydrogen peroxide. Sections were then mounted, dehydrated, and coverslipped with dibutyl phthalate xylene (Sigma-Aldrich).
The procedure for processing tissue from the mice followed similar steps except that: (1) the tissue was rinsed in 0.1% PBT (PBS with .01% Triton X-100) rather than PBS, (2) the concentration of the primary antibody, rabbit anti-cFOS, was higher (1:20,000), and (3) the tissue was incubated overnight in the ABC solution. Finally, the DAB reaction was carried out in a Trizma buffer. A second series of brain sections from each animal was stained with cresyl violet and used to delineate regions of interest for analysis.
2.4. Data Analysis
To analyze the behavioral data, Vital View files were converted into actograms via ClockLab (Actimetrics, Wilmette, IL, USA) and raw data were transferred into Microsoft Excel. The actograms provided visual confirmation of masking behavior during the light pulse, while Excel allowed for quantitative assessment of the data. To statistically compare activity between groups, the raw data were first converted into percentages (activity during the hour before sacrifice/24 hr of baseline activity) and then arcsine transformed. For each species the values from light pulsed and control animals were compared with independent-sample t-tests using SPSS.
Counts were conducted by two observers, one for the grass rat study and the other for the mouse study. Observers were blind to the experimental condition of the animals; selected sections were counted by both observers to ensure that the same criteria were being used in identification of labeled cells. A light microscope equipped with a drawing tube was used to produce bilateral maps of cFos+ cells from at least one section through each area. Counts were done on two sections for the SCN, vSPZ, IGL, DLG, the ventrolateral preoptic nucleus (VLPO), the lateral hypothalamus (LH), locus coeruleus (LC) and DR. Counting boxes described in earlier reports were used to delineate regions sampled in the VLPO (190μm by 190μm; [65]), vSPZ (215μm by 160μm;[66]), LH (1200μm by 700μm; [67–68]), LC (400μm by 700μm; [69]), and DR (150μm by 650μm; [69]). The remaining regions were outlined and all labeled cells within their borders were mapped and counted. The boundaries of the SCN and OPN were easily visualized, while the VMH, IGL and DLG were delineated with the aid of the nissl-stained tissue. Independent sample t-tests were used to determine if numbers of FOS+ cells differed between light-exposed and control groups; counts from each region, and from each species, were analyzed separately.
3. Results
3.1. Behavior
Exposure of grass rats to a 1 hr light pulse at ZT14 induced a marked increase in general activity. Specifically, among control animals, 4.69±0.67% of daily activity occurred during the hour beginning at ZT14, whereas among animals exposed to 1 hr of light at ZT14 this figure was significantly higher, at 15.72±4.59% (t(5)=2.74, p=0.041). The opposite response was observed in the mice. In this case, the percentages of daily activity occurring during the hour beginning at ZT14 were 17.66±3.52% among control mice but only 4.06±1.53% among light-exposed animals (t(8)=3.75, p=0.006). These results are consistent with previous findings [10].
3.2. cFOS expression in retinorecipent brain regions
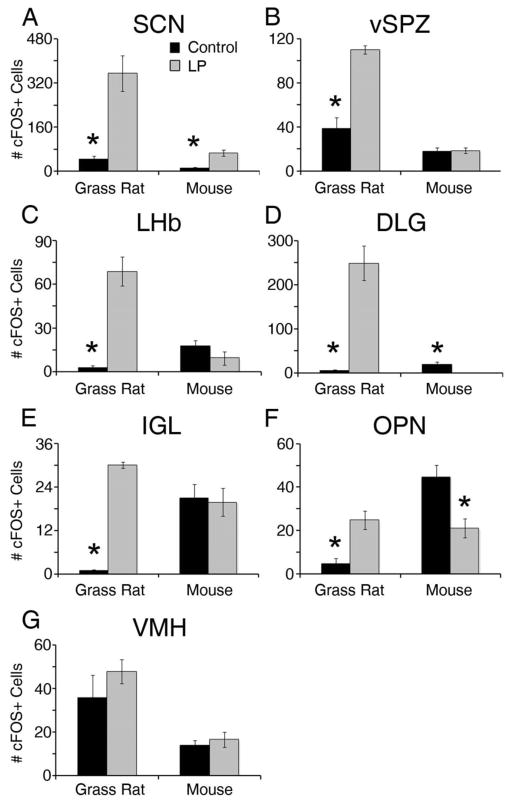
Several different patterns of cFOS responses to light in retinorecipient regions were observed in the brains of mice and grass rats (Figure 1 & 2). One was evident in the SCN, where the number of cFOS+ cells increased in response to the light pulse in both species (grass rat: t(5)=4.04, p=0.01; mouse: t(8)=4.77, p=0.001). In the VMH, by contrast, neither species responded to the light; (grass rat: (t(5)=1.10, p=0.32; mouse: t(8)=0.67, p=0.59). In three other retinorecipient areas that we examined, the vSPZ, IGL and LHb, light induced an increase in cFOS in grass rats and had no significant effect in mice (grass rat: vSPZ t(5)=7.57, p<0.01, IGL t(5)=31.22, p<0.001 and LHb (t(5)=5.67, p<0.01; mouse: vSPZ: t(8)=0.12, p=0.908, IGL: t(8)=0.22, p=0.828 and LHb: t(8)=1.33, p=0.220). Numbers of cFOS+ cells within the ventrolateral geniculate nucleus of grass rats and mice showed the same pattern as in the IGL (data not shown). Finally, light induced an increase in cFOS in both the DLG and OPN of grass rats (DLG: t(5)=3.61, p=0.012; OPN: t(5)=3.61, p=0.012), and it led to a decrease in cFOS in these regions in mice (DGL: t(8)=4.308, p=0.003 and OPN: t(8)=3.486, p=0.008).
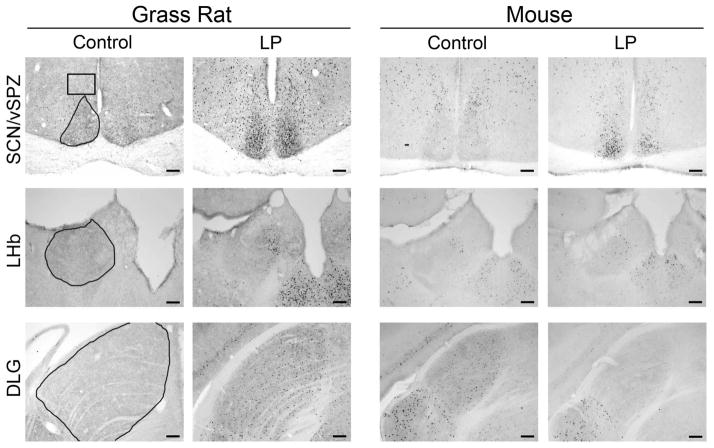
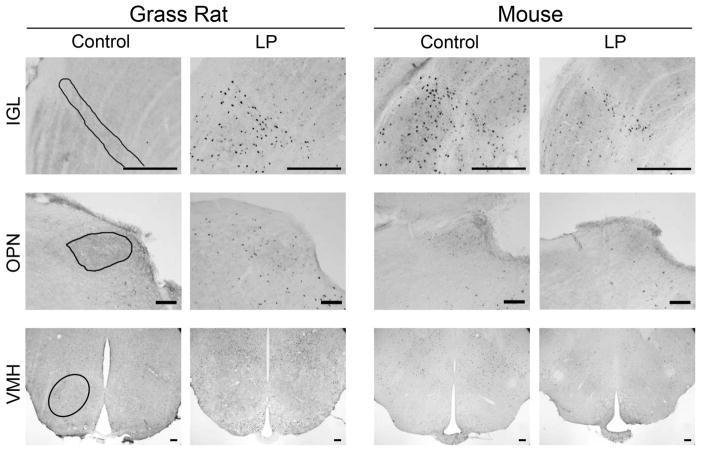
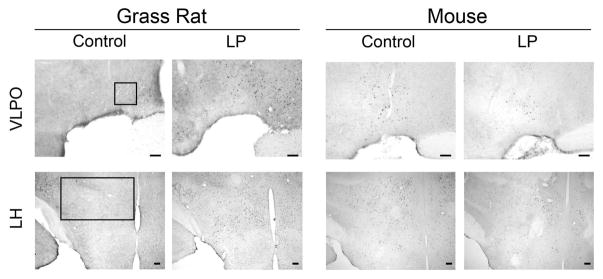
Figure 1.
Photomicrographs of cFOS in retinorecipient brain regions of grass rats and mice. In grass rats, on the left, cFOS was increased by the light pulse in all areas except the VMH, which remained unchanged. In the mice, on the right, cFOS was increased by the light pulse in the SCN, unchanged in the vSPZ, LHb, IGL, and VMH and decreased in the dLGN and OPN. Areas of interest are outlined in the first column; sampling areas are described in the text. Scale bar = 100μm.
Figure 2.
Patterns in cFOS expression in retino-recipient brain regions of light-exposed and control grass rats and mice. Panel A shows a significant increase in activation within the SCN in both species. Three regions, the vSPZ (B), LHb (C) and IGL (E) responded with a significant increase in grass rats but not in the mice. Two regions, the DLG (D) and OPN (F) had opposite responses to light exposure in the two species, with mice experiencing a decrease and grass rats an increase in activation. Panel G shows no response within the VMH of either species. An asterisk (*) indicates significance p < .05.
3.3. cFOS expression in arousal/sleep-related regions
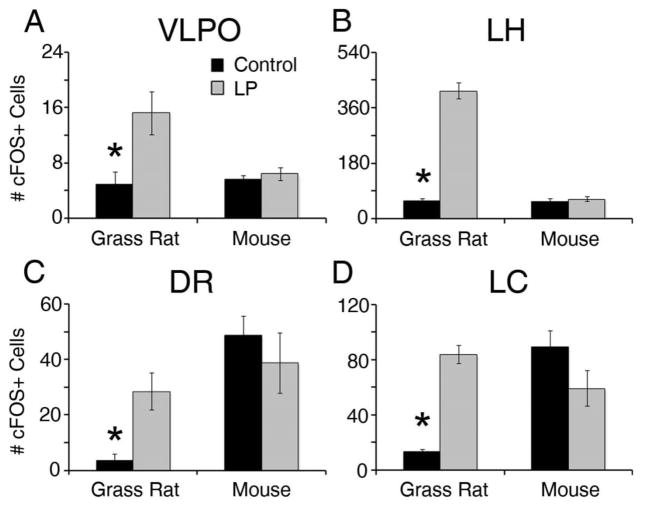
We examined regions that are associated with sleep and arousal because light can directly inhibit sleep in diurnal species and induce it in nocturnal ones. In the grass rat, all arousal-inducing areas that we examined showed an increase in cFOS+ cells after the light pulse (Figure 3 & 4); this included the DR (t(5)=7.67, p<0.01) and the LC (t(5)=7.11, p<0.01). Among mice, there was no response to a light pulse in the DR (t(8)=0.774, p=0.461) or the LC (t(8)=1.753, p=0.118). In the LH (Figure 3 & 4), where orexin neurons are found, control grass rats had significantly lower numbers of cFOS+ cells than grass rats exposed to the light pulse (t(5)=11.50, p<0.001). Among mice, cFOS did not differ in the two groups (t(8)=0.66, p=0.527). Finally, in the VLPO, a sleep-promoting area of the hypothalamus, light induced an increase in cFOS in the grass rats (t(5)=5.87, p<0.01) but had no effect in mice (t(8)=0.74, p=0.481).
Figure 3.
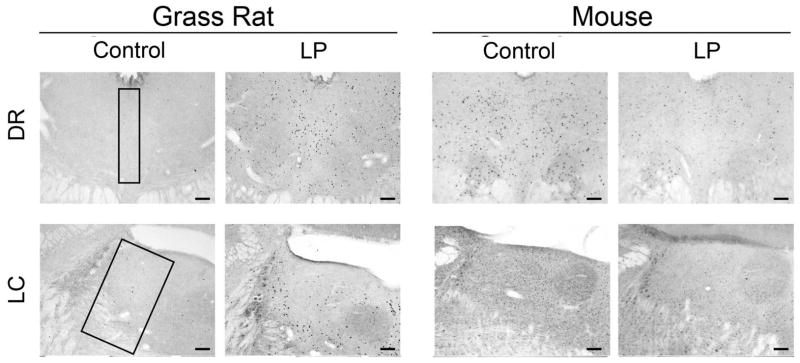
Photomicrographs of cFOS in arousal/sleep-related regions. In grass rats, on the left, cFOS was increased by the light in all areas. In mice, on the right, light had no effect on cFOS in any of these areas. Regions of interest are outlined in the first column and sampling regions are described in the text. Scale bar = 100μm.
Figure 4.
Patterns in cFOS expression in arousal/sleep-related regions. In the grass rat, cFOS was induced by light in the VLPO (A), LH (B), DR (C), and LC (D). No response was observed in any of these arousal/sleep-related regions in the mouse. An asterisk (*) indicates significance p < .05.
4. Discussion
4.1. Technical issues
In the current study brain responses of mice and grass rats to light were similar in some regions but very different in others. Several technical issues need to be considered when interpreting these data. First, we did not do a dose response curve, as Dkhissi-Benyahya et al. (2000) [70] have done with gerbils. We therefore cannot say anything about the saturation points of the behavioral and brain responses that we saw, or whether they are different from those of gerbils. Furthermore, with more sensitive methods (e.g. [71]) we might have detected effects of light in some brain regions that did not exhibit a response in the current study. However, our most central question was how different regions of the brains of our diurnal and nocturnal species would respond to a stimulus that produces an increase in activity in the former and a decrease in the latter, which is something that our approach enabled us to do.
A second potential problem that must be considered is that the data from the grass rat study were obtained from females whereas for the mouse study we used males. The patterns we observed are not likely to be due to the sex difference, however, as the effects of 1 h light at ZT 14 on behavior are the same in male and female grass rats, and different in both compared to male mice ([10]; current results); we are unaware of any studies that have compared these patterns in males and females of the latter species. In fact, to our knowledge, there are no published data on whether sex influences masking or cFOS responses to light in any nocturnal rodent. Only three of the studies that we have been able to find on cFos responses to light (Table 1†) have used females, and in those three there was no assessment of whether the sexes differed. Mechanisms underlying the same behaviors in males and females could be somewhat different, and in the discussion below we do not assume that comparisons between the cFOS responses of our female grass rats and male mice reflect patterns that we would see from within sex/across species comparisons. However, it seems most likely that the key patterns are the same in males and females of the same species.
4.2. cFOS expression in retinorecipent brain regions
When considering the cFOS responses of different retinorecipient areas in grass rats and mice, four general patterns become apparent. (1) In the SCN grass rats and mice responded to light in the same manner. This result provides assurance that the fundamental parameters of the experimental manipulation (e.g. light intensities) and methods used to assess cFOS in this study were sound. (2) In the VMH there was no response in either species, confirming the fact that light-induced changes in cFOS that we observed did not reflect a simple widespread reaction to the stimulus, and can therefore provide insight into more focused functional responses to photic stimuli that induce masking. (3) In three retinorecipient areas, the vSPZ, IGL and LHb, light induced an increase in cFOS in grass rats but had no effect in mice. Thus, there is a relationship between a behavioral response to light and photic induction of cFOS in these regions in our grass rats, whereas the behavioral and cFOS responses are dissociable in the mice, suggesting that masking of behavior by light does not depend on induction of cFOS in these areas in mice, though it might in grass rats. (4) Finally, in two brain regions, the DLG and OPN, the responses seen in mice and grass rats were in opposite directions. This raises the question of whether such cells might contribute to differences in functional responses to light in these two species.
The implications of these patterns are discussed in greater depth below, but first it should be noted that in some regions the cFOS responses observed in our study were different from those described in some earlier published reports, and that results among those studies are not always consistent. Table 1 summarizes key elements of experimental design and the results that are described in the research literature on cFOS responses to photic stimuli in other rodents. Many potentially important experimental parameters vary among these studies, including light intensity, time of day at which animals were exposed to light, the duration of the light pulse and the strain of mouse used in these studies.
4.2.1. SCN
In the SCN, we found that both species showed a clear increase in cFOS after the light pulse (Figure 1 & 2). This pattern of activation has been demonstrated before in both grass rats and mice pulsed with light during the dark phase of a 24 hr LD cycle as in the current study (Grass rat: [66,72]; Mouse: [42,73]), as well as when they are kept in DD and exposed to a pulse of light during the subjective night (Grass rats: [74]; Mouse: [27,75]). The focus of research on induction of cFOS in the SCN by light has been on its role in phase shifting of the endogenous circadian oscillator, and there is good evidence that it does play such a role in nocturnal rodents [77–78]. This is likely to be the case in grass rats as well, as effects of light on the circadian system in these animals are very similar to those seen in nocturnal species [78].
The possibility that light-induced cFOS in the SCN plays a role in masking has not been examined in any species, though the question of whether the SCN itself is important for masking has been addressed in numerous studies of SCN lesioned nocturnal rodents. One form of masking that is the same in nocturnal and diurnal species is the suppression of melatonin secretion by light at night, and this appears to depend on the SCN [63,79–80]. The role of the SCN in masking of behavior has been directly tested in only two studies of nocturnal animals (hamsters), and these produced conflicting results [20–21]. The presence of behavioral rhythms in SCN-lesioned animals maintained in a 24 hr LD cycle can also indicate the maintenance of masking, but here too, the results are mixed (Table 2); they are also complicated by the fact that SCN lesions may cause varying degrees of damage to the optic chiasm [96]. Although the nature of the role that the SCN might play in masking has not been definitively established in nocturnal species, Morin [5] makes a strong case that it is likely to be a central one. There are no data on these issues in diurnal species.
Table 2.
Masking in 12:12 LD condition for animals with suprachiasmatic nucleus lesions
| Author | Species | Strain/Species | Variable | Masking |
|---|---|---|---|---|
| Easton et al., 2004 [81] | Mice | C57B1/6J | GA, EEG, Temperature | N* |
| Tong et al., 2013 [82] | Mice | ddY | HR and Temperature | N |
| Stephan & Zucker, 1972 [83] | Rat | Sprague-Dawley | Drinking and Wheel running | N† |
| Coindet et al., 1975 [84] | Rat | EEG | N | |
| Mistlberger et al., 1983 [85] | Rat | Sprague-Dawley | EEG | Y |
| Liu et al., 2012 [86] | Rat | Sprague-Dawley | EEG | N |
| Ibuka & Kawamura, 1975 [87] | Rat | Albino | EEG | N |
| Ibuka et al., 1977 [88] | Rat | Wistar | EEG | N |
| Aguilar-Roblero et al, 1986 [89] | Rat | Wistar | Drinking | N |
| Scheer et al., 2001 [14] | Rat | Wistar | Drinking, HR, GA | N* |
| Amir et al., 2004 [90] | Rat | Wistar | Wheel Running | N* |
| Zhang et al., 2004 [91] | Rat | Wistar | GA & Temperature | Y |
| Hu et al. 2007 [92] | Rat | Wistar | GA | N |
| Angeles-Castellanos et al, 2010 [93] | Rat | Wistar | GA & Temperature | N* |
| Warren et al., 1994 [94] | Rat | Long Evans | GA, HR, Temperature | N |
| Wachulec et al, 1997 [95] | Rat | Long Evans | Drinking, Temperature, GA | 3Y 5N† |
| Mistlberger et al., 1992 [96] | Hamster | Golden Syrian | Wheel running | 10Y 2N† |
| DeCoursey et al. 1997 [97] | Ground Squirrels | Wheel running | Y | |
| Fuller et al, 1981 [98] | Squirrel Monkey | Drinking and Temperature | Y | |
| Sato & Kawamura, 1984 [98] | Chipmunk | Siberian | Wheel running | 1Y 3N |
Y Animals maintain masking response to light after lesion
N Animals no longer mask to light after lesion
Extensive damage to the Optic Chiasm
Used lack if rhythm as criteria for good lesion
4.2.2. vSPZ
The vSPZ is one of the three retinorecipient regions in which light produced a robust increase in cFOS expression in grass rats but had no effect in mice. The induction of cFOS in this region by light has been seen previously in grass rats [74], as well as nocturnal rodents, including hamsters, rats, and mice [33–34,55,99]. Thus, the lack of a response in our mice is somewhat surprising. It is not likely to be due to technical difficulties, as in these same animals we were able to see clear behavioral responses to the light, which also induced changes in cFos elsewhere in the brain. The differences among studies might be attributable to the fact that our strain of mice was not the same as those used in the two earlier studies [33–34]. In any case, the current results suggest that in our mice, the masking of activity by a photic stimulus can occur in the absence of a cFOS response of vSPZ neurons to that stimulus.
The question of whether the presence of a cFOS response of cells in the vSPZ reflects a role that this region may play in masking is more complex. It is an interesting area because it receives input from both the retina [32,100–102], and the SCN [103–105], and it projects to many of the same areas as the SCN [105–107]. It is thus well positioned to integrate direct photic signals from the retina with circadian signals, and to modulate the same functions that are regulated by the SCN. There is some evidence that the vSPZ may contribute to diurnality of the grass rat circadian system, as it exhibits rhythms in cFOS that are very different from those seen in nocturnal lab rats [108] and that persist in DD [66]. Lesions in this area also lead to a reduction in the ratio of activity during subjective day relative to night in these animals and to a decrease in the rate of reentrainment following a shift in the LD cycle [109]. The role of the vSPZ in masking, however, has not been directly tested in any species.
4.2.3. IGL
The second of the three retinorecipient regions in which light produced an increase in cFOS in grass rats but had no effect in mice is the IGL (Figure 1 & 2). Interestingly, cFOS is induced by light in the IGL of another diurnal species, the Octodon degus, [58]. There is good evidence that this is also the case in the hamster, but the data on rats and mice are inconsistent (Table 2) [36,38,43–47,48, 51,99,110]. This may be due to differences in the length of the light pulse to which animals were exposed, or to the strain of animal examined. Interestingly, Juhl et al. [50] found in rats that light triggered an increase in cFOS within the subset of IGL cells that contain enkephlin but not in neuropeptide Y-containing calls. One important question is whether light induces cFOS in the same subpopulations of IGL cells in diurnal grass rats as it does in nocturnal lab rats.
The patterns of responsiveness of grass rats and mice seen in the current study raise the question of whether the IGL might contribute to differences in their masking behavior. Several kinds of data suggest that this might be the case. As is the case with the SCN and vSPZ, the retinal input to the IGL comes from ipRGCs in mice [32], and these cells play an important role in masking [111]. Lesion studies have also produced interesting results relating the IGL to masking, in both nocturnal and diurnal animals. In nocturnal rodents, masking is retained under LD conditions after the IGL is lesioned [24,112–113] and in hamsters the masking response of wheel running to light is actually increased after the IGL has been destroyed [113]. The most striking effect of IGL lesions on masking, however, is that seen in diurnal grass rats, as Gall et al. [23] showed that destruction of the IGL completely reversed the direction of the masking response. That is, lesioned animals decreased their activity when presented with light, rather than increasing it. Results from lesion studies, as well as the cFOS response shown here, therefore suggest that the IGL plays an important role in the ability of grass rats to sustain a masking response typical of diurnal animals. It should be noted that the influence of light on cFOS within the IGL could be indirect, occurring, for example, via inputs that it receives from the from orexin cells in the lateral hypothalamus.
4.2.4. LHb
The LHb is the third retinorecipient brain region in which light increased cFOS in the grass rat but had no effect in mice. Only one other study that we are aware of has directly assessed effects of light on cFOS in this region in nocturnal mice, and that study found a large stimulatory effect [34]. On the other hand, in an LD cycle, cFOS in this region is higher during the night than during the day in nocturnal rodents, including mice, rats and hamsters [114]. It should also be noted that activity appears to induce a rise in cFOS in the habenula [114–116], and cFos in this region is associated with stress [117–118]. Perhaps differences in these parameters could account for the differences between our results with mice and those described by LeGates et al. [34].
The response of the habenula to light is of special interest because its roles in regulation of activity [115] and its projections to arousal-inducing areas of the brain [119–121] put it in a good position to mediate masking. In vivo electrophysiological studies in rats have shown that neurons in the habenula, particularly its lateral subregion, respond to acute presentation of light, and in vitro recordings reveal a circadian rhythm in its firing rate [122]. Though it is not yet clear whether the LHb contributes to masking, or to species differences in its manifestation, its responsiveness to light in grass rats (Figure 2) suggest that it is a region that should be explored further in efforts to better understand these issues.
4.2.5. DLG
The DLG is one of the two brain regions in which light induced an increase in cFOS in the grass rat and a decrease in mice (Figure 2). It should be noted that Prichard et al. (2002) saw no effect of a 2 hr pulse of light at midnight on cFOS in the DLG of rats. In contrast to these nocturnal rodents, grass rats exhibited a robust response to light, as has been reported in two other diurnal species, tree shrews [123] and Mongolian gerbils [124]. The DLG is an important part of the primary visual system [125–126], but it also plays a role in the masking behavior of mice, as lesions in this area enhance the inhibitory effect of light on activity in these animals [26]. The possibility that the DLG plays a role in masking in grass rats and other diurnal species has not yet been examined, but the data here suggest the possibility of interesting and important differences in the role that it might play in nocturnal and diurnal species.
4.2.6. OPN
The second region in which light increased cFOS in the grass rat and decreased it in mice was the OPN (Figure 2). In this case, the control mice appeared to have very high levels of cFOS, and light exposure diminished the number of cFOS+ cells by more than 50%. This result stands in contrast to that described in another nocturnal rodent by Prichard et al. [51], who reported that a 2 hr pulse of light at ZT19 induced a significant increase in cFOS within the OPN of lab rats; light during the inactive period had no effect in that study. The different responses of our mice and the rats of Prichard et al. [51] could be due to the times of day at which animals were sampled, or the OPN may not be the same with respect to the manner in which it responds to light in these two nocturnal species.
The focus of research on the OPN has been on its mediation of the pupillary light reflex [127] but the same characteristics that allow it to play that role are ones that could also enable it to contribute to masking. The OPN receives substantial input from melanopsin-containing cells in the retina [32], contains neurons that are capable of coding illumination levels [128] and it projects to at least one region that appears to play a role in masking, the IGL [129]. Additionally, removal of the OPN interferes with masking of REM sleep by darkness in albino rats [27]. The role of this region in masking of general activity by photic stimuli has not been examined in either nocturnal or diurnal species.
4.3. cFOS expression in Arousal/Sleep-Related Regions
In all four arousal/sleep-related areas examined here light induced an increase in cFOS in grass rats but had no effect in mice. The implications of these species differences are discussed below, but it should be noted that in this case there are few published data with which to compare our results.
4.3.1. VLPO
In the VLPO, a region of the hypothalamus known to promote sleep in some species [130–131], we found that the same light that stimulated general activity actually increased cFOS in grass rats (Figure 3). The reasons for this paradoxical response are not clear, but one possibility involves the internal circuitry of the VLPO. The subset of cells in this region that actually stimulate sleep in nocturnal rodents are known to contain galanin, and cFOS is elevated in those cells during sleep in nocturnal rats [132]. Thus, it may be that in grass rats light activates a different subset of cells, perhaps even inhibitory interneurons that suppress galanin-containing cells in the VLPO, which could lead to a reduction in sleep.
In mice, we found that while light decreased activity it did not affect cFOS in the VLPO. Previous experiments examining light-induced cFOS in this area in mice have produced results that are somewhat contradictory. Lupi et al. [37], using RT-PCR, saw a significant light-induced increase in mRNA for cFos when mice were pulsed for 1 hr with light. However, Tsai et al. [40] saw no effect of light on overall levels of cFOS in this region but they did when they focused specifically on the sleep-promoting galanin-positive cells there. It should be noted that the VLPO is innervated by fibers originating in the retina in grass rats and mice [32,101], though there is evidence that the density of that retinal input is higher in nocturnal lab rats than grass rats (Nunez, unpublished results). Several investigators have suggested that this pathway may play a role in masking [2,37] but to our knowledge no one has directly examined this possibility.
4.3.2. LH, DR, LC
The LH, DR and LC are of interest in understanding the neural mechanisms of masking as each plays an important role in induction and maintenance of arousal [133–135]. In grass rats, all three of these brain regions responded to light, which may reflect their contribution to the light-induced increase in activity seen in these animals. In mice, however, there was a dissociation between the effects of light on activity and on cFOS in these regions. That is, the light that inhibited activity had no effect of cFOS in the LH, DR or LC. This result, which has been reported by others [39], may reflect a delay in the decay of cFOS relative to a decline in neuronal activity. Light-induced cFOS has been seen previously in the LH and DR of grass rats [136], as well as the DR of another diurnal murid rodent, the Mongolian gerbil [124]. Interestingly, the retina projects directly to the LH of grass rats [101], and to the DR of Mongolian gerbils [137]. It is tempting to speculate that these pathways play a role in the induction of cFOS and positive masking in these diurnal species.
5. Conclusions
The acute behavioral responses of nocturnal and diurnal species to light exposure, which may be modulated by time of day, are typically completely different, with the nocturnal animals decreasing activity and diurnal ones increasing it (e.g. [10]). Although little is known about neural mechanisms that are responsible for these differences, the present data highlight some brain regions that could play important roles. Here, we found that when grass rats and mice held under identical conditions were pulsed with light that had diametrically opposed effects on behavior, most retinorecipient and arousal-related brain regions responded differently. The only exception was the SCN, which likely reflects the fact that the relationships between photic stimuli, circadian rhythms and SCN function are very similar in nocturnal and diurnal species [78]. The differences in cFOS responses to light in other retinorecipient regions are more likely to reflect neural mechanisms that contribute to species differences in masking. Data on lesions of the IGL have provided support for the hypothesis that this region plays an important role in maintenance of positive masking of activity by light in these diurnal animals [23]. The present data raise the possibility that differential responsiveness of cells within the vSPZ, LHb, OPN and DLG may also contribute to the constellation of adaptive responses to light that distinguish diurnal from nocturnal species. Future experimental work will be required to assess this possibility, to establish the role of these brain regions in masking behaviors.
Highlights.
Light induced changes in brains of diurnal grass rats and nocturnal mice.
cFos responses of the two species differed in several retinorecipient regions.
cFos was stimulated in sleep/arousal-related regions in grass rats but not mice.
Acknowledgments
This work was supported by the National Science Foundation grant IOS1051919 to LS, AAN and LY. DDS was supported by a National Institute of Health training grant (T32 MH070343). The funding source(s) had no involvement in the design of the study; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. We thank Alexandra Castillo-Ruiz, Andrew Gall, Jennifer Langel, Adam Stowie, and Thomas Groves for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aschoff J. Masking and parametric effects of high-frequency light-dark cycles. Jpn J Physiol. 1999;49(1):11–18. doi: 10.2170/jjphysiol.49.11. [DOI] [PubMed] [Google Scholar]
- 2.Redlin U. Neural basis and biological function of masking by light in mammals: Suppression of melatonin and locomotor activity. Chronobiology International. 2001;18(5):737–758. doi: 10.1081/cbi-100107511. [DOI] [PubMed] [Google Scholar]
- 3.Minors DS, Waterhouse JM. Masking in humans: the problem and some attempts to solve it. Chronobiol Int. 1989;6(1):29–53. doi: 10.3109/07420528909059140. [DOI] [PubMed] [Google Scholar]
- 4.Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harbor Symposia on Quantitative Biology. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Morin LP. Nocturnal light and nocturnal rodents: Similar regulations of disparate functions? Journal of Biological Rhythms. 2013;28(2):95–106. doi: 10.1177/048730413481921. [DOI] [PubMed] [Google Scholar]
- 6.Vivanco P, Rol MA, Madrid JA. Two steady-entrainment phase and graded masking effects by light generate different circadian chronotypes in Octodon degus. Chronobiology International. 2009;26(2):219–241. doi: 10.1080/07420520902768203. [DOI] [PubMed] [Google Scholar]
- 7.Vivanco P, Rol MA, Madrid JA. Pacemaker phase control versus masking by light: setting the circadian chronotype in dual Octodon degus. Chronobiology International. 2010a;27(7):1365–1379. doi: 10.3109/07420528.2010.502984. [DOI] [PubMed] [Google Scholar]
- 8.Vivanco P, Otalora BB, Rol MA, Madrid JA. Dissociation of the circadian system of Octodon degus by T28 and T21 light-dark cycles. Chronobiology International. 2010b;27(8):1580–1595. doi: 10.3109/07420528.2010.510228. [DOI] [PubMed] [Google Scholar]
- 9.Mrosovsky N. Masking: history, definitions, and measurement. Chronobiol Int. 1999;16(4):415–429. doi: 10.3109/07420529908998717. [DOI] [PubMed] [Google Scholar]
- 10.Shuboni DD, Cramm S, Yan L, Nunez AA, Smale L. Acute Behavioral Responses to Light and Darkness in Nocturnal Mus musculus and Diurnal Arvicanthis niloticus. Journal of Biological Rhythms. 2012;27(4):299–307. doi: 10.1177/0748730412449723. [DOI] [PubMed] [Google Scholar]
- 11.Butler MP, Silver R. Divergent photic thresholds in the non-image-forming visual system: entrainment, masking and pupillary light reflex. Proceedings of the Royal Society B-Biological Sciences. 2011;278(1706):745–750. doi: 10.1098/rspb.2010.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morin LP, Lituma PJ, Studholme KM. Two Components of Nocturnal Locomotor Suppression by Light. Journal of Biological Rhythms. 2010;25(3):197–207. doi: 10.1177/0748730410369890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pendergast JS, Yamazaki S. Masking Responses to Light in Period Mutant Mice. Chronobiology International. 2011;28(8):657–663. doi: 10.3109/07420528.2011.596296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheer FA, Ter Horst GJ, van Der Vliet J, Buijs RM. Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. Am J Physiol Heart Circ Physiol. 2001;280(3):H1391–1399. doi: 10.1152/ajpheart.2001.280.3.H1391. [DOI] [PubMed] [Google Scholar]
- 15.Redlin U, Mrosovsky N. Masking of locomotor activity in hamsters. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 1999;184(4):429–437. doi: 10.1007/s003590050342. [DOI] [PubMed] [Google Scholar]
- 16.Redlin U, Mrosovsky N. Nocturnal activity in a diurnal rodent (Arvicanthis niloticus): The importance of masking. Journal of Biological Rhythms. 2004;19(1):58–67. doi: 10.1177/0748730403260371. [DOI] [PubMed] [Google Scholar]
- 17.Refinetti R. Variability of diurnality in laboratory rodents. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 2006;192:701–714. doi: 10.1007/s00359-006-0093-x. [DOI] [PubMed] [Google Scholar]
- 18.Weinert D, Weinandy R, Gattermann R. Photic and non-photic effects on the daily activity pattern of Mongolian gerbils. Physiology & Behavior. 2007;90(2–3):325–333. doi: 10.1016/j.physbeh.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Cohen R, Smale L, Kronfeld-Schor N. Masking and Temporal Niche Switches in Spiny Mice. Journal of Biological Rhythms. 2010;25(1):47–52. doi: 10.1177/0748730409351672. [DOI] [PubMed] [Google Scholar]
- 20.Redlin U, Mrosovsky N. Masking by light in hamsters with SCN lesions. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 1999;184(4):439–448. doi: 10.1007/s003590050343. [DOI] [PubMed] [Google Scholar]
- 21.Li XD, Gilbert J, Davis FC. Disruption of masking by hypothalamic lesions in Syrian hamsters. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology. 2005;191(1):23–30. doi: 10.1007/s00359-004-0569-5. [DOI] [PubMed] [Google Scholar]
- 22.Youngstrom TG, Weiss ML, Nunez AA. Retinofugal projections to the hypothalamus, anterior thalamus and basal forebrain in hamsters. Brain Res Bull. 1991;26(3):403–411. doi: 10.1016/0361-9230(91)90014-b. [DOI] [PubMed] [Google Scholar]
- 23.Gall AJ, Smale L, Yan L, Nunez AA. Lesions of the Intergeniculate Leaflet Lead to a Reorganization in Circadian Regulation and a Reversal in Masking Responses to Photic Stimuli in the Nile Grass Rat. PLoS One. 2013;8(6):e67387. doi: 10.1371/journal.pone.0067387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redlin U, Vrang N, Mrosovsky N. Enhanced masking response to light in hamsters with IGL lesions. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 1999;184(4):449–456. doi: 10.1007/s003590050344. [DOI] [PubMed] [Google Scholar]
- 25.Edelstein K, Mrosovsky N. Behavioral responses to light in mice with dorsal lateral geniculate lesions. Brain Research. 2001;918(1–2):107–112. doi: 10.1016/s0006-8993(01)02966-3. [DOI] [PubMed] [Google Scholar]
- 26.Redlin U, Cooper HM, Mrosovsky N. Increased masking response to light after ablation of the visual cortex in mice. Brain Research. 2003;965(1–2):1–8. doi: 10.1016/s0006-8993(02)03844-1. [DOI] [PubMed] [Google Scholar]
- 27.Miller AM, Miller RB, Obermeyer WH, Behan M, Benca RM. The pretectum mediates rapid eye movement sleep regulation by light. Behav Neurosci. 1999;113(4):755–765. doi: 10.1037//0735-7044.113.4.755. [DOI] [PubMed] [Google Scholar]
- 28.Sisk CL, Stephan FK. Central visual pathways and the distribution of sleep in 24-hr and 1-hr light-dark cycles. Physiol Behav. 1982;29(2):231–239. doi: 10.1016/0031-9384(82)90009-9. [DOI] [PubMed] [Google Scholar]
- 29.Altimus CM, Guler AD, Villa KL, McNeill DS, Legates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci U S A. 2008;105(50):19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS One. 2008;3(9):e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102-+. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. Journal of Comparative Neurology. 2006;497(3):326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooks E, Waters E, Farrington L, Canal MM. Differential hypothalamic tyrosine hydroxylase distribution and activation by light in adult mice reared under different light conditions during the suckling period. Brain Struct Funct. 2011;216(4):357–370. doi: 10.1007/s00429-011-0318-9. [DOI] [PubMed] [Google Scholar]
- 34.LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594–598. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lima AG, Britto LRG, Hossokawa NM, Hamassaki-Britto DE. Static, but not optokinetic visual stimuli induce Fos expression in the retina and brain of retinal degeneration mice. Neuroscience Letters. 2003;342(1–2):9–12. doi: 10.1016/s0304-3940(03)00217-9. [DOI] [PubMed] [Google Scholar]
- 36.Lupi D, Cooper HM, Froehlich A, Standford L, McCall MA, Foster RG. Transgenic ablation of rod photoreceptors alters the circadian phenotype of mice. Neuroscience. 1999;89(2):363–374. doi: 10.1016/s0306-4522(98)00353-4. [DOI] [PubMed] [Google Scholar]
- 37.Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11(9):1068–1073. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- 38.Lupi D, Semo M, Foster RG. Impact of age and retinal degeneration on the light input to circadian brain structures. Neurobiol Aging. 2012;33(2):383–392. doi: 10.1016/j.neurobiolaging.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Mendoza J, Clesse D, Pevet P, Challet E. Food-reward signalling in the suprachiasmatic clock. Journal of Neurochemistry. 2010;112(6):1489–1499. doi: 10.1111/j.1471-4159.2010.06570.x. [DOI] [PubMed] [Google Scholar]
- 40.Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Bourgin P. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(−/−) mice. PLoS Biol. 2009;7(6):e1000125. doi: 10.1371/journal.pbio.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huerta JJ, Llamosas MM, Cernuda-Cernuda R, Garcia-Fernandez JM. Spatio-temporal analysis of light-induced Fos expression in the retina of rd mutant mice. Brain Research. 1999;834(1–2):122–127. doi: 10.1016/s0006-8993(99)01604-2. [DOI] [PubMed] [Google Scholar]
- 42.Masana MI, Benloucif S, Dubocovich ML. Light-induced c-fos mRNA expression in the suprachiasmatic nucleus and the retina of C3H/HeN mice. Brain Res Mol Brain Res. 1996;42(2):193–201. doi: 10.1016/s0169-328x(96)00031-9. [DOI] [PubMed] [Google Scholar]
- 43.Delogu A, Sellers K, Zagoraiou L, Bocianowska-Zbrog A, Mandal S, Guimera J, Lumsden A. Subcortical visual shell nuclei targeted by ipRGCs develop from a Sox14+-GABAergic progenitor and require Sox14 to regulate daily activity rhythms. Neuron. 2012;75(4):648–662. doi: 10.1016/j.neuron.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 44.Rusak B, Robertson HA, Wisden W, Hunt SP. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990;248(4960):1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- 45.Peters RV, Aronin N, Schwartz WJ. c-Fos expression in the rat intergeniculate leaflet: photic regulation, co-localization with Fos-B, and cellular identification. Brain Res. 1996;728(2):231–241. doi: 10.1016/0006-8993(96)00414-3. [DOI] [PubMed] [Google Scholar]
- 46.Aronin N, Sagar SM, Sharp FR, Schwartz WJ. Light regulates expression of a Fos-related protein in rat suprachiasmatic nuclei. Proc Natl Acad Sci U S A. 1990;87(15):5959–5962. doi: 10.1073/pnas.87.15.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park HT, Baek SY, Kim BS, Kim JB, Kim JJ. Profile of Fos-like immunoreactivity induction by light stimuli in the intergeniculate leaflet is different from that of the suprachiasmatic nucleus. Brain Res. 1993;610(2):334–339. doi: 10.1016/0006-8993(93)91419-s. [DOI] [PubMed] [Google Scholar]
- 48.Janik D, Mrosovsky N. Gene expression in the geniculate induced by a nonphotic circadian phase shifting stimulus. Neuroreport. 1992;3(7):575–578. doi: 10.1097/00001756-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Cha YJ, Lee JH, Baik TK, Park JS. c-Fos immunoreactivity in the neurons of the lateral geniculate nucleus in albino rats by light exposure after dark rearing. Korean J Ophthalmol. 2011;25(6):434–439. doi: 10.3341/kjo.2011.25.6.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juhl F, Hannibal J, Fahrenkrug J. Photic induction of c-Fos in enkephalin neurons of the rat intergeniculate leaflet innervated by retinal PACAP fibres. Cell Tissue Res. 2007;329(3):491–502. doi: 10.1007/s00441-007-0422-6. [DOI] [PubMed] [Google Scholar]
- 51.Prichard JR, Stoffel RT, Quimby DL, Obermeyer WH, Benca RM, Behan M. Fos immunoreactivity in rat subcortical visual shell in response to illuminance changes. Neuroscience. 2002;114(3):781–793. doi: 10.1016/s0306-4522(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 52.Janik D, Mikkelsen JD, Mrosovsky N. Cellular colocalization of Fos and neuropeptide Y in the intergeniculate leaflet after nonphotic phase-shifting events. Brain Res. 1995;698(1–2):137–145. doi: 10.1016/0006-8993(95)00878-t. [DOI] [PubMed] [Google Scholar]
- 53.Marchant EG, Morin LP. The hamster circadian rhythm system includes nuclei of the subcortical visual shell. J Neurosci. 1999;19(23):10482–10493. doi: 10.1523/JNEUROSCI.19-23-10482.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muscat L, Morin LP. Intergeniculate leaflet: contributions to photic and non-photic responsiveness of the hamster circadian system. Neuroscience. 2006;140(1):305–320. doi: 10.1016/j.neuroscience.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Van Reeth O, Zee PC, Takahashi JS, Turek FW. Fos protein expression in the circadian clock is not associated with phase shifts induced by a nonphotic stimulus, triazolam. Neurosci Lett. 1993;164(1–2):203–208. doi: 10.1016/0304-3940(93)90892-o. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Kornhauser JM, Zee PC, Mayo KE, Takahashi JS, Turek FW. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, fos expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience. 1996;70(4):951–961. doi: 10.1016/0306-4522(95)00408-4. [DOI] [PubMed] [Google Scholar]
- 57.Abe H, Honma S, Shinohara K, Honma KI. Circadian modulation in photic induction of Fos-like immunoreactivity in the suprachiasmatic nucleus cells of diurnal chipmunk, Eutamias asiaticus. J Comp Physiol A. 1995;176(2):159–167. doi: 10.1007/BF00239919. [DOI] [PubMed] [Google Scholar]
- 58.Krajnak K, Dickenson L, Lee TM. The induction of Fos-like proteins in the suprachiasmatic nuclei and intergeniculate leaflet by light pulses in degus (Octodon degus) and rats. J Biol Rhythms. 1997;12(5):401–412. doi: 10.1177/074873049701200502. [DOI] [PubMed] [Google Scholar]
- 59.Mahoney MM, Smale L, Lee TM. Daily immediate early gene expression in the suprachiasmatic nucleus of male and female Octodon degus. Chronobiol Int. 2009;26(5):821–837. doi: 10.1080/07420520903044265. [DOI] [PubMed] [Google Scholar]
- 60.Schumann DM, Cooper HM, Hofmeyr MD, Bennett NC. Light-induced Fos expression in the suprachiasmatic nucleus of the four-striped field mouse, Rhabdomys pumilio: A southern African diurnal rodent. Brain Res Bull. 2006;70(4–6):270–277. doi: 10.1016/j.brainresbull.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Morin LP, Studholme KM. Millisecond Light Pulses Make Mice Stop Running, then Display Prolonged Sleep-Like Behavior in the Absence of Light. Journal of Biological Rhythms. 2009;24(6):497–508. doi: 10.1177/0748730409349059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Studholme KM, Gompf HS, Morin LP. Brief light stimulation during the mouse nocturnal activity phase simultaneously induces a decline in core temperature and locomotor activity followed by EEG-determined sleep. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2013;304(6):R459–R471. doi: 10.1152/ajpregu.00460.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chellappa SL, Steiner R, Blattner P, Oelhafen P, Gotz T, Cajochen C. Non-visual effects of light on melatonin, alternedd and cognitive performance: Can blue-enriched light keep us alert? PLoS ONE. 2011;6(1):e16429. doi: 10.1371/journal.pone.0016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McElhinny TL. Masters Dissertation. Michigan State University; East Lansing, MI, USA: 1996. Reproductive Biology and Biological Rhythms in Arvicanthis niloticus. [Google Scholar]
- 65.Novak CM, Smale L, Nunez AA. Fos expression in the sleep-active cell group of the ventrolateral preoptic area in the diurnal murid rodent, Arvicanthis niloticus. Brain Res. 1999;818(2):375–382. doi: 10.1016/s0006-8993(98)01319-5. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz MD, Nunez AA, Smale L. Differences in the suprachiasmatic nucleus and lower subparaventricular zone of diurnal and nocturnal rodents. Neuroscience. 2004;127(1):13–23. doi: 10.1016/j.neuroscience.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 67.Martinez GS, Smale L, Nunez AA. Diurnal and nocturnal rodents show rhythms in orexinergic neurons. Brain Res. 2002;955(1–2):1–7. doi: 10.1016/s0006-8993(02)03264-x. [DOI] [PubMed] [Google Scholar]
- 68.Nixon JP, Smale L. Individual differences in wheel-running rhythms are related to temporal and spatiral patterns of activition of orexin A and B cells in a diurnal rodent (Arvicanthis niloticus) Neuroscience. 2004;127(1):25–34. doi: 10.1016/j.neuroscience.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 69.Castillo-Ruiz A, Nunez AA. Fos expression in arousal and reward areas of the brain in grass rats following induced wakefulness. Physiol Behav. 2011;103(3–4):384–392. doi: 10.1016/j.physbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dkhissi-Benyahya O, Sicard B, Cooper HM. Effects of Irradiance and Stimulus Duration on Early gene Expression (Fos) in the Suprachiasmatic Nucleus: Temporal Summation and Reciprocity. The Journal Neuroscience. 2000;20(20):7790–7797. doi: 10.1523/JNEUROSCI.20-20-07790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rieux C, Carney R, Lupi D, Dkhissi-Benyahya O, Jansen K, Chounlamountri N, Foster RG, Cooper HM. Analysis of Immunohistochemical Label of Fos Protein in the Suprachiasmatic Nucleus:Comparison of different Methods of Quantification. J Biol Rhtyhms. 2002;17(2):121–136. doi: 10.1177/074873002129002410. [DOI] [PubMed] [Google Scholar]
- 72.Katona C, Rose S, Smale L. The expression of Fos within the suprachiasmatic nucleus of the diurnal rodent Arvicanthis niloticus. Brain Res. 1998;791(1–2):27–34. doi: 10.1016/s0006-8993(97)01092-5. [DOI] [PubMed] [Google Scholar]
- 73.Colwell CS, Foster RG. Photic regulation of Fos-like immunoreactivity in the suprachiasmatic nucleus of the mouse. J Comp Neurol. 1992;324(2):135–142. doi: 10.1002/cne.903240202. [DOI] [PubMed] [Google Scholar]
- 74.Mahoney M, Bult A, Smale L. Phase response curve and light-induced fos expression in the suprachiasmatic nucleus and adjacent hypothalamus of Arvicanthis niloticus. J Biol Rhythms. 2001;16(2):149–162. doi: 10.1177/074873001129001854. [DOI] [PubMed] [Google Scholar]
- 75.Geusz ME, Fletcher C, Block GD, Straume M, Copeland NG, Jenkins NA, Day RN. Long-term monitoring of circadian rhythms in c-fos gene expression from suprachiasmatic nucleus cultures. Curr Biol. 1997;7(10):758–766. doi: 10.1016/s0960-9822(06)00334-4. [DOI] [PubMed] [Google Scholar]
- 76.Honrado GI, Johnson RS, Golombek DA, Spiegelman BM, Papaioannou VE, Ralph MR. The circadian system of c-fos deficient mice. J Comp Physiol A. 1996;178(4):563–570. doi: 10.1007/BF00190186. [DOI] [PubMed] [Google Scholar]
- 77.Wollnik F, Brysch W, Uhlmann E, Gillardon F, Bravo R, Zimmermann M, Herdegen T. Block of c-Fos and JunB expression by antisense oligonucleotides inhibits light-induced phase shifts of the mammalian circadian clock. Eur J Neurosci. 1995;7(3):388–393. doi: 10.1111/j.1460-9568.1995.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 78.Smale L, Lee T, Nunez AA. Mammalian diurnality: Some facts and gaps. Journal of Biological Rhythms. 2003;18(5):356–366. doi: 10.1177/0748730403256651. [DOI] [PubMed] [Google Scholar]
- 79.Perreau-Lenz S, Kalsbeek A, Garidou ML, Wortel J, van der Vliet J, van Heijningen C, Simonneaux V, Pevet P, Buijs RM. Suprachiasmatic control of melatonin synthesis in rats: inhibitory and stimulatory mechanisms. Eur J Neurosci. 2003;17(2):221–228. doi: 10.1046/j.1460-9568.2003.02442.x. [DOI] [PubMed] [Google Scholar]
- 80.Perreau-Lenz S, Kalsbeek A, Pevet P, Buijs RM. Glutamatergic clock output stimulates melatonin synthesis at night. Eur J Neurosci. 2004;19(2):318–324. doi: 10.1111/j.0953-816x.2003.03132.x. [DOI] [PubMed] [Google Scholar]
- 81.Easton A, Meerlo P, Bergmann B, Turek FW. The suprachiasmatic nucleus regulates sleep timing and amount in mice. Sleep. 2004;27(7):1307–1318. doi: 10.1093/sleep/27.7.1307. [DOI] [PubMed] [Google Scholar]
- 82.Tong M, Watanabe E, Yamamoto N, Nagahata-Ishiguro M, Maemura K, Takeda N, Nagai R, Ozaki Y. Circadian expressions of cardiac ion channel genes in mouse might be associated with the central clock in the SCN but not the peripheral clock in the heart. Biol Rhythm Res. 2013;44(4):519–530. doi: 10.1080/09291016.2012.704801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stephan FK, Zucker I. Circadian-rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic-lesions. Proceedings of the National Academy of Sciences of the United States of America. 1972;69(6):1583–&. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coindet J, Chouvet G, Mouret J. Effects of lesions of suprachiasmatic nuclei on paradoxical sleep and slow-wave sleep circadian-rhythms in rat. Neuroscience Letters. 1975;1(4):243–247. doi: 10.1016/0304-3940(75)90068-3. [DOI] [Google Scholar]
- 85.Mistlberger RE, Bergmann BM, Waldenar W, Rechtschaffen A. Recovery sleep following sleep deprivation in intact and suprachiasmatic nuclei-lesioned rats. Sleep. 1983;6(3):217–233. doi: 10.1093/sleep/6.3.217. [DOI] [PubMed] [Google Scholar]
- 86.Liu XG, Zhang BJ, Xu XH, Huang ZL, Qu WM. Lesions of suprachiasmatic nucleus modify sleep structure but do not alter the total amount of daily sleep in rats. Sleep and Biological Rhythms. 2012;10(4):293–301. doi: 10.1111/j.1479-8425.2012.00572.x. [DOI] [Google Scholar]
- 87.Ibuka N, Kawamura H. Loss of circadian rhythm in sleep-wakefulness cycle in the rat by suprachiasmatic nucleus lesions. Brain Res. 1975;96(1):76–81. doi: 10.1016/0006-8993(75)90574-0. [DOI] [PubMed] [Google Scholar]
- 88.Ibuka N, Inouye SI, Kawamura H. Analysis of sleep-wakefulness rhythms in male rats after suprachiasmatic nucleus lesions and ocular enucleation. Brain Res. 1977;122(1):33–47. doi: 10.1016/0006-8993(77)90660-6. [DOI] [PubMed] [Google Scholar]
- 89.Aguilar-Roblero R, Garcia-Hernandez F, Aguilar R, Arankowsky-Sandoval G, Drucker-Colin R. Suprachiasmatic nucleus transplants function as an endogenous oscillator only in constant darkness. Neurosci Lett. 1986;69(1):47–52. doi: 10.1016/0304-3940(86)90412-x. [DOI] [PubMed] [Google Scholar]
- 90.Amir S, Lamont EW, Robinson B, Stewart J. A circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN-controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis. J Neurosci. 2004;24(4):781–790. doi: 10.1523/jneurosci.4488-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang S, Zeitzer JM, Yoshida Y, Wisor JP, Nishino S, Edgar DM, Mignot E. Lesions of the suprachiasmatic nucleus eliminate the daily rhythm of hypocretin-1 release. Sleep. 2004;27(4):619–627. doi: 10.1093/sleep/27.4.619. [DOI] [PubMed] [Google Scholar]
- 92.Hu K, Scheer FA, Ivanov P, Buijs RM, Shea SA. The suprachiasmatic nucleus functions beyond circadian rhythm generation. Neuroscience. 2007;149(3):508–517. doi: 10.1016/j.neuroscience.2007.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Angeles-Castellanos M, Salgado-Delgado R, Rodriguez K, Buijs RM, Escobar C. The suprachiasmatic nucleus participates in food entrainment: a lesion study. Neuroscience. 2010;165(4):1115–1126. doi: 10.1016/j.neuroscience.2009.11.061. [DOI] [PubMed] [Google Scholar]
- 94.Warren WS, Champney TH, Cassone VM. The suprachiasmatic nucleus controls the circadian rhythm of heart rate via the sympathetic nervous system. Physiol Behav. 1994;55(6):1091–1099. doi: 10.1016/0031-9384(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 95.Wachulec M, Li H, Tanaka H, Peloso E, Satinoff E. Suprachiasmatic nuclei lesions do not eliminate homeostatic thermoregulatory responses in rats. J Biol Rhythms. 1997;12(3):226–234. doi: 10.1177/074873049701200304. [DOI] [PubMed] [Google Scholar]
- 96.Mistlberger RE. Nonphotic entrainment of circadian activity rhythms in suprachiasmatic nuclei-ablated hamsters. Behav Neurosci. 1992;106(1):192–202. doi: 10.1037//0735-7044.106.1.192. [DOI] [PubMed] [Google Scholar]
- 97.DeCoursey PJ, Krulas JR, Mele G, Holley DC. Circadian performance of suprachiasmatic nuclei (SCN)-lesioned antelope ground squirrels in a desert enclosure. Physiol Behav. 1997;62(5):1099–1108. doi: 10.1016/s0031-9384(97)00263-1. [DOI] [PubMed] [Google Scholar]
- 98.Fuller CA, Lydic R, Sulzman FM, Albers HE, Tepper B, Moore-Ede MC. Circadian rhythm of body temperature persists after suprachiasmatic lesions in the squirrel monkey. Am J Physiol. 1981;241(5):R385–391. doi: 10.1152/ajpregu.1981.241.5.R385. [DOI] [PubMed] [Google Scholar]
- 99.Todd WD, Gall AJ, Weiner JA, Blumberg MS. Distinct retinohypothalamic innervation patterns predict the developmental emergence of species-typical circadian phase preference in nocturnal Norway rats and diurnal nile grass rats. J Comp Neurol. 2012;520(14):3277–3292. doi: 10.1002/cne.23098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johnson RF, Morin LP, Moore RY. Retinohypothalamic projections in the hamster and rat demonstrated using cholera toxin. Brain Res. 1988;462(2):301–312. doi: 10.1016/0006-8993(88)90558-6. [DOI] [PubMed] [Google Scholar]
- 101.Gaillard F, Karten HJ, Sauve Y. Retinorecipient areas in the diurnal murine rodent Arvicanthis niloticus: A disproportionally large superior colliculus. Journal of Comparative Neurology. 2013;521(8):1699–1726. doi: 10.1002/cne.23303. [DOI] [PubMed] [Google Scholar]
- 102.Smale L, Boverhof J. The suprachiasmatic nucleus and intergeniculate leaflet of Arvicanthis niloticus, a diurnal murid rodent from East Africa. J Comp Neurol. 1999;403(2):190–208. doi: 10.1002/(sici)1096-9861(19990111)403:2<190::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 103.Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J, Silver R. Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J Comp Neurol. 2004;468(3):361–379. doi: 10.1002/cne.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morin LP, Goodless-Sanchez N, Smale L, Moore RY. Projections of the suprachiasmatic nuclei, subparaventricular zone and retrochiasmatic area in the golden hamster. Neuroscience. 1994;61(2):391–410. doi: 10.1016/0306-4522(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 105.Schwartz MD, Urbanski HF, Nunez AA, Smale L. Projections of the suprachiasmatic nucleus and ventral subparaventricular zone in the Nile grass rat (Arvicanthis niloticus) Brain Res. 2011;1367:146–161. doi: 10.1016/j.brainres.2010.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Canteras NS, Ribeiro-Barbosa ER, Goto M, Cipolla-Neto J, Swanson LW. The retinohypothalamic tract: comparison of axonal projection patterns from four major targets. Brain Res Rev. 2011;65(2):150–183. doi: 10.1016/j.brainresrev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 107.Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258(2):204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- 108.Nunez AA, Bult A, McElhinny TL, Smale L. Daily rhythms of Fos expression in hypothalamic targets of the suprachiasmatic nucleus in diurnal and nocturnal rodents. J Biol Rhythms. 1999;14(4):300–306. doi: 10.1177/074873099129000713. [DOI] [PubMed] [Google Scholar]
- 109.Schwartz MD, Nunez AA, Smale L. Rhythmic cFos expression in the ventral subparaventricular zone influences general activity rhythms in the Nile grass rat, Arvicanthis niloticus. Chronobiol Int. 2009;26(7):1290–1306. doi: 10.3109/07420520903415742. [DOI] [PubMed] [Google Scholar]
- 110.Kornhauser JM, Mayo KE, Takahashi JS. Light, immediate-early genes, and circadian rhythms. Behav Genet. 1996;26(3):221–240. doi: 10.1007/BF02359382. [DOI] [PubMed] [Google Scholar]
- 111.Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiology International. 2003;20(6):989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- 112.Cipolla-Neto J, Bartol I, Seraphim PM, Afeche SC, Scialfa JH, Peracoli AM. The effects of lesions of the thalamic intergeniculate leaflet on the pineal metabolism. Brain Res. 1995;691(1–2):133–141. doi: 10.1016/0006-8993(95)00654-9. [DOI] [PubMed] [Google Scholar]
- 113.Edelstein K, Amir S. The role of the intergeniculate leaflet in entrainment of circadian rhythms to a skeleton photoperiod. J Neurosci. 1999;19(1):372–380. doi: 10.1523/JNEUROSCI.19-01-00372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tavakoli-Nezhad M, Schwartz WJ. Hamsters running on time: is the lateral habenula a part of the clock? Chronobiol Int. 2006;23(1–2):217–224. doi: 10.1080/07420520500521947. [DOI] [PubMed] [Google Scholar]
- 115.Engber TM, Koury EJ, Dennis SA, Miller MS, Contreras PC, Bhat RV. Differential patterns of regional c-Fos induction in the rat brain by amphetamine and the novel wakefulness-promoting agent modafinil. Neurosci Lett. 1998;241(2–3):95–98. doi: 10.1016/s0304-3940(97)00962-2. [DOI] [PubMed] [Google Scholar]
- 116.Paul MJ, Indic P, Schwartz WJ. A role for the habenula in the regulation of locomotor activity cycles. Eur J Neurosci. 2011;34(3):478–488. doi: 10.1111/j.1460-9568.2011.07762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chastrette N, Pfaff DW, Gibbs RB. Effects of daytime and nighttime stress on Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus, the habenula, and the posterior paraventricular nucleus of the thalamus. Brain Res. 1991;563(1–2):339–344. doi: 10.1016/0006-8993(91)91559-j. [DOI] [PubMed] [Google Scholar]
- 118.Lehner M, Taracha E, Skorzewska A, Wislowska A, Zienowicz M, Maciejak P, Plaznik A. Sensitivity to pain and c-Fos expression in brain structures in rats. Neurosci Lett. 2004;370(1):74–79. doi: 10.1016/j.neulet.2004.07.089. [DOI] [PubMed] [Google Scholar]
- 119.Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H. Molecular characterization of the subnuclei in rat habenula. J Comp Neurol. 2012;520(18):4051–4066. doi: 10.1002/cne.23167. [DOI] [PubMed] [Google Scholar]
- 120.Andres KH, von During M, Veh RW. Subnuclear organization of the rat habenular complexes. J Comp Neurol. 1999;407(1):130–150. doi: 10.1002/(sici)1096-9861(19990428)407:1<130::aid-cne10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 121.Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187(1):19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- 122.Zhao H, Rusak B. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience. 2005;132(2):519–528. doi: 10.1016/j.neuroscience.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 123.Poveda A, Kretz R. c-Fos expression in the visual system of the tree shrew (Tupaia belangeri) J Chem Neuroanat. 2009;37(4):214–228. doi: 10.1016/j.jchemneu.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 124.Fite KV, Wu PS, Bellemer A. Photostimulation alters c-Fos expression in the dorsal raphe nucleus. Brain Res. 2005;1031(2):245–252. doi: 10.1016/j.brainres.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 125.Hendrickson AE, Wilson JR, Ogren MP. The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in Old World and New World primates. J Comp Neurol. 1978;182(1):123–136. doi: 10.1002/cne.901820108. [DOI] [PubMed] [Google Scholar]
- 126.Hoffmann KP, Stone J, Sherman SM. Relay of receptive-field properties in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1972;35(4):518–531. doi: 10.1152/jn.1972.35.4.518. [DOI] [PubMed] [Google Scholar]
- 127.Allen AE, Brown TM, Lucas RJ. A distinct contribution of short-wavelength-sensitive cones to light-evoked activity in the mouse pretectal olivary nucleus. J Neurosci. 2011;31(46):16833–16843. doi: 10.1523/jneurosci.2505-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Szkudlarek HJ, Orlowska P, Lewandowski MH. Light-induced responses of slow oscillatory neurons of the rat olivary pretectal nucleus. PLoS One. 2012;7(3):e33083. doi: 10.1371/journal.pone.0033083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morin LP, Blanchard J. Organization of the hamster intergeniculate leaflet: NPY and ENK projections to the suprachiasmatic nucleus, intergeniculate leaflet and posterior limitans nucleus. Vis Neurosci. 1995;12(1):57–67. doi: 10.1017/s0952523800007318. [DOI] [PubMed] [Google Scholar]
- 130.Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci. 2001;21(13):4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271(5246):216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 132.Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115(1):285–294. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 133.Mistlberger RE, Antle MC, Kilduff TS, Jones M. Food- and light-entrained circadian rhythms in rats with hypocretin-2-saporin ablations of the lateral hypothalamus. Brain Res. 2003;980(2):161–168. doi: 10.1016/s0006-8993(03)02755-0. [DOI] [PubMed] [Google Scholar]
- 134.Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76(1):130–141. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 135.Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci. 2013;7:28. doi: 10.3389/fnbeh.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Adidharma W, Leach G, Yan L. Orexinergic signaling mediates light-induced neuronal activation in the dorsal raphe nucleus. Neuroscience. 2012;220:201–207. doi: 10.1016/j.neuroscience.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fite KV, Birkett MA, Smith A, Janusonis SF, McLaughlin S. Retinal ganglion cells projecting to the dorsal raphe and lateral geniculate complex in Mongolian gerbils. Brain Research. 2003;973(1):146–150. doi: 10.1016/s0006-8993(03)02549-6. [DOI] [PubMed] [Google Scholar]