Abstract
Objective
The objective of this study was to investigate the existence of an equine pain face and to describe this in detail.
Study design
Semi-randomized, controlled, crossover trial.
Animals
Six adult horses.
Methods
Pain was induced with two noxious stimuli, a tourniquet on the antebrachium and topical application of capsaicin. All horses participated in two control trials and received both noxious stimuli twice, once with and once without an observer present. During all sessions their pain state was scored. The horses were filmed and the close-up video recordings of the faces were analysed for alterations in behaviour and facial expressions. Still images from the trials were evaluated for the presence of each of the specific pain face features identified from the video analysis.
Results
Both noxious challenges were effective in producing a pain response resulting in significantly increased pain scores. Alterations in facial expressions were observed in all horses during all noxious stimulations. The number of pain face features present on the still images from the noxious challenges were significantly higher than for the control trial (p = 0.0001). Facial expressions representative for control and pain trials were condensed into explanatory illustrations. During pain sessions with an observer present, the horses increased their contact-seeking behavior.
Conclusions and clinical relevance
An equine pain face comprising ‘low’ and/or ‘asymmetrical’ ears, an angled appearance of the eyes, a withdrawn and/or tense stare, mediolaterally dilated nostrils and tension of the lips, chin and certain facial muscles can be recognized in horses during induced acute pain. This description of an equine pain face may be useful for improving tools for pain recognition in horses with mild to moderate pain.
Keywords: experimental study, horses, pain behavior, pain evaluation, pain face
Introduction
There is as yet no universally implemented pain scale for assessing pain in horses. The attempts that have been made all include behavioural cues, as physiological measures cannot stand alone (Price et al. 2003; Pritchett et al. 2003; Bussieres et al. 2008; Lindegaard et al. 2010; Graubner et al. 2011). Challenges in pain assessment are numerous including problems with recognizing early or subtle signs of pain and possible suppression of pain behaviour due to potentially threatening stimuli (Taylor et al. 2002).
Humans are endowed with an evolutionarily developed skill for recognizing emotions through facial expressions (Deyo et al. 2004; Kadosh & Johnson 2007). These expressions change when experiencing pain, making them sensitive pain indicators. Even when adult persons are asked to conceal their pain, facial expressions will continue to leak that information (Prkachin & Mercer 1989). Furthermore, facial expressions are considered to be the most consistent pain expression in children (Poole & Craig 1992).
Charles Darwin predicted that nonhuman animals could exhibit similar facial expressions in response to emotional states as humans do (Darwin 1872), but only recently have facial expressions been proposed as a method for evaluating pain in animals (Flecknell 2010). A Mouse Grimace Scale (Langford et al. 2010) and later a rat and rabbit scale have been developed (Sotocinal et al. 2011; Keating et al. 2012). Facial expressions of pain in horses have previously been based on observations by experienced horse practitioners, rather than on systematic investigations (Fraser 1969; Sanford et al. 1986; Taylor et al. 2002). Love et al. (2011) used kinematic analysis to determine that certain facial expressions of horses changed during injections and these findings were very recently corroborated in a study describing the Horse Grimace Scale for horses undergoing castration (Dalla Costa et al. 2014). Facial expressions of pain may be a valuable addendum to existing pain evaluation tools. However, it is of great importance to differentiate changes in facial expressions due to pain from changes due to stress (Love 2009), analgesics, anaesthetics (Seibert et al. 2003; Ashley et al. 2005) and other interfering factors, such as the influence of humans.
The objectives of the present study were to investigate the facial expressions in horses during induced acute pain and to describe these facial cues in detail. In order to be able to identify the facial expressions of pain, this study was planned to preclude stress and to avoid treatment with anaesthetics or analgesics. Pain was induced using two noxious stimuli, a tourniquet on the antebrachium and topical application of capsaicin. These methods are well described in human pain research but are to the authors’ knowledge novel methods for pain induction in horses. Our hypotheses were that horses display facial expressions of pain similar to other mammals and that these expressions are moderated in the presence of humans.
Materials and methods
The experimental protocol was approved by the Danish Animal Experiments Inspectorate.
Study design
The study was conducted as a semi-randomized, controlled, crossover trial, which was randomized into two blocks on the parameter observer; in the ‘no-observer’ trials, the observer was not visible or audible to the horses. The treatment sequence was the same for all horses (Table1). Each horse participated in six trials, two control trials with no noxious stimulus and four pain trials with two different noxious stimuli; each noxious stimuli with and without an observer present. All trials were performed with the horses standing in a narrow, rectangular area of the stable, restrained by a neck collar and with a grey screen on the right side. The three youngest horses displayed very exploratory behaviour due to the degree of freedom allowed by the neck collar and wore an ordinary halter in some trials. The time of treatment was constant for each individual horse, with three horses before midday and three horses after midday. Two horses, one from each block, received a noxious stimulus every day for four consecutive days; four horses had one resting day between trials.
Table 1.
Block randomization and treatment sequences for all horses
| Trial | Block 1 (n = 3) | Block 2 (n = 3) |
|---|---|---|
| Control I (no noxious stimuli) | With observer | No observer |
| Control II (no noxious stimuli) | No observer | With observer |
| Tourniquet I (right thoracic limb) | With observer | No observer |
| Capsaicin I (right shoulder / left thigh) | No observer | With observer |
| Tourniquet II (left thoracic limb) | No observer | With observer |
| Capsaicin II (left shoulder / right thigh) | With observer | No observer |
Animals
Six healthy horses of different breeds, five mares and one gelding, aged 3–14 years were included in the study. The horses were housed in the research facility of the University of Copenhagen in 3 × 4 m stalls at a constant temperature of 13 ± 1 °C. They were fed a grain mixture twice daily as well as hay and water ad libitum and were turned out into a paddock for approximately 2 hours prior to the training and trial procedure each day. The horses were stabled at the research facility for at least 10 days prior to the study. During this period the horses were groomed and trained with positive reinforcement to accept standing in the trial area, wearing a neck collar. Horses were under constant observation during all trials.
Trials
Pain was induced using two noxious stimuli applied to the horses for 20 minutes. The time T0 started the moment the application was completed and finished at T20. Baseline values were measured before the initiation of the noxious event. The first noxious stimulus was an application of a tourniquet (a pneumatic blood pressure cuff) placed around the antebrachium as proximal as possible, applying pressure to the extensor and flexor muscles of the forelimb. The cuff was inflated to 240 mmHg while the limb was lifted. After fitting the cuff the horses were free to bear weight on the leg, which increased the pressure of the cuff. After 20 minutes the horses were examined and the cuff was deflated.
The second noxious stimulus was a topical application of capsaicin crème 10% (Capsaicin 62%, USP 31; Inga Pharmaceuticals, India, formulated by the university pharmacy, in Essex crème; Merck Sharp & Dohme B.V., Holland). The capsaicin créme was applied on the lateral aspect of the hindlimb, above the hock in the area of the proximal part of m. extensor digitalis lateralis of the first two horses. For safety reasons, the four other horses had capsaicin applied on the lateral aspect of the shoulder, caudoventrally to the scapula at the junction of m. deltoideus and m. tricipitis brachii. A 10 × 10 cm area was clipped bilaterally 8–10 days prior to the procedure to facilitate skin contact. The capsaicin cream was applied to the skin and covered with a plastic sheet and two layers of self-adhesive bandage (Animal Polster; Snögg AS, Norway) to secure occlusion and prevent the horses from getting in contact with the capsaicin. The equivalent contralateral area was covered with plastic and self-adhesive bandage to function as the control site. After 20 minutes, the horses were examined and the capsaicin cream was removed mechanically and cleansed with vegetable oil.
Control trials were performed with the horses standing undisturbed in the trial area. These trials lasted 30 minutes and T0 was the start of the video recording.
Clinical examination
A clinical examination was performed prior to applying the noxious stimuli (baseline), before removing it (T20) and 60 minutes after removal (T80). The clinical examination included: heart rate (HR), respiratory rate (fR), rectal and skin temperature (RT and ST, respectively) measurements. The skin temperature was measured with an infrared thermometer (Raytek Raynger MX4; Raytek, CA, USA) below the tourniquet and in the area of the capsaicin application. Skin sensitivity was evaluated with soft touch (cotton bud) and pin pricks, in the area of the treatment before and after the capsaicin challenge. Four horses had continuous electrocardiography recorded (Televet 100 ECG recorder; Jørgen Kruuse A/S, Denmark) and non-invasive mean arterial blood pressure (MAP) measured with a tail cuff (Mindray PM-9000 Vet monitor; Mindray, China) at baseline, T20 and T80.
Video recordings
Video recordings were performed with a camera (Canon Legria HF S21, Canon Inc., Tokyo, Japan) placed on a tripod on the left side of the horses. The camera was placed at a distance of two meters, allowing primarily the head of the horses within the video frame. The neck collar allowed the horses a wide range of head motion, occasionally out of the video frame. The observer hid behind a wall during the ‘no observer’ trials and stood approximately one meter from the horses, not facing them and behaving as neutrally as possible during the ‘with observer’ trials.
Observations of pain behaviour
During all experimental sessions the horses were observed for pain behaviour using a modified version of the composite measure pain scale (Lindegaard et al. 2010; Table2).
Table 2.
Composite measure pain scale modified from Lindegaard et al. (2010). The categories ‘location in the stall’ and ‘response to open door’ were left out as the horses were restrained in this study. ‘Response to approach’ and ‘response to grain’ were changed to comply with horses being restrained and were left out for the evaluation 10 minutes after initiation of the noxious stimuli as no observer was present
| Behaviour category | Scores | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Gross pain behaviour* | None | Occasional | Continuous | ||
| Weight bearing | Normal weight bearing | Foot intermittent of the ground/ resting more than other front limb | Continuously taking foot off the ground and trying to replace it | No weight bearing. Foot off the ground or toe just touching the ground | |
| Head position | Above withers or eating | Level of withers | Below withers | ||
| Response to approach | Looks at observer. ears forward | Does not look at observer, ears back | |||
| Response to treat | Takes treat with no hesitation | Looks at observer | No response | ||
| Subjective pain score | No apparent pain | Mild discomfort | Slight pain | Moderate pain | Severe pain |
Gross pain behaviour includes; pawing, swating, flehmen.
An ethogram of the components of the horses’ facial expressions was constructed based on the video recordings of the ‘no observer’ trials. The horses were obviously more restless during pain inductions making blinded observations impossible for the construction of the ethogram.
Quantifying the duration of the specific changes in facial expressions was not possible due to the subtleness of the expressions combined with the increased restlessness, therefore an assessment of the presence or absence of each pain feature was performed. The 4 ear movements and positions identified were quantified for statistical comparison as was the additional behaviours identified from the video recordings that were chewing, yawning, snorting, head shaking, eye blinking and moving out of the picture frame (Table3).
Table 3.
Description of the behaviours quantified from the video recordings as the proportion of time (prop. time), frequency or counts. All but the ‘contact seeking behaviour’ is quantified from the ‘no-observer’ trials
| Category | Definitions of behaviours |
|---|---|
| Ear movements | |
| Forward | Both ears facing forward (prop. time) |
| Attentive | One ear in a ‘fixed’ position facing forward or back, the other ear moving, alternating between facing back and forward. The ‘fixed’ ear occasionally changes position simultaneously with the other ear (prop. time) |
| Asymmetrical/low | Both ears are moving in different directions or placed in asymmetrical positions none of the ears are facing directly forward or back/a lowering of both ears (increased distance between them) facing the sides or slightly back. The ears may also be both asymmetrical and low (prop. time) |
| Back | Both ears facing back (prop. time) |
| Out of frame | The horse is moving the head up or down and is thereby not visible in the video frame (prop. time) |
| Contact seeking behavior (with-observer) | |
| Physical contact/ visual contact | Bending the neck and head towards the observer, nudging, pushing, gently biting the observer or looking at the observer (prop. time) |
| Eye blinking | Blinking is defined as any movement of the upper eyelid towards the lower eyelid or ‘flutter’ described as a fine fibrillar hesitating movement of the skin of the forehead in the region of the eyebrow over the inner canthus of the eye; This does not involve movement of the eyelids (Blount 1927; frequency) |
| Other behaviours | |
| Chewing | Chewing without feed in the mouth (counts) |
| Yawning | Opening of the mouth in a yawn, extending the head and neck, eyes rolls/closes and mouth closes (counts) |
| Snorting | Forceful exhalation through the nostrils while making a characteristic sound (counts) |
| Head shaking | Rotary movements of the head in a clockwise/anti-clockwise direction (counts) |
Video sequences and still images of the horses showing the pertinent facial expressions were presented to a scientific illustrator to depict important differences between horses in pain and in the control situation.
From the ‘with observer’ trials two observer-related behaviours were identified: visual contact with the observer and physical contact with the observer (Table3). All behaviours were recorded for 10 undisturbed minutes, starting 5 minutes after application of the noxious stimuli.
A ‘blinded’ evaluation of randomized still images from the video recordings of all ‘no observer’-trials was performed by one person. Still images were chosen over video sequences to avoid bias from the horses’ increased movements during pain inductions. The images were sampled from the first 5 minutes of each video, to avoid using the same sequence as was used for the statistical calculation of the occurrence of the behaviours identified from the video analysis. Six images, 3 control images and the first sharp image at 2, 3 and 4 minutes after pain inductions (minimum 1 minute after the observer left the horse undisturbed) of all horses were included. Images were excluded when the horses were obviously chewing, blinking, whinnying or attentive to something outside the stables or if the entire head was not present within the video frame. All images were evaluated for the presence or absence of each specific feature of the facial expression of pain described from the initial ethogram. The sum of features present in each image and the proportion of images comprising each feature were reported.
Statistical analysis
Comparison of physiological and behavioural parameters between the control trials and the two inductions were performed with a Wilcoxon matched-pairs signed rank test. For all directional hypotheses, a one-tailed test was used. All results are reported as the mean ± the standard deviation (SD). The comparison of the Kaplan–Meier survival curves were performed with the log-rank (Mantel-Cox) test. The statistical package Graph Pad Prism version 6.03 (GraphPad Software Inc., La Jolla, CA, USA) was used. All statistical analysis were performed for the trials with no observer, except for the analysis of the observer-related behaviour. Only five horses were included in the statistical analysis for the tourniquet trials with no observer because one horse was injured at pasture on the day of this trial.
Results
Physiological parameters
All physiological paramenters are reported as mean ± SD. Temperature, HR and fR did not differ significantly between baseline and T20 for any trials. Although not significant, the area under the curve for the heart rate (Fig.1) was lower for the control trial (791.3 ± 65.0) than for the pain trials (capsaicin 849.9 ± 74.6, tourniquet 844.9 ± 68.2). The MAP increased during the tourniquet trial from 70 ± 5 mmHg (baseline) to 80 ± 4 mmHg (T20) and decreased to values below baseline values 60 ± 8 mmHg (T80), this increase was not significant (p = 0.06). During the capsaicin trial there was a negligible increase in MAP from 76 ± 7 mmHg (baseline) to 76 ± 5 mmHg (T20; p = 0.5) which decreased to values below baseline values 70 ± 7 mmHg (T80). The skin temperatures prior to the capsaicin application (31.3 ± 1.7 °C) differed significantly (p = 0.03) to temperatures after removal of capsaicin (34.8 ± 0.5 °C) while the temperatures of the control site (contralateral) did not differ significantly (31.5 ± 1.5 °C before versus 32.3 ± 1.0 °C after). The skin sensitivity test evoked similar responses in the horses prior to and after the capsaicin challenge. The temperatures prior to the tourniquet application (21.3 ± 4.1 °C) differed significantly (p = 0.02) to temperatures at T20 (19.6 ± 3.1 °C) whereas temperatures of the contralateral leg did not differ significantly between T0 (22.8 ± 3.0 °C) and T20 (22.1 ± 2.4 °C).
Figure 1.
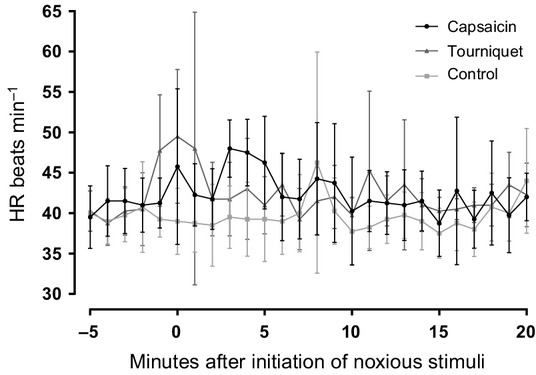
Heart rate (HR), calculated from the electrocardiogram of four horses. The time starts 5 minutes prior to application of the noxious stimuli to illustrate the immediate effect of the tourniquet, while the effect of the capsaicin is slow (2 minutes).
Behavioural evaluation (no observer trials)
During the control trials the horses mostly stood still. All horses became drowsy after 8.7 ± 7.1 minutes (Fig.2), evident by orbital closure and ears held back (Fig.3). After this initial drowsiness, the horses were periodically attentive towards the surroundings, evident by active ear and eye movements.
Figure 2.
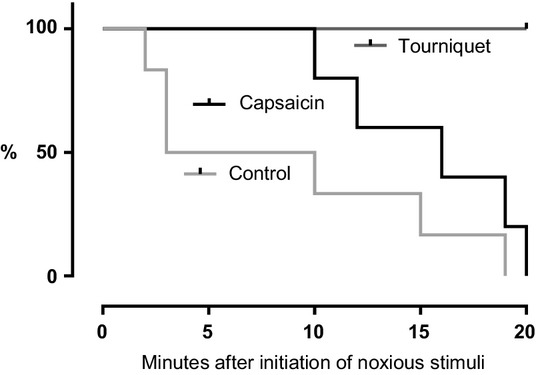
Time in minutes from application of the noxious stimuli (T0) until each of the horses exhibited behaviour associated with sleep, expressed as a proportion (%) of all horses in the group. Kaplan–Meier survivor function for the control trial and both pain trials.
Figure 3.

The picture illustrates a horse exhibiting signs associated with sleep during the control trial. Characteristics for this facial expression were orbital closure, ears held back and loose lips.
The application of capsaicin induced behavioural changes after approximately 2 minutes and the tourniquet induced immediate changes with increased agitation expressed by pawing, lateral head movements, stepping back and forth in the trial area and altered facial expressions. Following the capsaicin application the horses displayed behaviours interpreted as drowsiness after 15.4 ± 4.3 minutes. Drowsiness was never evident in horses after application of the tourniquet (p = 0.0002; Fig.2). The facial expressions of pain faded with increased drowsiness.
Composite measure pain scale scores for both the capsaicin and the tourniquet method differed significantly from the control trial at T10 and T30 (Fig.4). Both pain inductions produced an acute, moderate and reversible pain reaction.
Figure 4.
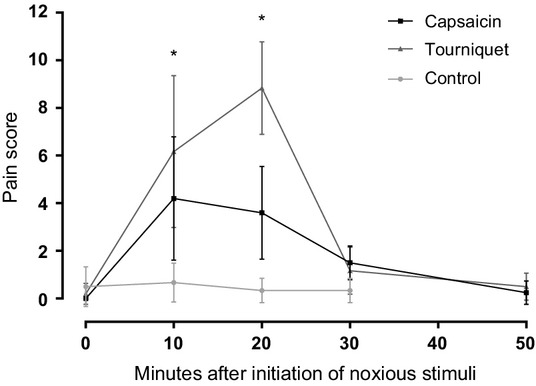
Mean pain score (±SD) from all horses using the composite measure pain scale in Table2. *Significant difference from control at T10 (p = 0.03) and T20 (p = 0.03). †Significant difference from control at T10 (p = 0.02) and T20 (p = 0.02).
Facial expression of pain
Facial expressions were altered in all horses during pain inductions and comprised alterations in ear positions, eyes, nostrils and muscle tension. The facial expressions were not static but changed whenever the horses reacted to something in the surroundings. Not all identified features of the pain face were present simultaneously at all times. The alterations in facial expressions are described separately for each feature of the face (Table4) and are depicted in illustrations (Fig.5a–c).
Table 4.
Description of the features of the equine pain face
| Pain face feature | Detailed description |
|---|---|
| Asymmetrical/low ears | Both ears are moving in different directions or are placed in asymmetrical positions with neither of the ears facing directly forward or back. There may be lowering of both ears (increased distance between them) with the opening of the ears facing the sides or slightly back. The ears may be both asymmetrical and low. |
| Angled eye | There is tension of the m. levator anguli oculi medialis (Fig.7). |
| Withdrawn and tense stare | The quality of the glance changes to become withdrawn and tense. |
| Nostrils – square-like | The nostrils are dilated mediolaterally; especially the medial wing of the nostril may be tense. This is most obvious during inspiration. |
| Tension of the muzzle | There is increased tonus of the lips and tension of the chin resulting in an edged shape of the muzzle. |
| Tension of the mimic muscles | There is tension of the muscles visible on the lateral aspect of the head, especially m. zygomaticus and m. caninus, but m. masseter may also be tense. |
Figure 5.
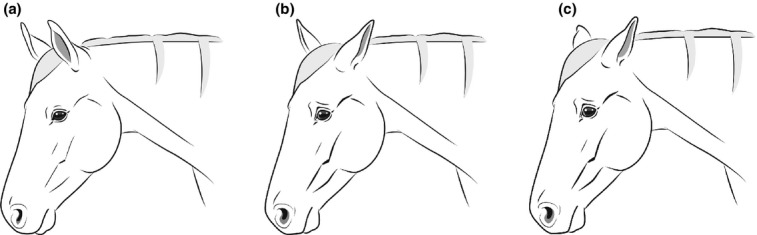
(a) Facial expression of a pain free, relaxed and attentive horse (Ill. Andrea Klintbjer). (b) Facial expression of a horse in pain, comprising all features of the pain face including asymmetrical ears (Ill. Andrea Klintbjer). (c) Facial expression of a horse in pain, comprising all features of the pain face including low ears (Ill. Andrea Klintbjer).
Ears
The distance between the bases of the ears increased during pain inductions because the ears tended to drop slightly down to each side of the head with a concomitant outward rotation of the ears (Fig.6). The movement pattern of the ears changed. During pain sessions, the horses spent significantly more time with ears moving ‘asymmetrically’ and/or ‘low’ and less time with attentive ears and ears forward (Table5).
Figure 6.

Horse during pain induction, displaying low, asymmetrical ears, an angled eye with an intense stare, and tension of the muzzle and the mimic muscles.
Table 5.
Comparison of behaviours between the pain inductions and the control trial (no observer). The ear movements, ‘out of frame’ and ‘contact seeking behaviour’ are reported as proportion of time (%). The eye blinking is reported as frequency (per second) and ‘other behaviours’ is reported as counts in 10 minutes
| Category | Control (n = 6)(mean ± SD) | Capsaicin (n = 6)(mean ± SD) | p | Control (n = 5) (mean ± SD) | Tourniquet (n = 5) (mean ± SD) | p |
|---|---|---|---|---|---|---|
| Ear movements | ||||||
| Forward | 22 ± 16% | 13 ± 11% | 0.06 | 24 ± 15% | 13 ± 15% | 0.02 |
| Attentive | 40 ± 10% | 11 ± 6% | 0.03 | 41 ± 9% | 15 ± 11 | 0.02 |
| Asymmetrical/low | 23 ± 14% | 54 ± 0.5% | 0.03 | 21 ± 13% | 51 ± 23 | 0.02 |
| Back | 16 ± 12% | 23 ± 16% | 0.2 | 14 ± 11% | 21 ± 16 | 0.3 |
| Out of frame | 2.2 ± 3.4% | 11 ± 15% | 0.06 | 2.2 ± 3.4% | 25 ± 16% | 0.02 |
| Contact seeking behaviour | 9.1 ± 8.5% | 22 ± 14.3% | 0. 06 | 8.8 ± 5.9% | 16.4 ± 14.2% | 0.06 |
| Eye blinking | 0.41 ± 0.15/s | 0.44 ± 0.11/s | 0.2 | 0.39 ± 0.14/s | 0.46 ± 0.13/s | 0.08 |
| Other behaviours | ||||||
| Chewing | 12 ± 8 | 14 ± 13 | 0.8 | 12 ± 9 | 14 ± 5 | 1 |
| Yawning | 2 ± 4 | 0.5 ± 0.8 | 0.8 | 2 ± 4 | 3 ± 6 | 1 |
| Snorting | 0 ± 0 | 0 ± 0 | – | 0 ± 0 | 0.6 ± 1.3 | 1 |
| Head shaking | 4 ± 8 | 5 ± 3 | 0.4 | 4 ± 9 | 4 ± 6 | 1 |
Bold values are significant, p < 0.05.
Eyes
During noxious stimulus, the muscles surrounding the eyes tightened, especially the m. levator anguli oculi medialis, giving the upper eyelid a very characteristic angled appearance which increased the incidence of exposing the sclera at the medial canthus of the eye in some of the horses (Fig. 7). The amount and/or frequency of the exposed sclera could not be determined from the video recordings. The stare became withdrawn and intense during pain inductions (Fig.8a) in contrast to the relaxed glance in the control trials (Fig.8b).
Figure 7.

Illustration of the eye with the indication of the size and position of the m. levator anguli oculi medialis (pink) (Ill. Andrea Klintbjer).
Figure 8.
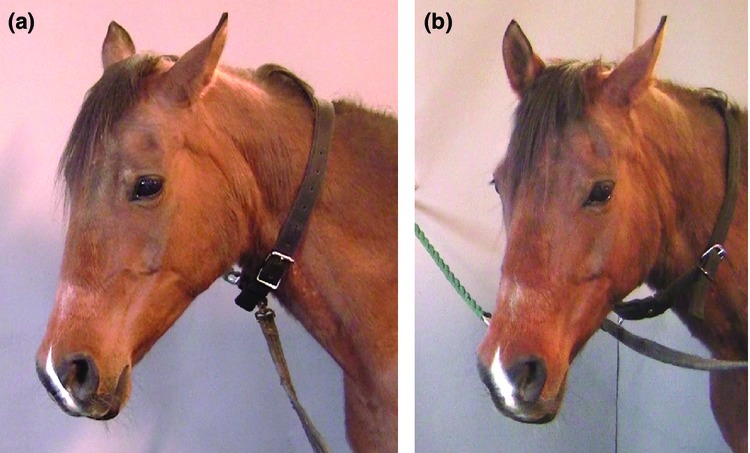
(a) Horse during pain induction displaying low ears, an angled eye with an intense stare, medio-laterally dilated nostrils and tension of the muzzle. (b) A horse during the control trial, relaxed with attentive ears and a relaxed stare.
Lower face
The nostrils dilated in a mediolateral manner during noxious stimulus; the shape of the nostrils changed from the normal elongated shape to a striking edged square shape. This was evident by the medial wing of the nostril expanding medially, either constantly or on inspiration, in combination with a widening of the nostrils in lateral direction (Fig.8a).
Increased tonus of the lips and tension of the chin during noxious events resulted in a more edged shape of the muzzle (Figs6 & 8a). In conjunction with this change in conformation to the rostral part of the face, increased tension of the m. zygomaticus and the m. caninus accentuated the appearance of these muscles on the lateral aspect of the head (Fig.6). M. masseter tightened in some of the horses as a result of clenched jaws.
Behavioural evaluation (blinded observer)
Fifty-one still images from the video recordings of the ‘no-observer’ trials were evaluated by a blinded observer for the presence of the features of the facial expressions of pain (Fig.9). The horses on the images from the control trials displayed zero to two features, although one horse in one image displayed four features (false positive). The horses on the images from the pain trials displayed three or more features; one horse displayed no features (false negative). The number of features in the control images were significantly lower than the number of features evident in the capsaicin trial (p = 0.0001) and the tourniquet trial (p < 0.0001). The feature most consistent with pain, as evaluated from the still images was the tense, withdrawn stare.
Figure 9.
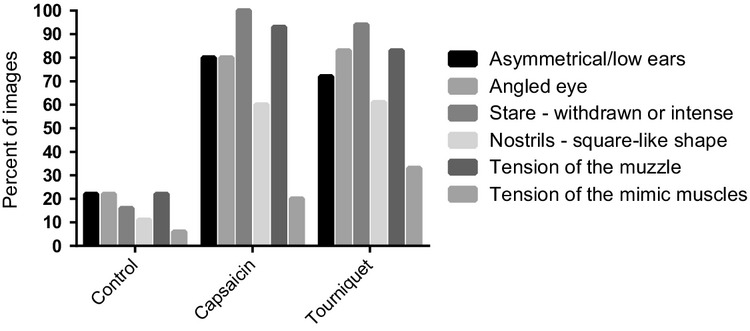
The proportion of images showing each pain face feature. The proportions are grouped into the control, capsaicin and tourniquet trials.
Other behaviours
The analysis of the non-mimic behaviours identified from the ‘no-observer’ videos (Table3) showed that the frequencies of these behaviours did not differ significantly between control trials and pain inductions (Table5).
Effect of observer
The horses did not seem to suppress the changes in facial expressions in the presence of the observer, although they were less pronounced whenever the horses tried to interact with the observer. The proportion of time spent on contact seeking behaviour increased during noxious stimulation compared to control trials, although this increase was not significant (Table5).
Discussion
All horses in this study displayed specific changes in their facial mimic during all pain inductions. Two different pain induction methods were chosen in order to cover a wider aspect of pain sensations and thereby get a more solid description of facial expressions of pain. As expected, the two noxious stimuli applied were effective in producing a pain response, evident by a decreased ‘time to doze off’ and an increased composite measure pain score. The intensity of the facial expressions differed within trials and between horses, possibly due to the different pain sensations of the two noxious stimuli.
The increased distance between the ears and the outward rotation of the ears observed in the present study during pain induction, has also been described in rats and mice (Langford et al. 2010; Sotocinal et al. 2011) and asymmetrical ear positioning has been associated with negative emotions in sheep (Boissy et al. 2011). Horses observed post-operatively have been shown to spend more time with the ears facing the sides (outward rotation) compared to pre-operatively (Price et al. 2003). Dalla Costa et al. (2014) found ears back as a pain indicator in horses while ears back did not differ between trials in this study.
A ‘brow lowering’ or ‘frown’ in humans is often interpreted as a ‘worried expression’ which is also described in painful horses (Fraser 1969). What humans interpret as a worried expression in the horse appears to be a result of the characteristic angled appearance of the eye due to the tension of the m. levator anguli oculi medialis. This facial action has not previously been described in details but the painful horse has been described to have ‘slightly puckered eyes’ (Fraser 1969) or to have a ‘tension’ above the eye (Dalla Costa et al. 2014). Dalla Costa et al. (2014) also found orbital tightening in painful horses which is inconsistent with the widening of the eyes and the increased exposure of the sclera found in this study. This orbital tightening and the backwards ears of castrated horses however, resembles the appearance of the horses dozing off in the present study, suggesting that the Horse Grimace Scale describes a combination of pain and fatigue as a consequence of the surgical stress response. Fatigue may be an important factor when evaluating horses post-surgically and it may explain the differences in facial expressions between the castrated horses and the horses in this study.
The change in the quality of the glance characterized by a withdrawn appearance of the eye with a tense stare has previously been described for equine pain behaviour (Fraser 1969; Sanford et al. 1986; Taylor et al. 2002; Sutton et al. 2013).
Pain in horses has previously been reported to result in flared, dilated or strained nostrils (Fraser 1969; Sanford et al. 1986; Dalla Costa et al. 2014). The medio-lateral dilation of the nostrils observed in this study has previously been reported as a result of kinematic analysis of facial movements during injections in horses (Love et al. 2011). Looking at the horses during pain inductions, on first impression, the nostrils were perceived just as dilated or strained. A thorough inspection revealed that the movements of the nostrils during inspiration originated from the middle of the medial and lateral wings. This resulted in a dilation of the nostrils in a square-like form which can be differentiated from the more rounded dilation seen during respiration in pain free horses. The movement of the medial wing of the nostril was characteristic and could be recognized during acute pain, even when the respiration rate was normal. The tightened lips have previously been linked to fear and pain response in horses (Casey 2007; Dalla Costa et al. 2014). The increased tension of the m. zygomaticus and the m. caninus seen in this study may be the same tension that is reported as ‘strained chewing muscles’ for castrated horses (Dalla Costa et al. 2014).
The horses in this study displayed increased interaction with the observer during pain stimulation. This is in contrast to previous research, reporting a reluctance to interact with humans when in pain (Price et al. 2003; Pritchett et al. 2003; Bussieres et al. 2008; Lindegaard et al. 2010; Graubner et al. 2011; Sutton et al. 2013). This discrepancy in behavioural response may be explained by the intensity of the noxious stimuli and the habituation prior to the study. The observer was a familiar person associated with positive reinforcement training, which has been shown to increase contact seeking behaviour (Sankey et al. 2010). Though the reluctance to be handled when in pain is obviously a very important observation, the contact-seeking behaviour may be interesting in relation to the early detection of pain by horse owners. Horses with acute or low grade pain may increase their contact-seeking behaviour when they percieve themselves to be in a safe environment.
The noxious stimuli used for the present study were chosen because they reliably induce pain in humans. Ischemic pain induction with a tourniquet is used as a model for deep tissue pain in man (Smith et al. 1966; Tuveson et al. 2006). The routine use of a tourniquet in horses is presumably painful as it causes a pronounced rise in blood pressure (Abrahamsen et al. 1989; Copland et al. 1989). A tourniquet pressure of 240 mmHg was chosen in this study because the blood pressure of the standing horse is similar to the blood pressure of humans, and for the submaximal effort tourniquet test in humans, a pressure of 200–300 mmHg has been applied (Smith et al. 1966; Pertovaara et al. 1982, 1984). The tourniquet is suggested to induce both pressure pain and ischemic pain due to accumulation of metabolites caused by the compromised circulation distal to the cuff (Pertovaara et al. 1984). As expected the tourniquet induced moderate pain in all horses although two horses with a very low start temperature of the induced leg (18.3 and 16.4 °C) were less affected. Some indications of reperfusion pain such as very low head positioning were noticed.
Capsaicin is the pungent constituent of chilli peppers. Applied to the skin, it causes neurogenic inflammation and a local, burning pain sensation (Farina et al. 2001) by activation of the nociceptive C-fibres (Baumann et al. 1991). Topically applied capsaicin is used as a pain model in humans (Baron et al. 1999) to mimic clinical pain (Le Bars et al. 2001) and a topical application of low concentration capsaicin cream has been reported to cause a pain reaction in horses (Seino et al. 2003). A 10% capsaicin cream evokes pain levels up to 10 on a VAS scale in humans (Robbins et al. 1998). Capsaicin 8% patches used for analgesia in humans with neuropathic pain evoke an increasing, burning pain sensation throughout the 60 minutes that the treatment lasts (Knolle et al. 2013). The apparently shorter lasting pain expression in the horses in this study may be explained by the use of a capsaicin cream and not the specially formulated matrix for continuous release. In the present study, the capsaicin effectively induced moderate acute pain and the horses recovered fully within hours. No hypersensitivity to light touch and pin pricks was noted. Both types of noxious stimuli applied were easy to administer and could be used for repeated inductions, which was important as the horses acted as their own controls in this study.
The findings of this study indicate that facial expressions of pain in horses can be considered a suitable tool for recognizing and evaluating pain in horses. If these facial expressions of acute pain in horses are as comprehensible as those reported for mice (Leach et al. 2012), they may be a potential addendum for a behaviour based pain evaluation scale to improve recognition of pain and horse welfare. It is uncertain whether the facial expressions of pain demonstrated in this study are sensitive for grading pain in a more quantitative manner, and further investigation into sensitivity and intra- and inter-observer reliability is warranted.
In conclusion, the present study demonstrates that horses exhibit facial expressions of pain during periods of induced acute pain. The equine pain face involves: ‘low’ and/or ‘asymmetrical’ ears, an angled appearance of the eyes, a withdrawn and/or tense stare, mediolaterally dilated nostrils and tension of the lips, chin and certain mimetic muscles and can potentially be incorporated to improve existing pain evaluation tools.
Acknowledgments
This study was funded by grants from University of Copenhagen (PhD scholarship) and Foreningen KUSTOS.
References
- Abrahamsen E, Hellyer PW, Bednarski RM. Tourniquet-induced hypertension in a horse. J Am Vet Med Assoc. 1989;194:386–388. [PubMed] [Google Scholar]
- Ashley FH, Waterman-Pearson AE, Whay HR. Behavioural assessment of pain in horses and donkeys: application to clinical practice and future studies. Equine Vet J. 2005;37:565–575. doi: 10.2746/042516405775314826. [DOI] [PubMed] [Google Scholar]
- Baron R, Wasner G, Borgstedt R. Effect of sympathetic activity on capsaicin-evoked pain, hyperalgesia, and vasodilatation. Neurology. 1999;52:923–932. doi: 10.1212/wnl.52.5.923. [DOI] [PubMed] [Google Scholar]
- Baumann TK, Simone DA, Shain CN. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- Blount W. Studies of the movements of the eyelids of animals: blinking. Exp Physiol. 1927;18:111–125. [Google Scholar]
- Boissy A, Aubert A, Desire L. Cognitive sciences to relate ear postures to emotions in sheep. Anim Welf. 2011;20:47–56. [Google Scholar]
- Bussieres G, Jacques C, Lainay O. Development of a composite orthopaedic pain scale in horses. Res Vet Sci. 2008;85:294–306. doi: 10.1016/j.rvsc.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Casey R. Clinical problems associated with the intensive management of performance horses. In: Waran N, editor. The Welfare of Horses. Secaucus, NJ, USA: Kluwer Academic Publishers; 2007. pp. 19–44. [Google Scholar]
- Copland VS, Hildebrand SV, Hill T. Blood-pressure response to tourniquet use in anesthetized horses. J Am Vet Med Assoc. 1989;195:1097–1103. [PubMed] [Google Scholar]
- Dalla Costa E, Minero M, Lebelt D. Development of the Horse Grimace Scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS ONE. 2014;9:e92281. doi: 10.1371/journal.pone.0092281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The Expression of the Emotions in Man and Animals. London, UK: John Murray; 1872. [Google Scholar]
- Deyo KS, Prkachin KM, Mercer SR. Development of sensitivity to facial expression of pain. Pain. 2004;107:16–21. doi: 10.1016/s0304-3959(03)00263-x. [DOI] [PubMed] [Google Scholar]
- Farina S, Valeriani M, Rosso T. Transient inhibition of the human motor cortex by capsaicin-induced pain. A study with transcranial magnetic stimulation. Neurosci Lett. 2001;314:97–101. doi: 10.1016/s0304-3940(01)02297-2. [DOI] [PubMed] [Google Scholar]
- Flecknell PA. Do mice have a pain face? Nat Methods. 2010;7:437–439. doi: 10.1038/nmeth0610-437. [DOI] [PubMed] [Google Scholar]
- Fraser JA. Some observations on behaviour of horses in pain. Br Vet J. 1969;125:150–151. [Google Scholar]
- Graubner C, Gerber V, Doherr M. Clinical application and reliability of a post abdominal surgery pain assessment scale (PASPAS) in horses. Vet J. 2011;188:178–183. doi: 10.1016/j.tvjl.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Kadosh KC, Johnson MH. Developing a cortex specialized for face perception. Trends Cogn Sci. 2007;11:367–369. doi: 10.1016/j.tics.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Keating SCJ, Thomas AA, Flecknell PA. Evaluation of EMLA cream for preventing pain during tattooing of rabbits: changes in physiological, behavioural and facial expression responses. PLoS ONE. 2012;7:1–11. doi: 10.1371/journal.pone.0044437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knolle E, Zadrazil M, Kovacs GG. Comparison of cooling and EMLA to reduce the burning pain during capsaicin 8% patch application: a randomized, double-blind, placebo-controlled study. Pain. 2013;154:2729–2736. doi: 10.1016/j.pain.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Bailey AL, Chanda ML. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- Leach MC, Klaus K, Miller AL. The assessment of post-vasectomy pain in mice using behaviour and the Mouse Grimace Scale. PLoS ONE. 2012;7:1–9. doi: 10.1371/journal.pone.0035656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegaard C, Thomsen MH, Larsen S. Analgesic efficacy of intra-articular morphine in experimentally induced radiocarpal synovitis in horses. Vet Anaesth Analg. 2010;37:171–185. doi: 10.1111/j.1467-2995.2009.00521.x. [DOI] [PubMed] [Google Scholar]
- Love EJ. Assessment and management of pain in horses. Equine Vet Educ. 2009;21:46–48. [Google Scholar]
- Love E, Gillespie L, Colborne G. 2011. Facial expression of pain in horses. In: Association of Veterinary Anaesthetists Spring 2011 Meeting.
- Pertovaara A, Kemppainen P, Johansson G. Ischemic pain nonsegmentally produces a predominant reduction of pain and thermal sensitivity in man: a selective role for endogenous opioids. Brain Res. 1982;251:83–92. doi: 10.1016/0006-8993(82)91276-8. [DOI] [PubMed] [Google Scholar]
- Pertovaara A, Nurmikko T, Pontinen PJ. 2 separate components of pain produced by the submaximal effort tourniquet test. Pain. 1984;20:53–58. doi: 10.1016/0304-3959(84)90810-8. [DOI] [PubMed] [Google Scholar]
- Poole GD, Craig KD. Judgments of genuine, suppressed, and faked facial expressions of pain. J Pers Soc Psychol. 1992;63:797. doi: 10.1037//0022-3514.63.5.797. [DOI] [PubMed] [Google Scholar]
- Price J, Catriona S, Welsh EM. Preliminary evaluation of a behaviour-based system for assessment of post-operative pain in horses following arthroscopic surgery. Vet Anaesth Analg. 2003;30:124–137. doi: 10.1046/j.1467-2995.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- Pritchett LC, Ulibarri C, Roberts MC. Identification of potential physiological and behavioral indicators of postoperative pain in horses after exploratory celiotomy for colic. Appl Anim Behav Sci. 2003;80:31–43. [Google Scholar]
- Prkachin KM, Mercer SR. Pain expression in patients with shoulder pathology: validity, properties and relationship to sickness impact. Pain. 1989;39:257–265. doi: 10.1016/0304-3959(89)90038-9. [DOI] [PubMed] [Google Scholar]
- Robbins WR, Staats PS, Levine J. Treatment of intractable pain with topical large-dose capsaicin: preliminary report. Anesth Analg. 1998;86:579–583. doi: 10.1097/00000539-199803000-00027. [DOI] [PubMed] [Google Scholar]
- Sanford J, Ewbank R, Molony V. Guidelines for the recognition and assessment of pain in animals. Vet Rec. 1986;118:334–338. doi: 10.1136/vr.118.12.334. [DOI] [PubMed] [Google Scholar]
- Sankey C, Richard-Yris M-A, Henry S. Reinforcement as a mediator of the perception of humans by horses (Equus caballus. Anim Cogn. 2010;13:753–764. doi: 10.1007/s10071-010-0326-9. [DOI] [PubMed] [Google Scholar]
- Seibert LM, Parthasarathy V, Trim CM. Proceedings of the American College of Veterinary Anesthesiologists 27th annual meeting, Orlando, Florida, 10–11 October 2002 – abstract. Vet Anaesth Analg. 2003;30:100–120. [Google Scholar]
- Seino KK, Foreman JH, Greene SA. Effects of topical perineural capsaicin in a reversible model of equine foot lameness. J Vet Intern Med. 2003;17:563–566. doi: 10.1111/j.1939-1676.2003.tb02479.x. [DOI] [PubMed] [Google Scholar]
- Smith GM, Egbert LD, Markowitz RA. An experimental method sensitive to morphine in man: the submaximum effort tourniquet technique. J Pharmacol Exp Ther. 1966;154:324–332. [PubMed] [Google Scholar]
- Sotocinal SG, Sorge RE, Zaloum A. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton GA, Paltiel O, Soffer M. Validation of two behaviour-based pain scales for horses with acute colic. Vet J. 2013;196:394–401. doi: 10.1016/j.tvjl.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Taylor PM, Pascoe PJ, Mama KR. Diagnosing and treating pain in the horse – Where are we today? Vet Clin North Am Equine Pract. 2002;18:1–19. doi: 10.1016/s0749-0739(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Tuveson B, Leffler AS, Hansson P. Time dependant differences in pain sensitivity during unilateral ischemic pain provocation in healthy volunteers. Eur J Pain. 2006;10:225–232. doi: 10.1016/j.ejpain.2005.03.010. [DOI] [PubMed] [Google Scholar]


