Abstract
OBJECTIVE
To determine the prevalence and sensitivity of CT Colonography (CTC) in the detection of non-polypoid adenomas using restricted criteria of height:width ratio of <50% and height elevation of ≤3mm.
MATERIAL AND METHODS
In ACRIN 6664, an institutional review board-approved, HIPAA-compliant study, a cohort of 2531 asymptomatic participants underwent CTC and screening colonoscopy. The CTC exams were interpreted by both two-dimensional and three-dimensional techniques. Non-polypoid adenomatous polyps identified by CTC or colonoscopy were retrospectively reviewed to determine which polyps met the restricted criteria. The prevalence of non-polypoid adenomas and prospective sensitivity of CTC were determined. Descriptive statistics are used to report the prevalence, size, and histology. Sensitivities for the non-polypoid (with 95% CIs) and polypoid lesions are compared with a two-sided Z test for two independent binomial proportions.
RESULTS
The retrospective review confirmed 21 non-polypoid adenomas, yielding a prevalence of 0.83% (21/2531 participants). 8 (38.1%) were advanced adenomas, many (50%, 4/8) secondary to large size (≥10mm) only. The overall per polyp sensitivity of CTC (combined 2D and 3D interpretation) for detecting non-polypoid adenomas ≥ 5mm (n=21), ≥ 6mm (n=16) and ≥10mm (n=5) were 0.76, 0.75, and 0.80, respectively, which was not statistically different from the sensitivity of detecting polypoid adenomas (p>0.37).
CONCLUSION
In this large screening population, non-polypoid adenomas had a very low prevalence (<1%), and advanced pathologic features were uncommon in polyps <10mm in diameter. The majority of non-polypoid adenomas are technically visible at CTC, with prospective sensitivities similar to polypoid adenomas using an interpretation approach combining both two-dimensional and three-dimensional review.
Recently there has been increasing interest in non-polypoid or flat adenomas of the colon. However, there is controversy regarding their prevalence, incidence of associated advanced pathologic features (size ≥1cm, villous components, or high-grade dysplasia), aggressiveness and diagnostic criteria used to define non-polypoid adenomas (1-29). Several reviews have discussed these issues (4, 18, 30, 31).
Non-polypoid adenomas were initially described in Japan with prevalence rates varying from 13 to 48% and were thought to be rare in western countries (2, 13, 20, 22, 28, 32). However, recent publications have reported their occurrence in western countries with prevalence rates from 7 to 55% (6, 8, 9, 17, 19, 26, 29, 33, 34). Some investigators have reported that these lesions may have an increased incidence of advanced histologic features or increased aggressiveness relative to polypoid adenomas, while others have shown the opposite (1, 9, 12-15, 17, 24).
Confounding many analyses, has been the discrepancy in the criteria used for defining a non-polypoid adenoma. Previous publications have used gross morphologic description consisting of a ratio of the height to width of no more than 50%. More recent investigations have utilized restricted criteria to include only lesions which protrude 3 mm or less above the mucosal surface (15, 35, 36).
CT Colonography (CTC) has been shown to be a feasible technique for colorectal cancer screening (37, 38). However, there are limited data on the sensitivity of CTC for detecting non-polypoid adenomas (5, 15, 16, 39-41). The purpose of this study is to provide further data on some of the controversial issues discussed previously, including the prevalence of these lesions in a large asymptomatic screening population, incidence of associated advanced pathologic features, per polyp sensitivity of CTC in the detection of non-polypoid adenomas using restricted criteria of height:width ratio of <50% and height elevation of ≤3mm.and potential cause for false negative CTC interpretations.
Materials and Methods
Demographics
This study includes a cohort of patients with non-polypoid adenomas in the National CT Colonography Trial of the American College of Radiology Imaging Network (37). The results of that study have been previously reported and will be summarized below.
A total of 15 sites participated in the study, all had complied with the provisions of the Health Insurance Portability Accountability Act, and approval was obtained from the institutional review board. 2600 asymptomatic participants 50 years of age or older with scheduled routine screening colonoscopy were recruited between February 2005 and December 2006.
Radiologist Experience and Training
Each radiologist was required to have experience interpreting at least 500 CTC examinations or participate in a training session which included 12 non-polypoid polyps and cancers. Training included the 3D and 2D evaluation of non-polypoid lesions (i.e., 2D/3D morphology, enlarged axial images for 2D review, lung and soft tissue/intermediate windows for evaluation of internal attenuation). In addition, all radiologists were required to pass a qualifying exam which included 4 non-polypoid polyps (42).
CT Colonography Technique
The preparation for CTC included stool tagging, laxative purgation, and fluid tagging. All examinations were performed with multidector-row CT scanners that had a minimum of 16 rows. Images were reconstructed to a slice thickness of 1.0 to 1.25 mm, with a reconstruction interval of 0.8 mm.
CT Colonography Interpretation
Each CTC was randomly assigned to one of the 15 site radiologists to be read blindly with the use of either a primary two-dimensional (2D) technique with three-dimensional endoluminal problem solving or a three-dimensional (3D) endoluminal technique with two-dimensional problem solving. Subsequently the exam was reviewed by a second blinded reviewer at one of the other sites using the opposite algorithm. There were no requirements specifying which window settings were to be used by the individual site radiologist. The location, size and morphology were recorded for each suspected abnormality ≥5mm in diameter. Morphology was recorded by the site radiologist as non-polypoid (flat; height:width ratio of <50%) or polypoid (sessile, pedunculated). The site radiologist did not use the 3mm height criterion, which was assessed by a central reader at the time of the central review (described below).
Colonoscopy
Colonoscopy was performed and reported according to standard clinical protocol at each site and was performed or supervised by an experienced staff gastroenterologist or surgeon, who was blinded to the CTC results. Polyps were measured using a biopsy forceps for size reference. Colonoscopy was considered the gold standard for the presence of a polyp and was performed without segmental unblinding. Dye-spraying technique was not required or routinely performed but could be used at the discretion of the endoscopist. All identifiable lesions were photographed. The site endoscopist reported the colonoscopy findings according to their institutional protocol. The site endoscopist was not given prospective criteria for terminology of polyp morphology. These clinical reports were reviewed by one central radiologist for morphologic descriptors that indicated non-polypoid or flat morphology (e.g., flat, carpet, minimally elevated, plaque-like, etc…).
Histology Review
Tissue samples from all lesions ≥5 mm in diameter were reviewed by an experienced gastrointestinal pathologist and included classification (eg, adenoma, hyperplastic, other), subtype (tubular, tubulovillous, villous) and histologic grade (low or high). Serrated histology was not classified during the central read as this terminology was not widely implemented at the time of the study. Size was determined from the pathology report unless the specimen was resected piecemeal, fulgurated or not removed, in which case colonoscopy estimate of size was used.
Cohort Selection
Among the 2531 ACRIN 6664 participants with complete data, 62 had lesions with size 5mm or larger classified as non-polypoid on CTC or described as non-polypoid on colonoscopy report that were visualized at colonoscopy. The 62 lesions were retrospectively reviewed to: confirm morphology, histology, determine prospective sensitivity of CTC for non-polypoid adenomas ≥5mm, determine potential reasons for failed detection at CTC, assess morphologic appearance, and evaluate the conspicuity on different CTC window settings.
Retrospective Central CTC Analysis
All CTC examinations with an adenomatous polyp ≥5mm in size coded as non-polypoid at colonoscopy or CTC and with a polyp confirmed at colonoscopy were retrospectively reviewed for polyp analysis by one radiologist with an experience of reading over 500 CTC exams. If the polyp was only seen by colonoscopy, the CTC exam was reviewed to determine if the polyp could be seen in retrospect. Polyps definitely seen at CTC (using standard matching rules)(37) were further analyzed using magnified multiplanar reformatted two-dimensional images, which showed the largest size of the lesion. The maximum width and height of the polyp was recorded on both the supine and prone acquisitions. The non-submerged index polyp surrounded by air was reviewed on three different window settings including lung (1500/-600), soft tissue (400/0) and colon (1000/0) and the conspicuity of the polyp on each window was noted (1=not visualized; 2=barely visualized; 3=adequately visualized; 4=excellent visualization). Because submerged polyps are difficult to visualize on lung windows, and given a wider window setting is required for visualization of submerged polyps, conspicuity was not compared on positions were the polyp was submerged. CTC datasets were also reviewed to determine potential causes for failed detection of polyps reported at colonoscopy (quality, perception, occult, characterization).
Central Determination of Non-polypoid Adenoma Criteria
Figure 1 shows the algorithm that was used as the non-polypoid adenoma criteria. In summary, central readers (one gastroenterologist and one radiologist) examined all adenomas identified by site gastroenterologists or radiologists as being of non-polypoid morphology. These central readers then strictly applied restricted criteria to select polyps that had both a height ≤3mm and height:width ratio of <50%. The central gastroenterologist utilized reports and endoscopic photos. If the images obtained at colonoscopy were not adequate to allow the central gastroenterologist to determine if the polyp had a non-polypoid morphology it was coded as indeterminate and the description on the site colonoscopy report was used. The central radiologist separately measured all lesions on CTC identified by the site radiologists and central gastroenterologist (if the polyp was visualized on CTC).
Fig. 1.
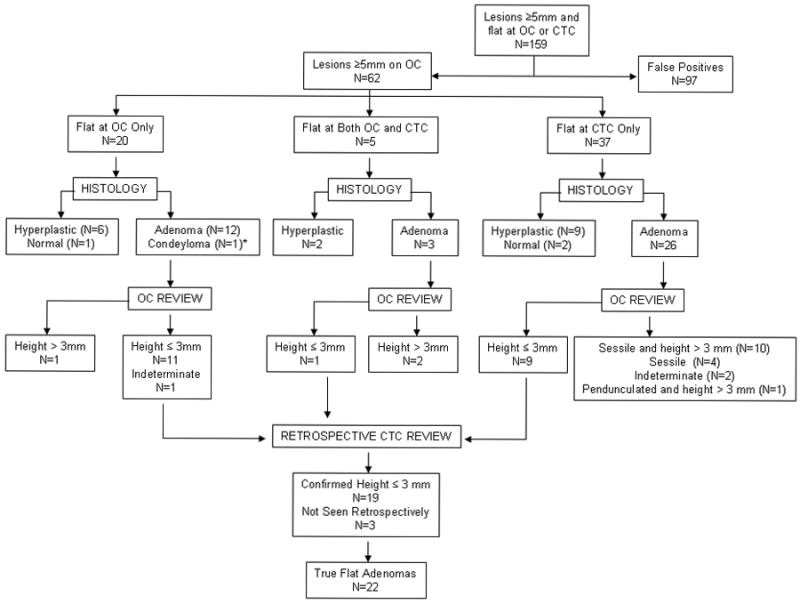
Algorithm for Polyp Classification
Comparison of Non-polypoid vs. Polypoid Sensitivities
To compare the sensitivity for detecting non-polypoid vs. polypoid adenomas and nonadenomas, the results for the combined primary 2D and primary 3D interpretations from two separate radiologists for the original ACRIN trial were calculated as these were not included in the original study (37). If the polyp was detected on either the primary or secondary interpretation it was considered to be detected. It should be noted that in the initial ACRIN trial only one interpretation (initial randomization) was reported, so this report uniquely describes the combined 2D and 3D sensitivities for the entire cohort of adenomas and for the non-polypoid adenomas.
Statistical Method
Descriptive statistics are used to report the prevalence, size, and histology of non-polypoid adenomas. The primary unit of analysis was the adenomatous polyp. 95% CIs for the estimates of sensitivity were presented as the exact confidence intervals (Clopper-Pearson). Sensitivities for the non-polypoid and polypoid lesions are compared with a two-sided Z test statistic for two independent binomial proportions. The significance level is set at 0.05. Statistical analyses were implemented with SAS 9.1.
Results
All adenomas ≥ 5 mm
The National CT Colonography Trial reported 374 adenomas or adenocarcinomas ≥5mm in diameter in 2531 adults. In this analysis, due to the review of the colonoscopy and pathology reports, one polyp that was not included in the primary article was measured as 5mm at colonoscopy and included as a non-polypoid polyp. Thus the total number of adenomas ≥ 5 mm reported here are 375 rather than the previously reported 374. Of the 375 adenomas ≥ 5 mm, 62 polyps visualized at colonoscopy were described by the site gastroenterologist or radiologist to be non-polypoid (5 described by both the site endoscopist and radiologist, 20 by the site endoscopist alone and 37 by the site radiologist alone). These 62 polyps were then analyzed by the central readers to determine if they met restricted criteria for non-polypoid adenomas (Figure 1).
Non-polypoid adenomas ≥ 5 mm
Of the 62/375 adenomas that were reviewed retrospectively, 21 lesions from 21 participants met our restricted criteria for a non-polypoid adenoma (Figure 1). The overall prevalence was 0.83% (21/2531 participants) at the participant level (13 male, 8 female, mean age = 60.4, range 50 - 71 years).
The mean diameter of the non-polypoid adenomas was 9.1mm (range 5-25mm) and the majority were located in the cecum (n=8) and transverse colon (n=6) with a few polyps in the ascending (n=3), descending (n=3) and sigmoid (n=1) colon. Examples of the appearances of these lesions are shown in Figures 2-5.
Fig. 2. Cecal Tubular Adenoma.
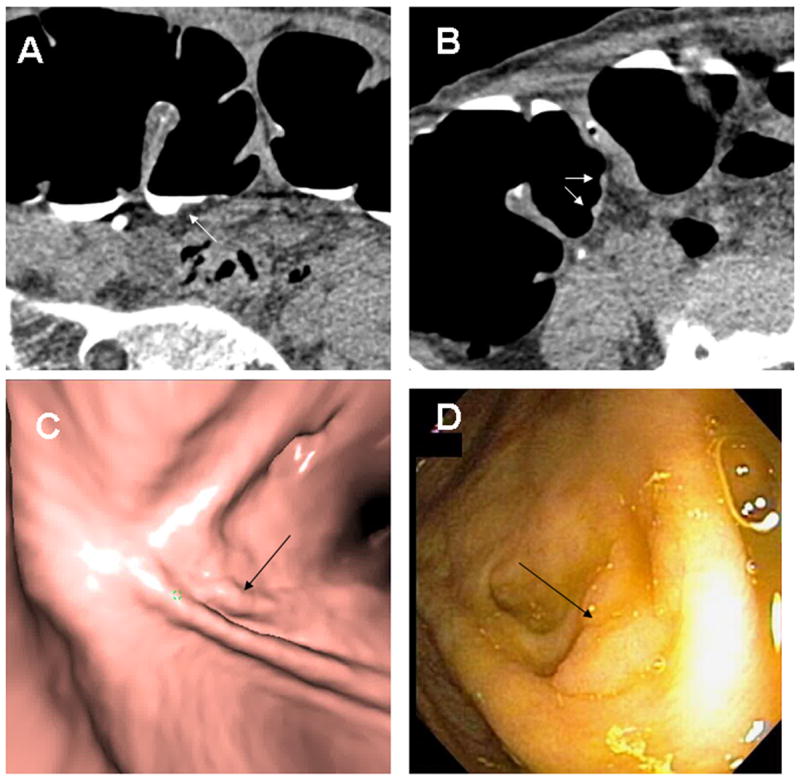
A 10 × 2 mm tubular adenoma with low-grade dysplasia is seen in retrospect at the base of a fold in the cecum (arrows) on supine 2D (A), prone 2D (B), 3D endoluminal (C) and colonoscopy (D). Note the polyp is submerged in tagged fluid on the supine images and would be obscured on supine endoluminal views without fluid subtraction. The linear configuration could be misinterpreted as a fold however this is immediately adjacent to a normal-appearing fold and only extends a short distance.
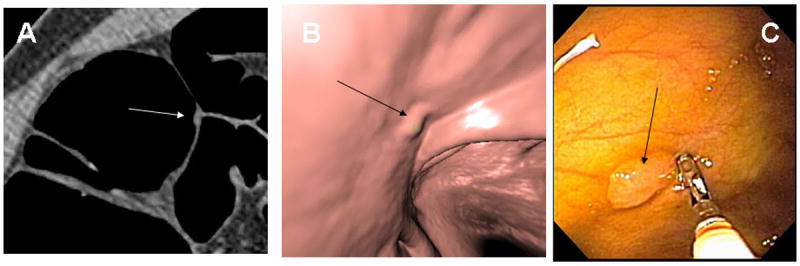
Fig. 5. Cecal Tubulovillous Adenoma.
8 × 2mm low-grade tubulovillous adenoma in the cecum (arrows) seen in retrospect on 2D lung window (A), soft tissue window (B), colon window (C) and 3D endoluminal (D) images. In this example the polyp is seen well on all three window settings.
Of these non-polypoid adenomas, 8/21 (38.1%) were advanced adenomas based on size of ≥1cm (n=5), high-grade dysplasia (n=1) or villous component (n=4). One large polyp possessed both high grade dysplasia and villous component, so only 3/16 polyps < 1cm in diameter had advanced pathologic features secondary to tubulovillous components, none had high-grade dysplasia and none were purely villous.
Using the methods described above, combining 2D and 3D CTC interpretations, the overall per polyp sensitivity of CTC for detecting non-polypoid adenomas ≥ 5mm, ≥ 6mm and ≥10mm was 0.76 (95% CI 0.54 to 0.90), 0.75 (95% CI 0.49 to 0.90) and 0.80 (95% CI 0.31 to 0.97), respectively. These results are similar to the sensitivities for all of the polypoid adenomas in the National CT Colonography Trial using combined 2D and 3D interpretation, which were 0.68 (95% CI 0.62 to 0.72, n=354), 0.75 (95% CI 0.69 to 0.80, n=253) and 0.85 (95% CI 0.0.78 to 0.90, n=123) for adenomas ≥ 5mm, ≥ 6mm and ≥10mm respectively (Table 2). There were no statistical significant differences between the sensitivities of non-polypoid and polypoid adenomas (p >0.37). The false negative rate for all non-polypoid polyps ≥ 5mm was 24% (5/21) with 95% CI 8-47%. Five of the six non-polypoid adenomas not seen prospectively could be seen in retrospect. Therefore, 95.2% (20/21) of non-polypoid adenomas in this group of patients technically could be seen. The most likely reason for failed detection in the five cases seen in retrospect was perceptual errors.
Table 2.
Sensitivity of Non-polypoid and Polypoid Polyps by Size (Combined 2D and 3D Interpretation)
| Non-Polypoid (n=21) | Polypoid (n=354) | Polypoid vs Non-polypoid p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Sensitivity | 95% CI Lower Bound | 95% CI Upper Bound | N | Sensitivity | 95% CI Lower Bound | 95% CI Upper Bound | |||
| Size | 21 | 0.7619 | 0.5397 | 0.8973 | 354 | 0.6751 | 0.6223 | 0.7238 | 0.37 | |
| Adenomas | ≥5 mm | |||||||||
| ≥6 mm | 16 | 0.7500 | 0.4918 | 0.9029 | 253 | 0.7470 | 0.6899 | 0.7967 | 0.98 | |
| ≥10 mm | 5 | 0.8000 | 0.3090 | 0.9728 | 123 | 0.8537 | 0.7816 | 0.9048 | 0.77 | |
| Non-Adenomas | ≥5 mm | 18 | 0.5556 | 0.3422 | 0.7502 | 155 | 0.4645 | 0.3643 | 0.5677 | 0.43 |
| ≥6 mm | 13 | 0.6154 | 0.3641 | 0.8172 | 96 | 0.5208 | 0.3927 | 0.6463 | 0.49 | |
| ≥10 mm | 2 | 0.0000 | 0.0000 | 0.8419 | 25 | 0.6400 | 0.4252 | 0.8203 | 0.31 | |
| All Lesions | ≥5 mm | 39 | 0.6667 | 0.5112 | 0.7928 | 509 | 0.6110 | 0.5607 | 0.6590 | 0.46 |
| ≥6 mm | 29 | 0.6897 | 0.5099 | 0.8260 | 349 | 0.6848 | 0.6252 | 0.7389 | 0.96 | |
| ≥10 mm | 7 | 0.5714 | 0.2298 | 0.8563 | 148 | 0.8176 | 0.7437 | 0.8738 | 0.24 | |
The individual sensitivity (a potential indicator of conspicuity) of detecting non-polypoid adenomas ≥ 5mm for primary 2D interpretation was 0.52 (11/21, 95% CI 0.30 to 0.74) and for primary 3D interpretation 0.33 (7/21, 95% CI 0.15 to 0.57), which was not significantly different (p=0.21). For non-submerged polyps, the polyp conspicuity rank for lung, soft tissue and colon window had mean values of 2.8, 2.7, and 2.9, respectively and were not significantly different from each other (p=0.074).
Non polypoid, non-adenomas ≥ 5 mm
There were18 non-adenomatous, non-polypoid polyps (17 hyperplastic polyps, 1 condyloma). The CTC sensitivity for detecting non-polypoid, non-adenomatous polyps ≥5mm, ≥6mm, ≥1cm in diameter using combined 2D and 3D interpretation was 0.56 (n=18, 95% CI 0.34, 0.75), 0.62 (n=13, 95% CI 0.36 to 0.82) and 0.00 (n=2, 95% CI 0 to 0.84) which was not significantly different from that for detecting polypoid, non-adenomatous polyps (p>0.31) (Table 2).
Carpet Lesions
Of our 21 non-polypoid adenomas, 5 could be considered carpet lesions with a diameter of ≥1cm. No non-polypoid adenomas meeting our restricted criteria of a 3mm height threshold measured >3cm in diameter.
Non polypoid lesions ≥ 5 mm seen at CTC but not colonoscopy
Ninety-seven polyps (mean size=10.8 mm, range 5 - 57mm) were coded as non-polypoid at CTC but were not detected at colonoscopy. None of these patients underwent repeat colonoscopy so these may represent either CTC false positives or colonoscopy false negatives.
DISCUSSION
Non-polypoid adenomas have been classified according to macroscopic criteria from the Japanese Research Society and Paris Classification (18, 21, 40). One potential reason mentioned for the wide variation in prevalence includes the variable criteria used for classification of non-polypoid adenomas. Initial reports of non-polypoid adenomas used criteria of a height to width ratio of ≤50%. However more recently, experts have recommended more strict criteria of a height of ≤3mm (15, 35, 36). In a large prospective study of 5107 adults, the maximum height averaged 2.2mm and was ≤3mm in 86% for all non-polypoid lesions between 6 and 30mm, (15).
Prevalence rates of non-polypoid adenomas using colonoscopy for detection in variably defined subject populations have ranged from 13 to 48% in Japan and 7-55% in Western countries. (2, 4, 6, 8, 9, 13, 17, 19, 20, 22, 26, 28, 29, 32-34). There is substantial controversy regarding the clinical significance of non-polypoid adenomas with varied reports of definition, prevalence, biologic behavior and appropriate screening techniques (4, 18, 30, 31).
The prevalence of non-polypoid or flat adenomas (superficial elevated) using restricted criteria was very low (0.83%) in our study. In other screening studies, the prevalence of non-polypoid adenomas was higher at 4.2% (n=1233 patients) (43) and up to 13.1% using a 3mm height criteria (15). There are several potential reasons for the lower prevalence seen in our study. First, we used more restrictive criteria (≤3mm height on both colonoscopy and CTC) than has been used in other studies. Secondly, we did not require the uniform use of dye spraying that has been suggested to enhance detection at colonoscopy. This has been mentioned as a potential cause for underestimation of prevalence in other studies and may have had an impact on our study results. Potentially some of the false positive CTC interpretations represented polyps that were missed at colonoscopy. Thirdly, we only included polyps that could be confirmed to have non-polypoid morphology on colonoscopy, as historically the morphologic classification has been based on the endoscopic appearance of these polyps. Therefore those polyps that appeared non-polypoid on CTC only were not included. Pickhardt et al. found in a study of 59 flat adenomas that 17 were flat on both techniques, 17 on colonoscopy only and 25 by CTC only (16). Finally, we only used the subjective description included in the colonoscopy report to identify non-polypoid polyps. It is possible that additional non-polypoid polyps were not described as such and could have been inadvertently excluded.
A higher percentage (38.1%) of our non-polypoid adenomas were advanced adenomas compared to prior studies with ranges of 7.3% -8.5% (43, 44). Similar to their studies, however, many of the advanced adenomas were secondary to their large size (≥1cm), not advanced histology. None of the advanced adenomas in our study contained carcinoma. In addition, only 3/16 non-polypoid adenomas ≤1cm had villous components and none had high grade dysplasia. In a large screening study of 3,536 persons, of the 9 non-polypoid advanced adenomas, none had high grade dysplasia (44). Other large CTC screen studies have also shown that non-polypoid lesions are less likely to have advanced histology or malignancy than polypoid lesions (15).
Despite the perception that these polyps may be more difficult to detect than routine polypoid lesions, there was no significant difference in detection based on morphology in our study. Prior studies have also shown excellent detection of flat polyps. For example, in a CTC screening study of 5107 individuals, Pickhardt et al. detected 954 polyps ≥6mm in size, 13.1% of which were non-polypoid using a 3mm height criteria. Since perceptual errors were postulated to be the most common reason for failure to detect the missed non-polypoid polyps, adequate training, excellent exam quality and meticulous interpretation technique should enhance detection. While we did not validate a specified window setting for the detection of non-polypoid polyps, window settings should be interactively changed during 2D interrogation to permit discrimination between fluid and stool tagging, air and soft tissue attenuation polyps.
To our knowledge, we report the first comparison of sensitivity for primary 2D to primary 3D interpretation for specifically detecting non-polypoid lesions in a blinded prospective study. Our study showed that the sensitivity for detection using primary 2D tended to be higher than for primary 3D interpretation, however the difference was not statistically significant. While most radiologists combine a primary 2D and 3D interpretation approach into their reading algorithm, the blinded prospective interpretation used in this study provides new data regarding the conspicuity of non-polypoid adenomas. These data emphasize that a combination of primary 2D and 3D interpretation techniques likely optimizes sensitivity as some of these lesions may be more conspicuous on one technique. Using a primary 3D interpretation approach, Pickhardt et al. showed no significant difference in the detection between non-polypoid and polypoid polyps (82.8% vs. 86.2% respectively) (16). Park et al reported a lower sensitivity of 66.7% (12/18) for detecting non-polypoid adenomatous lesions ≥6mm using a primary 3D approach, however, they evaluated smaller lesions with a height criterion of ≤2mm (40).
Carpet lesions or lateral spreading tumors represent a unique subgroup of non-polypoid polyps more commonly seen in the rectum and cecum. These are non-polypoid neoplasms that extend circumferentially rather than vertically. Different size criteria have been proposed including polyps ≥ 1cm (18, 45) or ≥ 3cm (15). Five of the non-polpoid adenomas meeting our restricted criteria could be considered carpet lesions with a diameter of ≥1cm. However, none of the non-polypoid adenomas meeting our restricted criteria of a 3mm height threshold measured ≥3cm in diameter. Pickhardt et al. also showed all of their carpet lesions ≥3cm exceeded 3mm in height (15).
Several limitations to our study should be acknowledged. Advanced endoscopic techniques such as dye spraying were not widely used in our study. Some investigators have recommended this technique to improve detection of flat lesions and the lack of utilization has been proposed as a reason for lower prevalence in western countries. Another limitation is that histologic criteria for non-polypoid adenomas (thickness less than twice the adjacent normal mucosa) that have been previously published were not specifically utilized in the central review of the polyps in the ACRIN 6664 study (7, 12-14, 21). Had the criteria been applied to all polyps prospectively, additional polyps may have been identified. We did not record the presence of serrated histology so we are unable to determine the prevalence of this finding in hyperplastic polyps. Retrospective analysis of endoscopic photos for height and morphologic assessment was subjective and limited by the lack of a forceps for internal calibration in most cases. Measurement of the polyp size on CTC was performed manually and an automated process may have provided more accurate quantitative measurement. Finally, a large number of non-polypoid polyps detected at CTC, were not seen at colonoscopy. Patients did not undergo repeat colonoscopy or have segmental unblinding as performed in other studies to further assess the false positive exams. Therefore, we are unable to determine the true specificity of CTC, as some of these polyps potentially may have been missed at colonoscopy.
In conclusion, in this large asymptomatic colorectal cancer screening group, non-polypoid adenomas had a very low prevalence (<1%). 38% of non-polypoid adenomas were advanced adenomas, many based on size ≥10mm. Advanced pathologic features such as villous components were uncommon in non-polypoid adenomas <1cm and high grade dysplasia absent. The majority of non-polypoid adenomas are technically visible at CTC with prospective sensitivities similar to polypoid polyps using a combined two-dimensional and three-dimensional interpretation approach.
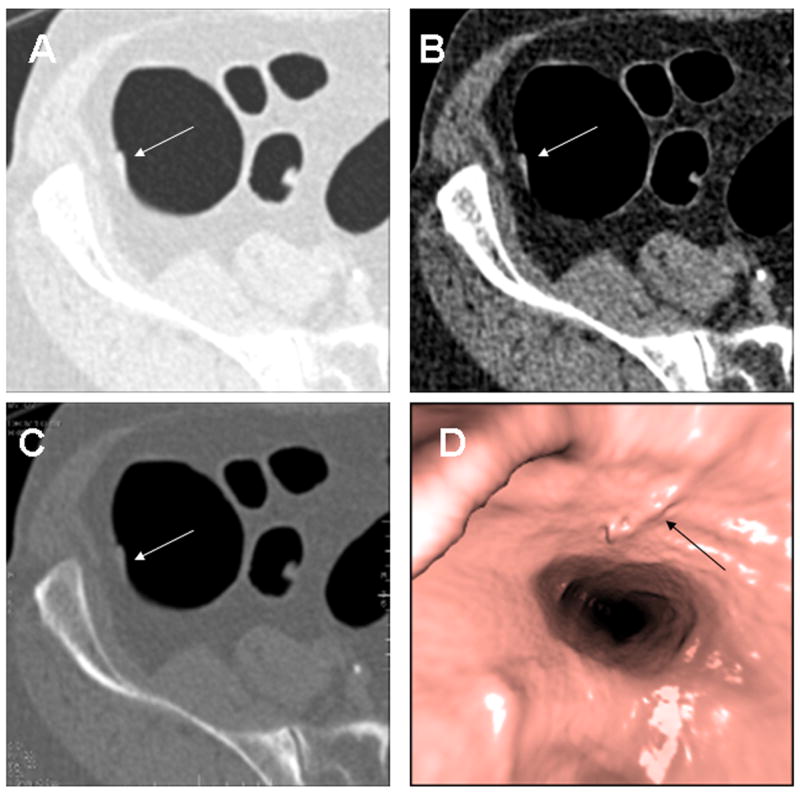
Fig. 3. Cecal Tubulovillous Adenoma.
9 × 3mm tubulovillous adenoma with low-grade dysplasia in the cecum (arrows) on 2D (A), 3D endoluminal (B) and colonoscopy (C). The polyp is near the base of a fold and was only seen on the prospective 2D interpretation technique.
Fig. 4. Ascending Colon Tubular Adenoma.
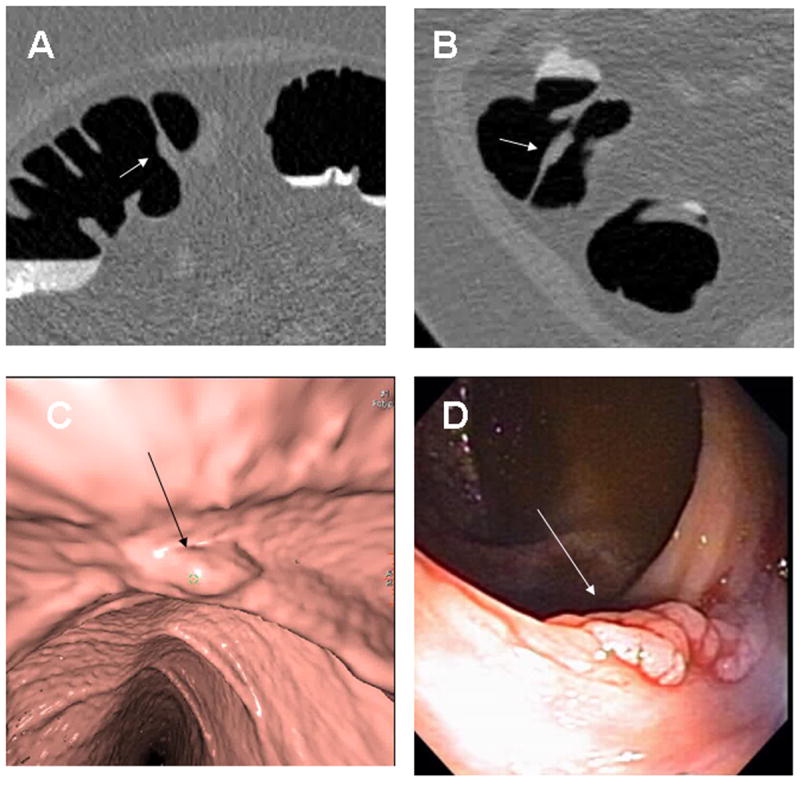
8 × 3mm tubular adenoma in the ascending colon along a fold (arrows) seen in retrospect on 2D supine (A), 2D prone (B), 3D endoluminal (C) and colonoscopy (D). On the 3D endoluminal views, the less than optimal distension causes some irregularity of the mucosa that potentially could have obscured the detection of the polyp. On the 2D views, the focal enlargement of the fold should raise suspicion for an associated polyp.
Table1.
Summary of Non-polypoid Adenomas
| Polyp # |
Size (mm) |
Histology | Grade | Location | Prospectively seen on 2D |
Prospectively seen on 3D |
Prospectively seen on combined 2D & 3D |
Retrospectively Seen |
Height Supine (mm) |
H:W Ratio Supine |
Height Prone (mm) |
H:W Ratio Prone |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | TVA | Low | Cecum | No | No | No | Yes | . | . | 2 | 0.33 |
| 2 | 8 | TVA | Low | Cecum | No | No | No | Yes | 3 | 0.30 | 3 | 0.27 |
| 3 | 5 | TA | Low | Cecum | Yes | Yes | Yes | Yes | 2 | 0.33 | 3 | 0.50 |
| 4 | 15 | TA | Low | Transverse | No | Yes | Yes | Yes | 3 | 0.50 | 3 | 0.60 |
| 5 | 10 | TA | Low | Cecum | No | No | No | Yes | 2 | 0.14 | 2 | 0.14 |
| 6 | 18 | TA | Low | Transverse | Yes | No | Yes | Yes | 3 | 0.38 | . | . |
| 7 | 5 | TA | Low | Transverse | No | No | No | No | . | . | ||
| 8 | 9 | TVA | Low | Cecum | Yes | No | Yes | Yes | 2 | 0.40 | 3 | 0.50 |
| 9 | 7 | TA | Low | Descending | Yes | No | Yes | Yes | 2 | 0.29 | 2 | 0.40 |
| 10 | 7 | TA | Low | Ascending | Yes | No | Yes | Yes | 2 | 0.33 | 2 | 0.29 |
| 11 | 5 | TA | Low | Descending | No | Yes | Yes | Yes | . | . | 3 | 0.75 |
| 12 | 5 | TA | Low | Sigmoid | Yes | No | Yes | Yes | 2 | 0.50 | 2 | 0.67 |
| 13 | 7 | TA | Low | Cecum | No | Yes | Yes | No | . | . | . | . |
| 14 | 6 | TA | Low | Descending | Yes | No | Yes | Yes | 2 | 0.50 | 2 | 0.50 |
| 15 | 11 | TA | Low | Transverse | No | Yes | Yes | Yes | . | . | 3 | 0.21 |
| 16 | 8 | TA | Low | Ascending | Yes | No | Yes | Yes | 1 | 0.17 | . | . |
| 17 | 8 | TA | Low | Ascending | No | No | No | Yes | 3 | 0.19 | 3 | 0.20 |
| 18 | 9 | TA | Low | Transverse | No | No | No | Yes | . | . | 3 | 0.33 |
| 19 | 5 | TA | Low | Transverse | Yes | Yes | Yes | Yes | 2 | 0.33 | 2 | 0.29 |
| 20 | 9 | TA | Low | Cecum | Yes | Yes | Yes | Yes | 3 | 0.43 | 2 | 0.33 |
| 21 | 25 | TVA | High | Cecum | Yes | No | Yes | Yes | 3 | 0.25 | 3 | 0.12 |
TA=Tubular adenoma
TVA=Tubulovillous adenoma
2D=Primary 2D interpretation
3D=Primary 3D interpretation
H:W=Height to width
Note: H:W ratio may be >50% at CTC as the morphologic appearance at colonoscopy was considered the gold standard.
Acknowledgments
Work funded by NIH/NIH ACRIN Grant # U01 CA79778 S2
Contributor Information
Jeff L. Fidler, Professor of Radiology, Mayo Clinic, Rochester, MN.
Zheng Zhang, Assistant Professor, Brown University, Providence, RI
Benjamin A. Herman, Brown University, Providence, RI.
Paul J. Limburg, Professor of Medicine, Mayo Clinic, Rochester, MN.
J.G. Fletcher, Professor of Radiology, Mayo Clinic, Rochester, MN
Abraham Dachman, Professor of Radiology, University of Chicago, Chicago, IL.
Jay P. Heiken, Professor of Radiology, Mallickrodt Institute of Radiology, St. Louis, MO
Mark D. Kuo, Scottsdale Medical Imaging Ltd, Scottsdale, AZ.
Christine O. Menias, Professor of Radiology, Mayo Clinic, Scottsdale, AZ.
Bettina Siewert, Associate Professor of Radiology, Beth Israel Deaconess Medical Center, Boston, MA.
Jugesh I. Cheema, Evansville Radiology, Evansville, IN.
Richard G. Obregon, Radiology Imaging Associates, Denver, CO.
Peter Zimmerman, Professor of Radiology, UCLA Medical Center, Los Angeles, CA.
Karen M. Horton, Professor of Radiology, Johns Hopkins University, Baltimore, MD.
Kevin Coakley, Clinical Radiologists, SC, Springfield, IL.
Revathy B. Iyer, Professor of Radiology, UT MD Anderston Cancer Center, Houston, TX.
Amy Hara, Professor of Radiology, Mayo Clinic, Scottsdale, AZ.
Robert A. Halvorsen, JR., Professor of Radiology, Medical College of Virginia, Richmond, VA.
Giovanna Casola, Professor of Radiology, UCSD Medical Center, San Diego, CA.
Judy Yee, Professor of Radiology, UCSF VA Medical Center, San Francisco, CA.
Lawrence J. Burgart, Abbott Northwestern Hospital, Minneapolis, MN
C. Daniel Johnson, Professor of Radiology, Mayo Clinic, Scottsdale, AZ.
References
- 1.Adachi M, Muto T, Morioka Y, et al. Flat adenoma and flat mucosal carcinoma (IIIb type) - a new precursor of colorectal carcinoma? Dis Colon Rectum. 1988;31:236–43. doi: 10.1007/BF02552553. [DOI] [PubMed] [Google Scholar]
- 2.Bond JH. Small flat adenomas appear to have little clinical importance in Western countries. Gastrointest Endosc. 1995;42:184–7. doi: 10.1016/s0016-5107(95)70082-x. [DOI] [PubMed] [Google Scholar]
- 3.Cairns A, Quirke P. Flat adenomas. Br J Surg. 1999;86(12):1489–90. doi: 10.1046/j.1365-2168.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 4.Fidler J, Johnson C. Flat polyps of the colon: accuracy of detection by CT colonography and histologic significance. Abdominal Imaging. 2009;34(2):157–71. doi: 10.1007/s00261-008-9388-4. [DOI] [PubMed] [Google Scholar]
- 5.Fidler JL, Johnson CD, MacCarty RL, Welch TJ, Hara AK, Harmsen WS. Detection of flat lesions in the colon with CT colonography. Abdominal Imaging. 2002;27(3):292–300. doi: 10.1007/s00261-001-0171-z. [DOI] [PubMed] [Google Scholar]
- 6.Fujii T, Rembacken BJ, Dixon MF, Yoshida S, Axon AT. Flat adenomas in the United Kingdom: are treatable cancers being missed? Endoscopy. 1998;30:437–43. doi: 10.1055/s-2007-1001304. [DOI] [PubMed] [Google Scholar]
- 7.Hart AR, Kudo S, Mackay EH, Mayberry JF, Atkin WS. Flat adenomas exist in asymptomatic people: important implications for colorectal cancer screening programmes. Gut. 1998;43:229–31. doi: 10.1136/gut.43.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurlstone DP, Cross SS, Adam I, et al. A prospective clinicopathological and endoscopic evaluation of flat and depressed colorectal lesions in the United Kingdom. Am J Gastroenterol. 2003;98:2543–9. doi: 10.1111/j.1572-0241.2003.07679.x. [DOI] [PubMed] [Google Scholar]
- 9.Jaramillo E, Watanabe M, Slezak P, Rubio C. Flat neoplastic lesions of the colon and rectum detected by high-resolution video endoscopy and chromoscopy. Gastrointest Endosc. 1995;42:114–22. doi: 10.1016/s0016-5107(95)70066-8. [DOI] [PubMed] [Google Scholar]
- 10.Kubota O, Kino I, Kimura T, Harada Y. Nonpolypoid adenomas and adenocarcinomas found in background mucosa of surgically resected colons. Cancer. 1996;77:621–6. doi: 10.1002/(sici)1097-0142(19960215)77:4<621::aid-cncr6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 11.Kudo S, Tamura T, Nakajima S, et al. Depressed type of colorectal cancer. Endoscopy. 1995;27:54–7. doi: 10.1055/s-2007-1005633. [DOI] [PubMed] [Google Scholar]
- 12.Mitooka H, Fujimori T, Maeda S, Nagasako K. Minute flat depressed neoplastic lesions of the colon detected by contrast chromoscopy using an indigo carmine capsule. Gastrointestinal Endoscopy. 1995;41(5):453–9. doi: 10.1016/s0016-5107(05)80003-3. [DOI] [PubMed] [Google Scholar]
- 13.Muto T, Kamiya J, Sawada T, Konishi F, Sugihara K, Kubota Y, et al. Small “flat adenoma” of the large bowel with special reference to its clinicopathological features. Dis Colon Rectum. 1985;28:847–51. doi: 10.1007/BF02555490. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien MJ, Winawer SJ, Zauber AG, et al. Flat adenomas in the National Polyp Study: is there increased risk for high-grade dysplasia initially or during surveillance? Clin Gastroenterol Hepatol. 2004;2(10):905–11. doi: 10.1016/s1542-3565(04)00392-1. [DOI] [PubMed] [Google Scholar]
- 15.Pickhardt PJ, Kim DH, Robbins JB. Flat (nonpolypoid) colorectal lesions identified at CT colonography in a U.S. screening population. Academic Radiology. 2010;17(6):784–90. doi: 10.1016/j.acra.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Pickhardt PJ, Nugent PA, Choi JR, Schindler WR. Flat colorectal lesions in asymptomatic adults: implications for screening with CT virtual colonoscopy. AJR American Journal of Roentgenology. 2004;183(5):1343–7. doi: 10.2214/ajr.183.5.1831343. [DOI] [PubMed] [Google Scholar]
- 17.Rembacken BJ, Fujii T, Cairns A, Dixon MF, Yoshida S, Chalmers DM, et al. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet. 2000;355:1211–4. doi: 10.1016/s0140-6736(00)02086-9. [DOI] [PubMed] [Google Scholar]
- 18.Ross AS, Waxman I. Flat and depressed neoplasms of the colon in Western populations. American Journal of Gastroenterology. 2006;101(1):172–80. doi: 10.1111/j.1572-0241.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 19.Saitoh Y, Waxman I, West AB, Popnikolov NK, Gatalica A, Watari J, et al. Prevalence and distinctive biologic features of flat colorectal adenomas in a North American population. Gastroenterology. 2001;120:1657–65. doi: 10.1053/gast.2001.24886. [DOI] [PubMed] [Google Scholar]
- 20.Sakashita M, Aoyama N, Maekawa S, et al. Flat-elevated and depressed, subtypes of flat early colorectal cancers, should be distinguished by their pathological features. Int J Colorectal Dis. 2000;15(5-6):275–81. doi: 10.1007/s003840000244. [DOI] [PubMed] [Google Scholar]
- 21.Soetikno R, Friedland S, Kaltenbach T, Chayama K, Tanaka S. Nonpolypoid (flat and depressed) colorectal neoplasms. Gastroenterology. 2006;130:566–76. doi: 10.1053/j.gastro.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki N, Price AB, Talbot IC, et al. Flat colorectal neoplasms and the impact of the revised Vienna Classification on their reporting: a case-control study in UK and Japanese patients. Scandinavian Journal of Gastroenterology. 2006;41:812–9. doi: 10.1080/00365520600610345. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki N, Talbot IC, Saunders BP. The prevalence of small. flat colorectal cancers in a western population. Colorectal Disease. 2004;6:15–20. doi: 10.1111/j.1463-1318.2004.00533.x. [DOI] [PubMed] [Google Scholar]
- 24.Togashi K, Konishi F, Koinuma K, Ishitsuka T, Kojima M, Okada M, et al. Flat and depressed lesions of the colon and rectum: pathogenesis and clinical management. Ann Acad Med Singapore. 2003;32:152–8. [PubMed] [Google Scholar]
- 25.Tsuda S, Veress B, Toth E, Fork FT. Flat and depressed colorectal adenomas in a North American population. Gastroenterology. 2001;120:1657–65. doi: 10.1053/gast.2001.24886. [DOI] [PubMed] [Google Scholar]
- 26.Tsuda S, Veress B, Toth E, Fork FT. Flat and depressed colorectal tumours in a southern Swedish population: a prospective chromoendoscopic and histopathological study. Gut. 2002;51:550–5. doi: 10.1136/gut.51.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe T, Muto T, Sawada T, Miyaki M. Flat adenoma as a precursor of colorectal carcinoma in hereditary nonpolyposis colorectal carcionma. Cancer. 1996;77:627–34. doi: 10.1002/(sici)1097-0142(19960215)77:4<627::aid-cncr7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T, Sawada T, Kubota Y, Adachi M, Saitoh Y, Masaki T, et al. Malignant potential in flat elevations. Dis Colon Rectum. 1993;36:548–53. doi: 10.1007/BF02049860. [DOI] [PubMed] [Google Scholar]
- 29.Wolber RA, Owen DA. Flat adenomas of the colon. Hum Pathol. 1991;22:70–4. doi: 10.1016/0046-8177(91)90064-v. [DOI] [PubMed] [Google Scholar]
- 30.Geboes K, DeHertogh G, B R, Geboes KP. Flat Adenomas, Significance, Detection, Treatment. Annals of Gastroenterology. 2010;23(4):266–9. [Google Scholar]
- 31.Lau PCP, Sung JJY. Flat adenoma in colon: two decades of debate. J Dig Dis. 2010;11(4):201–7. doi: 10.1111/j.1751-2980.2010.00439.x. [DOI] [PubMed] [Google Scholar]
- 32.Karita M, Cantero D, Okita K. Endoscopic diagnosis and resection treatment for flat adenoma with severe dysplasia. Am J Gastroenterol. 1993;88:1421–3. [PubMed] [Google Scholar]
- 33.Kiesslich R, von Bergh M, Hahn M, Hermann G, Jung M. Chromoendoscopy with indigo carmine improves the detection of adenomatous and nonadenomatous lesions in the colon. Endoscopy. 2001;33:1001–6. doi: 10.1055/s-2001-18932. [DOI] [PubMed] [Google Scholar]
- 34.Lanspa SJ, Rouse J, Smyrk T, Watson P, Jenkins JX, Lynch HT. Epidemiologic characteristics of the flat adenoma of Muto. A prospective study. Dis Colon Rectum. 1991;35:543–6. doi: 10.1007/BF02050533. [DOI] [PubMed] [Google Scholar]
- 35.Dachman AH, Zalis ME. Quality and consistency in CT colonography and research reporting. Radiology. 2004;230:319–23. doi: 10.1148/radiol.2302031113. [DOI] [PubMed] [Google Scholar]
- 36.Zalis ME, Barish MA, Choi JR, et al. CT colonography reporting and data system: a consensus proposal. Radiology. 2005;236(1):3–9. doi: 10.1148/radiol.2361041926. [DOI] [PubMed] [Google Scholar]
- 37.Johnson CD, Chen M-H, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. New England Journal of Medicine. 2008;359(12):1207–17. doi: 10.1056/NEJMoa0800996. Erratum appears in N Engl J Med. 2008 Dec 25;359(26):2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 39.Park SH, Ha HK, Kim MJ, et al. False-negative results at multi-detector row CT colonography: multivariate analysis of causes for missed lesions. Radiology. 2005;235(2):495–502. doi: 10.1148/radiol.2352040606. [DOI] [PubMed] [Google Scholar]
- 40.Park SH, Kim SY, Lee SS, et al. Sensitivity of CT colonography for nonpolypoid colorectal lesions interpreted by human readers and with computer-aided detection. AJR. 2009. American Journal of Roentgenology. 193(1):70–8. doi: 10.2214/AJR.08.2234. [DOI] [PubMed] [Google Scholar]
- 41.Park SH, Lee SS, Choi EK, et al. Flat colorectal neoplasms: definition, importance and visualization on CT colonography. AJR Am J Roentgenol. 2007;188:953–9. doi: 10.2214/AJR.06.0436. [DOI] [PubMed] [Google Scholar]
- 42.Fletcher JG, Chen M-H, Herman BA, et al. Can Radiologist Training and Testing Ensure High Performance in CT Colonography? Lessons From the National CT Colonography Trial. Am J Roentgenol. 2010;195(1):117–25. doi: 10.2214/AJR.09.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pickhardt PJ, Nugent PA, Choi JR, Schindler WR. Flat colorectal lesions in asymptomatic adults: implications for screening with CT virtual colonoscopy. AJR. 2004. American Journal of Roentgenology. 183(5):1343–7. doi: 10.2214/ajr.183.5.1831343. [DOI] [PubMed] [Google Scholar]
- 44.Kim DH, Pickhardt PJ, Taylor AJ. Characteristics of advanced adenomas detected at CT colonographic screening: implications for appropriate polyp size thresholds for polypectomy versus surveillance. AJR. 2007. American Journal of Roentgenology. 188(4):940–4. doi: 10.2214/AJR.06.0764. [DOI] [PubMed] [Google Scholar]
- 45.Hurlstone DP, Sanders DS, Cross SS, et al. Colonoscopic resection of lateral spreading tumours: a prospective analysis of endoscopic mucosal resection. Gut. 2004;53(9):1334–9. doi: 10.1136/gut.2003.036913. [DOI] [PMC free article] [PubMed] [Google Scholar]


