Abstract
Background
We describe baseline renal function and albumin excretion rate in patients enrolled in BARI 2D, a randomized clinical trial comparing revascularization and medical therapy with medical therapy alone and deferred or no revascularization, and the impact of glycemic control with either insulin providing or insulin sensitizing drugs, on 5 year mortality.
Methods
Study participants had T2DM, documented CAD, and creatinine < 2 mg/dl. Albuminuria status (albumin/creatinine ratio [ACR]) and estimated glomerular filtration rate (eGFR), utilizing the abbreviated MDRD equation, were determined at baseline. Univariate and multivariate relationships between baseline clinical characteristics and the presence of albuminuria and reduced eGFR rate were estimated.
Results
2146 subjects were included in the analysis. 43% of the cohort had evidence of kidney dysfunction at baseline: 23% had an eGFR ≥ 60 ml/min/1.73 m2 with either micro (>30 ACR; 17%) or macro (> 300 ACR; 6%) albuminuria. 21 % had a reduced eGFR < 60 ml/min/1.73 m2; 52 % with reduced eGFR had no albuminuria; 28 % had microalbuminuria and 20 % had macroalbuminuria. Race, smoking status, duration of diabetes, hypertension, HbA1c, triglycerides, vascular disease, abnormal ejection fraction, and reduced eGFR were associated with greater albuminuria. Age, sex, duration of diabetes, ACR, HbA1c, HDL, and number of hypertensive medications were associated with reduced eGFR.
Conclusion
Kidney dysfunction is common in older patients with T2DM and CAD; Albuminuria was present in 33%. Reduced eGFR was present in 21%, and half the patients with reduced eGFR had no evidence of albuminuria.
Introduction
Diabetic nephropathy, the leading cause of end-stage renal disease (ESRD) in the world, has been classically defined by the presence of proteinuria >0.5 gm/24hr (1–3). Other etiologies of chronic kidney disease, including those associated with minimal proteinuria, such as hypertensive nephrosclerosis, are also widely prevalent in the type 2 diabetes mellitus (T2DM) population (4–6). Identification of any form of nephropathy in patients with T2DM is important, since both albuminuria and reduced glomerular filtration rate (GFR) are independent risk factors for cardiovascular morbidity and mortality (7–19).
Many patients with chronic kidney disease (CKD) are unaware of their diagnosis, leading to delays in implementing treatment that can potentially lead to more effective prevention of ESRD and potentially to decreased morbidity and mortality from cardiovascular disease (1,20).
The current report describes the baseline prevalence and characteristics of kidney dysfunction in patients with T2DM and angiographically documented coronary artery disease (CAD) enrolled in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. A high prevalence of both albuminuric and non-albuminuric kidney dysfunction was demonstrated in the trial participants. These baseline data provide an opportunity to determine how the trial participants relate to broader T2DM populations and to observe associations among risk factors in this population.
Research Design and Methods
Study Design
BARI 2D is an international randomized clinical trial of 2368 subjects with T2DM and angiographically documented CAD comparing the impact of prompt revascularization and optimal medical therapy with optimal medical therapy alone with deferred revascularization as needed to relieve symptoms, and the impact of glycemic control with either insulin providing or insulin sensitizing drugs, on 5 year mortality. Details of the BARI 2D study, including baseline characteristics of the overall study population have been previously reported (21).
Subjects
Study participants were over age 25 years with T2DM and coronary artery disease documented by angiography and associated with objective signs of ischemia including a stress test or classic angina. Subjects with angiographically documented CAD that was considered to be amenable to coronary revascularization by at least one of the available methods were eligible for study entry. Additional entry criteria included the ability to perform all tasks related to glycemic control and risk factor management. Major exclusion criteria included prior cardiac revascularization within 12 months, definitive need for invasive coronary intervention, class III or IV congestive heart failure, serum creatinine concentration > 2.0 mg/dl prior to cardiac catheterization, hemoglobin A1c (HbA1c) > 13.0%, hepatic disease, current alcohol abuse, and pregnancy known or planned within the next 5 years.
Measurements
Serum creatinine was measured at each clinical site. Baseline GFR was estimated (eGFR) using the abbreviated Modified Diet in Renal Disease (MDRD) study equation, which includes age, sex, race, and serum creatinine measurement (22). Reduced eGFR was defined as < 60 ml/min/1.73m2. i.e. Stage III CKD. Urine specimens were collected at baseline and assayed for albumin and creatinine at the University of Minnesota Core Biochemistry Laboratory. Creatinine was measured using a modified Jaffe reaction on a Synchron CX3 Clinical System analyzer. Albumin was measured using the IMMAGE Immunochemistry System which uses a rate-nephelometry reaction. A baseline albumin creatinine ratio (ACR) was calculated for each subject. Albuminuria status for each participant was categorized as normoalbuminuric (ACR ≤ 30 mg/g), microalbuminuric (ACR>30 and ≤ 300 mg/g), or macroalbuminuric (ACR>300 mg/g).
Statistical Analysis
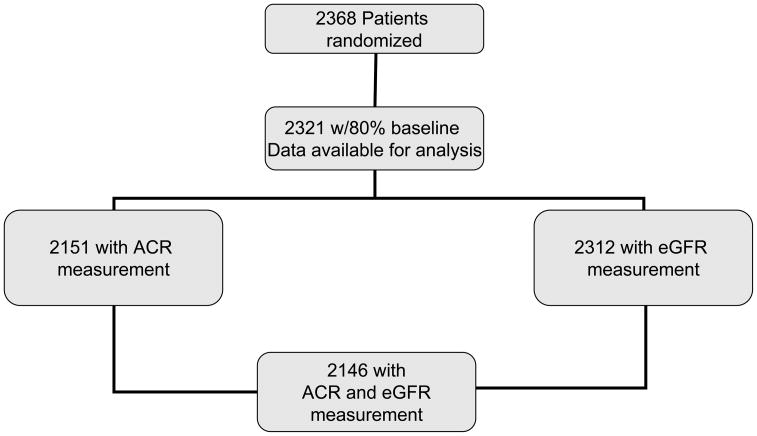
This analysis includes 2146 (91%) participants who had both ACR and estimated GFR (eGFR) measures. (Figure 1). Baseline demographic and clinical data are presented for these participants grouped according to albuminuria status and eGFR. Means and standard deviations are presented for continuous data and proportions for categorical. Continuous and categorical data were compared using analysis of variance F-statistics and chi-square statistics, respectively.
Figure 1.
BARI 2D Participants Breakdown
Regression Model specifics
Ordinal logistic regression was used to model albuminuria status, since it has a natural ordering (no albuminuria, microalbuminuria, macroalbuminuria). The odds ratio estimates obtained from this model compare the odds of more severe levels of albuminuria status to the odds of less severe levels (i.e. micro and macro compared to no albuminuria, macro compared to micro and no albuminuria). Logistic regression was used to identify demographic and clinical parameters associated with reduced eGFR at baseline. Patients were classified at study entry as either normal (eGFR: ≥ 60 ml/min/1.73m2) or reduced (eGFR: <60 ml/min/1.73m2).
Univariate and multivariable models for each of these outcomes are presented. A stepwise algorithm was used to identify those factors that were independently associated with the outcomes. A p-value of 0.05 was used as the criteria for both variable entry and exit from the models. Candidate variables for both multivariable models were age, sex, and race/ethnicity, diabetes duration, hypertension, vascular disease, cigarette smoking status, lower extremity ulcer, lower extremity amputation, laser treatment for diabetic retinopathy/macular edema, insulin use, systolic BP, diastolic BP, BMI, waist circumference, number of coronary arteries with stenoses, HbA1c, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, LV function, number and type of diabetes drugs, statin therapy, number of hypertensive drugs, and angiotensin converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBS) use. The reduced eGFR model was also performed after excluding age, sex, and race/ethnicity as they are components of eGFR. Additionally natural log transformed ACR was included in the reduced eGFR candidate list. Since left ventricular ejection fraction (LVEF) was missing in multiple cases, an indicator variable for LVEF missing was created and added to the models, so that the relationship between abnormal LVEF and the renal variables could be assessed, without losing multiple observations due to missing values. The independent variables from the models of albuminuria status and reduced eGFR were then used in a multivariate model of both albuminuria (ACR > 30) and reduced eGFR. Multivariate modeling takes into account the correlation between the two outcomes and enabled formal testing of differences between the associations of each of the independent variables and the outcomes of reduced eGFR and albuminuria. Odds ratio estimates of the renal outcomes are presented for the variables. The p-values for the test of global significance and the test for a difference between the outcome associations for each variable are also shown.
Results
The baseline characteristics of normoalbuminuric, microalbuminuric, and macroalbuminuric participants, categorized by eGFR (≥ or < 60 ml/min/1.73m2) (n=2146) are shown in Table 1. As compared to patients with normal ACR, patients with either microalbuminuria or macroalbuminuria were less likely to be non Hispanic white, had longer duration of diabetes mellitus, larger BMI, higher HbA1c, higher LDL-cholesterol, higher serum creatinine with lower eGFR, and higher systolic and diastolic blood pressures.
TABLE 1.
Baseline Characteristics of study participants by kidney disease status
| eGFR ≥60 | eGFR < 60 | ||||||
|---|---|---|---|---|---|---|---|
| Total (N=2146) | No Albuminuria (N=1217) | Micro Albuminuria (N=365) | Macro Albuminuria (N=121) | No Albuminuria (N=231) | Micro Albuminuria (N=125) | Macro Albuminuria (N=87) | |
| Demographic Features | |||||||
| Age††† | 62.3±8.9 | 60.9± 8.7 | 61.5± 8.6 | 61.6±8.8 | 68.1±7.8 | 67.6±7.5 | 63.8±8.8 |
| Sex, %††† | |||||||
| Male | 70.5 | 72.1 | 73.4 | 70.2 | 64.9 | 64.8 | 59.8 |
| Female | 29.5 | 27.9 | 26.6 | 29.8 | 35.1 | 35.2 | 40.2 |
| Race/Ethnicity, %** | |||||||
| White nonHispanic | 65.7 | 66.8 | 62.5 | 52.9 | 74.5 | 69.6 | 51.7 |
| Black nonHispanic | 16.6 | 15.6 | 18.4 | 29.8 | 11.7 | 14.4 | 21.8 |
| Hispanic | 12.6 | 12.5 | 13.4 | 12.4 | 10.0 | 11.2 | 20.7 |
| Asian nonHispanic | 4.4 | 4.4 | 5.2 | 5.0 | 3.0 | 4.0 | 5.7 |
| Other nonHispanic | 0.7 | 0.7 | 0.5 | 0.0 | 0.9 | 0.8 | 0.0 |
| Clinical Features | |||||||
| Duration of diabetes mellitus (yr)***††† | 10.4±8.6 | 8.9±8.0 | 11.0±8.3 | 13.8±8.0 | 11.3±9.3 | 15.1±9.9 | 15.4±9.5 |
| BMI (kg/m2)* | 31.7±5.9 | 31.5±5.8 | 32.0±6.1 | 32.3±7.0 | 31.1±5.3 | 32.7± 6.5 | 32.5±5.8 |
| Waist Circumference (cm) | |||||||
| Male* | 108.7±13.4 | 107.9±13.4 | 109.8±13.7 | 108.6±13.7 | 107.9± 11.9 | 112.1±12.5 | 113.1± 14.0 |
| Female | 105.8±14.9 | 105.5± 14.8 | 106.3±15.5 | 108.5±15.7 | 104.5± 15.4 | 107.1±, 14.9 | 104.8± 11.1 |
| HbA1c (%)***††† | 7.67±1.63 | 7.53±1.59 | 8.18±1.73 | 8.52±1.72 | 7.17±1.36 | 7.48±1.44 | 7.99±1.48 |
| Serum Creatinine (mg/dl)***††† | 1.04±0.27 | 0.94±0.19 | 0.97±0.19 | 0.96±0.21 | 1.37±0.21 | 1.43±0.22 | 1.51±0.26 |
| eGFR*** | 79.0±23.8 | 86.9±20.8 | 84.2±19.8 | 87.5±23.3 | 51.9±6.6 | 49.6±6.8 | 47.7±8.5 |
| Systolic BP (mmHg)***† | 131.8±20.3 | 128.5±19.0 | 136.2±20.0 | 146.3±21.7 | 127.9±17.0 | 134.0±19.0 | 147.9±26.2 |
| Diastolic BP (mmHG)***††† | 74.6±11.3 | 74.4±11.0 | 76.8±11.5 | 78.6±12.4 | 71.2±10.9 | 71.6±11.3 | 76.9±11.0 |
| LDL cholesterol (mg/dl)∞* | 96.7±33.6 | 96.6±33.0 | 98.9±35.9 | 99.4±35.6 | 93.1±30.5 | 89.8±30.2 | 104.2±38.5 |
| HDL cholesterol (mg/dl)∞** | 38.2±10.3 | 38.1±9.7 | 38.5±10.7 | 41.1±11.4 | 37.7±11.2 | 36.6±9.6 | 38.8±11.8 |
| Triglycerides (mg/dl)∞** | 181.0±135.1 | 173.5±121.9 | 190.2±140.7 | 214.9±204.8 | 178.6±152.9 | 195.4±113.7 | 185.3±135.2 |
Abbreviations: eGFR (estimated glomerular filtration rate)
Core lab value augmented with site estimate if core lab value missing
indicates trend comparison among albuminuria status categories (none, microalbuminuria, macroalbuminuria),
p < 0.05,
p < 0.01,
p < 0.001
indicates comparison between eGFR categories (eGFR < 60, eGFR ≥ 60)
p < 0.05,
p < 0.01,
p < 0.001
Medical history and prior treatment of the study participants are summarized in Table 2. Overall, there was a high frequency of hypertension (82.4%), hyperlipidemia requiring statin therapy (74.2%), and use of antiplatelet therapy (88.2 %). Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBS) were used in 74% of participants. Oral hypoglycemic agents were the most commonly utilized diabetic medications (80.5%). Insulin was utilized in 27.3%. Diet therapy alone was utilized in only 8.7%. Only 12% of participants were current smokers. As compared to patients with normal ACR, patients with microalbuminuria or macroalbuminuria were more likely to have hypertension, laser therapy for diabetic retinopathy/macular edema, and reduced left ventricular ejection fraction. Patients with micro- or macroalbuminuria were more likely to be receiving insulin therapy and were less likely to be managed with diet therapy alone.
TABLE 2.
Medical History and Treatment of study participants by kidney disease status
| eGFR ≥ 60 | eGFR < 60 | ||||||
|---|---|---|---|---|---|---|---|
| Total (N=2146) | No Albuminuria (N=1217) | Micro Albuminuria (N=365) | Macro Albuminuria (N=121) | No Albuminuria (N=231) | Micro Albuminuria (N=125) | Macro Albuminuria (N=87) | |
| Medical History | |||||||
| Hypertension(%)***††† | 82.4 | 77.9 | 87.3 | 89.2 | 86.5 | 89.4 | 93.0 |
| Laser Treatment for Diabetic Retinopathy/Macular Edema(%)***††† | 3.1 | 1.5 | 4.1 | 4.1 | 3.1 | 12.8 | 5.8 |
| Current Cigarette Smoker (%)††† | 11.9 | 12.6 | 14.5 | 15.7 | 5.6 | 4.8 | 12.6 |
| LVEF*** | 57.4±10.9 | 58.0±10.2 | 57.2±11.2 | 55.0±12.6 | 57.6±11.1 | 56.6±11.5 | 53.1±12.8 |
| Abnormal LVEF (< 50 %) (%)†† | 17.0 | 14.7 | 16.4 | 23.1 | 18.8 | 20.0 | 34.9 |
| Medical Treatment at baseline | |||||||
| ACEI or ARB(%)***††† | 77.2 | 72.0 | 82.4 | 86.0 | 85.3 | 85.6 | 82.8 |
| # BP medications***††† | 2.2±1.0 | 2.0±1.0 | 2.4±1.0 | 2.6±1.1 | 2.4±1.0 | 2.6±0.9 | 2.8±1.2 |
| Statins(%) | 74.2 | 72.9 | 76.9 | 76.0 | 73.6 | 76.0 | 78.2 |
| Glucose Control | |||||||
| Diet Alone (%)*** | 8.7 | 10.5 | 4.4 | 3.3 | 12.1 | 6.4 | 2.3 |
| Oral Hypoglycemic Agent (%)††† | 80.5 | 81.9 | 84.7 | 77.7 | 77.5 | 69.6 | 70.9 |
| Insulin(%)***††† | 27.3 | 21.5 | 32.1 | 38.8 | 24.2 | 44.8 | 54.0 |
Abbreviations: eGFR (estimated glomerular filtration rate) LVEF (left ventricular ejection fraction)
indicates trend comparison among albuminuria status categories (none, microalbuminuria, macroalbuminuria),
p < 0.05,
p < 0.01,
p < 0.001
indicates comparison between GFR categories (GFR < 60, GFR ≥ 60)
p < 0.05,
p < 0.01,
p < 0.001
Table 3 shows the percentage of participants who were achieving approved targets of diabetic therapy at baseline. For the total group < 10% had normal body mass index, 52% had systolic blood pressure < 130 mm Hg and 72% <140 mmHg, 39% had HbA1c <7.0% and 59% had LDL-cholesterol < 100 mg/dl. Patients with microalbuminuria or macroalbuminuria were less likely to have systolic blood pressure <130 or <140 mmHg or HbA1c <7.0%, but were more likely to be receiving ACEI/ARB therapy.
Table 3.
Proportion of study participants at targets assessed at baseline
| eGFR ≥60 | eGFR < 60 | ||||||
|---|---|---|---|---|---|---|---|
| Total (N=2146) | No Albuminuria (N=1217) | Micro Albuminuria (N=365) | Macro Albuminuria (N=121) | No Albuminuria (N=231) | Micro Albuminuria (N=125) | Macro Albuminuria (N=87) | |
| Systolic BP ≤130 mmHG (%)***† | 52.0 | 59.3 | 41.8 | 25.0 | 58.4 | 41.9 | 26.4 |
| Systolic BP ≤140 mmHG (%)*** | 72.4 | 79.1 | 61.5 | 43.3 | 82.7 | 67.7 | 43.7 |
| HbA1C < 7% (%)***†† | 39.1 | 42.1 | 29.0 | 16.5 | 53.2 | 44.4 | 25.3 |
| LDL Cholesterol < 100 mg/dl (%)† | 58.7 | 58.5 | 55.9 | 52.8 | 64.4 | 65.0 | 56.1 |
| LDL Cholesterol < 70 mg/dl (%) | 20.7 | 20.4 | 21.5 | 21.3 | 18.9 | 26.5 | 18.3 |
| Non-Smoker (%)††† | 88.1 | 87.4 | 85.5 | 84.3 | 94.4 | 95.2 | 87.4 |
| Aspirin Usage (%) | 88.1 | 88.9 | 89.0 | 87.6 | 87.4 | 83.2 | 83.9 |
| Use of ACEI or ARB (%)***††† | 77.2 | 72.0 | 82.4 | 86.0 | 85.3 | 85.6 | 82.8 |
| Use of Statins (%) | 74.2 | 72.9 | 76.9 | 76.0 | 73.6 | 76.0 | 78.2 |
| Normal BMI (20–25 kg/m2) (%) | 9.2 | 9.3 | 8.5 | 11.6 | 7.8 | 7.3 | 13.8 |
Abbreviations: eGFR (estimated glomerular filtration rate)
indicates trend comparison among albuminuria status categories (none, microalbuminuria, macroalbuminuria),
p < 0.05,
p < 0.01,
p < 0.001
indicates comparison between GFR categories (GFR < 60, GFR ≥ 60)
p < 0.05,
p < 0.01,
p < 0.001
The baseline characteristics of the study cohort according to stages of CKD utilizing the staging system recommended by the National Kidney Foundation are shown in Figure 2 (23, 24). Overall, 56% of the cohort had no evidence of kidney disease (normal albumin excretion and eGFR ≥ 60 ml/min/1.73m2). 43% of the cohort had evidence of CKD at baseline: 23% had eGFR (MDRD) ≥ 60 ml/min/1.73m2 with either micro (>30 ACR; 17%,) or macro (>300 ACR; 6%) albuminuria. 21% of the cohort had reduced eGFR, <60 ml/min/1.73m2; 52% with reduced eGFR had no albuminuria; 28% had micro albuminuria and 20 % had macroalbuminuria.
Multivariable analysis demonstrated that non-white ethnicity, smoking, longer duration of diabetes, reduced eGFR, higher triglycerides, and higher HbA1c were associated with more severe ACR status in BARI 2D participants (Table 4). Cardiovascular factors which were independently associated with higher levels of albuminuria included non-CAD/vascular disease, elevated systolic blood pressure, greater number of hypertension drugs, presence of lower extremity ulcers, and reduced left ventricular function (ejection fraction).
Table 4.
Factors Associated with More Severe Albuminuria Status
| N = 2030 | Univariate | Multivariable** | ||
|---|---|---|---|---|
| Odds Ratio*** (95% CI) | p_value | Odds Ratio*** (95% CI) | p_value | |
| Age | 1.01 (1.0–1.02) | 0.09 | 0.99 (0.97–1.00) | 0.29 |
| Female | 1.10 (0.90–1.34) | NS | 0.81 (0.64–1.03) | 0.09 |
| Race/Ethnicity | 0.001 | 0.028 | ||
| White Non-Hispanic | ---- | ----- | ||
| Black Non-Hispanic | 1.56 (1.23–1.99) | 1.18 (0.89–1.56) | ||
| Other Non-Hispanic | 1.30 (0.86–1.97) | 1.41 (0.90–2.20) | ||
| Hispanic | 1.36 (1.03–1.79) | 1.53 (1.13–2.07) | ||
| Smoking Status | 0.002 | |||
| Never Smoked | --- | --- | ||
| Current Smoker | 1.29 (0.95–1.76) | 0.11 | 1.75 (1.22–2.5) | |
| Former Smoker | 1.24 (1.01–1.53) | 0.04 | 1.45 (1.14–1.83) | |
| Duration of diabetes (every 5 years) | 1.26 (1.20–1.33) | < 0.001 | 1.20 (1.13–1.27) | < 0.001 |
| Non-Coronary Artery Disease* | 1.74 (1.41–2.13) | < 0.001 | 1.31 (1.04–1.64) | 0.02 |
| Lower extremity ulcer | 2.88 (1.62–5.13) | < 0.001 | 2.18 (1.15–4.12) | 0.02 |
| Systolic BP (every 10 mmHG) | 1.33 (1.27–1.39) | < 0.001 | 1.36 (1.29–1.43) | <0.001 |
| Number of Hypertension Drugs | 1.56 (1.42–1.71) | < 0.001 | 1.42 (1.29–1.58) | 0.005 |
| HgbA1c | 1.25 (1.18–1.32) | < 0.001 | 1.25 (1.18–1.34) | <0.001 |
| Log(Triglycerides) | 1.28 (1.09–1.50) | < 0.001 | 1.31 (1.10–1.57) | 0.003 |
| LVEF | ||||
| Normal LVEF (≥ 50) | --- | ---- | ||
| Abnormal LVEF** | 1.61 (1.27–2.04) | < 0.001 | 1.46 (1.12–1.89) | 0.005 |
| Unknown LVEF | 1.30 (0.78–2.16) | NS | 1.27 (0.73–2.20) | NS |
| Reduced eGFR | 2.36 (1.90–2.92) | < 0.001 | 2.24 (1.75–2.85) | < 0.001 |
Abbreviations: eGFR (estimated glomerular filtration rate), LVEF (left ventricular ejection fraction) CI(confidence interval), NS (not significant)
Non-Coronary Artery Disease includes documented carotid disease, carotid surgery, carotid stent, non-coronary vascular surgery, or intermittent claudication.
All variables listed included in the ordinal logistic mode.
Odds Ratio of More Severe Albuminuria Status compared to less severe
Age, sex, longer duration of diabetes, higher ACR, lower HDL, and greater number of hypertensive medications were independently associated with reduced eGFR (Table 5). Since age, sex, and race/ethnicity are components of the MDRD eGFR formula, multivariable analysis was also performed after exclusion of these variables. Similar findings were noted (Table 5). Longer duration of diabetes, higher ACR, lower HDL, greater number of hypertensive medications, current smoking status and lower diastolic blood were independently associated with reduced eGFR. There were no important differences in the variables associated with reduced eGFR in albuminuric patients compared to patients with no albuminuria.
Table 5.
Factors Associated with Reduced GFR (GFR < 60 ml/min/1.73m2)
| N =2030 | I. Univariate | II. Multivariable* | III. Multivariable Model with age, race, and gender** | |||
|---|---|---|---|---|---|---|
| Odds Ratio*** (95% CI) | p-value | Odds Ratio*** (95% CI) | p_value | OR*** (95% CI) | p-value | |
| Current Smoker | 0.46 (0.30–0.70) | < 0.001 | 0.52 (0.34–0.79) | 0.03 | 0.77 (0.49–1.21) | 0.26 |
| Duration of diabetes (every 5 yrs) | 1.23 (1.16–1.31) | < 0.001 | 1.18 (1.10–1.27) | <0.001 | 1.08 (1.01–1.17) | 0.037 |
| HbA1c | 0.88 (0.82–0.94) | < 0.001 | 0.80 (0.74–0.87) | <0.001 | 0.86 (0.78–0.94) | < 0.001 |
| HDL (every 5 mg/dl) | 0.93 (0.85–1.01) | 0.07 | 0.92 (0.87–0.97) | 0.006 | 0.98 (0.96–0.99) | < 0.001 |
| Diastolic BP (every 5 mmHG) | 0.90 (0.86–0.95) | < 0.001 | 0.92 (0.87–0.97) | 0.002 | 0.97 (0.92–1.03) | 0.35 |
| Glycemic Drugs | <0.001 | 0.07 | 0.07 | |||
| 0 or 1 non-insulin | --- | --- | ||||
| 2 or more non-insulin | 0.95 (0.73–1.23) | 0.74 (0.56–0.99) | 0.84 (0.62–1.13) | |||
| Insulin | 1.55 (1.19–2.01) | 0.98 (0.71–1.36) | 1.20 (0.86–1.69) | |||
| Number of Hypertension Drugs | 1.46 (1.31–1.63) | < 0.001 | 1.29 (1.15–1.44) | <0.001 | 1.28 (1.13–1.44) | <0.001 |
| Log(ACR) | 1.29 (1.21–1.36) | <0.001 | 1.29 (1.21–1.38) | < 0.001 | 1.30(1.21–1.39) | < 0.001 |
| Female Gender | 1.56(1.19–2.04) | 0.0013 | ||||
| Age(every year) | 1.08(1.06–1.09) | <0.001 | ||||
| Race | ||||||
| White | -- | 0.34 | ||||
| Black | 0.76(0.54–1.09) | |||||
| Asian | 0.97(0.53–1.79) | |||||
| Other | 1.20(0.78–1.86) | |||||
All variables listed included in the logistic model, excluding age, race, and gender
All variables listed included in the logistic model
Odds Ratio of Reduced GFR compared to Normal GFR
CI: Confidence interval
Variables with similar relationships to ACR > 30 and reduced eGFR were vascular disease, number of hypertension drug classes, duration of diabetes, triglycerides, and reduced LVEF (Table 6). Smoking status, systolic blood pressure, number of diabetes drugs and HgbA1c % had different associations. Patients with higher systolic blood pressure and those on insulin or more than one non-insulin diabetes drug were more likely to have albuminuria. These two variables had no relationship with reduced eGFR. As was seen in the separate models, smokers and patients with higher HgbA1c were more likely to have an ACR > 30, but were less likely to have reduced eGFR.
Table 6.
Factors Associated with Multivariate Analysis of Albuminuric Status and reduced eGFR
| N = 2030 | OR for Albuminuria (ACR > 30) | OR for reduced eGFR (eGFR < 60) | Global test for significance | p-value for equality between albuminuria OR and reduced eGFR OR |
|---|---|---|---|---|
| Smoking Status | <0.001 | 0.02 | ||
| Current Smoker vs never | 1.63* | 0.51** | ||
| Former Smoker vs never | 1.42** | 1.02 | ||
| Non-coronary Artery Disease | 1.36* | 1.35* | 0.006 | 0.95 |
| Lower extremity ulcer | 2.97** | 1.25 | 0.01 | 0.06 |
| Systolic BP, per 10 mmHG increase | 1.32*** | 1.03 | <0.001 | <0.001 |
| Number of Hypertension Drug Classes, per 1 drug class increase | 1.43*** | 1.36*** | <0.001 | 0.52 |
| Duration of Diabetes, per 5 years | 1.14*** | 1.23*** | <0.001 | 0.11 |
| Diabetes Drugs | <0.001 | 0.001 | ||
| 2 or more drug classes vs 0,1 drug class | 1.57** | 0.80 | ||
| Insulin vs 0,1 drug classes | 1.82*** | 1.12 | ||
| HgbA1c per 1% increase | 1.19*** | 0.81*** | <0.001 | <0.001 |
| HDL, per 5 mg/dl increase | 1.04 | 0.95 | 0.13 | 0.04 |
| Ln(Triglycerides) | 1.43*** | 1.27* | <0.001 | 0.41 |
| LV Ejection Fraction | ||||
| Abnormal (EF < 50%) | 1.42* | 1.48** | 0.004 | 0.84 |
Abbreviations: eGFR (estimated glomerular filtration rate), LV (left ventricular), OR(odds ratio)
Model also included age, race/ethnicity, and gender for the albuminuria outcome. An indicator variable for missing LV Ejection Fraction was also included.
p <0.05,
p < 0.01,
p < 0.001
Discussion
We identified a high prevalence of kidney dysfunction in middle-aged to older people with T2DM and angiographically documented CAD who were enrolled in the BARI 2D clinical trial. At the time of enrollment, hypertension was present in 82%. Albuminuria was present in 33%: 23% with microalbuminuria and 10% with macroalbuminuria. Reduced eGFR was present in 21%, and only half of these patients had albuminuria. Our findings extend and confirm observations from other cohorts of patients with T2DM that have reported that kidney dysfunction is not only highly prevalent in older patients with T2DM, but it is heterogenous. The DEMAND study, a global cross-sectional study of patients with T2DM reported that 50% of participants had either microalbuminuria or macroalbuminuria, 20% had eGFR<60 ml/min/1.73m2), and 80% were hypertensive (25). In the HOPE study 32% of patients with T2DM had microalbuminuria at baseline (26). So et al evaluated 4421 newly referred Chinese patients with T2DM with no prior history of macrovascular disease. In this cohort 26.3% had microalbuminuria, 12.7% had macroalbuminuria, and 12% had eGFR<60 ml/min/1.73m2. These subjects were younger (58 ± 13 yr) and had a shorter duration of diabetes mellitus (6.9 ±6.3 yr) than the BARI 2D study population (27).
Our finding, that 43% of patients enrolled in BARI 2D, all of whom have CAD, had either albuminuria, reduced eGFR or both, highlights the strong associations between renal and cardiac disease. Both prospective and retrospective studies have shown that microalbuminuria is an independent risk factor for cardiovascular disease in patients with T2DM with an estimated doubling of both cardiovascular and total mortality (7–14). Microalbuminuria is also a major predictor for diabetic nephropathy, which accounts for approximately 40 to 60% of incident cases of ESRD in the U.S (1–3). Reduced glomerular filtration rate has also been identified as an independent cardiovascular risk factor in prospective and epidemiologic studies (15–19).
There was significant heterogeneity with respect to the manifestations of kidney dysfunction in the BARI 2D cohort. The most common renal abnormality was microalbuminuria with normal eGFR (17%), followed by reduced eGFR (GFR< 60 ml/min; stage 3 CKD) without albuminuria (11%). Half of the subjects with reduced eGFR had normal albumin excretion. These findings reflect the fact that patients with T2DM are a heterogeneous group of patients who are generally older and have more comorbid conditions at diagnosis compared with adults with type 1 diabetes (1, 28). Other etiologies for kidney disease, which are not associated with increased urine albumin excretion, may be present such as renal vascular disease, hypertensive nephrosclerosis, and atheroembolic renal disease. Up to one-third of adults with newly diagnosed T2DM already have CKD (4–6,28), and data suggest that in many of these patients CKD initially developed either due to the hyperglycemia prior to the time of diagnosis or secondary to hypertension and other factors (28).
Currently utilized cutoff values for defining micro- and macroalbuminuria are arbitrary, while the risks associated with albuminuria are continuous, with increasing risk noted even in the “high normal” range of albuminuria (10–13). The relatively high prevalence of patients with normal albumin excretion in the current study may be partly due to a high frequency of ACE inhibitor and ARB use (77.2%) in the BARI 2D population. Similar observations have been made by Mac Isaac et al who reported renal parameters in 629 type II diabetic patients (6). In those patients with GFR < 60, 38% had microalbuminuria and 28% had macroalbuminuria, while 43/109 (39%) had normal levels of albumin excretion. Of note, 74% of patients in this series were receiving either ACE inhibitor or ARB therapy (6). Of the 443 participants with eGFR <60 ml/min/1.73m2 in the BARI 2D trial, 28% had microalbuminuria and 20% had macroalbuminuria, while 52% had normal levels of albumin excretion. In the DEMAND study, a cross-sectional study evaluating 32,208 type 2 diabetic patients from 33 countries (average age 61 yr), reduced GFR was noted in 17% of patients with normal values of urinary albumin excretion (25). The NHANES III cross sectional study reported that 13% of adults with T2DM had reduced GFR of < 60 ml/min/1.73m2 (5). Among the T2DM participants with reduced GFR, approximately 40% had abnormally increased urinary albumin excretion. The absence of both abnormal albumin excretion and diabetic retinopathy was noted in ~30%. The results did not change substantially after exclusion of participants receiving ACE inhibitors (5).
It is estimated that after 10 years, 10–28% of T2DM patients will develop microalbuminuria and 25–45% of patients with microalbuminuria will develop macroalbuminuria over the subsequent 5–10 years (1,28,29). The UKPDS study reported on incident/newly diagnosed patients with T2DM. In this population (n=5097), 6.5% had microalbuminuria and 0.7% macroalbuminuria. The patients were younger than the BARI 2D cohort with a mean age of 52 years. Long-term follow-up of the subjects documented an approximately 2% annual conversion from normal albumin excretion to microalbuminuria, and 2.8% from microalbuminuria to macroalbuminuria (30–32). Other studies have supported the association between declining GFR coincident with or following the onset of macroalbuminuria in diabetic nephropathy (1,24,29).
The recognition of both reduced GFR and albuminuria as independent predictors of the development of ESRD and of cardiovascular and all- cause mortality has been highlighted in several studies (33–36). Reduced creatinine clearance was independently associated with an increased risk of developing ESRD in a large Japanese population reported by Iseki et al (33). Subjects with a low creatinine clearance who had proteinuria on dipstick urinalysis had a significantly higher-risk of developing ESRD than patients with a low creatinine clearance not associated with proteinuria (33). In the NHANES III cohort, moderately decreased eGFR and albuminuria were independent predictors of cardiovascular and all-cause mortality in the general population (34). During this same time interval (1988–1994 to 1999–2004) the prevalence of both albuminuria and decreased GFR increased in the United States, according to the most recent NHANE surveys (37). Reduced eGFR and microalbuminuria were risk factors for cardiovascular death, independent of each other and of traditional risk factors in the HUNT II study (35). In patients with vascular disease, both decreased eGFR with normal albumin excretion and microalbuminuria with normal eGFR were independent predictors of future vascular events. The combination of decreased eGFR with albuminuria was associated with the highest risk of vascular events and all cause mortality (35).
We indentified multiple modifiable variables that were associated with the presence of either albuminuria or reduced eGFR. Prior studies have documented that a comprehensive approach to the treatment of these modifiable risk factors is associated with favorable improvements in both renal and cardiovascular outcomes (38–41). Higher HbA1c and smoking history were associated with albuminuria and had an inverse association with reduced eGFR in BARI 2D participants. These findings suggest that conditions associated with reduced eGFR without albuminuria, such as aging, hypertension, and interstitial kidney diseases may be less impacted by hyperglycemia and smoking history than proteinuric kidney diseases, such as diabetic nephropathy. The current study, however, represents cross-sectional baseline data. Other prospective longitudinal observational studies in people with type II diabetes have reported that hemoglobin A1c and heavy smoking were significantly associated with an increased rate of decline in glomerular filtration rate (32).
The strength of the BARI 2D study is the large, well-described population of patients with T2DM and angiographically documented CAD. Limitations of the current study include the determination of ACR from a single urine collection, such that our data did not permit confirmation of persistent microalbuminuria by the generally accepted criterion requiring that 2 out of 3 determinations are abnormal (23,24,28,29). The large number of samples collected and the relatively high diagnostic accuracy of ACR determined on a single urine collection should minimize analytical errors associated with day-to-day variations and albumin excretion (42, 43). In addition, the high prevalence of ACE/ARB use in the BARI 2D population likely contributes to an underestimation of the true prevalence of microalbuminuria in the BARI 2D study. GFR was not directly measured in the current study and was determined by the modified MDRD equation, based on a single determination of serum creatinine concentration. This method of estimating GFR has been validated in similar subjects, particularly in identifying those with reduced GFR (22–24). In the current study, patients with reduced GFR (<60 ml/min/1.73m2) tended to be older and were more likely to be female. Serum creatinine, however, was elevated (1.37–1.51 mg/dl, compared to 0.94–0.97 mg/dl in patients with GFR > 60 ml/min/1.73m2 (Table 1), consistent with a reduction in GFR. Additionally, the BARI 2D study population included only subjects with T2DM with documented CAD, such that this population is not a random sampling of the population of people with T2DM.
The five year average patient follow-up of the BARI 2D study will allow multiple issues concerning renal outcomes to be addressed, including: 1) determination of the development of micro- or macro albuminuria in the 55% of patient with normal kidney function at baseline, 2) determination of rates of progression of kidney disease in the 45% of patients with baseline kidney disease, 3) assessment of the time dependent effects of control of known risk factors for progression of kidney disease, such as control of blood pressure, glucose levels and hyperlipidemia, 4) whether or not insulin sensitizing anti-diabetic medications are associated with better preservation of kidney function than insulin releasing anti-diabetic medications, 5) whether or not there are different rates of progression of kidney disease in patients with reduced GFR without albuminuria, as compared to those with both reduced GFR and albuminuria. These data will also provide important information on the impact of the different stages of kidney disease and its progression on cardiovascular outcomes in T2DM with known CAD.
Acknowledgments
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) is funded by the National Heart, Lung and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, Nos. U01 HL061744, U01 HL061746, U01 HL061748, and U01 HL063804. Significant supplemental funding is provided by GlaxoSmithKline, Collegeville, PA, Bristol-Myers Squibb Medical Imaging, Inc., North Billerica, MA, Astellas, Pharma US, Inc., Deerfield, IL, Merck & Co., Inc., Whitehouse Station, NJ, Abbott Laboratories, Inc., Abbott Park, IL, and Pfizer, Inc., New York, NY.
Generous support is given by Abbott Laboratories Ltd., MediSense Products, Mississauga, Canada, Bayer Diagnostics, Tarrytown, NY, Becton, Dickinson and Company, Franklin Lakes, NJ, J.R. Carlson Labs, Arlington Hts., IL, Centocor, Inc., Malvern, PA, Eli Lilly and Company, Indianapolis, IN, LipoScience, Inc., Raleigh, NC, Merck Sante, Lyon, France, Novartis Pharmaceuticals Corporation, East Hanover, NJ, and Novo Nordisk, Inc. Princeton, NJ.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, or the National Institutes of Health.
References
- 1.Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes care. 2005;28:176–188. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System. Excerpts from the USRDS 2007 annual data report: Atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2008;51(Suppl 1):S1. doi: 10.1053/j.ajkd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Van Dijk PC, Jager KJ, Stengel B, et al. Renal replacement therapy for diabetic end-stage renal disease: Data from 10 registries in Europe (1991–2000) Kidney Int. 2005;67:1489. doi: 10.1111/j.1523-1755.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 4.Pham TT, Sim JJ, Kujubu DA, et al. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol. 2007;27:322. doi: 10.1159/000102598. [DOI] [PubMed] [Google Scholar]
- 5.Kramer HJ, Nguyen QD, Curhan G, et al. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA. 2003;289:3273–3277. doi: 10.1001/jama.289.24.3273. [DOI] [PubMed] [Google Scholar]
- 6.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, et al. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care. 2004;27:195–200. doi: 10.2337/diacare.27.1.195. [DOI] [PubMed] [Google Scholar]
- 7.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity onset diabetes. N Engl J Med. 1984;310:356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 8.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. Arch Intern Med. 1997;157:1413–1418. [PubMed] [Google Scholar]
- 9.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 10.Hillege HL, Fidler V, Diercks GFH, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 11.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 12.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 13.Romundstad S, Holmen J, Kvenild K, et al. Microalbuminuria and all-cause mortality in 2,089 apparently healthy individuals: a 4.4-year follow-up study. The Nord-Trondelag Health Study (HUNT), Norway. Am J Kidney Dis. 2003;42:466. doi: 10.1016/s0272-6386(03)00742-x. [DOI] [PubMed] [Google Scholar]
- 14.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 15.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 16.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 17.Henry RM, Kostense PJ, Bos G, et al. Mild renal insufficiency is associated with increased cardiovascular mortality: the Hoorn study. Kidney Int. 2002;62:1402–1407. doi: 10.1111/j.1523-1755.2002.kid571.x. [DOI] [PubMed] [Google Scholar]
- 18.Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–35. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 19.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events in the elderly. N Engl J Med. 2005;352:2049–60. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 20.Garg Amit X, Kiberd Bryce A, William Clark, et al. Albuminuria and renal insufficiency prevalence guides population screening: Results from the NHANES III. Kidney International. 2002;61:2165–2175. doi: 10.1046/j.1523-1755.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- 21.BARI 2D Investigators. Baseline Characteristics of Patients with Diabetes and Coronary Artery Disease Enrolled in the BARI 2D Trial. Am Heart J. 2008;156:528–536. doi: 10.1016/j.ahj.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 23.Eknoyan G, Hostetter T, Bakris GL, et al. Proteinuria and other markers of chronic kidney disease: A position statement of the national kidney foundation (NKF) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Am J Kidney Dis. 2003;42:617. doi: 10.1016/s0272-6386(03)00826-6. [DOI] [PubMed] [Google Scholar]
- 24.K/DOQI Clinical Practice Guidelines and Clinical Practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(Suppl 2):S17. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Parving H-H, Lewis JB, Ravid M, et al. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: A global perspective. Kidney International. 2006;69:2057–2063. doi: 10.1038/sj.ki.5000377. [DOI] [PubMed] [Google Scholar]
- 26.Gerstein HC, Joyce C, Mann J, et al. Prevalence and Determinants of Microalbuminuria in High-Risk Diabetic and Nondiabetic Patients in the Heart Outcomes Prevention Evaluation Study. Diabetes Care. 2000;23(Suppl 2):B35–B39. [PubMed] [Google Scholar]
- 27.So WY, Kong A, Ronald CW, et al. Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care. 2006;29 (9):2046–2052. doi: 10.2337/dc06-0248. [DOI] [PubMed] [Google Scholar]
- 28.Kramer H, Molitch ME. Screening for kidney disease in adults with diabetes. Diabetes Care. 2005;28:1813–1816. doi: 10.2337/diacare.28.7.1813. [DOI] [PubMed] [Google Scholar]
- 29.American diabetes Association: Standards of medical care and diabetes-2006. Diabetes Care. 2006;29 (Supp1):S4–42. [PubMed] [Google Scholar]
- 30.Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63:225. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 31.UK Prospective Diabetes Study Group. UK Prospective Diabetes Study (UKPDS): Urinary albumin excretion over 3 years in diet-treated type 2 (non-insulin-dependent) diabetic patients and association with hypertension, hyperglycaemia, and hypertriglyceridaemia. Diabetologia. 1993;36:1021. [PubMed] [Google Scholar]
- 32.Rossing K, Christensen PK, Hovind P, et al. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004;66:1596. doi: 10.1111/j.1523-1755.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- 33.Iseki K, Kozen K, Chiho I, et al. Relationship between predicted creatinine clearance and proteinuria and the risk of developing ESRD in Okinawa, Japan. Am J Kidney Dis. 2004;44:806–814. [PubMed] [Google Scholar]
- 34.Astor BD, Hallan SI, Miller EM, et al. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167:1226–1234. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 35.Hallan S, Astor B, Romundsrad S, et al. Association of kidney function and albuminuria with cardiovascular mortality in older versus younger individuals. Arch Intern Med. 2007;167:2490–2496. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 36.Viek ALM, van der Geaaf Y, Spiering W, et al. Cardiovascular events and all cause mortality by albuminuria and decreased Khmer filtration rate in patients with vascular disease. J Intern Med. 2008:1–10. doi: 10.1111/j.1365-2796.2008.01970.x. [DOI] [PubMed] [Google Scholar]
- 37.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 38.Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 39.Gaede P, Vedel P, Parving H-H, et al. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353:617. doi: 10.1016/S0140-6736(98)07368-1. [DOI] [PubMed] [Google Scholar]
- 40.Dahl-Jorgensen K, Bjoro T, Kierulf P, et al. Long-term glycemic control and kidney function in insulin-dependent diabetes mellitus. Kidney Int. 1992;41:920. doi: 10.1038/ki.1992.140. [DOI] [PubMed] [Google Scholar]
- 41.Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications (DCCT) Research Group. Kidney Int. 1995;47:1703. doi: 10.1038/ki.1995.236. [DOI] [PubMed] [Google Scholar]
- 42.Nathan DM, Rosenbaum C, Protasowicki VD. Single-void samples can be used to estimate quantitative proteinuria. Diabetes Care. 1987;10:414. doi: 10.2337/diacare.10.4.414. [DOI] [PubMed] [Google Scholar]
- 43.Schwab SJ, Dunn FL, Feinglos MN. Screening for microalbuminuria. A comparison of single sample methods of collection and techniques of albumin analysis. Diabetes Care. 1992;15:1581. doi: 10.2337/diacare.15.11.1581. [DOI] [PubMed] [Google Scholar]