Abstract
Objectives
Recent work indicates that the feedback negativity (FN) and P3 components from gambling feedback tasks can be understood as mixtures of functionally distinct processes occurring separately in theta and delta frequency bands. The current study was conducted to assess whether dissociable processes occurring in the theta and delta bands would similarly account for activity underlying N2 and P3 components in a go/no-go task.
Methods
The current study measured EEG signals from 66 participants during a go/nogo task, and a time-frequency (TF) principal components analysis (PCA) decomposition approach was used to extract theta and delta measures from condition averages.
Results
Theta and delta measures separately increased in relation to response inhibition, and were uniquely related to the N2 and P3 components, as predicted.
Conclusions
Findings support the view that the theta and delta measures indexed separable processes related to response inhibition, and better indexed the processes underlying N2 and P3 components in this go/no-go task
Significance
Theta and delta measures may index separable functional processes across other common ERP tasks, and may represent an improved target for research relative to standard time-domain components.
Keywords: response inhibition, time-frequency, EEG, theta, delta
Introduction
Time-frequency (TF) approaches to assessing event-related potential (ERP) activity have provided evidence that many common ERP components contain a mixture of overlapping TF components which can index separable underlying processes. Substantial work has focused on ERPs from target detection tasks, where theta (3-7 Hz) and delta (0-3 Hz) TF activity have been shown to underlie common time-domain ERP components such as the P3 (Başar-Eroglu and Demiralp, 2001; Başar et al., 1999; Başar et al., 2001; Demiralp et al., 2001a; Demiralp et al., 2001b; Porjesz et al., 2005; Yordanova et al., 2000; Makeig et al., 2002). Additional work has demonstrated that the error-related negativity (ERN; Gehring et al., 1993; Falkenstein et al., 1991) is well characterized by theta TF measures (Gehring and Willoughby, 2004; Trujillo and Allen, 2007; Yordanova et al., 2004; Cavanagh et al., 2011; Cavanagh et al., 2009a; Cohen and Cavanagh, 2011). Using a recently developed approach based on principal components analysis (PCA) of reduced interference distributions (RID) from Cohen's class of TF transforms (Bernat et al., 2005), recent work has provided improved TF specificity for indexing theta and delta contributions to P3 (Bernat et al., 2011; Bernat et al., 2007; Gilmore et al., 2010), ERN (Bernat et al., 2005; Hall et al., 2007), and most recently the feedback negativity (FN; Bernat et al., 2011; Nelson et al., 2011; Bernat et al., 2012). This recent work with the FN was notable in indexing highly overlapping theta and delta activations, supporting the idea that they index functionally distinct processes, and providing a clear model of how the theta and delta phase dynamics and amplitude combine in an ordered way to produce activity generally measured in traditional time-domain components. The current study focuses on response inhibition ERPs recorded during a go/no-go task. The first goal was to assess for separable theta and delta processes underlying the N2-P3 in response inhibition. The second goal was to assess whether the phase and amplitude dynamics observed to underlie the FN would occur similarly in the go/no-go task, suggesting that these dynamics may be an important approach to measuring common ERP components associated with cognitive control.
Separating Overlapping Theta and Delta Activity with Time-Frequency Analysis
There have been numerous applications of time-frequency approaches to ERP data which have added considerable support to the idea that theta and delta are separable processes underlying ERPs (Demiralp et al., 2001a; Demiralp et al., 2001b; Başar, et al., 1999; Başar et al., 2000), as well as to the characterization of midline frontal theta activity associated with a number of different tasks (Cavanagh, et al., 2011; Cohen et al., 2008; Cohen et al., 2007; Cavanagh et al., 2009b). The current report utilizes a recent TFPCA analysis approach based on the reduced interference distribution from Cohen's class of time-frequency transforms (Bernat et al., 2005). This approach has proven effective in parsing out frequency-independent components that occur in similar time windows from several common components such as the ERN (e.g. Bernat et al., 2005; Hall et al., 2007), the FN (Bernat et al., 2011; Nelson et al., 2011; Bernat et al., 2012), and the P3 (Bernat et al., 2011; Bernat et al., 2007; Gilmore et al., 2010). Thus, because this TF-PCA approach has been effective for assessing delta and theta for several common ERP components, application to the no-go N2 and no-go P3 is likely to provide useful new information about activity underlying these commonly studied components.
A new finding that emerged from the recent TF-PCA work with the FN and P3 (Bernat et al., 2011) was an ordered description of how the phase (positive and negative peaks) and amplitude for both theta and delta combined in traditional time-domain components, suggesting a clear description of how these dynamics can complicate inferences from time-domain measures, as follows. For the time-domain FN and P3, the faster oscillation of theta contributed increased amplitude to the negative polarity of the FN component, but positive amplitude at P3, due to the phase reversal of theta during these two components. On the other hand, the slower changing phase of delta contributed positive amplitude to both the FN and P3 components. This theta and delta phase dynamic had a crucial relationship to gain-loss experimental effects when observed in the time domain; the increased delta activity to gain feedback corresponded to an enhanced positivity at both FN and P3, while enhanced theta activity for losses produced opposite effects at FN (increased negative amplitude) and P3 (increased positive amplitude). Together theta and delta combined additively at FN to create a large gain-loss difference, but in a subtractive manner at P3 resulting in a non-significant gain-loss difference (Bernat et al., 2011). A primary goal of the current study is to assess these theta phase and amplitude dynamics in relation to data from a go/no-go task in order to evaluate whether these dynamics may contribute to the commonly assessed N2 and P3 components, as was found for the FN and P3 components. Such effects could have implications for understanding how theta and delta contribute to ERPs more broadly, and provide an assessment of separable theta and delta functional processes during response inhibition.
Go/No-Go and Response Inhibition
Two conventional time-domain ERP components elicited during a go/no-go task, the no-go N2 and no-go P3, are thought to index the cognitive and motor processes underlying response inhibition (Kopp et al., 1996; Pfefferbaum et al., 1985; Kok, 1986). The no-go N2 is a negative potential with a midline frontal distribution that peaks between 250 and 350 ms after the presentation of a stimulus associated with response inhibition (Eimer, 1993). While there is some debate as to whether the no-go N2 is an electrocortical index of response inhibition (Kok, 1986) or simply an indicator of response conflict or target detection (Nieuwenhuis et al., 2003; Donkers and Boxtel, 2004), recent research has indicated that the no-go N2 may have two functional components with separate neural generators, one involving response inhibition (mediated by the dorsal lateral and ventral prefrontal cortices), and another associated with conflict monitoring (anterior cingulate cortex; Lavric et al., 2004). The no-go P3 is also increased for demands of response inhibition, but peaks between 300 and 500 ms post-stimulus, and has a more central or anterior distribution compared to the parietal P3 response related to response commission (Pfefferbaum et al., 1985; Kopp et al., 1996; Fallgatter et al., 1997). Reliable no-go N2 and no-go P3 components have also been elicited by the suppression of covert responses (e.g., counting some stimuli but not counting others; Pfefferbaum, 1985), indicating that the effects are not solely due to overlapping motor potentials or the withholding of a covert motor response. While the no-go N2 has generally been associated with response inhibition, the findings for the no-go P3 have been a matter of debate: despite work suggesting the no-go N2 and no-go P3 having similar neural sources identified through source localization (Bokura et al., 2001), several studies have found no effect of response type on the P3 when go and no-go trials are equiprobable (Lavric et al., 2004; Falkenstein et al., 1999), and as the P3 response is notoriously sensitive to stimulus frequency (Yamaguchi and Knight, 1991), it is possible that the experimental effects are due in part to the rarity of no-go trials. In sum, the no-go N2 and no-go P3 reflect both overt and covert inhibition of prepotent responses and index general executive control processes.
In addition to time-domain measures, time-frequency approaches have also been implemented with ERP data from go/no-go tasks, although the information is relatively scarce. Reports have documented increased mid-frontal theta-band activity during demands of response inhibition (Yamanaka and Yamamoto, 2009; Barry, 2009; Kamarajan et al., 2004; Kamarajan et al., 2006; Kirmizi-Alsan et al., 2006), indicating that theta activity may reflect inhibitory processes. In line with findings indicating that joint theta and delta activity underlies several ERP components (Başar et al., 1999; Karakas et al., 2000a, 2000b), some studies report delta-band activity underlying the nogo N2 and P3 responses, with the delta-no-go activity having a more central topographic distribution compared to delta-go data (Barry, 2009; Kirmizi-Aslan et al., 2006; Kamarajan et al., 2004; Kamarajan et al., 2006). Recently, Müller and Anokhin (2012) reported increased inter-electrode phase coherence in both theta and delta between F3-F4, indicative of functional integration between bilateral prefrontal channels during response inhibition.
These TF applications with go/no-go data are largely based on trial-level data, and it is important to explain that TF approaches can be applied to both trial-level or condition averaged data. While TF applications using averaged data will contain mostly phase-locked information, applications to trial-level data will contain all activity, phase-locked and non phase-locked. Broadly, the majority of TF applications in the literature have focused on trial-level data, with the goal of assessing the new information available at the trial-level that cannot be modeled in the condition averaged data from which common time-domain measures are taken (N2, P3, etc.). The work reported here, however, is focused on application of TF analysis using condition averaged data, with the goal of re-representing widely studied time-domain N2 and P3 components in terms of TF theta and delta activity. The idea is to use exactly the same averaged data in both time-domain and time-frequency approaches to show direct correspondence between the measures across the two domains. This approach has recently contributed important new information to the understanding of the FN and P3 time-domain components observed in a common gambling feedback task (Bernat et al., 2011), as detailed above. In this regard, one goal of the current report is to evaluate the idea that common time-domain components generally index mixtures of separable TF components, and thus TF decomposition may be crucial to understanding widely reported effects using time-domain components.
Current Study
Because functionally distinct theta and delta components have been shown to have unique contributions to the FN-P3 complex, we sought to evaluate whether these measures, and their associated phase dynamics, would be similarly observed for an N2-P3 complex observed in a go/no-go task. Positive findings would provide two new pieces of information. First, this would extend previous work from gambling feedback data, and suggest that separable yet overlapping theta and delta processes are active during response inhibition. Second, if the theta/delta phase dynamics are shown to underlie the go/no-go N2 and P3, as was shown for the FN and feedback-P3, this would support the suggestion that this dynamic may be at work more generally in common ERP measures and could be important to evaluate across data from other experimental paradigms. To assess this, the current study compared TF theta and delta measures with conventional time-domain N2 and P3 ERP measures in data from a go/no-go task using the TF-PCA approach described above (Bernat et al., 2005), in the same manner as applied previously to gambling feedback data (Bernat et al., 2011). Relative to previous reports of amplitude increases for both N2 time-domain and TF theta components in relation to no-go stimuli, we hypothesized that response inhibition (no-go) would produce a larger theta response than response commission (go). Relative to consistent reports of increased amplitude of time-domain P3 component measures, we hypothesized that no-go trials would be associated with increased delta band activity, relative to go stimuli. We hypothesized that due to increases in both theta and delta associated with no-go stimuli (unlike the gambling-feedback theta and delta effects, which were in opposing directions), the combination of separable phase dynamics would result in an attenuated go/no-go effect at N2 and an enhanced go/no-go effect at P3 as measured in the time-domain. In summary, the two main goals of the current study were: 1) to assess whether theta and delta TF measures index independent processes occurring during response inhibition, and 2) to identify whether theta and delta have unique contributions to the N2-P3 complex (similar to the FN-P3 decomposition).
Methods
Participants
Participants were 78 undergraduate and community participants recruited from introductory psychology classes at the Florida State University and through Craigslist. Participants were compensated with course credit or money ($10.00/hour). Twelve subjects were excluded from the dataset: six due to software problems during recording, and six due to excessive artifacts (>33% of trials rejected using the methods described below). Participants were screened for normal or corrected to normal vision, neurological disorders, and previous traumatic brain injuries. Thus, the final sample contained 66 participants (31 females, age: M = 20.9, SD = 4.9).
Experimental Procedure
Testing was conducted in a sound-attenuated, dimly lit room, with the stimuli presented centrally on a 21” CRT color monitor at a viewing distance of 100 cm. Participants completed a set of mood and personality questionnaires prior to testing and then performed five tasks (a gambling feedback task, a reinforcement learning task, a Flanker task, a Novelty-Oddball task, and a go/no-go task), the one reported here being the last for each participant. Participants took a brief break after the third task. Together, the recording session lasted between 1.5 and 2 hours. Behavioral responses were made using a PST Serial Response Box and stimuli were delivered using E-Prime 1.1 (Psychology Software Tools, Inc.).
The experimental task was a go/no-go task similar to the design used by Roche and colleagues (2005), where two different white letters were displayed sequentially on a black background, and participants were asked to make a button press when the stimulus presented followed a different stimulus (go trials), but to withhold their response when the stimulus repeated the one that immediately preceded it (no-go trials; e.g., the fourth letter in the string H-K-H-H-K). No-go trials were pseudo-randomly interspersed throughout the task. Three blocks of 144 trials were completed, with 108 go trials and 36 no-go trials in each block (75% and 25%, respectively). The letters used were X-Y, O-P, and D-U, and a different pair was used for each block. Stimulus duration was 300 ms, the response window was 1150 ms, and the inter-trial-interval was 900 ms. Participants completed a set of 20 practice trials prior to recording, but no electrocortical data was recorded. After each block participants were presented with their overall accuracy and were allowed to rest until manually initiating the next block.
Electroencephalographic Recordings
Recordings were collected using a 128-channel Synamps RT amplifier (Neuroscan, Inc.) in conjunction with a Neuroscan 128-channel Quik-cap (sintered Ag-Ag/Cl; non-standard layout [NSL] channel positions). Problems with adequate scalp connection for 10 channels around the ears necessitated removing these channels from the data, which resulted in a total of 112 channels available for analysis. Horizontal and vertical ocular activity was recorded using electrodes positioned on the outer canthus of both eyes and above and below the left eye, respectively. Impedances were kept below 10 k . EEG signals were referenced to the vertex electrode during recording, and rereferenced to averaged mastoid sites offline. Data were collected with an analog 0.05 to 200 Hz bandpass filter, and digitized at 1000 Hz using Neuroscan Acquire 4.5 (Neuroscan, Inc.).
Data Preprocessing
Continuous EEG recordings were epoched from 1000 ms pre- to 2000 ms post-stimulus, and re-referenced to averaged mastoid sites. Data were then corrected for ocular artifacts using an algorithm developed by Semlitsch, Anderer, Schuster, and Presslich (1986), implemented in the Neuroscan Edit 4.5 software (Neuroscan, Inc.). Finally, data were downsampled to 128 Hz using the Matlab resample function (Mathworks, Inc.). These 3 second epochs were baseline-corrected for the 150 ms preceding stimulus onset. Two methods of data cleaning were undertaken. First, a two-step method for trial-level rejection was employed: 1) whole trials were rejected if activity at two frontal sites (equivalent to F3 and F4 from the 10-20 system) exceeded ± 75 μV in either the pre- (-1000 to -1 ms) or post-stimulus (1 to 2000 ms) time windows (relative to one another), and 2) activity at individual sites that exceeded ± 100 μV within each trial (based on the same pre- and post-stimulus time regions) were also omitted from analysis. Using these criteria, 9.7% of trials were excluded. Additionally, across all subjects and electrodes, a total of 10 electrodes (out of 7392) became disconnected during recording, and were omitted from the analysis. Trials in which an incorrect response was made (i.e., an error of omission on go trials or an error of commission on no-go trials) were excluded from analysis, with 7.75% of trials removed. After preprocessing, the data were averaged across trials within stimulus class (i.e., go, no-go) individually for each participant. Go trials were segmented into three groups of every third go (e.g., a sequence of go-go-go-go would be grouped as 1, 2, 3, 1, respectively) to equate the number of trials between go and no-go conditions submitted to the principal components analysis (see below), and go trials were then averaged together post-PCA for statistical analysis.
Data Reduction
Time-domain components: N2 and P3
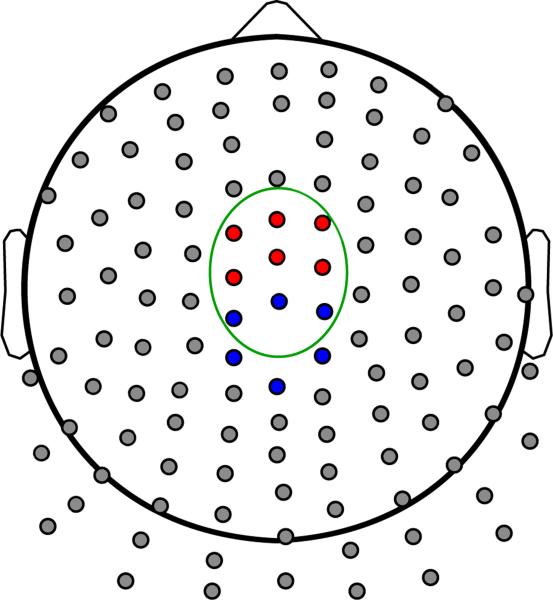
The time-domain components were identified through visual inspection of grand average ERP waveforms. The time-domain N2 was defined as the maximal negative deflection in the ERP occurring between 179.69 and 390.63 ms post-stimulus, relative to a -101.56 to -7.81 ms baseline, and the P3 was defined as the maximum positive deflection occurring between 250 to 492.19 ms post-stimulus, relative to the same baseline (where ms corresponded to bins of a 128 Hz resampled signal). For statistical analyses, activity during the N2 and P3 were quantified as the average of nine frontocentral sites which were most proximal topographically to the N2 and P3 no-go minus go differences (see Figure 1).
Figure 1.
Channel layout of the Neuroscan 128-Channel NSL Quik-Cap. Clusters of channels used for statistical analysis are highlighted. Red: Frontal cluster for theta; blue: central cluster for delta; channels within green oval: fronto-central cluster for N2 and P3 ERPs.
Time-frequency components: theta and delta
As noted above, one common problem with traditional ERP measures is the substantial spatial and temporal overlap between components (Spencer et al., 1999), and because the N2 and P3 overlap in time and topographic distribution, joint TF analysis was used to parse activity that has differing spatial and/or temporal information. This method has been shown to effectively extract separable frequency components that overlap in time from several common ERP components (Bernat et al., 2005; Bernat et al., 2007; Bernat et al., 2011; Hall et al., 2007).
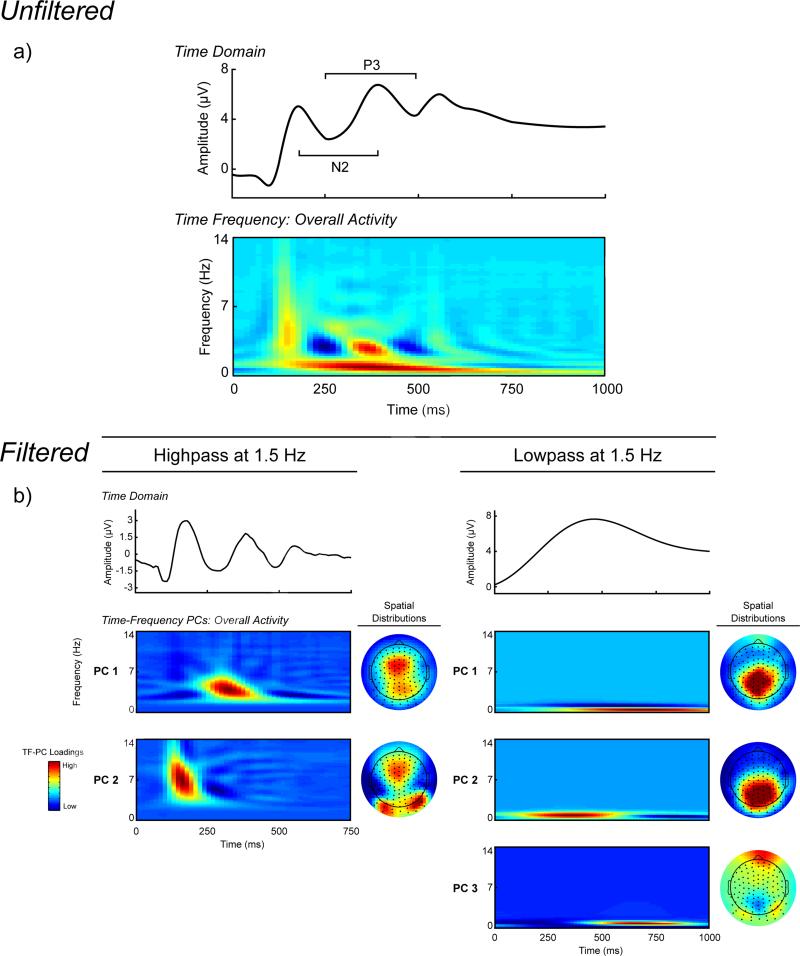
The data was pre-filtered in order to isolate the relatively weaker higher frequency theta activity from the lower frequency delta activity for the PCA (cf. Bernat et al., 2011). The theta and delta filter cutoffs were chosen based on visual inspection of the unfiltered averaged TF energy representation across all subjects and stimuli. The separation of the prominent lower frequency component and the higher frequency activity in the N2-P3 time window is evident in the unfiltered averaged TF representation in Figure 2, and occurred at 1.5 Hz. The condition averaged stimulus-locked signals were filtered independently using separate highpass and lowpass 3rd order Butterworth filters set at 1.5 Hz for the high frequency and low frequency data, respectively. Next, the filtered signals were transformed into TF energy representations using the binomial RID variant of Cohen's class of TF transformations using the full epochs to provide sufficient data to resolve low frequency activity (Bernat et al., 2005). PCA was applied to the TF transforms of the theta and delta filtered signals separately. This TF-PCA approach (based on the covariance matrix with a Varimax rotation; Bernat et al., 2005) was applied to each TF representation with a 0 to 14 Hz frequency window and 0 to 750 ms or 0 to 1000 ms post-stimulus time window for high and low frequency signals, respectively, to identify the underlying components in the dataset that accounted for the greatest variance across all TF data points.
Figure 2.
Time-domain and time-frequency (TF) decomposition of stimulus-locked ERPs. All ERPs and TF representations are plotted as the average across corresponding channel clusters (see Figure 1). Row A, waveform plot. Averaged unfiltered stimulus-locked ERP across all trials. TF-representation plot. Averaged unfiltered TF representation of the ERP across all trials. Row B, waveform plots. Averaged ERP activity across all trials, frequency-filtered using a 3rd order Butterworth filter to isolate higher-frequency (1.5 Hz highpass) and lower frequency (1.5 Hz lowpass) activity. TF representation plots. TF representations of the high- and low-frequency data principal component scores across all trials, sorted by percentage variance accounted for post-Varimax rotation. Topographic maps. Scalp topography distributions for the mean of the TF-PCA energy for each TF representation. For the high-frequency data the first PC has a frontal distribution, while the second PC has a mixture of bilateral occipital and frontal distributions. The first two PCs for the low-frequency data share a centro-parietal distribution, while the third PC is mainly frontal.
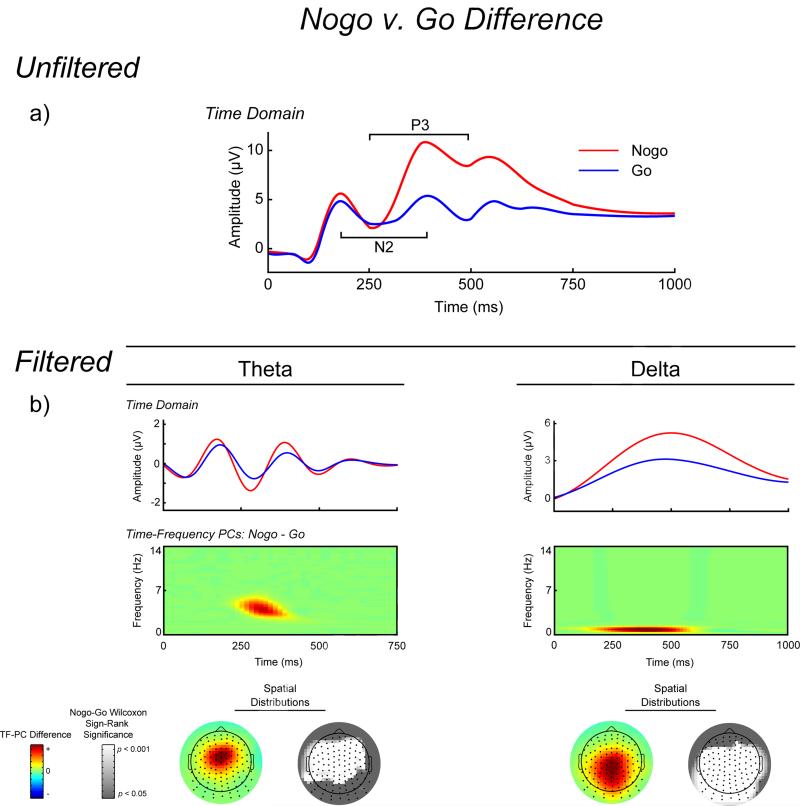
For the higher frequency data, two components were extracted based on the Scree test, both accounting for a total of 28.5% of variance in the decomposition (see Figures 2 and 3). PC1 occurred during the N2-P3 complex (~275 to 400 ms) in the theta range (3 to 5.5 Hz), and PC2 occurred earlier during the P2 ERP (~120 to 200 ms) and at a higher frequency than PC1 (~5 to 9 Hz). Of interest to the present study is PC1 (Theta) for the following reasons: 1) it exhibited an experimental difference (Wilcoxon Signed-Rank tests comparing activity in no-go vs. go trials: Zs = 6.136 and 0.208, ps < 0.001 and = 0.836 for PC1 and PC2, respectively), and 2) its time course corresponded best to the N2-P3 ERPs. Three PCs were extracted using the Scree test for the lower frequency data, accounting for a total of 93.6% of the variance in the data. PC1 and PC3 were maximal between 600 and 800 ms, and PC2 occurred during the N2-P3 components (~250 to 450 ms). PC2 (Delta) was chosen for further analysis for the following reasons: 1) it showed the largest no-go minus go difference (Wilcoxon Signed-Rank tests comparing activity in no-go vs. go trials: Zs = 6.455, 6.532, and 2.993, ps < 0.001, < 0.001, and = 0.003 for PCs 1–3, respectively), and occurred closest in time to the N2-P3 complex (PCs 1 and 3 represented post-P3 activity). Theta and delta were quantified as the average of six fronto-central and central sites, respectively, that were most topographically proximal to the center of the no-go minus go difference, and these averages were used as the unit of analysis (see Figure 1).
Figure 3.
Time-domain and time-frequency (TF) representations of the no-go minus go differences. All signals and TF-representations are plotted as the average across corresponding channel clusters (see Figure 1). Row A, waveform plot. Averaged unfiltered stimulus-locked ERPs for no-go (red) and go (blue) trials separately. Evident on the no-go trials is the negativity peaking around ~300 ms (no-go N2) and the subsequent positivity (no-go P3). Row B, waveform plots. Average ERPs for no-go and go trials separately, frequency filtered with a 3rd order Butterworth filter to capture the activity in the theta and delta TF-energy PCs (3.25-4.75 and 0.5-1.0 Hz, respectively). These plots depict the increase for no-go activity in both theta and delta frequency bands. TF representation plots. No-go minus go difference representations for the PCs loadings of averaged TF activity. Again, both theta and delta are sensitive to no-go stimuli. Colored topographic maps. Scalp topography for the mean no-go minus go difference of the TF-PCA loadings for theta and delta. Increased no-go theta is maximal at frontal sites, while delta has a more centro-parietal increase for no-go stimuli. Grayscale topographic maps. These topographic maps depict the statistical significance of the no-go minus go Wilcoxon Signed-Ranks test for each channel, where theta showed the most significance for frontal channels and the difference in delta was maximal at central-parietal sites.
Data Analysis Plan
Due to their robustness against departures from normality, nonparametric statistical tests were used. To evaluate the effect of stimulus on the ERPs and TF-energy PCs, we preformed Wilcoxon Signed-Rank tests comparing no-go and go trials. The bivariate association between TF-theta and TF-delta was estimated using Spearman's rank-order correlation for both the grand average across trials and the difference scores between no-go and go component measures. Next, regression analyses were performed to assess whether the TF components contributed uniquely to, and accounted for, the time-domain N2 and P3 components. For each regression, theta and delta scores served as the predictor variables, with N2 and P3 alternately serving as the dependent variable. For two of the models, the overall grand average values were used for the time-domain component (i.e., N2 or P3) and the theta and delta scores, and for the remaining two, the no-go and go difference scores were used for both the ERP components and TF scores. Thus, the four models were: 1) the overall N2 peak as the dependent variable, and overall theta and delta measures as the predictors, 2) the overall P3 peak as the dependent variable, and overall theta and delta scores as the predictors, 3) the no-go minus go N2 difference score as the dependent variable, and the no-go minus go difference scores for theta and delta as the predictors and finally, 4) the no-go minus go P3 difference score as the dependent variable and the theta and delta no-go minus go difference scores were used as predictors.
Results
Behavioral Results
Correct responses to go stimuli had a mean reaction time (RT) of 445.06 ms (SD = 75.63 ms), while incorrect responses to no-go stimuli (false alarms) had a mean RT of 451.89 ms (SD = 104.49). Since the number of go and no-go stimulus were different, accuracy rates were calculated within each stimulus type (i.e., sum of correct omissions/sum of no-go trials, and sum of correct commissions/sum of go trials). The mean ratio of correct responses in the go condition was 97.45% (SD = 2.98), while the mean ratio of correct omissions for the no-go condition was 76.64% (SD = 15.00), indicating that participants performed fairly well, although the no-go condition may have been more difficult.
Impact of Response Type on Time-Domain and Time-Frequency Components
Time-domain N2 and P3 and TF-theta and TF-delta effects are presented in Figure 3. Wilcoxon Signed Rank tests comparing no-go and go trials indicated that while there was no effect of stimulus on the N2 peak at frontal-central sites (Z = -0.361, p = 0.718), no-go trials elicited a larger P3 than go trials at central sites (Z = 6.832, p < .001). For the TF-PCs, both theta and delta were larger for no-go stimuli than go stimuli (Zs = 6.136 and 6.532, respectively, ps < .001).
Relationship Between TF Theta and Delta
Spearman rank order correlations were performed for both grand average and go minus no-go difference to examine the relationship between TF theta and delta. For the grand averages, theta and delta evidenced a moderate correlation (Spearman's rho (64) = .40, p < .001), suggesting that while delta and theta shared a moderate amount of variance, they did not simply index the same underlying process. The correlation between theta and delta no-go minus go difference scores was also moderate (Spearman's rho (64)= .27, p = .029), similarly suggesting separable processes.
Predicting ERP Component Activity using TF Theta and Delta
Table 1 shows four separate ordinary least squares (OLS) regressions predicting, alternately, N2 or P3 peak with theta and delta to investigate the relationship between time-domain and TF representations of N2 and P3. For predicting the grand average N2 scores, grand average theta and delta scores accounted for 46.72% of the variance, and critically, both accounted for unique variance. For the P3 grand average measure, theta and delta explained 83.84% of the variance, and again, each TF component accounted for unique variance in P3. For the no-go minus go difference models, theta and delta difference scores accounted for 37.60% of variance in the N2 component scores, with both theta and delta having unique and near equal contributions to the model. Finally, theta and delta difference scores accounted for 74.10% of the variance in the no-go minus go P3 difference scores, with both accounting for unique variance in the model. Regression assumptions of residual normality, independence of residuals, and homoscedasticity were satisfied.1
Table 1.
Regression Parameters from Models Predicting Time-Domain N2 and P3 with TF-Theta and TF-Delta
| Time-Domain Measure | Model ParametersOverall Activity (and df) | No-Go Minus Go Difference | |
|---|---|---|---|
| N2 Peak | F (2, 63) | 27.63*** | 18.98*** |
| R2 | .47 | .38 | |
| Theta t (63) | –4.77*** | –5.46*** | |
| Delta t (63) | 7.25*** | 5.31*** | |
| P3 Peak | F (2, 63) | 163.39*** | 90.13*** |
| R2 | .84 | .74 | |
| Theta t (63) | 4.96*** | 4.12*** | |
| Delta t (63) | 13.17*** | 8.67*** | |
p ≤ 0.0001
Discussion
This study investigated theta and delta TF activity during a complex response inhibition task, based on previous research demonstrating that indexing theta and delta activity during common ERP component windows measures can offer some important advantages relative the traditional time-domain measures (e.g. FN and P3; Bernat et al., 2011). The positive results from the current study provide information relative to the two primary aims. First, results extend previous work with gambling feedback data to this go/no-go task, revealing that theta and delta measures were both increased for demands of response inhibition, and indexed separable but overlapping processes occurring during conventional electrocortical indices of response inhibition (i.e., N2 and P3). This provides motivation for future work to better understand the functional role of these theta and delta processes during response inhibition and decision making. Second, results bolster the view that indexing these co-occuring but separable processes operating in theta and delta frequency ranges may offer an important new way to look at traditional time-domain component measures based on typical condition averaged ERP data. The current findings support the view that common time-domain ERP measures (e.g., N2 and P3), contain a mixture of at least these two processes (which can vary independently from task to task), obscuring effects and complicating inferences if not disaggregated.
Functional Aspects of Theta and Delta Dynamics During Response Inhibition
The observed increase in medial-frontal theta activity is consistent with previous observations with go/no-go task data (Yamanaka and Yamamoto, 2009; Barry, 2009; Kamarajan et al., 2004; Kamarajan et al., 2006; Kirmizi-Alsan et al., 2006), as well as other control-related processes such as response error and feedback processing (Bernat et al., 2011; Gehring and Willoughby, 2004; Trujillo and Allen, 2007; Yordanova et al., 2004; Cavanagh et al., 2011; Cavanagh et al., 2009a; Cavanagh, et al., 2011; Cohen et al., 2007; Cavanagh et al., 2009b). This is in addition to the large number of studies demonstrating increases in traditional time-domain N2/ERN/FN components in these tasks (Pfefferbaum et al., 1985; Gehring et al., 1993; Miltner et al., 1997). Source imaging of these effects have indicated the anterior cingulate cortex (ACC) as a primary generator of these theta-related ERP components (Gehring and Willoughby, 2004; Miltner et al., 1997; Cohen, 2011; Luu and Tucker, 2001; Luu et al., 2004; Luu, et al., 2003; Wang et al., 2005; Bokura et al., 2001; Pandey et al., 2012). The observed theta increases for no-go stimuli are consistent with an initial detection of demands of motor inhibition associated with no-go stimuli, similar to the well documented no-go N2 component (Pfefferbaum et al., 1985; Falkenstein et al., 1999; Kok, 1986). Together with previous work, the present results add weight to the notion of a common ACC-based frontal midline theta underlying a number of ERP negativities (e.g., ERN, FN, N2; cf. Cavanagh et al., 2011). These findings are consistent with recent observations of a broader salience network identified via fMRI, in which the ACC plays a central role (Seeley et al.. 2007).
Previous empirical work has detailed delta band activity associated with a myriad of cognitive functions, including reward processing (Bernat et al., 2011; Nelson et al., 2011), target detection (Schürmann et al., 1995; Gilmore et al., 2010), commission of motor errors (Cavanagh et al., 2011; Yordanova et al., 2004), and reward magnitude (Bernat et al., 2012). In contrast to the delta effects exhibited in a gambling-feedback task, where theta and delta were sensitive to different stimuli (theta-loss and delta gain; Bernat et al., 2011), the delta activation detailed in the current report was sensitive to the same experimental effect as theta (i.e., greater for no-go stimuli). This delta activity may dually reflect motor/cognitive inhibition (Smith et al., 2008) and stimulus context updating, similar in function to the P3a (Polich, 2007). From the ERPs presented in Figure 1, delta activity can be observed to occur throughout the ERP window, and while TF-PC analysis indicated that this delta activity significantly contributed to both the N2 and P3 components, regression models indicated that delta clearly contributes most to P3 amplitude. These different delta-related findings suggest that delta activity is sensitive to differing manipulations across tasks. Thus, while delta may index a relatively singular process within a certain paradigm, the present results suggest that delta most likely does not represent a similar process across tasks as is suggested for theta. From this, there appear to be two separable, but highly overlapping, co-activations operating throughout the N2 and P3 ERP components, both of which are sensitive to, but carry unique variance relating to, demands of response inhibition
Implications for ERP Measurement
The positive results in the current report strengthen the notion that theta and delta phase dynamics play a crucial role in the morphology of ERPs, often obscuring effects observed in traditional time-domain component measures, and bolster the view that time-frequency analyses may offer an important new look at traditional time-domain component measures from condition averaged ERP data. The standard N2 and P3 measures observed here did not accurately characterize the multiple processes occurring during (i.e., theta and delta) related to response inhibition which occurred during the ERP window, similar to what was shown for the FN and P3 components during a gambling feedback task (Bernat et al., 2011). This bolsters the idea that superimposed theta and delta processes may underlie ERP components across a broad array of tasks. In detail, the rapid oscillatory rise and fall of theta activity corresponds to a polarity-shift from a negative deflection during the N2 to a positive deflection at the P3, while delta contributes a unidirectional positivity throughout the ERP response. Moreover, in the current report, regression models indicated that theta and delta contributed uniquely in the opposite direction at N2, creating a non-significant no-go minus go difference (subtractive effects of the opposing polarities during no-go trials), but uniquely in the same direction at P3, leading to a rather robust no-go/go difference (additive effects of the joint positivity of theta and delta during no-go trials). This relationship of TF phase dynamics to ERP measures contributes substantially to the time-domain experimental effects, such that if both theta and delta are larger for the same stimuli (e.g., as in the current report) they combine to produce a muted N2 effect and inflated P3 effect, and conversely, if theta and delta are sensitive to the same stimuli in opposite directions or to different stimuli (e.g., delta-gain and theta-loss; Bernat et al., 2011). The co-activation of theta and delta and their separate relations to ERP components indicate that dividing ERPs into components based on peaks and troughs may artificially separate overlapping, but separable, processes (e.g., theta and delta) operating throughout the ERP response. Thus, TF EEG phase dynamics have a crucial role in the structure of ERP components, and decomposing ERPs into TF representations can help avoid the problems of summed overlapping frequency information on ERP morphology (superposition principle; Schürmann et al., 2001).2
Future Directions and Limitations
A central future direction motivated by these findings is to investigate the functional aspects and neural generators of theta and delta during response inhibigion. For example, now that both theta and delta have been shown to be sensitive to response inhibition, it would be interesting to assess the role and coordination of each in terms of behavioral indices, such as reaction time and accuracy. In terms of neuronal generators, while an a priori hypothesis of ACC sources underlying theta activity would be expected from previous reports detailing the sources of no-go N2 and other medial-frontal negativities, as mentioned above, the neuronal generators of delta and the no-go P3 are less clear. Another measure of interest, not evaluated in the present study, is phase-synchrony (Aviyente et al, 2011; Tallon-Baudry et al. 1996; Lachaux et al., 1999). This includes intertrial phase synchrony (i.e., quantification of the consistency of brief responses from trial to trial at a single electrode) and inter-regional phase synchrony (i.e., indexing short- and long-range functional integration via between-channel synchronization within trials). Such work could reveal whether theta and delta have separable contributions in terms of these EEG measures regarding response inhibition, as the present amplitude results suggest. It would also be of interest to further assess other frequency bands (e.g., alpha, beta, gamma) to better detail the TF-varying activation underlying conventional ERP components in this and other tasks. Finally, it will be of interest to evaluate theta and delta contributions to conventional components in additional task paradigms, to further understand contributions from these frequency bands from both a function and measurement perspective.
Highlights.
Conventional go/no-go time-domain N2 and P3 measures each contain mixtures of independent and temporally overlapping medial-frontal theta and centro-parietal delta activity.
Theta and delta activity increase independently during no-go response inhibition.
The phase of theta and delta activity detail a principled mapping between time-frequency and conventional time-domain component measures.
Acknowledgement
This work was supported by the National Institute of Mental Health Grant MH080239 (PI: Bernat).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Three tests were performed to check the regression assumptions of normality, residual independence, and constant variance of the residuals. The Shapiro-Wilk test was used to test whether the residuals from all four models reported in Table 1 satisfied normality, with the null hypothesis that the data were normally distributed. All four tests were not significant (Ws > 0.98, ps > 0.27), indicating that the null hypothesis of normality could not be rejected. Durbin-Watson tests were performed for each regression model to evaluate whether the residuals were independent. With the exception of positive autocorrelation for the P3 difference model (p = 0.029), all p-values were above 0.12, indicating the null hypothesis of residual independence could not be rejected. Since the D-statistic of the P3 difference model was only 1.5, the instance of autocorrelation was not very strong, indicating there was no serious need for correction. To check for constant variance, OLS models were fitted with the predicted values from the regression models in Table 1 predicting the absolute value of the residuals, with the null hypothesis of constant residual variance (Faraway, 2005). All models and predictors were not significant (ps > .19), indicating that the null hypotheses of constant variance cannot be rejected. Thus, the assumptions of all four regression models were largely satisfied.
We use the term ‘superposition principle’ to describe the idea that ERPs consist of the superimposed activation from several frequency bands, but stop short of addressing the question of the nature of the underlying EEG activity, in terms of arbitrating between contributions from time-locked phasic bursts or ongoing oscillations of neuronal populations, as that is out of the scope of the current report.
References
- Aviyente S, Bernat EM, Evans WS, Sponheim SR. A phase synchrony measure for quantifying dynamic functional integration in the brain. Hum Brain Mapp. 2011;32:80–93. doi: 10.1002/hbm.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RJ. Evoked activity and EEG phase resetting in the genesis of auditory Go/NoGo ERPs. Biol Psychol. 2009;80:292–299. doi: 10.1016/j.biopsycho.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Başar-Eroglu C, Demiralp T. Event-related theta oscillations: an integrative and comparative approach in the human and animal brain. Int J Psychophysiol. 2001;39:167–195. doi: 10.1016/s0167-8760(00)00140-9. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroglu C, Karakas S, Schurmann M. Brain oscillations in perception and memory. Int J Psychophysiol. 2000;35:95–124. doi: 10.1016/s0167-8760(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Malone SM, Williams WJ, Patrick CJ, Iacono WG. Decomposing delta, theta, and alpha time-frequency ERP activity from a visual oddball task using PCA. Int J Psychophysiol. 2007;64:62–74. doi: 10.1016/j.ijpsycho.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Baskin-Sommers A. Time-frequency theta and delta measures index separable components of feedback-processing. Int J Psychophysiol. 2012;85:341. doi: 10.1111/psyp.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Steele VR, Gehring WJ, Patrick CJ. Externalizing psychopathology and gain-loss feedback in a simulated gambling task: dissociable components of brain response revealed by time-frequency analysis. J Abnorm Psychol. 2011;120:352–364. doi: 10.1037/a0022124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Williams WJ, Gehring WJ. Decomposing ERP time-frequency energy using PCA. Clin Neurophysiol. 2005;116:1314–1334. doi: 10.1016/j.clinph.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Kobayashi S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clin Neurophysiol. 2001;112:2224–2232. doi: 10.1016/s1388-2457(01)00691-5. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJ. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci. 2009a;29:98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJ. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. NeuroImage. 2009b;49:3198–3209. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJ. Theta lingua franca: a common mid- frontal substrate for action monitoring processes. Psychophysiology. 2011;49:220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. Error-related medial frontal theta activity predicts cingulate-related structural connectivity. NeuroImage. 2011;55:1373–1383. doi: 10.1016/j.neuroimage.2010.12.072. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Cavanagh JF. Single-trial regression elucidates the role of prefrontal theta oscillations in response conflict. Frontiers in Psychology. 2011;2:30. doi: 10.3389/fpsyg.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. NeuroImage. 2007;35:968–978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Ridderinkhof KR, Haupt S, Elger CE, Fell J. Medial frontal cortex and response conflict: evidence from human intracranial EEG and medial frontal cortex lesion. Brain Res. 2008;1238:127–142. doi: 10.1016/j.brainres.2008.07.114. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Istefanopulos Y, Başar-Eroglu C, Başar E. Wavelet analysis of oddball P300. Int J Psychophysiol. 2001a;39:221–227. doi: 10.1016/s0167-8760(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Comerchero M, Polich J. Wavelet analysis of P3a and P3b. Brain Topogr. 2001b;13:251–267. doi: 10.1023/a:1011102628306. [DOI] [PubMed] [Google Scholar]
- Donkers FC, van Boxtel GJ. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and Cognition. 2004;56:165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol Psychol. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroenceph Clin Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol (Amst) 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Brandeis D, Strik WK. A robust assessment of the NoGo-anteriorisation of P300 microstates in a cued continuous performance test. Brain Topogr. 1997;9:295–302. doi: 10.1007/BF01464484. [DOI] [PubMed] [Google Scholar]
- Faraway JJ. Linear models with r. Chapman and Hall/CRC; Boca Raton: 2005. [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Dochin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Willoughby AR. Are all medial frontal negativities created equal? Toward a richer empirical basis for theories of action monitoring. In: Ullsperger M, Falkenstein M, editors. Errors, conflicts, and the brain. Current opinions on performance monitoring. Max Planck Institute of Cognitive Neuroscience; Leipzig, Germany: 2004. pp. 14–20. [Google Scholar]
- Gilmore CS, Malone SM, Bernat EM, Iacono WG. Relationship between the P3 event-related potential, its associated time-frequency components, and externalizing psychopathology. Psychophysiology. 2010;47:123–132. doi: 10.1111/j.1469-8986.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychol Sci. 2007;18:326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjez B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, et al. The role of brain oscillations as functional correlates of cognitive systems: a study of frontal inhibitory control in alcoholism. Int J Psychophysiol. 2004;51:155–180. doi: 10.1016/j.ijpsycho.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjez B, Jones K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, et al. Event-related oscillations in offsprings of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry. 2006;59:625–634. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakaş S, Erzengin ÖU, Başas E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin Neurophysiol. 2000a;11:1719–1732. doi: 10.1016/s1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- Karakaş S, Erzengin ÖU, Başas E. The genesis of human event-related responses explained through the theory of oscillatory neural assemblies. Neurosci Lett. 2000b;285:45–48. doi: 10.1016/s0304-3940(00)01022-3. [DOI] [PubMed] [Google Scholar]
- Kirmizi-Alsan E, Bayraktaroglu Z, Gurvit H, Keskin YH, Emre M, Demiralp T. Comparative analysis of event-related potentials during Go/NoGo and CPT: decomposition of electrophysiological markers of response inhibition and sustained attention. Brain Res. 2006;1104:114–128. doi: 10.1016/j.brainres.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Kok A. Effects of degradation of visual stimulation on components of the event-related potential (ERP) in go/nogo reaction tasks. Biol Psychol. 1986;23:21–38. doi: 10.1016/0301-0511(86)90087-6. [DOI] [PubMed] [Google Scholar]
- Kopp B, Mattler U, Goertz R, Rist F. N2, P3 and the lateralized readiness potential in a nogo task involving selective response priming. Electroenceph Clin Neurophysiol. 1996;99:19–27. doi: 10.1016/0921-884x(96)95617-9. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavric A, Pizzagalli DA, Forstmeier S. When ‘go’ and ‘nogo’ are equally frequent: ERP components and cortical tomography. Eur J Neurosci. 2004;20:2483–2488. doi: 10.1111/j.1460-9568.2004.03683.x. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM. Regulating action: alternating activation of midline frontal and motor cortical networks. Clin Neurophysiol. 2001;112:1295–1306. doi: 10.1016/s1388-2457(01)00559-4. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, et al. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. J Cogn Neurosci. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Müller V, Anokhin AP. Neural synchrony during response production and inhibition. PLoS One. 2012;7:1–11. doi: 10.1371/journal.pone.0038931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LD, Patrick CJ, Collins P, Lang AR, Bernat EM. Alcohol impairs brain reactivity to explicit loss feedback. Psychopharmacology (Berl) 2011;218:419–428. doi: 10.1007/s00213-011-2323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/Nogo task: effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Pandey AK, Kamarajan C, Tang T, Chorlian DB, Roopesh BN, Manz N, et al. Neurocognitive deficits in male alcoholics: an ERP/sLORETA analysis of the N2 component in an equal probability go/nogo task. Biol Psychol. 2012;89:170–182. doi: 10.1016/j.biopsycho.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopell BS. ERPs to response production and inhibition. Electroenceph Clin Neurophysiol. 1985;60:423–434. doi: 10.1016/0013-4694(85)91017-x. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjez B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Roche RA, Garavan H, Foxe JJ, O'Mara SM. Individual differences discriminate event-related potentials but not performance during response inhibition. Experimental Brain Res. 2005;160:60–70. doi: 10.1007/s00221-004-1985-z. [DOI] [PubMed] [Google Scholar]
- Schürmann M, Başar-Eroglu C, Kolev V, Başar E. A new metric for analyzing single-trial event-related potentials (ERPs): application to human visual P300 delta response. Neurosci Lett. 1995;197:167–170. doi: 10.1016/0304-3940(95)11912-g. [DOI] [PubMed] [Google Scholar]
- Schürmann M, Başar-Eroglu C, Kolev V, Başar E. Delta responses and cognitive processing: single-trial evaluations of human visual P300. Int J Psychophysiol. 2001;29:229–239. doi: 10.1016/s0167-8760(00)00144-6. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Movement-related potentials in the Go/NoGo task: the P3 reflects both cognitive and motor inhibition. Clin Neurophysiol. 2008;119:704–714. doi: 10.1016/j.clinph.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin EA. componential analysis of the ERP elicited by novel events using a dense electrode array. Psychophysiology. 1999;36:409–414. doi: 10.1017/s0048577299981180. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 1996;16:4240–4229. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo LT, Allen JJ. Theta EEG dynamics of the error-related negativity. Clin Neurophysiol. 2007;118:645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci. 2005;25:604–613. doi: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. P300 generation by novel somatosensory stimuli. Electroenceph Clin Neurophysiol. 1991;78:50–55. doi: 10.1016/0013-4694(91)90018-y. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Yamamoto Y. Single-trial eeg power and phase dynamics associated with voluntary response inhibition. J Cogn Neurosci. 2009;22:714–727. doi: 10.1162/jocn.2009.21258. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Devrim M, Kolev V, Ademoglu A, Demiralp T. Multiple time-frequency components account for the complex functional reactivity of P300. Neuroreport. 2000;11:1097–1103. doi: 10.1097/00001756-200004070-00038. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Falkenstein M, Hohnsbein J, Kolev V. Parallel systems of error processing in the brain. NeuroImage. 2004;22:590–602. doi: 10.1016/j.neuroimage.2004.01.040. [DOI] [PubMed] [Google Scholar]