Abstract
The unique circuitry of the hippocampus is thought to support the encoding and retrieval of context-rich episodic memories. Given the neuroanatomical differences between the hippocampal subfields, determining their functional roles during representation of contextual features in humans is an important yet unaddressed research goal. Prior studies suggest that during the acquisition of information from the environment, the dentate gyrus (DG) and CA3 subfields rapidly differentiate competing contextual representations, whereas CA1, situated downstream from CA3/DG, is believed to process input from both CA3 and neocortical areas via the temporoammonic pathway. To further explore the functionality of these roles, we used high-resolution fMRI to investigate multivariate response patterns within CA3/DG and CA1 during the processing of spatial context. While undergoing functional imaging, participants viewed videos of virtual environments and were asked to discriminate between similar, yet geometrically distinct cities. We manipulated a single contextual feature by systematically morphing the city configurations from one common geometric shape to another, resulting in four cities—two distinctively shaped cities and two intermediate “morphed” cities. Pattern similarity within CA3/DG scaled with geometric changes to the environment. In contrast, CA1 pattern similarity, as well as interregional pattern similarity between CA1 and parahippocampal cortex, increased for the regularly shaped configurations compared to the morphs. These results highlight different roles for subfields CA3/DG and CA1 in memory and advance our understanding of how subcomponents of the human hippocampal circuit represent contextual features of memories.
Keywords: hippocampus, human episodic memory, navigation, CA1, CA3
INTRODUCTION
The hippocampus plays a critical role in representing contextual details that underlie our memory for events (Diana, Yonelinas, & Ranganath, 2007; Eichenbaum, Yonelinas, & Ranganath, 2007; Smith & Mizumori, 2006; Yonelinas, Kroll, Dobbins, Lazzara, & Knight, 1998). Part of this role is widely assumed to involve binding features of an event to a specific spatial context (memory for “where” something occurred, such as at a café), referred to more generally as memory for context (Diana et al., 2007; Tulving, 2002). Yet how the human hippocampus represents and differentiates spatial context during episodic memory encoding and retrieval remains largely unknown. Given the hypothesized importance of the primary hippocampal subfields, CA3, CA1 & Dentate Gyrus (DG) (Amaral & Witter, 1989), to aspects of episodic memory performance (Guzowski, Knierim, & Moser, 2004; Katz, Kath, Spruston, & Hasselmo, 2007; Rolls & Kesner, 2006; Yassa & Stark, 2011), determining what roles the different subfields play when processing spatial context in humans remains critical in advancing our understanding of the neural basis of memory.
Anatomical tracing studies suggest differences between afferent input to CA3 and CA1; specifically, a significant percentage of CA3 input comes from its recurrent collaterals, whereas the primary sources of CA1 input come from both CA3 and neocortical areas (via the temporoammonic pathway) (Amaral & Insausti, 1990). Thus, due to the recurrent collaterals, CA3/DG would appear best suited to complete/differentiate competing inputs (Levy, 1989), while CA1 may be in a unique position to combine differentiated input from CA3 and generalized input from neocortical areas (Katz et al., 2007; van Kesteren, Ruiter, Fernandez, & Henson, 2012), such as parahippocampal and retrosplenial cortex. Therefore, we might predict that CA3/DG and CA1 would show distinct roles in representation of spatial context during the formation of memories.
Although past studies have identified differences in subfield activation during verbal and object-related memory (Bakker, Kirwan, Miller, & Stark, 2008; Brown, Hasselmo, & Stern, 2014; Kirwan, Jones, Miller, & Stark, 2007; Lacy, Yassa, Stark, Muftuler, & Stark, 2010; Zeineh, Engel, & Bookheimer, 2000; Zeineh, Engel, Thompson, & Bookheimer, 2003), these studies were not designed to examine how the subfields represent spatial context, a critical component of episodic memory. Furthermore, because rodent studies have suggested segregated input of spatial and non-spatial context into the hippocampus via entorhinal cortex (Hargreaves, Rao, Lee, & Knierim, 2005) and different coding schemes for spatial vs. non-spatial information within the hippocampus (J. K. Leutgeb et al., 2005), it is important to establish how and in what manner spatial context is processed within the human hippocampal subfields.
To address this question, we scanned participants as they performed a contextual discrimination task, which involved viewing immersive video clips from four distinct virtual reality cities. We employed high-resolution fMRI to target the hippocampus and utilized multivariate pattern similarity (MPS) analyses (Kriegeskorte & Bandettini, 2007; Kriegeskorte, Goebel, & Bandettini, 2006) to examine internal contextual representation within the hippocampal subfields. Both patient and neuroimaging studies have demonstrated the importance of the hippocampus in tasks involving spatial scene perception, allocentric processing, and spatial memory (Astur, Taylor, Mamelak, Philpott, & Sutherland, 2002; Barense, Henson, Lee, & Graham, 2010; Graham et al., 2006; Hartley et al., 2007; King, Burgess, Hartley, Vargha-Khadem, & O'Keefe, 2002). By inducing small systematic manipulations to the boundary shape of the virtual cities (i.e., morphing from a circle to a square), we were able to examine the response sensitivity to parametric changes to spatial context. In accordance with prior work in rodents using a related paradigm (J. K. Leutgeb et al., 2005; Wills, Lever, Cacucci, Burgess, & O'Keefe, 2005), we hypothesized that CA3/DG and/or CA1 would show parametric changes in neural representations as a function of geometric dissimilarity. Second, we tested for subfield differences in the trial-to-trial representation of regular geometries (circle, square) in comparison to the perceptually ambiguous morph-shaped cities, based on the hypothesis that CA1 might serve as a locus of integration of stored geometrical templates (i.e., regular cities) represented in neocortical regions (Op de Beeck, Wagemans, & Vogels, 2001) with input from CA3 (Remondes & Schuman, 2004). Our results provide novel evidence that hippocampal subfields serve distinct roles during the extraction of spatial contextual details and assimilation of these details with preexisting cortical representations of shapes in support of episodic memory.
METHODS
Participants
17 participants (10 female) were recruited from the UC Davis community. All participants were native English speakers with normal or corrected to normal vision between the ages of 18 and 30. Each volunteer was paid for the entire session, which lasted approximately 3 hours. All participants gave informed written consent before participation. One participant was removed from the study due to excessive head movement in the scanner.
Task design
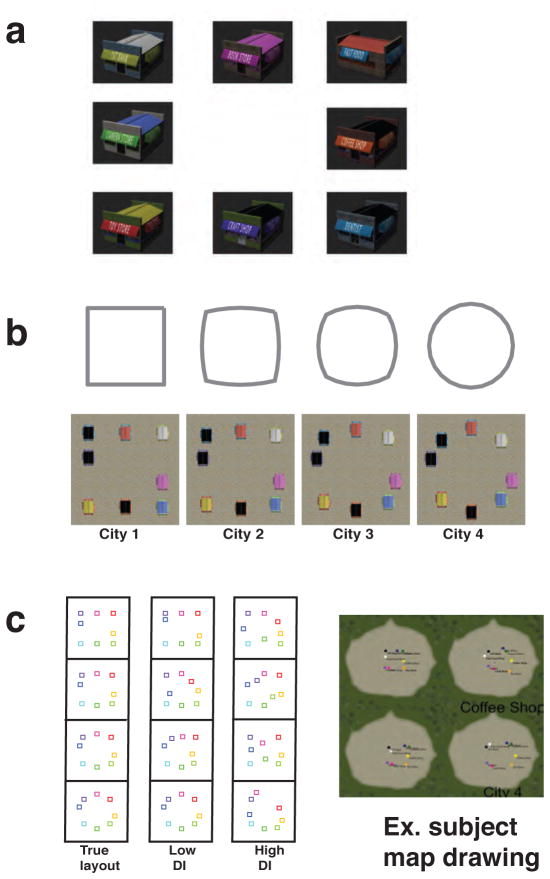
Four virtual environments were constructed using the Unity software package. We modeled a ‘morphed’ design manipulation used in past rodent studies (J. K. Leutgeb et al., 2005; Wills et al., 2005) by systematically varying the positioning of eight unique stores to produce four cities arranged along a circle-square spectrum (Fig. 1b). Two of these configurations were a square-shaped and circular-shaped layout (City 1 and City 4 respectively). The other two cities were a circle-square and square-circle morph (City 2 and City 3)(Fig. 1a). While city configurations involved a progressive transformation between circle and square, we held landmark identity and ordinal position constant.
Figure 1. Virtual reality spatial discrimination paradigm.
(a) Each city contained an identical set of 8 stores (1st bank, Book store, Fast food, Camera store, Coffee shop, Toy store, Craft shop, Dentist). Before the start of the experiment, each participant previewed the storefronts in a series of three video montages. (b) Aerial view of the four geometrically morphed virtual cities shows how the city store positions changes as a function of morphing for the four different cities. (c) After the context discrimination task (i.e., scanner task) participants performed the post-scan map-drawing task. Lower distortion index (MDI) indicates more accurate map-drawings.
Store positions were calculated using the Matlab function polymorph (http://www.mathworks.com). Each polygon contained eight points, which were shifted approximately equal distance between neighboring morphs. For instance, the Euclidean distance shift between the position of store A in City 1 and its position in City 2 was equivalent to the distance between the position of store B in City 1 and store B’s position in City 2 (Fig. 1a). Consequently, in this example, the total Euclidean distance calculated from point shifts from City 1 to City 2 was equal to the total distance between City 2 and City 3.
First-person navigation video clips were recorded within the cities, with each video clip (20 seconds long) corresponding to a single trial. Each video was captured from a single city (Fig. 1a,b) and all video clips contained a unique, non-repeated navigation route.
Behavioral methods
Participants were presented video clips in the scanner and were explicitly told to study the environment and attempt to mentally extract the city configuration. To ensure that attention was focused on the city geometry, we asked participants to compare the geometric configuration of the current trial to the configuration from the prior video trial in a manner similar to continuous recognition paradigms (e.g., Viskontas, Knowlton, Steinmetz, & Fried, 2006). The cities were designed to require the participants to assess the configuration holistically rather than approximate the geometry of the city based upon a single view, which we determined during behavioral piloting. After the first trial of each run, participants indicated with a button press whether the current city was the “same” or “different” than that of the former trial. Beforehand, participants were explicitly instructed that each city contained an identical set of eight stores (‘1st Bank', 'Book Store', 'Camera Store', 'Coffee Shop', 'Toy Store', 'Craft Shop', 'Dentist', 'Fast Food Shop'). Participants were also informed that the ordinal sequence of the stores would remain constant across cities but that each city would possess a different geometric configuration
On the first trial of each run, participants were instructed to simply watch the video clip and try to learn the city layout. On successive trials, participants were asked to make a button box response: ‘Press button 1 if you believe the city depicted in the video is the same as the city depicted in the video which occurred immediately prior, and press button 2 if you believe it to be a different city’. Participants were instructed to make a response within the 20 s time frame of the video and process the spatial information provided in the clip after making a response. Between trials, participants performed a 5 s long active baseline task in order to reduce rest related activity within the medial temporal lobe (Stark & Squire, 2001) which consisted of an arrow pointing either left or right; participants pressed button 1 for left or 2 for right. A total of 96 video clips were divided across four runs, and after omitting the opening trial, we collected 92 trials of interest from each participant.
Participants were explicitly told that there were four cities but were given no other descriptive information about the configurations of the cities (i.e., circle or square). Immediately following the scan, we evaluated participant knowledge of the spatial geometries of each city by asking participants to construct maps (Wolbers & Buchel, 2005) of the four cities as accurately as possible using in-house map-drawing software (Fig. 1c). Each map was then oriented by store serial order and projected into template space through affine transformation. All except a single participant correctly recalled store serial order; this participant simply transposed two of the stores. In order to assess city geometry accuracy, we calculated the ratio of map-to-template area difference to total template area (distortion index) for each city. Distortion indices were used to ascertain which maps corresponded to the square city (city 1) and circle city (city 4). Next, scores of the remaining maps were used to match participant drawings to square-morph city (city 2) and circle-morph city (city 3). We then created a single composite map-drawing distortion index (MDI) score for the purposes of assessing overall map-drawing performance. Because several participants were only able to recall three city maps, their morph-city MDI scores were calculated using a single morph city map.
Trials were presented in pseudo-randomized order that ensured an equal distribution of same and different trials (46 Same, 46 Different trials), equal numbers of environment type (City 1, City 2, City 3, City 4; 23 trials each), and equal numbers of city pair combinations (e.g., City 1 followed by City 2, City 2 followed by City 3, etc.). Run order was counterbalanced across participants. Both the scan task and map-drawing task were programmed and employed through the Psychophysics Toolbox (http://psychtoolbox.org/).
Outside of the scanner, each participant successfully completed a practice module, which consisted of mock versions of both the continuous recognition and map-drawing tasks. Prior to beginning the experiment, participants were first acclimated to the serial order of the city configuration by viewing a video montage containing 360 degree rotating presentations of each of the eight stores on a black background within the scanner. The temporal order of this montage matched the serial order of stores along the city perimeters. The purpose of the acclimation phase was to ensure heightened processing of city configuration over that of store order and descriptor information during task.
fMRI Methods
Participants were scanned at the University of California-Davis Imaging Center in Davis, CA using a Siemens 64-channel 3 Tesla “Skyra” MRI System. Sequences were acquired perpendicular to the long-axis of the hippocampus. High-resolution functional (BOLD) images were collected using an echo-planar imaging (EPI) sequence [repetition time (TR) = 3000 ms, echo time (TE) = 29 ms, slices = 36, field of view (FOV) = 192 mm, bandwidth = 1462 Hz/pixel, resolution = 1.5 X 1.5 X 2mm]. High-resolution structural images were acquired using a T2-weighted turbo-spin echo (TSE) sequence [TR= 4200 ms, TE= 93 ms, FOV =20 cm, flip angle = 139°, bandwidth = 199 Hz/pixel, voxel size = 0.4 x 0.4 x 1.9mm]. Additionally, coplanar matched-bandwidth EPI sequences [TR = 3000 ms, TE = 38 ms, slices = 36, FOV = 245 mm, flip angle = 90°, bandwidth = 1446 Hz/pixel] were collected and utilized to improve registration of the EPI images to the high-resolution structural scan (Ekstrom et al., 2009; Zeineh et al., 2000).
The functional images were slice-time and head-motion corrected using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). The coplanar matched-bandwidth structural scan was co-registered to the mean of the realigned functional scans. Subsequently, the T2 high-resolution image was then co-registered to the matched-bandwidth image. Individual trial responses were estimated using a voxel-wise, general linear model (GLM) that produced a summary image for each of the 92 trials of interest. Specifically, each trial (i.e., a 20 s video period) was entered as a regressor, which then allowed for the resulting trial specific beta images to be sorted by condition (e.g. S-ALL, D-ALL) and statistically compared. Each model incorporated movement and run information as nuisance regressors.
We constrained our analyses to the medial temporal lobe (MTL) and targeted the following areas commonly implicated in spatial context representation (Eichenbaum et al., 2007): CA1, CA3, DG and the extra-hippocampal parahippocampal cortex (PHC)(Fig. 1). Entorhinal cortex (EC) was omitted from analyses due to imaging signal dropout, an issue present in other high-resolution imaging studies targeting the MTL using similar MRI acquisition sequences (e.g., Duncan, Tompary, & Davachi, 2014). In accordance with the methodology from other high-resolution fMRI projects, the “CA3/DG” ROI was defined to include subfields CA3, CA2 and DG because spatial resolution limitations prevent finer division (Chen, Olsen, Preston, Glover, & Wagner, 2011; Ekstrom et al., 2009; LaRocque et al., 2013; Zeineh et al., 2000). Boundaries of these regions were traced by hand on the T2-weighted TSE structural scans using the ‘FSLview’ module of FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) and following guidelines similarly utilized in past reports (Duvernoy, 1998; Ekstrom et al., 2009; Zeineh et al., 2000). For the group searchlight analysis, participant specific information maps were warped into a common space. Cross-participant alignment was achieved using diffeomorphic metric mapping method and implementing participant-specific weighted ROIs. All procedures were carried out in Matlab with in-house scripts that employed the Advanced Normalization Tools framework (Avants, Epstein, Grossman, & Gee, 2008). Normalization procedures warped all participants’ T2 images to that of a template participant and used the resulting deformation maps to additionally carry along all information maps into common space. These morphed maps were then subjected to one of three types of group analysis: Contrast, linear trend analysis, and seed-based interregional pattern coherence analysis. The searchlight analysis involved extracting voxel-wise multivariate pattern similarity (MPS) scores within the searchlight sphere, which was conducted for each condition of interest within each participant. We established significant clusters by thresholding at a value of two-tailed p value < 0.05 and at minimum of 4 contiguous voxels (determined through permutation testing to exceed the false positive rate at p < .05 [corrected]) (Forman et al., 1995).
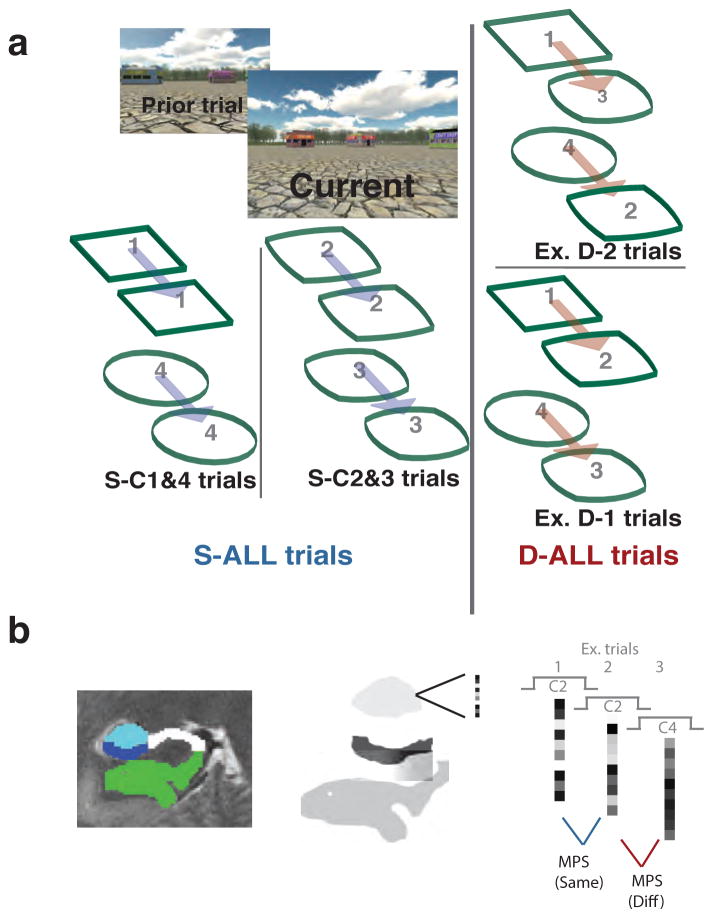
Both ROI and searchlight analyses utilized a metric of neural similarity, MPS, congruent with the representational similarity analysis approach (Kriegeskorte et al., 2006). Neural activation patterns were defined for each of the 92 trials of each participant using general linear model estimates. All trials were analyzed regardless of response accuracy; however, no-response trials were excluded. MPS was calculated on a trial-by-trial basis by correlating ROI-bound voxel patterns for each pair of neighboring trials (i.e. 4 runs X 23 = 92 pattern similarity scores in total). Outliers were controlled for at the voxel level by replacing extreme values with ROI mean values and additionally, at the MPS level, by omitting extreme scores greater than 1.5 X the interquartile range. Trial-wise MPS was approximated using Pearson correlations of voxelwise BOLD activity between temporally adjacent pairs of trials (Fig. 2b). Fischer-z transforms were employed on correlation values when entered into all statistical tests (Fischer, 1928). MPS was calculated within ROI in the ROI-centric analysis, and in the searchlight analysis, MPS was calculated within a 3 voxel-wide radius pseudospherical shape chosen to accommodate the protracted dimensions of the human MTL. In both cases, MPS scores were parsed by behavioral conditions (all same trials [S-ALL], all different trials [D-ALL], different trials differing by one morph [D-1], different trials differing by two morphs [D-2], same city 1 and 4 trials [S-C1&4], and same city 2 and 3 trials [S-C2&3]; Fig. 2a) and averaged within participant. Participants responded substantially faster to cities differing by at least three geometrical morph-iterations (D-3) vs. all other city comparisons (D-3: 7.57s (1.83) vs. D-1: 13.47s (2.4), D-2: 10.85s (2.08), and S-ALL: 14.37s (1.97)). D-3 judgments also only comprised a small number of trials (N=7). Thus, we did not include D-3 city comparisons in our final analyses, although including them did not change our results significantly overall.
Figure 2. Experimental conditions and pattern similarity analysis.
(a) The “same” trials (S-ALL) were broken down into specific conditions by degree of geometric regularity. S-C1&4 (i.e., Square, Circle) condition embodied recognizable regular geometric templates when compared to the S-C2&3 trials, which involved morphed geometric shapes (i.e., City 2: Square-circle; City 3: Circle-square). Different trials (D-ALL) were further parsed by degree of discrimination difficulty, which scaled with between-city geometric similarity. Therefore cities involved in D-1 trials were more easily discriminated than D-2 trials. (b) Subfields were manually traced and included CA3/DG (teal), CA1 (blue) and PHC (green). Multivariate pattern similarity analysis was utilized to compare adjacent trials and generate trial specific pattern similarity scores.
Interregional pattern coherence was calculated for each participant by computing Pearson’s r between each node and each seed-of-interest (CA1 & CA3/DG) using the fluctuating trial-to-trial MPS scores, parsed by condition of interest (i.e. S-C1&4 and S-C2&3). The analysis is similar to a beta series functional connectivity analyses (Rissman, Gazzaley, & D'Esposito, 2004), and though somewhat novel, a similar technique was effectively implemented in a prior study to assess hippocampal-cortical pattern similarity coherence (Ritchey, Yonelinas, & Ranganath, 2014).
RESULTS
Behavioral performance
All participants discriminated the spatial configurations significantly above chance (accuracy: 73% [standard deviation [SD]=12%]) and overall, displayed higher accuracy when judging two trials as different compared to identification of trials as same (S-ALL: 68% correct [SD=16%]; D-ALL: 79% correct [SD=11%]; t15 = 3.57, p = 0.0028). D prime (d’) scores, which account for participants using more conservative or liberal strategies to disambiguate same/different cities (Macmillan & Creelman, 2005), were also significantly greater than chance (mean d': 1.37 (0.80); t15 = 6.83; p < 0.0001) and did not vary as a function of learning over the course of experimental runs (repeated measures one way ANOVA: F3,42 = 1.7, p = 0.181). Consistent with the idea that participants actively processed differences in spatial geometry of the cities, performance varied as a function of increasing geometrical dissimilarity (d‘ D-1: 0.96 (0.76); d’ D-2: 1.52 (1.03); d’ D-3: 1.54 (.71); F2,30 = 17.83, p < 0.0001). This indicated that the greater the geometrical dissimilarity, the greater the ability of participants to discriminate cities as different. In addition to detecting geometrically different cities more readily, participants also drew maps of regular cities (City 1&4) more accurately than morph cities (City 2&3)(Fig. 1B&D). Specifically, participants’ map-drawing distortion indices (MDI) (excluding one outlier participant [Z-score = 3.68]), revealed a significant difference in MDI between the four cities (F3,42 = 10.48, p < 0.0001), with post hoc comparisons showing that the MDI for morph cities was higher than regular cities (Table 1). In sum, our behavioral results indicate that participants were readily able to discriminate same and different cities, that this ability varied as a function of differences in spatial geometry, and that morphed cities resulted in less stable representations than geometrically regular cities.
Table 1. Participants successfully detected change in context.
Detecting change in context (different trials) resulted in higher performance than detecting preservation of context (same trials). Performance was also higher for same city 1&4 trials (S-C1&4) compared to same city 2&3 (S-C2&3). Importantly, in all conditions, participants performed significantly above chance levels. To assess spatial learning, participants were asked create maps of each of the four cities. Mean DI scores describe the overall amount of map-drawing distortion.
| Behavioral results | ||
|---|---|---|
| Disrimination task | m | std |
|
|
||
| Same (CRT) | 0.68 | 0.16 |
| Diff (CRT) | 0.79 | 0.11 |
| Same C1&4(CRT) | 0.73 | 0.14 |
| Same C2&3(CRT) | 0.62 | 0.19 |
| d’ | 1.37 | 0.80 |
| Map-Drawing | ||
| DI-C1 | 0.0832 | 0.0503 |
| DI-C2 | 0.1238 | 0.0586 |
| DI-C3 | 0.1409 | 0.0573 |
| DI-C4 | 0.1071 | 0.0478 |
A role for CA3/DG in differentiating same from different spatial contexts
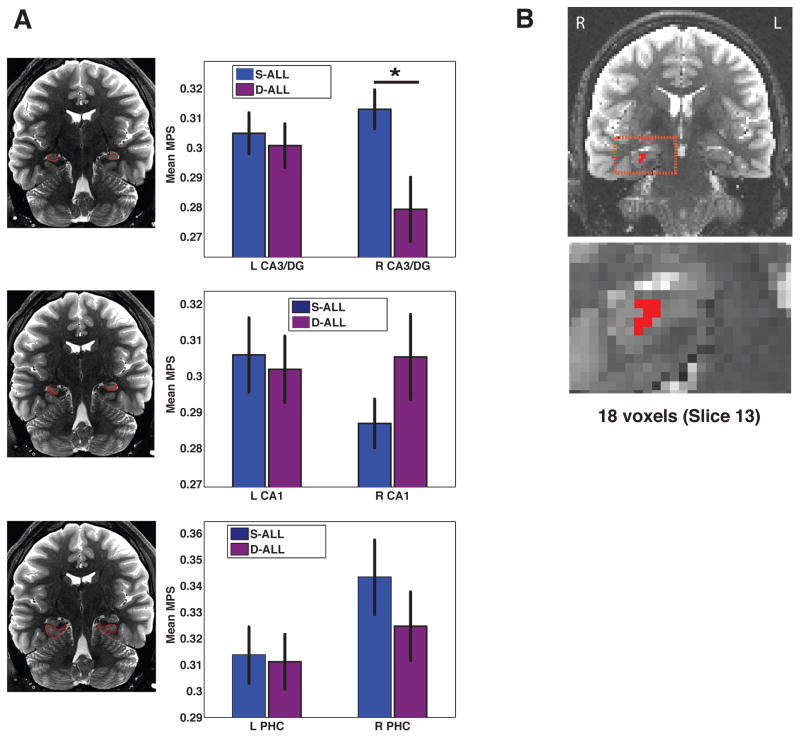
We performed a region-of-interest (ROI) analysis on multivariate pattern similarity (MPS) values to assess sensitivity to change in environmental input in subfields CA3/DG, CA1, and PHC. We identified a single subfield within the hippocampus showing differences in MPS as a function of geometric similarity, right CA3/DG (Fig. 3a). A repeated measures ANOVA with factors of Hemisphere (2; Left, Right), Subfield (3; CA3/DG, CA1, PHC) and Condition (2; same vs. different trials) revealed a significant main effect of subfield (F3,45 = 4.79 p <0.01) and a significant Hemisphere X Subfield X Condition interaction (F3,45 = 4.00 p<0.05). Critically, this interaction was not driven by performance; controlling for individual differences in performance, by including map-drawing MDI scores as a covariate in an ANCOVA, produced a comparable Hemisphere X Subfield X Condition interaction effect (F2,28 = 4.06 p < 0.05). Post hoc t-tests revealed that mean pattern similarity for same trials (S-ALL) was significantly greater than mean MPS for different trials (D-ALL) within right CA3/DG (t15 = 2.63 p < 0.05) but not within left CA3/DG, bilateral PHC or bilateral CA1. Furthermore, we found that these MPS differences were not related to univariate parameter estimates. An ANOVA revealed a main effect of subfield (F2,30 = 39.8 p < 0.0001), with parameter estimates higher overall in parahippocampal gyrus than hippocampus proper; though unlike MPS effects, no condition-related univariate interaction effects reached significance. Furthermore, no other univariate effects were significant in this analysis; see Supplementary Table 1 for more details. Again, including only correct trials in this analysis yielded similar results.
Figure 3. Role for CA3/DG in differentiation of same vs. different trials.
(a) When viewing videos of city navigation, S-ALL multivariate pattern similarity was significantly greater than D-ALL within the right CA3/DG subregion. (b) A parallel searchlight analysis identified a cluster within right CA3/DG.
As an additional means of assessing subfield specific changes in pattern similarity, we conducted a searchlight analysis (Kriegeskorte et al., 2006) throughout the MTL which allowed us to localize fine-scale multivariate patterns. This analysis identified two clusters in CA3/DG, an 18-voxel cluster localized within the right CA3/DG (max-t value= 3.49)(Fig. 3b) exhibiting greater pattern similarity for S-ALL compared to D-ALL trials and a second, smaller cluster within left CA3 also showing S-ALL > D-ALL pattern similarity (maximum t-value=3.04, 7 voxels). Overall these results suggest that CA3/DG activation patterns code for changes in city geometry; when participants viewed the same city, pattern similarity was higher CA3/DG than when they viewed different cities. Furthermore, our analyses revealed that these effects could not be accounted for by differences in participant performance or univariate effects alone.
Differences in pattern similarity as a function of contextual input
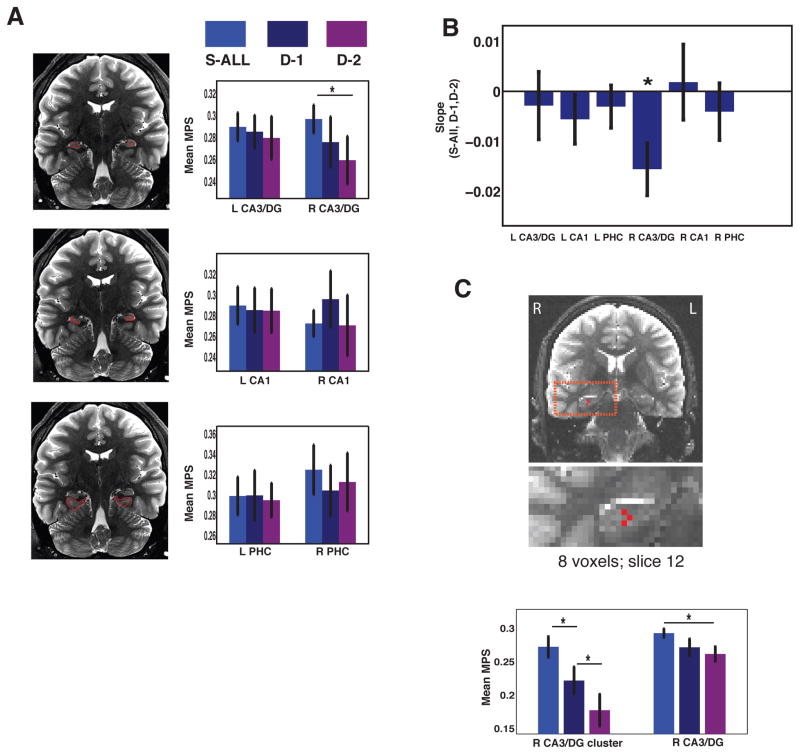
To better understand whether changes in CA3/DG scaled with parametric changes in spatial context, we compared different trials based on those that differed by a single city-iteration (D-1) versus two city-iterations (D-2; see Fig. 2a). Based on our findings of higher MPS for same vs. different trials in right CA3/DG, we conducted a repeated measures ANOVA focusing specifically within the right hemisphere with factors of subfield (CA3/DG, CA1, PHC) and condition (S-ALL, D-1, and D-2). We found a significant main effect of Subfield (F2,30 = 9.75, p < 0.005) and a significant Subfield X Condition interaction effect (Fig. 4a, F4,60 = 3.87, p < 0.01). A linear trend analysis revealed a significant decrease in pattern similarity across the three conditions (i.e., same > D-1 > D-2 trials) for CA3/DG (t15 = 3.78, p < 0.005) but not for other regions (Fig. 4b). A searchlight analysis based on the same linear model confirmed these findings, revealing a significant cluster in right CA3/DG (9 voxels; max-t = −2.58, p < 0.05, corrected) (Fig. 4c) with no other significant clusters. These data suggest that CA3/DG not only exhibited pronounced sensitivity to changes in spatial context, but more specifically, CA3/DG displayed decreased pattern similarity as a function of geometrical dissimilarity. These findings are consistent with previous findings from rodent work, suggesting that CA3/DG plays a role in differentiating (pattern separating) more dissimilar inputs (i.e., different geometries) and completing overlapping inputs (i.e., the same geometry) (for a review, see: Yassa & Stark, 2011).
Figure 4. CA3/DG response scales parametrically with city dissimilarity.
(a) Multivariate pattern similarity response scales with changes in city geometry, an effect localized to the CA3/DG subregion. (b) Slope of S-ALL, D-1 and D-2 was significant across all participants. (c) Searchlight linear trend analysis identified a cluster within right CA3/DG.
Role for CA1 in integration of CA3 input with cortical geometric percepts
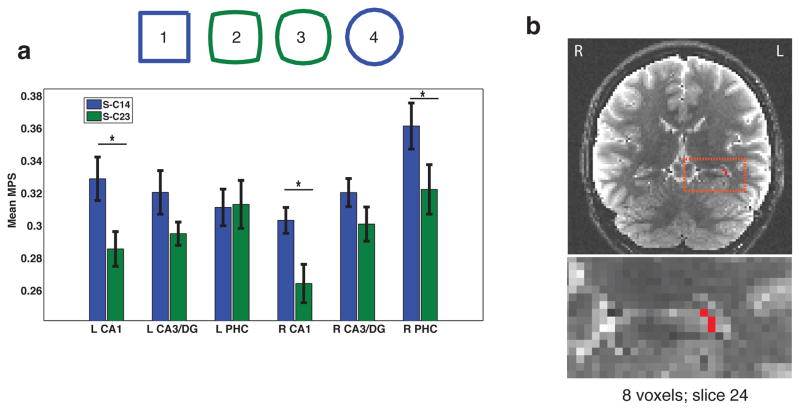
Prior theoretical and empirical work suggests that CA1 may integrate cortical input via the temporoammonic path with current input from CA3/DG (Katz et al., 2007; Remondes & Schuman, 2004). As we noted in our behavioral results, map-drawing performance was better for the geometrically regular compared to morph cities and discrimination performance was higher on trials involving the same city judgments for the geometrically regular cities (i.e., correctly judging whether the video clip involved the same square or circle-shaped city, termed S-C1&4) compared to trials involving correctly judging the morphed cities as same (S-C2&3)(Table 1; Fig. 2a). Thus, we hypothesized that geometrically regular cities might be important in anchoring how participants formed representation of both the regular and morphed cities. Past research has demonstrated a bias in inferotemporal cortex toward representing objects as either square or circular (Op de Beeck et al., 2001) while parahippocampal cortex is a key node in processing regularities in scene-specific spatial context (Epstein, Harris, Stanley, & Kanwisher, 1999). Because cortical areas communicate directly with CA1 via the temporoammonic pathway (Amaral & Insausti, 1990), we hypothesized that regular geometric shapes stored in cortical areas might interact to a greater extent with CA1 representations for regular cities compared to the morphs. To assess whether subfield ROIs exhibited differential representational sensitivity to city type, we divided the S-ALL condition into two component groups, S-C1&4 (same trials involving the City 1 or City 4, i.e. circle or square-shaped cities) or S-C2&3 (same trials involving City 2 or City 3, i.e. circle-square morph and square-circle morph trials). A repeated measures Hemisphere (2) X Subfield (3) X City type (2) ANOVA revealed a significant Hemisphere X Subfield X City type interaction (F2,30 = 4.16, p < 0.05) (Fig. 5a). Importantly, when performing the identical analysis but using univariate parameter estimate scores, we found no significant main or interaction effects (Subfield by City type: F2,30 = 0.29, p = 0.75; Hemisphere X Region X City type: F2,30 = 0.26, p = 0.78); see also Supplementary Table 1. Simple effect comparisons revealed that both left and right CA1 showed significantly higher MPS for S-C1&4 trials when compared to S-C2&3 trials (Left CA1: t15 = −2.98, p < 0.01; Right CA1: t15 = −2.63, p < 0.05), with right PHC also showing higher MPS for S-C1&4 compared to S-C2&3 (t15 = 2.86; p < 0.05). Neither left nor right CA3/DG demonstrated significantly higher mean MPS scores for S-C1&4 over S-C2&3, (Left CA3/DG: F1,14 = 2.01, p = 0.18; Right CA3/DG: F1,14 = 1.15, p = 0.30). Repeating the same analysis but limited to only correctly judged same trials (rather than all trials) did not change our results. Furthermore, a follow up searchlight analysis converged with the above ROI analysis by revealing a cluster primarily within the left CA1 subfield showing higher MPS for regular compared to morph cities (maximum t-value=4.00, 8 voxel cluster (Fig. 5b).
Figure 5. CA1 response to regular compared to morphed city shapes.
(a) CA1 and right PHC showed higher multivariate pattern similarity than CA3/DG for regular compared to morphed cities. (b) In support of the ROI analysis, our searchlight analysis revealed an 8 voxel cluster centered within left CA1.
Overall, our results showed that MPS was greater for geometrically regular cities (cities 1 and 4) compared to the morphs (cities 2 and 3), an effect primarily restricted to CA1. These results confirm that trial-by-trial representational patterns within CA1 were particularly sensitive to regular geometric percepts, suggesting a bias in CA1, but not CA3/DG, toward more stable representations for the regular geometric cities compared to the morphs. Importantly, these findings could not be accounted for by differences in performance nor univariate activation patterns alone.
CA1 shows increased pattern similarity coherence with PHC for regular compared to morphed cities
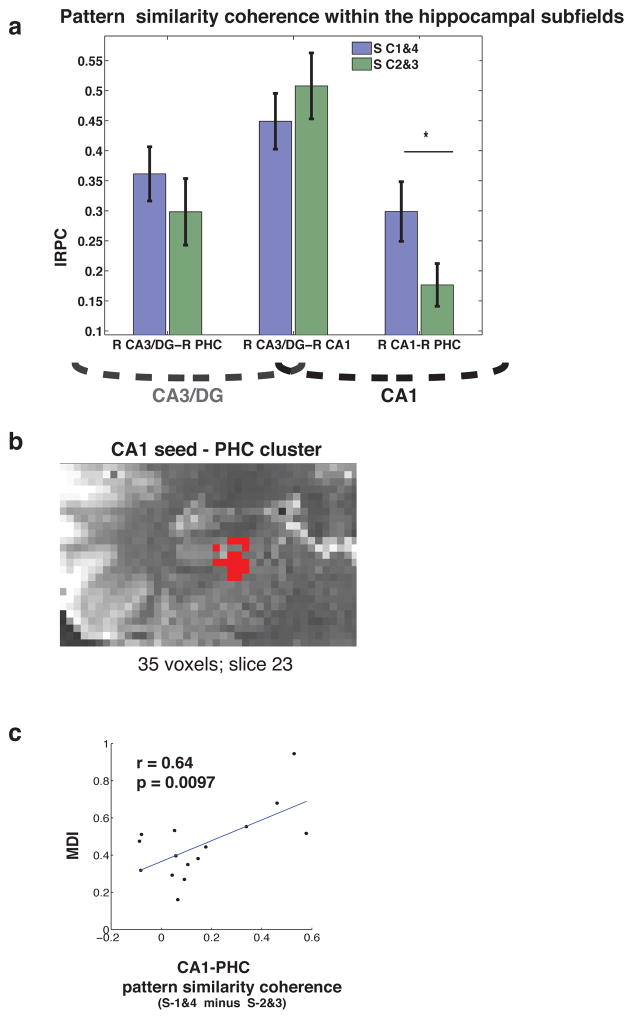
Our results above suggest that CA1 exhibits a possible relationship with long-term stored contextual frames by representing, more strongly, regular compared to morphed spatial contexts. Critical to determining whether this input might come via the temporoammonic pathway involves showing greater interactions between CA1 and cortical areas, such as parahippocampal cortex, during processing of the regular compared to morphed cities. To test this idea we analyzed interregional pattern coherence (IRPC) —essentially, the covariation of pattern similarity between brain regions — during regular and morph same city trials. A repeated measures ANOVA with factors Condition (S-C1&4, S-C2&3) and Seed-region (Left CA1, Left CA3/DG, Right CA1, Right CA3/DG) revealed a significant main effect of seed-region (F1,15 = 21.96, p < 0.001) and a Condition X Seed-region interaction effect (Fig. 6a,b: F1,15 = 8.37, p < 0.05). Post hoc comparisons revealed that this interaction effect was driven, in part, by the fact that right CA1 exhibited greater IRPC with right PHC for S-C1&4 trials than S-C2&3 (t15 = 3.17, p < 0.01). This finding suggested that CA1 displayed significantly higher coherence with PHC on regular compared to morphed city trials. Whereas post hoc comparisons revealed that CA3/DG-CA1 coherence exceeded both CA3/DG-PHC (t15 = 2.52, p = 0.02) and CA1-PHC (t15 = 4.41, p = < .001), there was no difference between conditions for CA3/DG-CA1 connectivity (t15 = −0.93, p = 0.36). It should be noted that we employed only correct trials in our analysis due to increased signal to noise for correct compared to incorrect responses, which could be a greater factor influencing interregional effects. Employing all trials (correct and incorrect), as we did in other analyses, led to similar overall results (significant Subregion by Condition interaction, F1,15 = 5.87, p < .05).
Figure 6. Correlation between CA1 and PHC responses for regular compared to morphed cities.
(a) CA1-PHC exhibited greater interregional coherence (IRC) during S-C1&4 trials than for morph trials. (b) A seed-based searchlight coherence analysis, contrasting S-C1&4 with S-C2&3 coherence, yielded similar results, revealing a 35 voxel cluster in right PHC. (c) Higher CA1-PHC IRC predicted worse overall map-drawing performance across participants.
Past studies have also suggested that morphed spatial environments may result in “hysteresis” of hippocampal representations such that those for morphed shapes may lag somewhat behind those of the more salient shapes (J. K. Leutgeb et al., 2005). In the same fashion that strong schematic labels can lead to worse memory performance (Brod, Werkle-Bergner, & Shing, 2013; Sloutsky & Fisher, 2004), this idea would predict that participants exhibiting higher MPS for the regular shapes (square and circle) might encode less accurate map details due to an inability to suppress the more rigid template representations. Conversely, it may be that strong representations of the regular shapes augment allocentric representation integration and therefore effectively improve map-drawing accuracy. To address these competing possibilities, we correlated map-drawing scores (i.e., participant map-drawing distortion indices) with S-C1&4 vs. S-C2&3 IRPC for Right CA1-PHC. We found that Right CA1-PHC coherence significantly correlated with map-drawing performance (r = 0.64, p < 0.01) (Fig. 6c) such that greater CA1-PHC IRPC predicted overall poorer map recall. Interestingly, we did not find a significant correlation between map-drawing performance and S-C1&4 vs. S-C2&3 MPS within either left or right CA1, though we did observe a trending relationship within right PHC (r = 0.44, p = 0.09). Taken together, our results suggest that neocortical-CA1 interactions (via PHC) play a potential role in communicating geometrical templates, perhaps to integrate new traces embodying novel contextual representations.
DISCUSSION
Based on past anatomical tracing work (Amaral & Insausti, 1990; Cenquizca & Swanson, 2007) and computational models of hippocampal subfield function (Katz et al., 2007; Levy, 1989), we hypothesized that CA3/DG and CA1 subfields of the hippocampus would show functional differences reflecting their theoretical contributions to episodic memory and navigation. Using a novel measure of representational change within the hippocampal subfields, we found that the right CA3/DG subfield was sensitive to changes in spatial-contextual input (Fig. 3). Our results also showed that the CA3/DG region exhibited a graded response pattern, with the degree of MPS scaling with spatial dissimilarity. Thus, as the spatial environments became more dissimilar, right CA3/DG MPS decreased in parallel (Fig. 4). While past studies in humans have suggested a role for CA3/DG in the differentiation of more or less similar visual stimuli during an object recognition task (Azab, Stark, & Stark, 2014; Bakker et al., 2008; Lacy et al., 2010; Yassa & Stark, 2011), our results provide a critical extension to these findings by showing that parametric changes in spatial geometry result in comparable changes to patterns of activation within CA3/DG. These findings are overall compatible with a pattern completion/pattern separation perspective on CA3/DG function because “same” trials” showed a higher degree of pattern similarity (pattern completion) than different trials. In contrast, cities involving different geometries showed increasingly lower pattern similarity (pattern separation), consistent with a role for CA3/DG in both processes (Guzowski et al., 2004; Yassa & Stark, 2011).
In CA1, where we did not observe sensitivity to trial-by-trial change in spatial context, we did find significant MPS differences when participants viewed the same regular geometric city compared to the same morphed city. Our task involved four different cities: two of these cities (City 1 and City 4) were regular shapes (circle and square) while the other two cities (City 2 and City 3) were morphed versions such that City 2 was closer in shape to City 1 and City 3 was closer in shape to City 4. Comparing same trials involving Cities 1 and 4 to same trials involving Cities 2 and 3 revealed significantly higher similarity of hippocampal representation during the processing regular shapes within the left and right CA1 subfields. One possible explanation for the increase in multivariate pattern similarity for geometrically regular cities is that in order for the hippocampus to integrate spatial information into stable, recollectable maps, it may be advantageous to integrate this information with task-relevant sensory features, such as stored geometric templates (i.e., squares and circles).
We found further support for the interpretation that CA1 might be playing a role in integrating shape templates with input from CA3/DG using an interregional pattern similarity analyses. This analysis revealed that CA1-PHC IRPS was significantly greater for the regular compared to the morph cities, which might be expected if cortically stored shape information were input via the temporoammonic pathway. In contrast, although CA3/DG-CA1 IRPS was higher overall than that observed for CA1-PHC, IRPS was not higher for regular vs. morphed city recognition judgments in CA3/DG-CA1 (or any other subfield-subfield comparisons), suggesting that CA3-CA1 likely interacted in a condition-independent fashion during spatial recognition. Consistent with a role in integrating cortically stored shape information with existing information actively being processed within the hippocampal circuit, the CA1 subfield is uniquely positioned to receive both direct input from entorhinal cortex via the temporoammonic path as well as direct input from CA3 (Amaral & Witter, 1989; Witter, 1993). Highlighting the relevancy of this pathway, Brun and colleagues showed that even in the absence of CA3 input to CA1 (following CA3 lesions in rodents), direct perforant path input is sufficient to generate stable place fields (Brun et al., 2008). This suggests that cortical input into CA1 may serve as a primary source of spatial information (Brun et al., 2008; Brun et al., 2002). How would this lead to the emergence of CA1 vs. CA3 representational differences? Compared to CA3 cells, which are biased toward coding for the immediate spatial environment, CA1 cells more flexibly code for a broader array of task relevant features, such as the addition of non-spatial features (Eichenbaum, Dudchenko, Wood, Shapiro, & Tanila, 1999). Rodent remapping studies show that CA3 cells respond more “coherently” to contextual manipulation than CA1 cells (Lee, Yoganarasimha, Rao, & Knierim, 2004; S. Leutgeb, Leutgeb, Treves, Moser, & Moser, 2004; Vazdarjanova & Guzowski, 2004). In contrast, CA1 cells display properties indicative of a distinctly conjunctive coding scheme. For example, neurons within CA1, when compared to CA3, are more likely to possess multiple place fields (Muller & Kubie, 1987), and remapping studies suggest CA1 may be more sensitive to common features across environments as opposed to global contextual differences between environments (S. Leutgeb et al., 2004). Additionally, recent work in both rodents and humans has implicated the CA1 field in the process of assimilating new information into prior memories (Larkin, Lykken, Tye, Wickelgren, & Frank, 2014; Schlichting, Zeithamova, & Preston, 2014). These findings are again consistent with a role for CA1 in extracting common features across stimuli, which, based on our results, we suggest may occur via anchoring of pre-existing representational “schemas” such as specific shapes (Tse et al., 2007).
Additional evidence highlighting the importance of neocortical-to-CA1 connectivity comes from recent navigation studies that suggest a role for retrosplenial cortex and parahippocampal cortex, rather than hippocampus, in accurate map-drawing following exploration of a route (Wolbers & Buchel, 2005; Zhang, Copara, & Ekstrom, 2012). Furthermore, recent high-resolution imaging has also suggested that the CA1 subfield and neocortical-to-CA1 connectivity are important to memory retrieval success (Brown et al., 2014; Duncan et al., 2014). Because CA1 area receives input from parahippocampal cortex via entorhinal cortex and the temporoammonic pathway (Amaral & Insausti, 1990), one possibility is that CA1 may play a role in anchoring new visuospatial input onto existing templates (see also: J. K. Leutgeb et al., 2005; Remondes & Schuman, 2004), referred to as “schemas” (Tse et al., 2007; van Kesteren et al., 2012). This schema information, depending on the experimental paradigm, may be primarily verbal, object-oriented, or a combination of the two. Consistent with this idea, we note that the squares and circles used in our paradigm would tap into an existing verbal code compared to the morphs. Thus, across both verbal and more visually-spatially oriented paradigms, interactions with cortical areas, including retrosplenial, parahippocampal and/or prefrontal cortex, could facilitate input of neural instantiations of schemas into CA1 (McKenzie, Robinson, Herrera, Churchill, & Eichenbaum, 2013; van Kesteren et al., 2012). This opens the possibility, however, that the cortically influenced CA1 code may compete with the spatially constrained CA3 input. Consistent with this idea, we observed worse map-drawing performance in participants with higher CA1-PHC IRPC, suggesting that cortical input may, in some instances, compete with the processed spatial representation received from CA3.
One important caveat is that while our coherence metric, IRPC, suggests that CA1 and PHC showed similar levels of increases and decreases in pattern similarity across conditions, it does not imply directionality (e.g., that PHC is driving CA1 effects). Nor does it provide direct insight into whether either CA1 or PHC plays a greater role in representing geometrically regular shapes. Past literature, however, shows that patients with hippocampal damage are unimpaired at remembering and drawing simple shapes (Baddeley, Allen, & Vargha-Khadem, 2010; Corkin, 2002) although impaired at representing novel, complex shapes (Jones-Gotman, 1986). Monkey electrophysiological studies further suggest that regular shapes may be stored in inferotemporal cortex (i.e. Op de Beeck et al. 2001), and, as outlined above, past literature supports the idea that important aspects of geometric representation (i.e., grid cell representations) are communicated to CA1 via ERC and the temporoammonic pathway (Brun et al., 2008). Thus, based on these findings, we favor the interpretation that shape representations in CA1 are inherited from cortical areas via PHC and the temporoammonic pathway, although our IRPC results themselves cannot speak directly to this issue.
The CA1 region has also been characterized as serving a role in match-mismatch detection to novel input patterns (Kumaran & Maguire, 2006; Lisman, 1999). Several imaging studies have provided support for such models based on evidence of BOLD amplitude changes within CA1 (Dudukovic, Preston, Archie, Glover, & Wagner, 2011; Duncan, Ketz, Inati, & Davachi, 2012). Our findings did not reveal significant differences in mean signal changes (parameter estimates) as a function of condition within CA1 or any other subfield, although our paradigm differs from these prior studies in that it did not involve the quick presentation of series of verbalizable stimuli. We also did not explicitly probe an explicit expectation response as was done previously, which could be important to detecting match or mismatch related signals in CA1. Instead, we used a virtual reality paradigm to simulate realistic and quantifiable manipulations specifically focused on spatial context, using stimuli made up 20 s long video clips. Interestingly, our CA3/DG effect, which showed lower MPS for parametrically different geometrically shaped cities, might, in principle, align better with a match-mismatch process. This interpretation finds support from models arguing that CA3 may be similarly capable of match-mismatch detection given that it receives converging inputs from DG and entorhinal cortex via the perforant path (Mizumori & Leutgeb, 1999; Vinogradova, 2001). Future studies involving direct comparison of object vs. spatial context processing using imaging techniques capable of resolving subfield function will be needed to fully address this issue.
Prior imaging studies in humans have reported activation differences between CA3 and CA1 in terms of their contributions to linear vs. non-linear response signals during sensory processing (Bakker et al., 2008; Lacy et al., 2010). These results, and subsequent computational models, suggest that CA3 demonstrates a pattern completion response in the face of relatively small changes in input, whereas in response to more explicit alterations, CA3 demonstrates a pattern separation response. Additionally, these prior imaging studies (Bakker et al., 2008; Lacy et al., 2010) argue that CA1, like CA3/DG, plays a role in completion and separation but displays a more graded, linear response compared to CA3/DG. While we did find separation/completion effects in CA3/DG, we did not find clear pattern separation/completion effects within CA1. However, this previous work utilized an experimental design that involved rapidly presented novel, identical and familiar lure pictures of objects to participants in the scanner. In contrast, the current virtual reality paradigm required participants to discriminate spatial-context during a relatively long period of exploration, which required participants to process distributed spatial features from different viewpoints and reach a subjective threshold to allow for a discrimination-based response. A possible advantage of using geometrical cities is that this method allows clear parameterizing of “sameness” or “differentness.” This paradigm is also more comparable to prior rodent studies involving morphed shapes, with MPS bearing more similarity to cellular remapping than the BOLD adaptation technique used in the aforementioned imaging studies. Yet, as mentioned above, coding of objects and otherwise verbalizable material may lead to different representational properties in the hippocampus than spatial context, consistent with some proposals that verbal and spatial material are handled differently within the hippocampal circuitry (Igloi, Doeller, Berthoz, Rondi-Reig, & Burgess, 2010). Furthermore, rodent studies suggest that distinct pathways involving medial and lateral entorhinal cortex, respectively, handle spatial and non-spatial information input into the hippocampus (Hargreaves et al., 2005). This suggests the possibility that these inputs may subsequently be processed differently within the hippocampal circuitry. Again, future studies involving direct comparison of object vs. spatial context processing using imaging techniques capable of resolving subfield function will be needed to fully address this issue.
Overall, our results suggest that specific subfields of the hippocampus process distinctive dimensions of information. CA3/DG codes dynamically changing spatial contextual features, while CA1, due to its position downstream from CA3 and relative absence of recurrent connectivity, is better suited to integrate context-specific spatial features with stored mnemonic input. Our findings weigh in on the outstanding question regarding the fundamental purpose of the temporoammonic pathway, which connects layer III of the entorhinal cortex to CA1, by suggesting a distinct role for CA1 in the integration of CA3 input with cortical representation. Our results thus provide new insight into how the human hippocampal subfields differentially contribute to episodic memory.
Supplementary Material
Acknowledgments
This work was supported by NINDS R01NS076856, the Sloan Foundation, and the Hellman Young Investigator Award. We thank the UC-Davis memory group for comments on this manuscript.
References
- Amaral DG, Insausti R. The Hippocampal formation. In: Paxinos G, editor. The Human Nervous System. San Diego: Academic Press; 1990. pp. 711–755. [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31(3):571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132(1):77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab M, Stark SM, Stark CE. Contributions of human hippocampal subfields to spatial and temporal pattern separation. Hippocampus. 2014;24(3):293–302. doi: 10.1002/hipo.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Allen R, Vargha-Khadem F. Is the hippocampus necessary for visual and verbal binding in working memory? Neuropsychologia. 2010;48(4):1089–1095. doi: 10.1016/j.neuropsychologia.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319(5870):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Henson RN, Lee AC, Graham KS. Medial temporal lobe activity during complex discrimination of faces, objects, and scenes: Effects of viewpoint. Hippocampus. 2010;20(3):389–401. doi: 10.1002/hipo.20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brod G, Werkle-Bergner M, Shing YL. The influence of prior knowledge on memory: a developmental cognitive neuroscience perspective. Front Behav Neurosci. 2013;7:139. doi: 10.3389/fnbeh.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TI, Hasselmo ME, Stern CE. A High-resolution study of hippocampal and medial temporal lobe correlates of spatial context and prospective overlapping route memory. Hippocampus. 2014;24(7):819–839. doi: 10.1002/hipo.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun VH, Leutgeb S, Wu HQ, Schwarcz R, Witter MP, Moser EI, et al. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron. 2008;57(2):290–302. doi: 10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, et al. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296(5576):2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56(1):1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Olsen RK, Preston AR, Glover GH, Wagner AD. Associative retrieval processes in the human medial temporal lobe: hippocampal retrieval success and CA1 mismatch detection. Learn Mem. 2011;18(8):523–528. doi: 10.1101/lm.2135211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S. What's new with the amnesic patient H.M.? Nat Rev Neurosci. 2002;3(2):153–160. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dudukovic NM, Preston AR, Archie JJ, Glover GH, Wagner AD. High-resolution fMRI reveals match enhancement and attentional modulation in the human medial temporal lobe. J Cogn Neurosci. 2011;23(3):670–682. doi: 10.1162/jocn.2010.21509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Ketz N, Inati SJ, Davachi L. Evidence for Area CA1 as a Match/Mismatch Detector: A High-Resolution fMRI Study of the Human Hippocampus. Hippocampus. 2012 doi: 10.1002/hipo.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Tompary A, Davachi L. Associative Encoding and Retrieval Are Predicted by Functional Connectivity in Distinct Hippocampal Area CA1 Pathways. J Neurosci. 2014;34(34):11188–11198. doi: 10.1523/JNEUROSCI.0521-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Hippocampus: Functional Anatomy, Vascularization, and Serial Sections with MRI. Berlin: Springer; 1998. [Google Scholar]
- Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron. 1999;23(2):209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Bazih AJ, Suthana NA, Al-Hakim R, Ogura K, Zeineh M, et al. Advances in high-resolution imaging and computational unfolding of the human hippocampus. Neuroimage. 2009;19:19. doi: 10.1016/j.neuroimage.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23(1):115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Fischer RA. The General Sampling Distribution of the Multipe Correlation Coefficient. Proceedings of the Royal Society of Mathematical, Physical, and Engineering Sciences A. 1928;121:654–673. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Graham KS, Scahill VL, Hornberger M, Barense MD, Lee AC, Bussey TJ, et al. Abnormal categorization and perceptual learning in patients with hippocampal damage. J Neurosci. 2006;26(29):7547–7554. doi: 10.1523/JNEUROSCI.1535-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44(4):581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308(5729):1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F, et al. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17(1):34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igloi K, Doeller CF, Berthoz A, Rondi-Reig L, Burgess N. Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proc Natl Acad Sci U S A. 2010;107(32):14466–14471. doi: 10.1073/pnas.1004243107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Gotman M. Right hippocampal excision impairs learning and recall of a list of abstract designs. Neuropsychologia. 1986;24(5):659–670. doi: 10.1016/0028-3932(86)90005-9. [DOI] [PubMed] [Google Scholar]
- Katz Y, Kath WL, Spruston N, Hasselmo ME. Coincidence detection of place and temporal context in a network model of spiking hippocampal neurons. PLoS Comput Biol. 2007;3(12):e234. doi: 10.1371/journal.pcbi.0030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Burgess N, Hartley T, Vargha-Khadem F, O'Keefe J. Human hippocampus and viewpoint dependence in spatial memory. Hippocampus. 2002;12(6):811–820. doi: 10.1002/hipo.10070. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Jones CK, Miller MI, Stark CE. High-resolution fMRI investigation of the medial temporal lobe. Hum Brain Mapp. 2007;28(10):959–966. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Bandettini P. Analyzing for information, not activation, to exploit high-resolution fMRI. Neuroimage. 2007;38(4):649–662. doi: 10.1016/j.neuroimage.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Goebel R, Bandettini P. Information-based functional brain mapping. Proc Natl Acad Sci U S A. 2006;103(10):3863–3868. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biol. 2006;4(12):e424. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2010;18(1):15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MC, Lykken C, Tye LD, Wickelgren JG, Frank LM. Hippocampal output area CA1 broadcasts a generalized novelty signal during an object-place recognition task. Hippocampus. 2014;24(7):773–783. doi: 10.1002/hipo.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocque KF, Smith ME, Carr VA, Witthoft N, Grill-Spector K, Wagner AD. Global similarity and pattern separation in the human medial temporal lobe predict subsequent memory. J Neurosci. 2013;33(13):5466–5474. doi: 10.1523/JNEUROSCI.4293-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Yoganarasimha D, Rao G, Knierim JJ. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430(6998):456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Treves A, Meyer R, Barnes CA, McNaughton BL, et al. Progressive transformation of hippocampal neuronal representations in “morphed” environments. Neuron. 2005;48(2):345–358. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305(5688):1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Levy WB. A computational approach to the hippocampal function. In: Hawkins RD, Bower GH, editors. Computational models of learning in simple neural systems. Orloando, FL: Academic; 1989. [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22(2):233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman DC. Detection theory: A user’s guide. Mahwah, New Jersey: Lawrence Erlbaum Associates, Inc; 2005. [Google Scholar]
- McKenzie S, Robinson NT, Herrera L, Churchill JC, Eichenbaum H. Learning causes reorganization of neuronal firing patterns to represent related experiences within a hippocampal schema. J Neurosci. 2013;33(25):10243–10256. doi: 10.1523/JNEUROSCI.0879-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJ, Leutgeb S. Interpreting neural representations of aged animals. Hippocampus. 1999;9(5):607–608. doi: 10.1002/(SICI)1098-1063(1999)9:5<607::AID-HIPO17>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7(7):1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Beeck H, Wagemans J, Vogels R. Inferotemporal neurons represent low-dimensional configurations of parameterized shapes. Nat Neurosci. 2001;4(12):1244–1252. doi: 10.1038/nn767. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431(7009):699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23(2):752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Yonelinas AP, Ranganath C. Functional Connectivity Relationships Predict Similarities in Task Activation and Pattern Information during Associative Memory Encoding. J Cogn Neurosci. 2014;26(5):1085–1099. doi: 10.1162/jocn_a_00533. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79(1):1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Schlichting ML, Zeithamova D, Preston AR. CA subfield contributions to memory integration and inference. Hippocampus. 2014 doi: 10.1002/hipo.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloutsky VM, Fisher AV. When development and learning decrease memory. Evidence against category-based induction in children. Psychol Sci. 2004;15(8):553–558. doi: 10.1111/j.0956-7976.2004.00718.x. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006;16(9):716–729. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98(22):12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, et al. Schemas and memory consolidation. Science. 2007;316(5821):76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- van Kesteren MTR, Ruiter DJ, Fernandez G, Henson RN. How schema and novelty augment memory formation. Trends in Neurosciences. 2012;35(4):211–219. doi: 10.1016/j.tins.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24(29):6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11(5):578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- Viskontas I, Knowlton B, Steinmetz PN, Fried I. Differences in Mnemonic Processing by Neurons in the Human Hippocampus and Parahippocampal Region. Journal of Cognitive Neuroscience. 2006;20:1654–1662. doi: 10.1162/jocn.2006.18.10.1654. [DOI] [PubMed] [Google Scholar]
- Wills TJ, Lever C, Cacucci F, Burgess N, O'Keefe J. Attractor dynamics in the hippocampal representation of the local environment. Science. 2005;308(5723):873–876. doi: 10.1126/science.1108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP. Organization of the entorhinal-hippocampal system: a review of current anatomical data. Hippocampus. 1993;3(Spec No):33–44. [PubMed] [Google Scholar]
- Wolbers T, Buchel C. Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. J Neurosci. 2005;25(13):3333–3340. doi: 10.1523/JNEUROSCI.4705-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends in neurosciences. 2011;34(10):515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Dobbins I, Lazzara M, Knight RT. Recollection and familiarity deficits in amnesia: convergence of remember-know, process dissociation, and receiver operating characteristic data. Neuropsychology. 1998;12(3):323–339. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Bookheimer SY. Application of cortical unfolding techniques to functional MRI of the human hippocampal region. Neuroimage. 2000;11(6 Pt 1):668–683. doi: 10.1006/nimg.2000.0561. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299(5606):577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
- Zhang H, Copara MS, Ekstrom AD. Differential Recruitment of Brain Networks following Route and Cartographic Map Learning of Spatial Environments. PLoS One. 2012;7(9):e44886. doi: 10.1371/journal.pone.0044886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.