Abstract:
Primary graft dysfunction (PGD) following lung transplantation is clinically similar to the acute respiratory distress syndrome. Because alcohol abuse independently increases the incidence of acute respiratory distress syndrome in at-risk individuals, we hypothesized that donor alcohol use is correlated with an increased risk of PGD. As a pilot study, we collected alcohol use histories using a validated instrument, the Alcohol Use Disorder Identification Test questionnaire, from 74 donors and correlated these with the development of PGD in corresponding recipients. Nineteen percent (14/74) of donors were classified as heavy alcohol users, as defined by the Alcohol Use Disorder Identification Test scores ≥8. In the 1st 4 days post-transplantation, similar percentages of recipients developed grade 3 PGD on at least 1 day (heavy alcohol user = 29% [4/14] versus lighter alcohol user = 27% [16/60]); however, recipients receiving a lung from a heavy alcohol user were more likely to have multiple and consecutive days of grade 3 PGD, especially in the 1st 48 hours post-transplant. Both median length of stay in the intensive care unit and hospital were somewhat longer in the heavy alcohol user group (9 versus 7 days and 19.5 versus 17.5 days, respectively). If these preliminary findings are validated in a multi-center study, they would have important implications not only for our understanding of the pathophysiology of PGD but also for the development of novel treatments based on the evolving evidence from experimental and clinical studies on how alcohol abuse renders the lung susceptible to acute edematous injury.
Key Indexing Terms: Primary graft dysfunction, Lung transplantation, Lung donor, Recipient, AUDIT, Alcohol use
Primary graft dysfunction (PGD) is the leading cause of death in the immediate period following lung transplantation and has an incidence of 15% to 25%.1 Unfortunately, there are no effective therapies, and even with supportive care, the mortality can be as high as 43%.2,3 It had previously been assumed that the factors predisposing to PGD pertain to the surgical procedure or to the allograft recipient.4 However, compelling experimental and clinical evidence suggests that donor-related risks factors are also important in the development of PGD. For example, it is now recognized that donor characteristics, such as age, smoking and mismatches with the donor in sex or race, contribute to poor recipient outcomes.4 We recently demonstrated a potential association between elevated donor levels of receptor for advanced glycation end-products and the development of PGD.5 In addition, other studies have shown that donor biomarkers such as interleukin (IL)-8,6 vascular endothelial growth factor7 and certain gene expression profiles8 are associated with an increased risk of developing PGD. Importantly, because there are no proven medical treatments for PGD, identification of additional biomarkers to accurately identify allografts that are at higher risk for developing this serious condition are needed.9
The features of PGD represent essentially a form fruste of the acute respiratory distress syndrome (ARDS), the most severe form of acute lung injury (ALI), which occurs within the unique context of lung transplantation.10 In the past 15 years, a strong independent association between alcohol abuse and ARDS has been identified, and our group at Emory University has been at the forefront of investigating the mechanisms by which alcohol abuse renders the lung susceptible to acute edematous injury. Specifically, alcohol abuse independently and significantly increases the risk of ARDS 2- to 4-fold in critically ill individuals.11,12 Although the mechanisms underlying this association are still being investigated, we have clear evidence from experimental models and clinical studies that chronic alcohol ingestion causes oxidative stress and depletes the pool of the antioxidant glutathione within the alveolar space.13,14 Alcohol-induced oxidative stress causes previously unrecognized alveolar epithelial dysfunction, including increased paracellular permeability, decreased liquid clearance, impaired surfactant production and decreased cell viability.13,15–17
This “alcoholic lung” phenotype is clinically silent in that the physiological perturbations identified in both experimental models and in otherwise healthy alcoholic individuals are not readily detectable without sophisticated measurements and do not manifest as significant lung dysfunction until an acute inflammatory stress, such as sepsis or aspiration, unmasks them. Specifically, alcohol abuse alone does not cause lung injury but rather it significantly lowers the threshold for its development. This previously unrecognized association between alcohol abuse and ARDS/ALI was identified only when prospective studies were done using accurate alcohol use assessments such as the Alcohol Use Disorder Identification Test (AUDIT).12 However, this type of prospective investigation has not heretofore been applied in the unique context of lung transplantation and PGD. Therefore, we sought to determine if donor alcohol abuse likewise increased the risk of PGD. As a 1st step and a means of identifying biological plausibility, we demonstrated that chronic alcohol use by the donor exacerbated airway injury in allograft recipients using an experimental rat model of heterotopic tracheal transplantation.18 We then initiated a single-center pilot study within our Emory Alcohol and Lung Biology Center, in collaboration with the McKelvey Center for Lung Transplantation at Emory University, to collect preliminary data in support of such an association that could stimulate the design and implementation of a larger multi-center study. Such information would have enormous implications for both the selection of lung allograft donors and for the development of novel therapeutic approaches to mitigate the devastating consequences of PGD.
We reasoned that this study was important to the lung transplant community, which includes donors, recipients and the tens of thousands of healthcare professionals involved in lung transplantation. Unfortunately, despite advances in lung transplantation techniques and identification of appropriate selection criteria, our ability to predict which lung allograft recipients will develop PGD is at present imprecise,19 and our limited understanding of the fundamental mechanisms driving the development of PGD has frustrated our attempts to identify effective therapies beyond supportive care. In light of recent experimental and clinical evidence revealing the strong and independent association between alcohol abuse and ALI, we felt that there were compelling reasons to examine whether donor alcohol abuse increased the risk of PGD. To address this hypothesis, we initiated a pilot study at Emory University through our Alcohol and Lung Biology Center to study the relationship between donor alcohol use and the development of PGD in lung transplant recipients.
PATIENTS AND METHODS
Donor and Recipient Characteristics
Eligibility
Between February 2007 and January 2009 at Emory Hospital in Atlanta, GA, 88 consecutive lung transplant recipients and their lung donors were considered for eligibility. Exclusion criteria included being re-transplanted (n = 4), no consent (n = 2) and improper lung preparation (n = 3). Two subjects received a lung from the same donor; 1 subject was randomly selected for inclusion in the analysis. Therefore, in total, there were 78 eligible lung allograft recipients, of whom 74 had their donor's alcohol use quantified using the AUDIT. All analyses are based on these 74 recipients.
Donor Lung Criteria
Lung donors were recruited from brain-dead patients consented for lung donation. Donor lungs used for transplantation at our institution had to meet the following inclusion criteria at the time of organ recovery: (1) compatible ABO blood group, (2) PaO2/FiO2 > 300 mm Hg, (3) chest radiograph without focal or significant findings consistent with pneumonia or lung contusion and (4) adequate bronchoscopic assessment was performed to ensure that no obvious aspiration was present. Explanted lungs were preserved using Perfadex (Vitrolife, Gothenburg, Sweden). The allograft ischemia time was recorded for all lung transplants.
Lung Recipients Criteria
Recipients listed at our institution received transplants according to their clinical priorities. Clinical data were available for all lung transplant recipients. This study was approved by the institutional review board at Emory University.
Immunosuppressive Therapy
All recipients received a standard immunosuppression protocol following transplantation, consisting of induction with IL-2 receptor antagonist and maintenance with a 3-drug combination with the calcineurin inhibitor tacrolimus, azathioprine and steroids as described previously.20
Transplant Infection Prophylaxis
All recipients received antibiotics up to 5 days after surgery, and subsequent antibiotic therapy length was determined based on final donor culture and intra-operative bronchial cultures obtained by swab at the time of surgery. All recipients received prophylaxis after transplantation against cytomegalovirus, Pneumocystis jirovecii and Aspergillus spp. as previously described.21
PGD Treatment
PGD treatment involved administration of diuretics, prolonged mechanical ventilation with adjusted FiO2 and positive end-expiratory pressure and inhaled NO as required.
Predictor and Outcomes Definitions
Definition of Alcohol Abuse
The AUDIT is a 10-question questionnaire that queries recent alcohol use, alcohol dependence symptoms and alcohol-related problems and has a sensitivity >90% to distinguish hazardous and harmful alcohol use whether the information is self-reported or obtained from a surrogate add.22,23 Respondents with scores ≥8 are categorized as heavy alcohol users, and those with scores <8 are categorized as light alcohol users.
Scoring of PGD
PGD was scored from 0 to 3 using chest radiographs and PaO2/FiO2 ratios per the guidelines of the International Society for Heart and Lung Transplantation. Scores were assigned at regular and/or specified intervals during the 1st 96 hours after lung transplantation.24 Before removal of their endotracheal tube, all recipients received a bronchoscopy to assess bronchial anastomosis and bronchoalveolar lavage fluid was obtained and submitted for culture. Furthermore, for recipients fitting clinical criteria of PGD, trans-bronchial biopsies were performed to rule out additional potential causes for abnormal radiographs/poor oxygenation.
Covariates Examined
Potential confounding variables were examined that could influence any observed association between donor alcohol abuse and subsequent PGD. These included:
Donor variables: Age, sex, race, smoking history (>1 pack-year) and cause of death.
Recipient variables: Age, race, sex and underlying lung disease, length of stay (LOS) in the intensive care unit (ICU) and overall hospital LOS.
Surgical variables: Transplant procedure, utilization of pulmonary cardiopulmonary bypass, ischemia time and duration of mechanical ventilation
LOS: LOS in the ICU and the overall hospital LOS.
Statistics
As this was a single-center pilot study with a limited number of participants, we recognized that it was unlikely that we would be able to identify a statistically significant difference in the incidence of PGD in allograft from donors with or without alcohol abuse unless that difference was very large. Therefore, the primary goal was to make comparisons that could be used to design a larger multi-center trial if our results suggested that donor alcohol abuse could be associated with a clinically meaningful difference in the incidence of PGD. In this context, we planned from the outset to present the descriptive results of this pilot study. Specifically, we report the number and percentage of subjects in each category. In addition, we calculated the odds ratio of developing PGD when receiving an allograft from a donor with alcohol abuse versus receiving an allograft from a donor without alcohol abuse using logistic regression and using a generalized linear mixed model methodology to account for multiple measurements per patient over time. Covariate adjusted odds ratios were estimated by a series of 2-variable models (ie, alcohol status and one of the potentially confounding covariates). Lung transplant donor and recipient demographic and clinical characteristics other than PGD outcomes were examined for their relationship to donor alcohol abuse status (donor and recipient age: Wilcoxon rank sum test; all categorical variables: Fisher's exact test; ICU and hospital LOS: log-rank test). All tests were 2-sided. All analyses were completed using SAS v9 for Windows 7 Enterprise (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
The most common indication for transplant was chronic obstructive pulmonary disease (COPD) (50% of all transplants) followed by idiopathic pulmonary fibrosis (41%). Sixty-five of 74 of all transplants (88%) included in the analyses were bilateral lung transplants. The median age of these donors was 30.5 (range, 12–62) years. The most common cause of death among donors was traumatic head injury (47.0%).
Donor and Recipient Demographics by Donor Alcohol Use Category
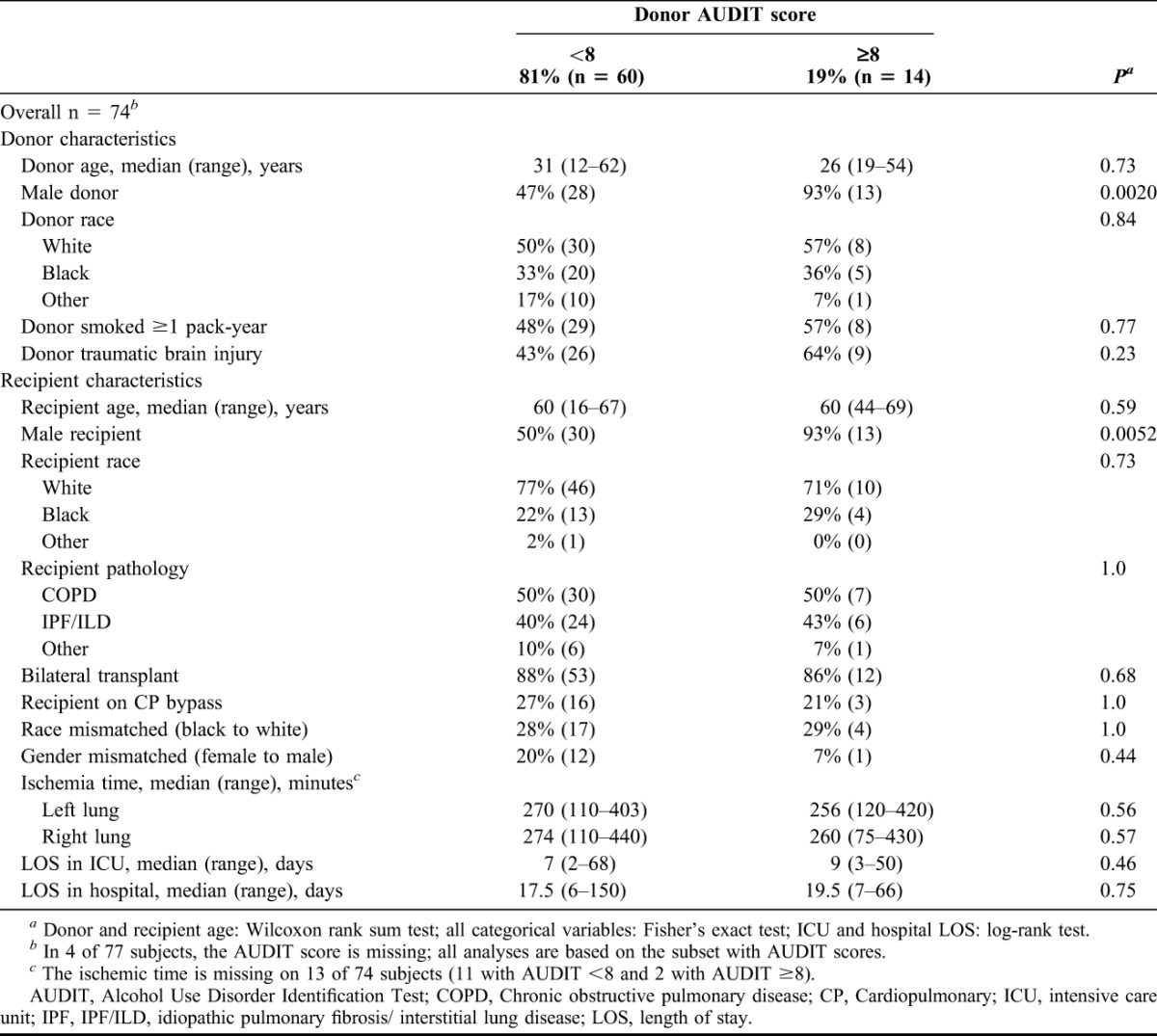
Fourteen of the recipients (19%) received a lung from a donor with heavy alcohol use (Table 1). The 2 donor alcohol use groups were similar for age, racial distribution and smoking history. The heavy alcohol use group had somewhat more deaths because of traumatic injury (64% versus 43%; P = 0.23) and a higher proportion of males (93% versus 47%; P = 0.002). Recipients in the 2 groups were similar for age, racial distribution, indication for transplant, transplant type, use of cardiopulmonary bypass, racial mismatch (African American to Caucasian) and ischemia time. Recipients of lungs from heavy drinkers were more likely to be male (93% versus 50%; P = 0.005). A gender mismatch (female to male) was somewhat less likely in the heavy alcohol use group 7% versus 20%, although this difference was not statistically significant. Median LOS in the ICU and in the hospital was 2 days longer in the heavy alcohol use group.
TABLE 1.
Lung transplant recipient and donor characteristics by donor alcohol status; Emory Transplant Center, February 2007 to January 2009
Development of PGD
Using the consensus ISHLT definitions, PGD grades of 0, 1, 2 and 3 at 48 hours (T48) after lung transplantation occurred in 43%, 27%, 14% and 17% of recipients, respectively.
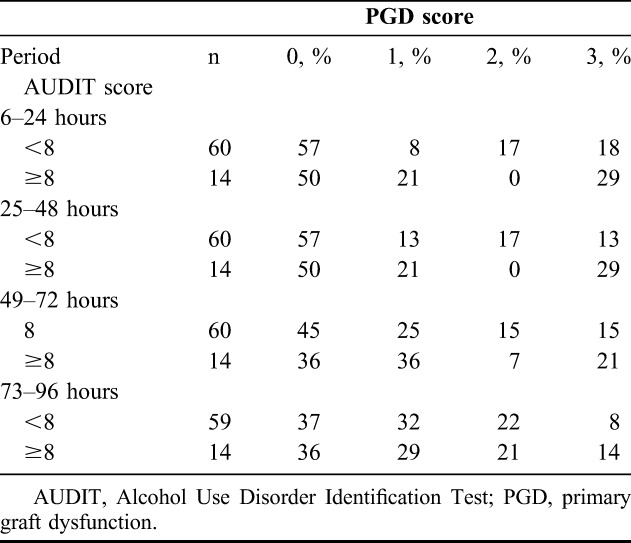
A little over a quarter of recipients in both groups had grade 3 PGD on at least 1 day during the 1st 4 days post-transplant (Table 2). However, PGD was more persistent among recipients of lungs from heavy alcohol users, with 50% of these PGD cases lasting 4 days (compared with 13% in the light alcohol group). Additionally, their PGD was more likely to occur on consecutive days. However, these differences between the 2 groups are based on a small number of cases with grade 3 PGD. On each of the 1st 4 post-transplant days, the incidence of grade 3 PGD was higher in the heavy alcohol use group (Table 3). The greatest difference between the light and heavy groups was apparent on the 1st 2 days (light versus heavy: 18% versus 29% at 6–24 hours; 13% versus 29% at 25–48 hours), with small differences thereafter (15% versus 21% at 49–72 hours; 8% versus 14% at 73–96 hours). On the 1st 2 days, there also was a substantial difference in the incidence of grade 2 PGD (17% versus 0% on both days).
TABLE 2.
Grade 3 PGD by donor alcohol status; Emory Transplant Center, February 2007 to January 2009
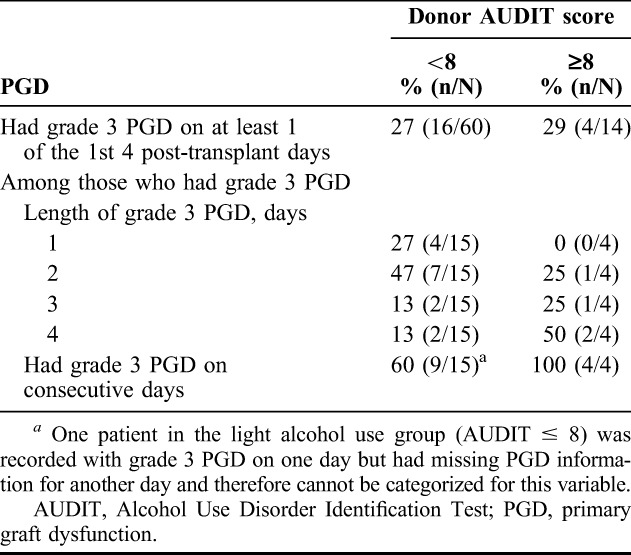
TABLE 3.
Relationship between lung transplant recipient PGD score and donor alcohol status, by period after transplant; Emory University Hospital Lung Transplant Service, February 2007 to January 2009
Odds Ratio of Developing PGD in Allograft From Alcoholic Versus Non-Alcoholic Donors
Using a method that takes into account the multiple days of grade 3 PGD measurement on each person, we compared the 2 groups for their odds of developing grade 3 PGD. Without controlling for any potential confounders, the odds ratio was 1.8, with a 95% confidence that the true value falls in the interval 0.6 and 5.6. Controlling either for the donor factors of age, traumatic brain injury (TBI) and smoking or for the recipient factors of diagnosis, race mismatch, gender mismatch, cardiopulmonary bypass (CPB) and type of transplant (bilateral or not) had minimal effect on the odds ratio (range 1.7–1.9). On average, the rate of PGD 3 per day was 22% in the heavy alcohol use group compared with 14% in the lighter use group.
DISCUSSION
We found that 1/5 of the lung donors at our center in this study had heavy alcohol intake when classified by validated instruments, such as AUDIT. In addition, while the risk of developing grade 3 PGD at least once in the 1st days post-transplantation seemed similar between the 2 groups, the number and duration of episodes of PGD 3 also seem greater when the allograft came from a donor with heavy alcohol use. For example, as disclosed in Table 2, PGD 3 lasting 4 days was present in 2/4 versus 2/15 of recipients who experienced any PGD 3. In parallel, we observed somewhat longer ICU and hospital LOSs if the donor had an AUDIT ≥8, and the recipients of allografts from donors with alcohol abuse had more grade 3 PGD at 48 hours post-transplantation. These findings build on previous observations that lung allograft recipients with grade 3 PGD within the 1st 48 hours following transplant had significantly decreased long-term survival, as well as longer ICU and hospital stays, when compared with recipients who had grade 1 or 2 PGD.25
Taken together, our findings in this pilot study are consistent with the multi-center studies showing that alcohol abuse increases the risk of developing ARDS approximately 4-fold in critically ill individuals and raise concern that donor alcohol abuse could significantly increase the risk of PGD following lung transplantation. Our preliminary findings in this single-center pilot study are provocative because they suggest that donor alcohol abuse may have an adverse effect on outcome following transplantation that cannot be predicted by our current risk-stratification criteria. Therefore, we believe that it is imperative for the lung transplant community to perform a larger multi-center study to either validate or refute an association between donor alcohol abuse and PGD because either result will be valuable as we work to improve outcomes following lung transplantation.
Our findings are consistent with a growing body of experimental and clinical evidence that excessive alcohol use renders the lung susceptible to injury. The initial link between alcohol abuse and ARDS was identified in a retrospective analysis of a clinical database of 351 subjects admitted to an ICU with an acute illness that placed them at risk for ARDS.11 This association was later confirmed in a prospective, multi-center study of 220 patients admitted with severe sepsis.12 In the initial study, the relative risk was approximately 2:1. However, in the latter study in which a validated case definition of an alcohol use disorder was used, the relative risk of ARDS in alcoholic individuals was approximately 3.7:1. Therefore, it is reasonable to be concerned that there is an increased relative risk of PGD following transplantation of lung allografts from donors with significant alcohol abuse and that a larger multi-center study would confirm our preliminary findings in this pilot study. In fact, even if we had determined in this pilot study that donor alcohol abuse significantly increased the relative risk of PGD at the P < 0.05 level, we would still need to confirm this finding in a larger multicenter study to ensure that the association was indeed generalizable.
To our knowledge, the AUDIT questionnaire has not been used in other clinical studies of lung donors, particularly to assess whether their antemortem alcohol use was associated with poorer post-transplant outcomes in lung allograft recipients. Although the standardized United Network for Organ Sharing questionnaires given to the surrogates of all potential organ donors include questions about alcohol use, those questions do not allow for specific and accurate classification of alcohol use disorders. Our group and others have used the AUDIT questionnaire in similar studies of critically ill individuals that have identified the increased risk of ALI in patients with alcohol use disorders.12 Importantly, in many cases we have relied on surrogates to answer the AUDIT questionnaires for their loved ones, and this use of surrogates has been independently validated by other investigators.26 Therefore, although the use of the AUDIT questionnaire in the particular context of lung transplantation is unique, its validated use in other clinical studies of critical illness and ALI support its use in this and future clinical studies of PGD and other post-transplant outcomes.
The main limitation of our pilot study was that it was a single-center study with a small number of alcoholic donors and a moderately small total number of subjects. Therefore, we recognized from the outset that it was very likely that our study would be underpowered to identify a statistically significant association between donor alcohol abuse and the subsequent development of PGD. In addition, donor alcohol use was assessed by donor surrogates. Although this could potentially lead to greater misclassification of alcoholics than with self-reports, if anything one might expect surrogate questionnaires to underestimate the true incidence of alcohol abuse by donors. If so, then the true impact of alcohol abuse could be even greater than estimated by our study. Furthermore, because of the small numbers of subjects in this study, we could not simultaneously control for multiple confounding factors related to both donor and recipient characteristics. Finally, we presented donor alcohol effects only on PGD and length of initial hospital stay; other outcomes, such as infections, oxidative stress, re-hospitalizations and long-term outcomes, were not examined.
However, if these preliminary findings are validated in a larger multi-center study, they would have important implications. As there are approximately 2,000 lung transplants performed in the United States annually, donor alcohol abuse may be responsible for a substantial number of cases of PGD and its associated morbidity and mortality. Therefore, we believe that this pilot study and its findings are provocative and that the increased risk of PGD in the context of donor alcohol abuse is consistent with extensive experimental and clinical evidence that overwhelmingly implicates alcohol abuse in the development of ALI in other settings.
The role of donor-derived factors in the pathogenesis of PGD was highlighted by a report of significant association of PGD among shared lung, kidney and heart recipients from the same donor.27 Despite optimization of donor and recipient selection criteria and advances in surgical techniques, PGD remains essentially refractory to treatment and is the primary cause of mortality in the immediate post-transplant period.1,2,28,29 As a result, a considerable body of research has focused on identifying donor risk factors that are associated with poor outcomes following transplantation. Elevated donor pre-transplant levels of biomarkers, such as receptor for advanced glycation end-products,5 IL-86 and vascular endothelial growth factor,7 are associated with increased risk or severity of PGD in recipients in the post-transplant period. This current study suggests that donor alcohol abuse is a factor that may have a greater impact on the risk of PGD than any other single factor identified to date. Therefore, if our results are validated in a larger, multi-center study, they could have important implications not only for lung donor screening but also for the generation of novel therapeutic interventions. Specifically, we could capitalize on the ever-increasing knowledge of the mechanisms by which alcohol abuse renders the lung susceptible to injury to design and test treatments to limit the incidence and/or severity of PGD.
There is considerable experimental evidence that a link between alcohol abuse and PGD is biologically plausible. For example, in an experimental rat model of transplantation, we determined that tracheal allografts from alcohol-fed donor rats developed more airway obliteration following heterotopic transplantation than tracheal allografts from control-fed rats.18 These experimental findings argue that alcohol use, independently of factors such as smoking or other illicit drug use renders the airway susceptible to damage following transplantation. This experimental model in the context of airway transplantation builds on extensive earlier studies from our group on the effects of alcohol on lung epithelial function. For example, we determined that alveolar epithelial type II cells that were isolated from alcohol-fed rats had decreased surfactant production and were more susceptible to oxidant-mediated injury.13 We also determined that alcohol ingestion alters alveolar epithelial barrier function in vivo, as reflected by increased protein leak across the alveolar barrier and decreased alveolar liquid clearance.16 A common mechanism seems to be that alcohol ingestion dramatically decreases alveolar epithelial levels of glutathione, a critical antioxidant within the alveolar space, and increases both endotoxin-mediated acute edematous injury in isolated lungs that were perfused ex vivo13,30 and sepsis-mediated ALI in vivo.17 Importantly, young and otherwise healthy subjects who meet criteria for alcohol abuse also have profoundly decreased levels of glutathione in their alveolar space.14 However, although chronic glutathione replacement in the alcohol diet in experimental animal models prevents glutathione depletion and thereby maintains alveolar epithelial function,13,16,17,30,31 it is unlikely that glutathione replacement alone could rescue the alcoholic lung in the context of ALI. For 1 reason, we determined that N-acetylcysteine, the only approved glutathione precursor available for human use, does not maintain the critical mitochondrial glutathione pool during alcohol feeding and does not preserve surfactant production.31 This is consistent with previous clinical trials in which N-acetylcysteine therapy was minimally efficacious in patients with established ARDS, although those trials were not directed toward patients with a history of alcohol abuse.32,33 In addition, the glutathione depletion within the airway is just 1 marker of the chronic oxidative damage that prolonged alcohol abuse inflicts on the airway, and this damage cannot be immediately reversed simply by the acute administration of glutathione supplements. Taken together, our experimental and clinical studies have identified that alcohol abuse causes previously unrecognized oxidative stress and epithelial dysfunction within the lung but that clinically significant therapeutic interventions will require a more comprehensive strategy than simple glutathione replacement.
CONCLUSIONS
Our data suggest that donor alcohol abuse may increase the risk of PGD following lung transplantation. Although this is at present a preliminary finding from a relatively small single-center study, it is consistent with the clearly established link between alcohol abuse and ARDS and is supported by a large body of experimental evidence over the past 2 decades showing that alcohol renders the lung susceptible to acute edematous injury. The results from this pilot study therefore provide a compelling argument to design and conduct a multi-center study to determine whether there is a true association between donor alcohol abuse and PGD and the magnitude of such an association if it exists. Based on the experimental and clinical evidence linking alcohol abuse to ARDS and ALI, there is in fact every reason to believe that PGD, which is essentially ALI/ARDS in the context of lung transplantation, is associated with donor alcohol abuse. The confirmation (or refutation) of such an association is critical to the lung transplant community and would have important implications for the evaluation and risk-stratification of donor-recipient pairs. Perhaps even more importantly, it would provide novel insights into the mechanisms that predispose a lung allograft recipient to develop PGD and therefore could lead to the design and testing of novel therapies to decrease the impact of this dreaded complication.
ACKNOWLEDGMENTS
The authors thank LifeLink of Georgia (organ procurement organization).
Footnotes
Supported by Emory University Research Committee award (2006073 to A.P.) and Emory Alcohol and Lung Biology Center (National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism [NIAAA] P-50 AA 13575701).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Diamond J, Lee JC, Kawut SM, et al. ; Lung Transplant Outcomes Group. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med 2005;171:1312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christie JD, Kotloff RM, Pochettino A, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest 2003;124:1232–41. [DOI] [PubMed] [Google Scholar]
- 4.Whitson BA, Nath DS, Johnson AC, et al. Risk factors for primary graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg 2006;131:73–80. [DOI] [PubMed] [Google Scholar]
- 5.Pelaez A, Force SD, Gal AA, et al. Receptor for advanced glycation end products in donor lungs is associated with primary graft dysfunction after lung transplantation. Am J Transplant 2010;10:900–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher AJ, Donnelly SC, Hirani N, et al. Elevated levels of interleukin-8 in donor lungs is associated with early graft failure after lung transplantation. Am J Respir Crit Care Med 2001;163:259–65. [DOI] [PubMed] [Google Scholar]
- 7.Krenn K, Klepetko W, Taghavi S, et al. Recipient vascular endothelial growth factor serum levels predict primary lung graft dysfunction. Am J Transplant 2007;7:700–6. [DOI] [PubMed] [Google Scholar]
- 8.Ray M, Dharmarajan S, Freudenberg J, et al. Expression profiling of human donor lungs to understand primary graft dysfunction after lung transplantation. Am J Transplant 2007;7:2396–405. [DOI] [PubMed] [Google Scholar]
- 9.Shargall Y, Guenther G, Ahya VN, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part VI: treatment. J Heart Lung Transplant 2005;24:1489–500. [DOI] [PubMed] [Google Scholar]
- 10.de Perrot M, Liu M, Waddell TK. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med 2003;167(4):490–511. [DOI] [PubMed] [Google Scholar]
- 11.Moss M, Bucher B, Moore FA, et al. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA 1996;275:50–4. [PubMed] [Google Scholar]
- 12.Moss M, Parsons PE, Steinberg KP, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med 2003;31:869–77. [DOI] [PubMed] [Google Scholar]
- 13.Holguin F, Moss I, Brown LA. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest 1998;101:761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moss M, Guidot DM, Wong-Lambertina M, et al. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med 2000;161:414–9. [DOI] [PubMed] [Google Scholar]
- 15.Molina PE, Hoek JB, Nelson S, et al. Mechanisms of alcohol-induced tissue injury. Alcohol Clin Exp Res 2003;27:563–75. [DOI] [PubMed] [Google Scholar]
- 16.Guidot DM, Modelska K, Lois M, et al. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol 2000;279:L127–35. [DOI] [PubMed] [Google Scholar]
- 17.Velasquez A, Bechara RI, Lewis JF, et al. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis-mediated lung dysfunction in ethanol-fed rats. Alcohol Clin Exp Res 2002;26:1245–51. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell PO, Guidot DM. Alcohol ingestion by donors amplifies experimental airway disease after heterotopic transplantation. Am J Respir Crit Care Med 2007;176(11):1161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arcasoy SM, Fisher A, Hachem RR, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part V: predictors and outcomes. J Heart Lung Transplant 2005;24:1483–8. [DOI] [PubMed] [Google Scholar]
- 20.Pelaez A, Lyon GM, Force SD, et al. Efficacy of oral ribavirin in lung transplant patients with respiratory syncytial virus lower respiratory tract infection. J Heart Lung Transplant 2009;28:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naik PM, Lyon GM, III, Ramirez A, et al. Dapsone-induced hemolytic anemia in lung allograft recipients. J Heart Lung Transplant 2008;27:1198–202. [DOI] [PubMed] [Google Scholar]
- 22.Neumann T, Neuner B, Gentilello LM, et al. Gender differences in the performance of a computerized version of the alcohol use disorders identification test in subcritically injured patients who are admitted to the emergency department. Alcohol Clin Exp Res 2004;28:1693–701. [DOI] [PubMed] [Google Scholar]
- 23.Moss M, Burnham EL. Alcohol abuse in the critically ill patient. Lancet 2006;368:2231–42. [DOI] [PubMed] [Google Scholar]
- 24.Christie JD, Carby M, Bag R, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2005;24:1454–9. [DOI] [PubMed] [Google Scholar]
- 25.Prekker ME, Nath DS, Walker AR, et al. Validation of the proposed International Society for Heart and Lung Transplantation grading system for primary graft dysfunction after lung transplantation. J Heart Lung Transplant 2006;25:371–8. [DOI] [PubMed] [Google Scholar]
- 26.Donovan DM, Dunn CW, Rivara FP, et al. Comparison of trauma center patients self-reports and proxy report on the alcohol use identification test (AUDIT). J Trauma 2004;5:873–82. [DOI] [PubMed] [Google Scholar]
- 27.Oto T, Excell L, Griffiths AP, et al. Association between primary graft dysfunction among lung, kidney and heart recipients from the same multiorgan donor. Am J Transplant 2008;8:2132–9. [DOI] [PubMed] [Google Scholar]
- 28.Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant 2007;26:1004–11. [DOI] [PubMed] [Google Scholar]
- 29.Burton CM, Iversen M, Milman N, et al. Outcome of lung transplanted patients with primary graft dysfunction. Eur J Cardiothorac Surg 2007;31:75–82. [DOI] [PubMed] [Google Scholar]
- 30.Lois M, Brown LA, Moss IM, et al. Ethanol ingestion increases activation of matrix metalloproteinases in rat lungs during acute endotoxemia. Am J Respir Crit Care Med 1999;160:1354–60. [DOI] [PubMed] [Google Scholar]
- 31.Guidot DM, Brown LA. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats. Alcohol Clin Exp Res 2000;24:1070–6. [PubMed] [Google Scholar]
- 32.Bernard GR. N-acetylcysteine in experimental and clinical acute lung injury. Am J Med 1991;91:54S–9S. [DOI] [PubMed] [Google Scholar]
- 33.Jepsen S, Herlevsen P, Knudsen P, et al. Antioxidant treatment with N-acetylcysteine during adult respiratory distress syndrome: a prospective, randomized, placebo-controlled study. Crit Care Med 1992;20:918–23. [DOI] [PubMed] [Google Scholar]


