Abstract
Phosphodiesterase 4D (PDE4D) has recently been implicated as a proliferation-promoting factor in prostate cancer and is over-expressed in human prostate carcinoma. However, the effects of PDE4D inhibition using pharmacological inhibitors have not been examined in prostate cancer. These studies examined the effects of selective PDE4D inhibitors, NVP-ABE171 and cilomilast, as anti-prostate cancer therapies in both in vitro and in vivo models. The effects of PDE4D inhibitors on pathways that are critical in prostate cancer and/or downstream of cyclic AMP (cAMP) were examined. Both NVP-ABE171 and cilomilast decreased cell growth. In vitro, PDE4D inhibitors lead to decreased signaling of the sonic hedgehog (SHH), Androgen Receptor (AR), and MAPK pathways, but growth inhibition was best correlated to the sonic hedgehog pathway. PDE4D inhibition also reduced proliferation of epithelial cells induced by paracrine signaling from co-cultured stromal cells that had activated hedgehog signaling. In addition, PDE4D inhibitors decreased the weight of the prostate in wild-type mice. Prostate cancer xenografts grown in nude mice that were treated with cilomilast or NVP-ABE171 had decreased wet weight and increased apoptosis compared to vehicle treated controls. These studies suggest the pharmacological inhibition of PDE4D using small molecule inhibitors is an effective option for prostate cancer therapy.
Implications
PDE4D inhibitors decrease the growth of prostate cancer cells in vivo and in vitro, and PDE4D inhibition has therapeutic potential in prostate cancer.
Introduction
Prostate cancer is the second leading cause of cancer deaths among men and the most commonly diagnosed non-cutaneous male cancer (1). Although there are well-defined histologic alterations that have been identified in prostate cancer, the nature of this genetically heterogeneous disease has limited the identification of novel oncogenes that can be used as therapeutic targets. Using a transposon insertional mutagenesis screen, our laboratory previously identified phosphodiesterase 4D (PDE4D) as a novel proliferation-promoting factor for prostate cancer (2). PDE4D was expressed in prostate cancer cell lines, but not primary prostate epithelial cells and knockdown of PDE4D significantly decreased prostate cancer cell growth and migration in vitro. Furthermore, knockdown of PDE4D by shRNA significantly reduced xenograft wet weights and xenograft cell proliferation in vivo. Subsequent studies that performed whole-genome sequencing of human prostate tumors detected internal rearrangements of the PDE4D gene in approximately 20% of the tumors sequenced (3). These results are consistent with results in other cancers that have rearrangements that are predicted to produce overexpression of short PDE4D isoforms (4).
Phosphodiesterases (PDEs) hydrolyze cyclic AMP (cAMP) and GMP (cGMP) and play a major role in cellular signaling. The PDE4 gene family has four isoforms: PDE4A, PDE4B, PDE4C, and PDE4D. In addition, the PDE4D gene has many variants attributed to alternative splicing and the use of multiple promoters (5, 6). PDE4D enzyme degrades cAMP, but does not affect cGMP (5). The distribution pattern of PDE4D variants varies within both cells and tissues (7). In the prostate, PDE4D expression was observed in both the stroma and in epithelium and its hydrolytic activity was restricted to the cytosolic compartment (8). In human prostate tumor microarrays representative of multiple stages of prostate cancer, PDE4D expression was most prominent in the epithelial cells (2). PDE4D was over-expressed in prostate adenocarcinoma samples when compared to benign prostatic hyperplasia samples in a human prostatic tissue microarray (2).
Previous studies have demonstrated the importance of cAMP signaling in prostate cancer. However, it remains unclear whether cAMP signaling promotes or inhibits prostate cancer progression. Transient increases in cAMP have been shown to be mitogenic in LNCaP prostate cancer cells by activation of extracellular signal-related kinase 1/2 (ERK1/2) while prolonged increases in cAMP led to growth arrest and neuroendocrine differentiation (9). In contrast, increased cAMP levels in androgen insensitive PC3 cells resulted in growth inhibition (10). cAMP/protein kinase A (PKA) activity has also been shown to affect the activity of the androgen receptor (AR) leading to increased AR-responsive gene expression in androgen-independent conditions (11, 12).
In addition, cAMP/PKA activity negatively regulates the hedgehog pathway (HH) in some cell types including leukemia cells and basal cell carcinomas and increasing cAMP signaling leads to growth arrest and death (13, 14). While the effects of PKA on the hedgehog pathway have not been investigated in prostate cancer cells, the hedgehog pathway has been shown to be an important driver of the prostate cancer phenotype in DU145 and PC3 (15). Furthermore, both DU145 and PC3 cells are growth-inhibited by PDE4D knockdown (2). In prostate cancer, increased expression of hedgehog pathway components is correlated with high Gleason grade (grade 7-8) and lymph node metastasis (16). In prostate development, epithelial cells secrete sonic hedgehog which binds to the mesenchymal smoothened (Smo) receptors which is essential for the formation of prostate buds (17). While it is clear that the hedgehog pathway is a critical pathway in prostate cancer progression, it remains unclear whether paracrine, autocrine or both mechanisms of hedgehog signaling are important for driving prostate cancer progression (18). However, based upon the effects of cAMP signaling the literature suggests that alterations in PDE4D expression seen in the prostate cancer could potentially affect many signaling pathways including MAPK, AR, and hedgehog. This suggests that pharmacological inhibition of PDE4D could affect several pathways that are linked to prostate cancer.
Phosphodiesterases have historically been attractive experimental targets for a wide variety of conditions including: erectile dysfunction (PDE5), depression (PDE4D), asthma, chronic obstructive pulmonary disorder (COPD) (PDE4D), and inflammation (PDE4D, PDE7) (19). Currently, the FDA-approved PDE inhibitors include a variety of PDE5 inhibitors utilized in the treatment of erectile dysfunction and a non-subtype selective PDE4 inhibitor, roflumilast, utilized in combination therapy for COPD (in combination with brochodilator)(20). First generation PDE4D inhibitors, such as rolipram, were initially developed and tested in the treatment of respiratory disorders, and second generation PDE4D inhibitors such as NVP-ABE171 (21) and cilomilast (Ariflo, SB207499) were designed to have an improved potency and subtype selectivity compared to first generation inhibitors (22). NVP-ABE171 was reported to have increased potency and selectivity for PDE4D compared to cilomilast, although both small molecule inhibitors were more selective for PDE4D than other PDE4 family members (21). NVP-ABE171 has been tested with in vitro and in vivo models of airway inflammation and was generally effective and well tolerated in rats and mice (21). Among PDE4D selective inhibitors, cilomilast was the most extensively tested in human clinical trials (23), but cilomilast was not FDA-approved due to a lack of efficacy in Phase III clinical studies for respiratory diseases. However, these clinical trials established doses (15 mg twice daily) of cilomilast that were safe for human use, with mild, transient side effects (22).
Currently, the effects of PDE4D in cancer are not well understood and only few studies examined the role of PDE4D and its inhibitors in cancer therapy. PDE4D inhibitors have not been tested in prostate cancer models. Studies in A549 lung cancer cells demonstrated that transforming growth factor-β (TGF-β) stimulation increased PDE4D expression and activity which promoted epithelial to mesenchymal transition, which could be attenuated with PDE4D inhibitor rolipram (24). A second study revealed that hypoxia via hypoxia inducible factor-α (HIF1-α) regulated PDE4D in a lung cancer model and that in vivo treatment with first generation PDE4D inhibitor, rolipram, decreased A549 xenograft weight and proliferation (25). Recently, a third study indicated that PDE4D expression increased in melanoma and endometrial carcinomas and that a 26B, a novel PDE4D inhibitor, decreased in vitro growth of HGC- 27 gastric carcinomas cells and A375 melanoma cells (26).
Based on our previous studies that showed that stable knockdown of PDE4D in cell lines reduced the growth of the prostate cancer epithelial cells and the published literature demonstrating in vivo efficacy on lung, melanoma, and gastric cancer xenografts, we hypothesized that the second-generation PDE4D inhibitors NVP-ABE171 and cilomilast would reduce the growth of prostate cancer cells. We chose to use two second-generation PDE4D inhibitors based on their selectivity for PDE4D as well as their previous use in other disease models with limited side effects observed in both humans and animal models. The results of the current study demonstrate that PDE4D inhibitors are effective at reducing growth of prostate cancer cells both in vivo and in vitro. In addition, PDE4D inhibition also reduced growth of benign prostate cells and modestly decreased the overall prostate weight in wild type C57BL-6 mice.
Materials and Methods
Cell lines
Prostate cell lines BPH1, (LNCaP (ATCC), and LNCaP-C4 (27)) were cultured in 5% heat-inactivated FBS (Sigma), 100 μg/mL penicillin-steptomycin (pen-strep) (Gibco), 1X HEPES (Gibco), 1X RPMI 1640 (CellGro) with 10-8 Mol/L dihydrotestosterone (Sigma) or in RPMI 1640 (ATCC) 10% FBS (Omega Scientific) with 100μg/mL pen-strep. UGSM-2 and Gli3-/-(18, 28) cells were cultured in 10% heat-inactivated FBS (Sigma), 100 μg/ml pen-strep (CellGro), 1X ITS (Lonza) with 10-8 Mol/L dihydrotestosterone (Sigma). BHPrE1 and NHPrE1 cells were cultured in DMEM/F12 5% FBS, 0.4% bovine pituitary extract (Hammnod Cell Tech, Windsor, CA), 0.005 μg/mL epidermal growth factor, 1 μg/mL ITS (Lonza), and 100 μg/mL pen-strep (29). P2 cells were cultured as previously described (30). For reduced serum experiments, 1% heat-inactivated FBS was used with the appropriate media.
Small molecule inhibitors
PDE4D selective inhibitors were obtained commercially (cilomilast from Selleck Chemicals and ChemPacific, NVP-ABE171 from SynphaBase) and dissolved in DMSO for in vitro experiments. Cyclopamine was obtained commercially (Toronto Research Chemicals or Enzo Life Sciences) and diluted in DMSO for in vitro experiments. For in vivo experiments, PDE4D selective inhibitors were diluted first in EtOH, followed by olive oil for a final concentration of 10% EtOH, 90% olive oil. PDE4D inhibitors were administered daily by oral gavage at a dose of 1 mg/kg NVP-ABE171 or 25 mg/kg cilomilast.
Cell growth and proliferation assays
Prostate cancer cell lines were plated at 2,000 cells per well in 96-well plates in the presence of PDE4D selective inhibitors (NVP-ABE171, cilomilast) or vehicle-only (DMSO) control. Cells were grown for 72 hours with daily changes of the growth media, which contained vehicle or PDE4D inhibitor. Following the growth period, relative cell numbers were quantified using a Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega).
Western Blots
Blots were performed as previously described (31). Primary antibodies for pERK1/2 and total ERK1/2 were obtained from Cell Signaling.
Microculture assays
5,000 to 7,500 cells were plated in microculture devices as previously described (32). LNCaP or LNCaP-C4 cells were cultured with either UGSM-2, UGSM-2 + exogenous sonic hedgehog (Shh, Curis), or Gli3-/- UGSM cells (UGli3-./-)(33). Drugs were added on day 1 and replenished until day 5. Following the growth period, cell proliferation was quantified using an EdU assay kit (Invitrogen) or RNA was extracted using DynaBeads (Invitrogen) (33). EdU assay images were obtained on an inverted Nikon Elipse Ti using a 4x objective. Fluorescent nuclear counts and GPF intensities were determined using ImageJ v1.38 (NIH). % EdU (+) cells were obtained by dividing total EdU (+) cells to total cell number (Hoescht-nuclear stain) X 100. Cell proliferation was assessed for significant differences by Wilcoxon Mann-Whitney test. Significant differences have a p-value <0.05.
qRT-PCR
A StepOne Plus (Applied Biosystems) or an iCycler (Biorad) thermocycler was used to perform qRT-PCR. Primers used were Ptch1, Gli1 (34) androgen receptor (AR, forward 5′-TTGGATGGCTCCAAATCAC-3′ and reverse 5′-GCAATGATACGATCGAGTTC-3′), prostate specific antigen (PSA, forward 5′-CATCAGGAACAAAAGCGTGA-3′ and reverse 5′-ATATCGTAGAGCGGGTGTGG-3′), and transmembrane protease, serine 2 (TMPRSS2, forward 5′-CCATTTGCAGGATCTGTCTG-3′ and reverse 5′-GGATGTGTCTTGGGGAGCAA-3′). Control housekeeping genes primers for 18S rRNA dimethyladenosine transferease (DIMT1 forward 5′-gaatgggatggtctagta-3′ and reverse 5′-TGGACTGAACAGTGAATT-3′), or GAPDH (34) were used and data are presented as 2̂(ΔCt).
Xenografts and animal experiments
All experiments using mice were performed using protocols approved by the University of Wisconsin Institutional Animal Care and Use Committees. LNCaP-C4 cells (1 × 105) were combined with matrigel (BD Biosciences) and grafted under the kidney capsule of male Nu/Nu mice (Charles River). After 1 week of xenograft growth, mice were given PDE4D selective inhibitors or vehicle-only control by gavage once daily for 6 weeks. After the treatment period, grafts were excised, weighed, and photographed. Xenografts were fixed in formalin, processed, and embedded in paraffin blocks. For experiments in wild-type mice, six-week-old C57BL/6 mice (Charles River) were treated daily with vehicle, cilomilast, or NVP-ABE171 for six weeks. After the treatment, mice were sacrificed and overall body weight and prostate weight were measured. Prostates were formalin fixed, processed, and embedded in paraffin blocks.
Immunohistochemistry
Immunohistochemistry was performed as previously described using antibodies for E-cadherin (Cell Signaling), smooth muscle actin (Sigma), p63 (Santa Cruz Biotechnology), Ki67 (Vector Laboratory, VP-K452) or p21 (Santa Cruz Biotechnology, sc-397) (31). Slides were dewaxed, rehydrated with a graded alcohol series, antigen retrieval was performed with antigen unmasking solution (Vector Labs), and endogenous peroxidases were quenched by incubation in H2O2. Slides were blocked with 2.5% serum, incubated with primary antibody overnight, washed, and incubated with biotinylated secondary antibody (Vector Labs) followed by incubation with ABC reagent (Vector Labs), and color development with DAB (Vector Labs). Slides were counterstained with haematoxylin, dehydrated, and mounted. TUNEL assays were performed as specified by the manufacturer using the DeadEnd Colormetric TUNEL assay system (Promega) and were counterstained with haematoxylin. Slides were imaged with a 20x objective on a Leica DMLB microscope and acquired using QCapture Software (QImaging Software). Labeling indices were calculated by blinded individuals who counted the number of positive cells (Ki-67, p21, TUNEL) and the total number of cells. Data are presented as the percentage of positive cells.
Results
To examine the effects of PDE4D inhibition on prostate cancer, LNCaP-C4 cells were treated with increasing concentrations of PDE4D inhibitors cilomilast and NVP-ABE171 (Figure 1). After 72 hours the effects on cell growth were assayed in a cell titer assay and demonstrated that there are dose-dependent decreases in the growth of LNCaP-C4 cells with both cilomilast (Figure 1A) and NVP-ABE171 (Figure 1B). Cilomilast treatment decreased growth with doses of 5 μM, but NVP-ABE171 was more potent and decreased growth with doses as low as 50 nM. PDE4D inhibition by cilomilast (Figure 1C) or NVP-ABE171 (Figure 1D) decreased the growth of BPH1, an SV-40 immortalized benign prostate cells at doses of 5 μM and 50 nM, respectively. In addition, dose dependent decreases in growth with increasing doses of NVP-ABE171 were also observed in P2 (Pten+/-) mouse prostate cancer cells (Figure 1E). The impact of PDE4D inhibition was also tested in normal spontaneously immortalized (untransformed) prostate epithelial cell lines BHPrE1 (Figure 1F) and NHPrE1 (Figure 1G) which were unaffected by either cilomilast or NVP-ABE171 treatment. These data suggested that PDE4D inhibition has a modest effect on growth in vitro of a transformed prostate cell lines.
Figure 1. LNCaP-C4 cells have decreased growth when treated with PDE4D inhibitors cilomilast and NVP-ABE171.
LNCaP-C4 prostate cancer cells in reduced serum media were treated with PDE4D selective inhibitors for 72 hours with A) cilomilast or B) NVP-ABE171 or vehicle-only (DMSO) control. BPH1 benign prostatic hyperplasia cells were treated with vehicle control (DMSO), C) cilomilast or D) NVP-ABE171 in complete media. E) P2 cells were treated with the indicated dose of NVP-ABE171 for 72 hours in reduced serum media. F) BHPrE1 or G) NHPrE1 cells were treated with 5 μM cilomilast or 50 nM NVP-ABE171 for 72 hours in reduced serum media. The graphs depict relative cell numbers for the different treatment groups with data normalized to vehicle-only controls and are presented as percent of vehicle control. Statistically significant differences from the vehicle-only controls were determined by ANOVA followed by Bonferroni's test for multiple comparisons and are indicated on the graph with *P<0.05. Bars indicate the standard error.
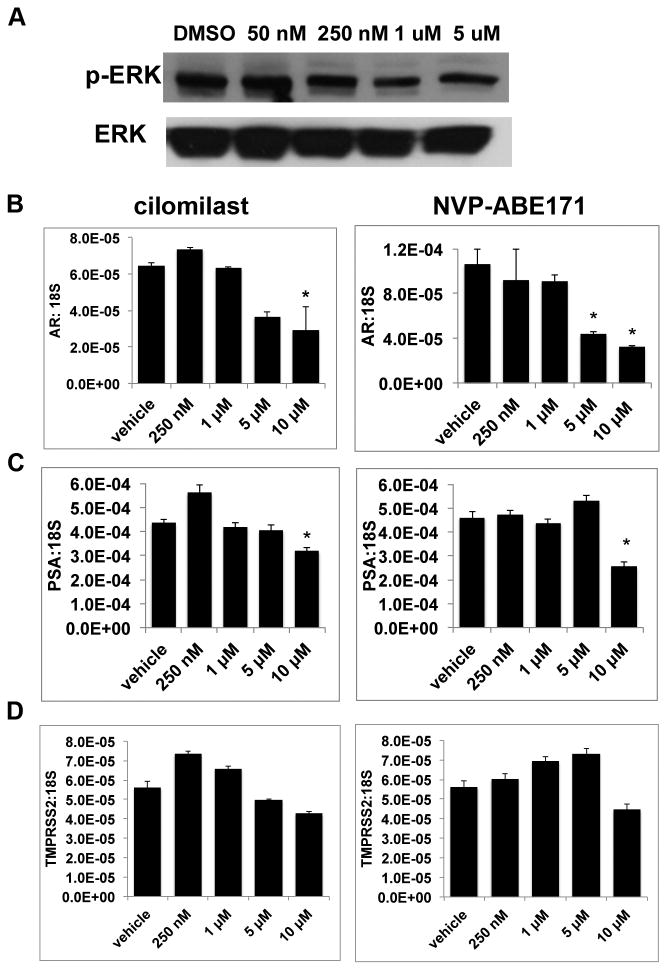
The main effector of cAMP is PKA which has impacts on multiple cell signaling pathways that are important in cancer (reviewed in (35)). Therefore, the effects of PDE4D inhibition on PKA regulated pathways were examined in LNCaP-C4 cells (Figure 2). Effects on the mitogen activated protein kinase (MAPK) pathway, which is regulated by PKA (35), were examined by measuring phosphorylation of downstream effector extracellular signal related kinase (ERK) (Figure 2A) by Western blot. While NVP-ABE171 decreased phosphorylated ERK (p-ERK) protein, the decrease is observed at 250 nM, a dose five-fold greater than the dose that produced growth inhibition. In addition, expression of androgen receptor, which is a primary therapeutic target of prostate cancer treatment and has previously been reported to be regulated by cAMP (36), was decreased by high doses of cilomilast and NVP-ABE171 (5-10 μM), but not at the low doses of NVP-ABE171 where growth was inhibited (Figure 2B). Similar to AR, expression of AR target gene prostate specific antigen (PSA) decreased with high doses of cilomilast and NVP-ABE171 (Figure 2C). A second AR target gene, transmembrane protease, serine 2 (TMPRSS2), was unaffected by either of the PDE4D inhibitors (Figure 2D).
Figure 2. PDE4D inhibition affects PKA mediated pathways.
LNCaP-C4 cells were treated with vehicle-only control (DMSO), cilomilast, or NVP-ABE171 for 72 hours in reduced serum media. A) Representative Western blot from whole cell lysates of LNCaP-C4 cells treated with indicated doses of NVP-ABE171.B-D) RNA was isolated, reverse transcribed, and qRT-PCR was performed to evaluate gene expression. Expression of B) Androgen Receptor and its target genes C) PSA and D) TMPRSS2 were assessed with cilomilast and NVP-ABE171 treatment. Data are presented as a representative experiment selected from a minimum of three independent experiments. Bars represent the average of three replicates and error bars are the SEM. ANOVA with Tukey's post test was performed and statistical significance (p<0.05) compared with vehicle control is indicated by an *.
Previous studies have demonstrated negative regulation of the hedgehog signaling pathway via PKA in other cell types (37, 38) and the role of the hedgehog pathway has been well established in prostate cancer (15). The effects of cilomilast and NVP-ABE171 treatment on the hedgehog pathway were measured using qRT-PCR to measure expression of Gli1 and Patched1 (Ptch1), genes that are upregulated in response to hedgehog pathway activation. Doses of cilomilast (5 μM) and NVP-ABE171 (50 nM) that resulted in growth inhibition also resulted in a statistically significant decrease in the expression of sonic hedgehog target genes Ptch1 and Gli1 (Figure 3A-B). The potential for complimentary effects between PDE4D inhibition and hedgehog inhibitor cyclopamine was examined. LNCaP-C4 cells were treated with doses of NVP-ABE171 (25 nM) or cyclopamine (0.5 μM) that did not affect growth on their own (Figure 3C). However, in combination sub-optimal doses of NVP-ABE171 and cyclopamine were able to decrease growth compared to control or either treatment alone.
Figure 3. PDE4D inhibition decreased autocrine hedgehog signaling.
LNCaP-C4 cells in reduced serum media were treated with vehicle-only control (DMSO), or the indicated concentrations of cilomilast, or NVP-ABE171 for 72 hours and RNA was collected. A) PTCH1, B) GLI1, and 18S transcript levels were evaluated using qRT-PCR. Statistical analysis was evaluated with ANOVA using GraphPad Prism. *P<0.05, (n=3 per data point). In conditions where LNCaP-C4 cell proliferation was reduced by NVP-ABE171 and cilomilast, Ptch1 and Gli1 mRNA expression was reduced. C) LNCaP-C4 cells were treated with vehicle-only control, 25 nM NVP-ABE171 (NVP), 0.5 μM cyclopamine (cyclo), or the combination of 25 nM NVP-ABE171 and 0.5 μM cyclopamine in reduced serum media. Cell growth was assessed after 72 hours of treatment. Statistically significant differences were determined by ANOVA followed by Bonferroni's test for multiple comparisons and are indicated on the graph with *P<0.05 (n=6 per data point). Bars indicate the standard error.
Since there are conflicting reports regarding the importance of autocrine and paracrine hedgehog signaling in prostate cancer, the connection between PDE4D and the sonic hedgehog pathway was explored further using a relevant model of stromal-epithelial signaling. LNCaP cells have been previously reported to lack autocrine hedgehog signaling capacity, however the contributions of autocrine and paracrine hedgehog signaling in various cell lines are still controversial (34). LNCaP or LNCaP-C4 cells were plated in microculture devices (33) with mesenchymal cell lines UGSM-2 (wild type mouse UGM cells), UGSM-2 cells with Shh (ligand that binds to and activates Smo receptor), or UGli3-/- cells (mesenchymal cells with constitutively active Shh signaling) (18). Proliferation of LNCaP or LNCaP-C4 cells in co-culture with the indicated mesenchymal cells treated with PDE4D inhibitors NVP-ABE171 or cilomilast, were measured by the incorporation of EdU (Figure 4A-B). Co-culture using mesenchymal cells with activated hedgehog signaling, either by the addition of shh or genetic (UGli3-/-), resulted in increased proliferation of both LNCaP and LNCaP-C4 cells. In addition, UGli3-/- cells are also unresponsive to common hedgehog inhibitors such as cyclopamine that target activation of the cell surface receptor Smo (33). Proliferation of LNCaP and LNCaP-C4 co-cultured with UGSM-2 cells was unaffected by cilomilast and NVP-ABE171. However, both NVP-ABE171 and cilomilast significantly inhibited proliferation induced by sonic hedgehog activation by co-culture with UGSM2+ Shh and UGli3-/- stromal cells (Fig 4A-B). RNA was isolated from the mesenchymal cells that were co-cultured with the LNCaP epithelial cells to measure the effects of PDE4D inhibitors on hedgehog target genes Gli1 and Ptch1 (Figure 4C-D). As anticipated, activation of hedgehog signaling (UGSM-2 +Shh and UGli3-/-) increased Gli1 and Ptch1 mRNA expression in the mesenchymal cells. Inhibition of PDE4D by cilomilast and NVP-ABE171 significantly decreased the expression of Gli1 and Ptch1 in UGSM-2 cells treated with Shh or in UGli3-/- cells, which suggests that PDE4D may be affecting prostate cancer growth by modulating hedgehog paracrine signaling that occurs via stromal and epithelial interactions.
Figure 4. Inhibition of PDE4D reduced growth and affected gene expression induced by Shh signaling in epithelial and stromal cells.
A) LNCaP or B) LNCaP-C4 cells were cultured in a microculture device with either UGSM-2, UGSM-2 + Shh, or Gli3-/- UGSM cells. Drugs (vehicle, cilomilast, or NVP-ABE171) were added at day 1 and replenished until day 5. Proliferation was assessed by EdU. Statistically significant differences were determined by ANOVA followed by Bonferroni's test for multiple comparisons and are indicated on the graph with *P<0.05 (n=6 per data point). Bars indicate the standard error. C-D) LNCaP cells were cultured in a microculture device with either UGSM-2, UGSM-2 + Shh, or Gli3-/- UGSM cells. Drugs were added at day 1 and replenished until day 5. RNA was extracted from the mesenchymal cells and C) Gli1 and D) Ptch1 expression was examined. Statistically significant differences were determined by ANOVA followed by Bonferroni's test for multiple comparisons and are indicated on the graph with *P<0.05 (n=6 per data point, relative to appropriate vehicle control). Error bars indicate the standard error.
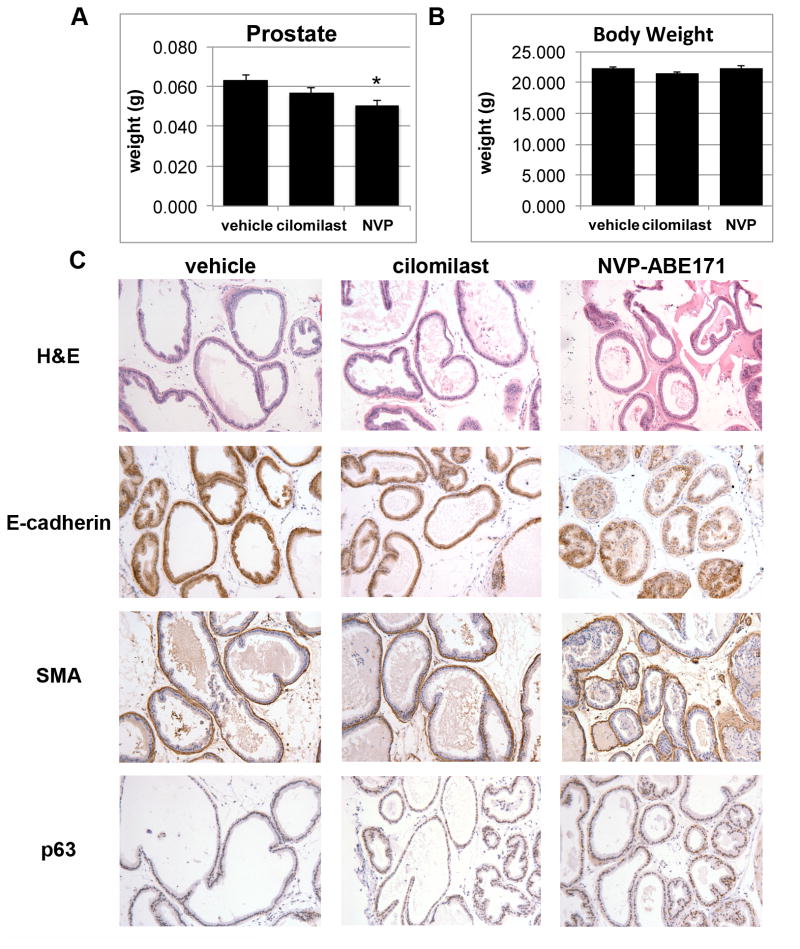
Although, data from the LNCaP and LNCaP-C4 indicated that PDE4D inhibition affected growth and signaling in prostate cancer cell lines, the effects of PDE4D inhibition on the normal prostate were unknown. In the course of experiments it was also observed that the prostates of the PDE4D inhibitor treated nude mice that were used as hosts in xenografts experiments appeared smaller (data not shown). The effects of PDE4D inhibition were examined in wild type C57BL/6 mice. Male C57BL/6 mice were treated daily with vehicle, cilomilast, or NVP-ABE171 for six weeks. The prostate wet weight (Figure 5A) and total body weight (Figure 5B) were measured at sacrifice. NVP-ABE171 treated mice has a statistically significant reduction in prostate weight and cilomilast treatment resulted in a similar trend, but the overall body weight and wet weights of the lungs, liver, spleen and seminal vesicles of the mice were not impacted (Figure 5A-B and data not shown). IHC was performed on prostate sections to determine the effects of PDE4D inhibition on morphology and organization (Figure 5C). The overall morphology and organization of the prostate appeared normal in the cilomilast and NVP-ABE171 treated prostates as assessed by hematoxylin and eosin (H&E) staining. In addition, markers for stromal (smooth muscle actin) and epithelial (E-cadherin) compartments were unchanged. While there was a trend to an increase in the basal epithelial subtype (p63), this increase was not statistically significant. Neuroendocrine markers, neuropilin and chromogranin A were examined due to a previous report that in an in vitro model PDE inhibitors caused a neuroendocrine differentiation (9). However, positive staining for neuroendocrine markers (as compared with a positive control) was not observed in any of the control, cilomilast or NVP-ABE171 treated prostates (data not shown). The expression of hedgehog activated genes, Shh, Gli1, and Ptch1 in the mouse prostate was not significantly affected by cilomilast or NVP-ABE171 treatment (data not shown). However, this was not unexpected since the level of hedgehog signaling in the adult mouse prostate is quite low compared to the human prostate or the developing prostate (39).
Figure 5. PDE4D inhibition decreased prostate weight but did not alter prostate morphology.
A-B) Male C57BL/6 mice were treated with vehicle-only (10% EtOH, 90% olive oil) vehicle (v), cilomilast (c) or NVP-ABE171 (N) by oral gavage daily (n=5-6 per condition). After six weeks animals were sacrificed and prostate were collected. Average weights of the A) prostate and B) total animal are represented in the graphs and error bars represent the SEM. Statistical analysis was performed using an ANOVA followed by Tukey's test for individual comparisons (* NVP-ABE171 P<0.05 versus vehicle control). C) IHC was performed on prostates that were fixed and paraffin sectioned. Sections were stained by H&E to assess overall morphology and IHC was performed to examine epithelial (E-cadherin), stromal (SMA- smooth muscle actin), and basal cell (p63) markers.
The effects of PDE4D inhibition were also measured in vivo using a prostate cancer xenograft model. LNCaP-C4 cells were implanted under the kidney capsule of nude mice. Host mice received daily oral doses of vehicle, or PDE4D inhibitors cilomilast or NVP-ABE171 for six week. Images of the xenografts from vehicle and treated animals are shown in Figure 6A. PDE4D inhibition with cilomilast or NVP-ABE171 resulted in a statistically significant decrease in the weight of the LNCaP-C4 xenograft (Figure 6B). Compared to the effects in vitro where the decrease in growth compared with control were only decreased by approximately 50%, the effect of PDE4D inhibitors in vivo was robust where cilomilast and NVP-ABE171 decreased xenograft wet weight 85% and 70%, respectively. In order to identify a potential mechanism to explain how PDE4D inhibitors decreased the size of LNCaP-C4 xenografts, xenografts were fixed and paraffin embedded to examine proliferation (Ki-67), apoptosis (TUNEL), and senescence (p21) markers by IHC (Figure 6C-D). Labeling indices were performed to compare the number of positive cells between treatment conditions (Figure 6D). NVP-ABE171 treatment resulted in a statistically significant decrease in Ki-67 positive cells (proliferation). Both cilomilast and NVP-ABE171 treatment resulted significant increase in TUNEL positive cells (apoptosis). Neither cilomilast nor NVP-ABE171 led to a change in the senescence marker p21.
Figure 6. PDE4D inhibitors increased apoptosis and decreased proliferation of LNCaP-C4 xenografts.
LNCaP-C4 prostate cancer cells were suspended in matrigel and transplanted under the kidney capsule of male nude mice hosts. Mice were treated with vehicle-only (10% EtOH, 90% olive oil) control, cilomilast or NVP-ABE171 by gavage (n=7-11 for each respective group). A) After 6 weeks of treatment, the xenografts were excised, weighed, and photographed. B) The average wet weights of the xenografts are shown with bars indicating the standard error. Statistical analysis was determined by ANOVA followed by Tukey's test for individual comparisons, *P<0.05 for cilomilast, P<0.05 for NVP-ABE171 versus vehicle control. C) The xenografts LNCaP-C4 xenografts were excised, fixed in formalin, and embedded in paraffin blocks. IHC with Ki67 (proliferation), TUNEL (apoptosis), or p21 (senescence) was performed. Representative Ki67 and TUNEL photomicrographs for each treatment group are shown. D) Labeling index was assessed for Ki67, TUNEL, and p21. Statistical analysis was determined by ANOVA followed by Tukey's test for individual comparisons, *P<0.05 for NVP-ABE171 versus vehicle-only control for Ki67. *P<0.01 for cilomilast, P<0.001 for NVP-ABE171 versus vehicle-only control for TUNEL.
Discussion
Resistance to existing prostate cancer therapies is a constant problem in prostate cancer as current treatments are not curative. Therefore, targeting novel pathways may provide alternatives to existing treatments for prostate cancer. Previous studies in our laboratory have identified PDE4D as a proliferation-promoting factor that has increased expression in prostate cancer (2). Since phosphodiesterases are good targets for small molecule inhibitors, the effects of PDE4D inhibitors were investigated as potential therapies for prostate cancer. To date, there have been no studies published which investigate PDE4D inhibitors in prostate cancer, although others have investigated the potential for use of PDE4D inhibitors in cancer therapy in vivo using animal models of cancer of lung cancer (25) and in vitro studies have shown efficacy of PDE4D inhibitors in lung, melanoma, and leukemia (25, 26, 40). The results of these studies suggest that PDE4D inhibition has the potential to be an effective prostate cancer therapy.
These studies demonstrate the efficacy of PDE4D inhibitors in both in vivo and in vitro prostate cancer models. In vitro moderate inhibition of LNCaP-C4 and P2 cell growth was observed and in vivo dramatic decreases in LNCaP-C4 xenografts wet weight were observed PDE4D inhibitor treated mice. It is unclear why the in vivo response to PDE4D inhibitors was so strong compared to the in vitro response, however; these results highlight the importance of examining the effect of potential therapeutics using multiple experimental approaches (41). While in vitro models of cell culture are models of growth in an isolated cell population, they provide a framework where the mechanism of action of a particular drug or pathway can be interrogated. In the current study, in vitro models identified effect of PDE4D inhibitors on several PKA mediated pathways (SHH, AR, and MAPK). Since in vivo studies add complexity, including interaction with a relevant microenvironment (including extracellular matrix, multiple additional cell types, paracrine signaling), growth in a 3D environment they provide valuable insights into how a drug may perform in conditions relevant to the disease being targeted. Data from co-culture experiments (Figure 4) provide additional complexity compared in vitro mono-culture and demonstrated that PDE4D inhibitors affected multiple cell types (epithelial and stromal) found in the prostate cancer microenvironment. These data suggest that PDE4D inhibitors may exert their effects by modulating paracrine signaling and influencing the prostate microenvironment. The in vivo studies with LNCaP-C4 xenografts, which are subject to the host microenvironment, demonstrated dramatic decrease in tumor size and increased apoptosis in response to PDE4D inhibitors. Ultimately, the complimentary data from in vivo and in vitro studies support the idea that overall PDE4D inhibitors may be efficacious as an anti-prostate cancer therapy.
Although, PDE4D inhibition affected multiple cell types the effects of PDE4D inhibition appear to have some selectivity for cancer and/or transformed rapidly proliferating immortalized cells. In vitro studies with the small molecule inhibitors revealed that both NVP-ABE171 and cilomilast decreased growth of the LNCaP-C4, and P2 prostate cancer cell lines as well as decreasing growth of the benign BPH1 cell line. BPH1 cells were immortalized with SV40 large T-antigen (42), which is known to inactivate multiple tumor suppressors including Rb and p53 and can have transforming effects on cells (reviewed in (43)) which may be responsible for the efficacy of PDE4D inhibitors on BPH1 cell growth. This is also supported by the data that demonstrated spontaneously immortalized prostate cells (NHPrE1 and BHPrE1) that express high levels of tumor suppressors p53, RB, and p21 (29), were unaffected by either cilomilast or NVP-ABE171 treatment. This is further supported by the in vivo data, where PDE4D inhibition had dramatic effects prostate cancer xenografts, but only modest effect on the wild-type prostate. In addition, in vivo the effect of the inhibitors was not apparent in many other tissues (lungs, liver, spleen or seminal vesicles) and the overall weight of the animals was not impacted. These data suggest an overall selectivity for transformed cell populations both in vivo and in vitro.
In vitro mechanistic studies revealed that PDE4D inhibition affected several PKA-mediated pathways, but only high doses of NVP-ABE171 lead to a decrease in MAPK and AR signaling pathways. However, the inhibition of hedgehog signaling pathway was best correlated with growth inhibition, which was observed at doses as low as 50 nMNVP-ABE171. A previous study used purified PDE4A, B, C, and D to determine the IC50 of both NVP-ABE171 and cilomilast. NVP-ABE171 had the lowest IC50 for PDE4D (1.5 nM), the next lowest was PDE4B (34 nM) and the IC50 values for PDE4A and PDE4C were both greater than 600 nM (21). For cilomilast the results showed a similar trend for IC50, but with less potency where the IC50 for PDE4D and PDE4B was the lowest at 63 nM and 288 nM, respectively. As a comparison, the prototypical PDE4 inhibitor rolipram is most potent for inhibition of PDE4A (IC50 of 3 nM) followed by PDE4B (130 nM) and PDE4D (240 nM)(44). The In vitro studies demonstrated growth inhibition by NVP-ABE171 effects doses as low as 40-50 nM and cilomilast doses of 5 μM, which have the potential to inhibit both PDE4D and PDE4B. Further experiments will be required to determine the relative contribution of each PDE4 isoform to the effects of NVP-ABE171 and cilomilast. Often times inhibition in whole cells or in vivo requires a higher concentration of the drug, which is consistent with treatments used in this study. This suggests that the results observed are likely due to the inhibition of PDE4D, but the impact of PDE4B cannot be completely excluded. The role of the hedgehog pathway is well-characterized in the context of prostate development, where paracrine signaling occurs from epithelial cells that secrete shh that binds to Smo on the mesenchymal cells and ultimately promotes prostatic budding and development (17). In the adult mouse prostate hedgehog activity is diminished compared with the developing prostate, but in the human adult prostate hedgehog signaling remains relatively high (39). Both human prostate tumors and mouse models of prostate cancer have increased hedgehog expression and activity, particularly in aggressive and androgen-independent cancers (15, 45). However, in prostate cancer it is unclear whether the paracrine hedgehog signaling remains intact or if there is autocrine signaling that occurs. Studies examining prostate tumor histology have demonstrated that the localization of hedgehog pathway components can be altered in tumors (16, 46). Epithelial expression of SMO, PTCH, and SHH increased and the expression of stromal hedgehog components GLI1, SMO, and PTCH were decreased (46). In contrast a study by Shigemura et al. demonstrated that GLI1 expression was increased in prostate cancer fibroblasts compared to normal fibroblasts (47). Additional studies by Shaw and colleagues demonstrated that either paracrine and autocrine hedgehog signaling in stroma can promote prostate cancer growth in vivo (18). Overall, the data suggests there may be heterogeneity in both the cell type (epithelial vs. stromal) and mechanism of hedgehog signaling (paracrine vs. autocrine) in prostate cancer. Collectively, these studies demonstrate hedgehog signaling is increased in prostate cancers and suggest that hedgehog signaling could be a potential therapeutic target in prostate cancer. Recently, the first clinical hedgehog inhibitor, vismodegib, received FDA approval for treatment of basal cell carcinoma (48) and clinical trials in prostate cancer are ongoing (NCT01163084). Interestingly, NVP-ABE171 and cilomilast were able to reduce expression of hedgehog activated genes Gli1 and Ptch1 in LNCaP-C4 epithelial cells. Furthermore, in co-cultures proliferation induced in LNCaP or LNCaP-C4 cells by hedgehog pathway activation (either by agonist treatment (UGSM+ shh) or genetic activation (Ugli3-/-)) was partially inhibited by PDE4D inhibition. In stromal cells with activated hedgehog signaling expression of hedgehog target genes was reduced by both NVP-ABE171 and cilomilast treatment. These results suggest that PDE4D inhibitors could affect both paracrine and autocrine sonic hedgehog signaling. These data suggests that PDE4D inhibition can at least in part overcome the effects of activated Shh signaling in both epithelial and stromal signaling. Interestingly, since the genetic activation of Shh is downstream of the surface receptor Smo, the UGli3-/- cells are unresponsive to many Shh inhibitors that work via Smo inhibition and these data suggest that PDE4D inhibition may be a novel pathway that could be effective on Smo inhibitor resistant cells. The Shh pathway is a relevant target in prostate cancer therapy and is increased in advanced or metastatic prostate cancer (15). Recently, sonic hedgehog signaling was implicated in growth of the stromal cells in the prostate observed in the mouse prostate after castration (49). The data from the current study suggest that the effects of PDE4D on the hedgehog pathway may be at least partially responsible for the decreases in growth observed in vitro, although it is unknown whether the effect of PDE4D inhibitors on hedgehog signaling occurs through direct or indirect mechanisms. In addition, in the context of the literature these studies suggest that therapies targeting PDE4D and/or the hedgehog pathway potentially provide a unique mechanism of action by targeting paracrine interactions.
For in vivo studies, doses of drugs and oral delivery method were chosen based on previous animal models in which NVP-ABE171 or cilomilast were used (21). Results of these studies suggest that PDE4D inhibitors, cilomilast and NVP-ABE171, are efficacious and safe in vivo using a relevant xenograft mouse model of prostate cancer. In vivo studies with the small molecule PDE4D inhibitors using the LNCaP-C4 xenograft model demonstrated that administration of NVP-ABE171 or cilomilast for 6 weeks decreased the wet weight of the xenografts. The data demonstrated that both NVP-ABE171 and cilomilast significantly increased apoptosis, while NVP-ABE171 also decreased proliferation. These results suggested that PDE4D inhibition in vivo is efficacious as an anti-prostate cancer therapy in LNCaP-C4 xenografts by causing an increase in apoptosis. In addition to decreasing prostate xenograft size, in wild-type mice PDE4D inhibitor treatment decreased prostate weight without an impact on total body weight. Additionally, the data indirectly suggest that PDE4D inhibition is not affecting the level of androgens or AR signaling, since the histology of the H&E from the prostate appears normal and does not demonstrate the atrophy and loss of ducts observed in castrated mice (50). Also, the wet weight of seminal vesicles, which are highly androgen sensitive, was unchanged by PDE4D inhibition (data not shown). Further studies are required to identify if there are a subset of patients that are likely to benefit from these inhibitors. In addition, based upon the impacts PDE4D inhibition on several key pathways in prostate cancer, hedgehog and AR, these results also suggest future studies to examine the effects of PDE4D inhibitors in combination with AR or hedgehog inhibitors. The results of this work suggest that there may be a precedent for further pre-clinical exploration of PDE4D inhibitors in prostate cancer, particularly in those model systems that have elevated PDE4D levels.
Acknowledgments
The authors would like to thank Joseph Wenninger, Nathaniel Feirer, and Brenton Severson for help with gavaging the mice and Laura Steinke and Meghan Learned for help with labeling indices analysis.
Financial Support: This study was supported by award W81XWH-05-1-053 to PCM from the Department of Defense Congressionally Mandated Research Program, grant CA140217 to PCM from the NIH/NCI, and grant DK091193 to PCM from the NIH/NIDDK. GLP was supported by NIH postdoctoral training grant T32 CA157322. KDPH was supported by postdoctoral fellowship W81XWH-10-1-0571 from the Department of Defense Congressionally Mandated Research Program and CA141798 from the NIH/NCI/NIDDK.
Footnotes
Author Contributions: Conception and design: KDPH, GLP, MD, PCM
Acquisition of data: GLP, KDPH,MD, KF, RLM
Analysis and interpretation of data: GLP, KDPH, MD
Writing of the manuscript: GLP, KDPH, and PCM,
Review and/or revision of the manuscript: MD, WB, and DJB
Technical, or material support: WB and DJB
Study supervision: WB, DJB, and PCM
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA: a cancer journal for clinicians. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Rahrmann EP, Collier LS, Knutson TP, Doyal ME, Kuslak SL, Green LE, et al. Identification of PDE4D as a proliferation promoting factor in prostate cancer using a Sleeping Beauty transposon-based somatic mutagenesis screen. Cancer Res. 2009;69:4388–97. doi: 10.1158/0008-5472.CAN-08-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baca SC, Prandi D, Lawrence MS, Mosquera JM, Romanel A, Drier Y, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153:666–77. doi: 10.1016/j.cell.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Natrajan R, Mackay A, Lambros MB, Weigelt B, Wilkerson PM, Manie E, et al. A whole-genome massively parallel sequencing analysis of BRCA1 mutant oestrogen receptor-negative and -positive breast cancers. J Pathol. 2012;227:29–41. doi: 10.1002/path.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houslay MD. PDE4 cAMP-specific phosphodiesterases. Progress in nucleic acid research and molecular biology. 2001;69:249–315. doi: 10.1016/s0079-6603(01)69049-4. [DOI] [PubMed] [Google Scholar]
- 6.Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. The Journal of biological chemistry. 2003;278:5493–6. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekaran A, Toh KY, Low SH, Tay SK, Brenner S, Goh DL. Identification and characterization of novel mouse PDE4D isoforms: molecular cloning, subcellular distribution and detection of isoform-specific intracellular localization signals. Cell Signal. 2008;20:139–53. doi: 10.1016/j.cellsig.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Uckert S, Oelke M, Stief CG, Andersson KE, Jonas U, Hedlund P. Immunohistochemical distribution of cAMP- and cGMP-phosphodiesterase (PDE) isoenzymes in the human prostate. European urology. 2006;49:740–5. doi: 10.1016/j.eururo.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 9.Bang BE, Ericsen C, Aarbakke J. Effects of cAMP and cGMP elevating agents on HL-60 cell differentiation. Pharmacology & toxicology. 1994;75:108–12. doi: 10.1111/j.1600-0773.1994.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 10.Bang YJ, Kim SJ, Danielpour D, O'Reilly MA, Kim KY, Myers CE, et al. Cyclic AMP induces transforming growth factor beta 2 gene expression and growth arrest in the human androgen-independent prostate carcinoma cell line PC-3. Proc Natl Acad Sci U S A. 1992;89:3556–60. doi: 10.1073/pnas.89.8.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadar MD. Androgen-independent Induction of Prostate-specific Antigen Gene Expression via Cross-talk between the Androgen Receptor and Protein Kinase A Signal Transduction Pathways. Journal of Biological Chemistry. 1999;274:7777–83. doi: 10.1074/jbc.274.12.7777. [DOI] [PubMed] [Google Scholar]
- 12.Xie Y, Wolff DW, Lin MF, Tu Y. Vasoactive intestinal peptide transactivates the androgen receptor through a protein kinase A-dependent extracellular signal-regulated kinase pathway in prostate cancer LNCaP cells. Molecular pharmacology. 2007;72:73–85. doi: 10.1124/mol.107.033894. [DOI] [PubMed] [Google Scholar]
- 13.Ji Z, Mei FC, Johnson BH, Thompson EB, Cheng X. Protein kinase A, not Epac, suppresses hedgehog activity and regulates glucocorticoid sensitivity in acute lymphoblastic leukemia cells. The Journal of biological chemistry. 2007;282:37370–7. doi: 10.1074/jbc.M703697200. [DOI] [PubMed] [Google Scholar]
- 14.Makinodan E, Marneros AG. Protein kinase A activation inhibits oncogenic Sonic hedgehog signalling and suppresses basal cell carcinoma of the skin. Experimental dermatology. 2012;21:847–52. doi: 10.1111/exd.12016. [DOI] [PubMed] [Google Scholar]
- 15.Sheng T, Li C, Zhang X, Chi S, He N, Chen K, et al. Activation of the hedgehog pathway in advanced prostate cancer. Molecular cancer. 2004;3:29. doi: 10.1186/1476-4598-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azoulay S, Terry S, Chimingqi M, Sirab N, Faucon H, Gil Diez de Medina S, et al. Comparative expression of Hedgehog ligands at different stages of prostate carcinoma progression. J Pathol. 2008;216:460–70. doi: 10.1002/path.2427. [DOI] [PubMed] [Google Scholar]
- 17.Podlasek CA, Barnett DH, Clemens JQ, Bak PM, Bushman W. Prostate Development Requires Sonic Hedgehog Expressed by the Urogenital Sinus Epithelium. Dev Biol. 1999;209:28–39. doi: 10.1006/dbio.1999.9229. [DOI] [PubMed] [Google Scholar]
- 18.Shaw A, Gipp J, Bushman W. The Sonic Hedgehog pathway stimulates prostate tumor growth by paracrine signaling and recapitulates embryonic gene expression in tumor myofibroblasts. Oncogene. 2009;28:4480–90. doi: 10.1038/onc.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006;147(Suppl 1):S252–7. doi: 10.1038/sj.bjp.0706495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163:53–67. doi: 10.1111/j.1476-5381.2011.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trifilieff A, Wyss D, Walker C, Mazzoni L, Hersperger R. Pharmacological profile of a novel phosphodiesterase 4 inhibitor, 4-(8-benzo[1,2,5]oxadiazol-5-yl-[1,7]naphthyridin-6-yl)-benzoic acid (NVP-ABE171), a 1,7-naphthyridine derivative, with anti-inflammatory activities. The Journal of pharmacology and experimental therapeutics. 2002;301:241–8. doi: 10.1124/jpet.301.1.241. [DOI] [PubMed] [Google Scholar]
- 22.Giembycz MA. An update and appraisal of the cilomilast Phase III clinical development programme for chronic obstructive pulmonary disease. British journal of clinical pharmacology. 2006;62:138–52. doi: 10.1111/j.1365-2125.2006.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chong J, Poole P, Leung B, Black PN. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. The Cochrane database of systematic reviews. 2011:CD002309. doi: 10.1002/14651858.CD002309.pub3. [DOI] [PubMed] [Google Scholar]
- 24.Kolosionek E, Savai R, Ghofrani HA, Weissmann N, Guenther A, Grimminger F, et al. Expression and Activity of Phosphodiesterase Isoforms during Epithelial Mesenchymal Transition: The Role of Phosphodiesterase 4. Molecular Biology of the Cell. 2009;20:4751–65. doi: 10.1091/mbc.E09-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pullamsetti SS, Banat GA, Schmall A, Szibor M, Pomagruk D, Hanze J, et al. Phosphodiesterase-4 promotes proliferation and angiogenesis of lung cancer by crosstalk with HIF. Oncogene. 2013;32:1121–34. doi: 10.1038/onc.2012.136. [DOI] [PubMed] [Google Scholar]
- 26.Lin DC, Xu L, Ding LW, Sharma A, Liu LZ, Yang H, et al. Genomic and functional characterizations of phosphodiesterase subtype 4D in human cancers. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1218206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang SM, Ozen M, et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000 Jul 1;44:91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. 44(2) [DOI] [PubMed] [Google Scholar]
- 28.Shaw A, Papadopoulos J, Johnson C, Bushman W. Isolation and characterization of an immortalized mouse urogenital sinus mesenchyme cell line. The Prostate. 2006;66:1347–58. doi: 10.1002/pros.20357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang M, Strand DW, Fernandez S, He Y, Yi Y, Birbach A, et al. Functional Remodeling of Benign Human Prostatic Tissues In Vivo by Spontaneously Immortalized Progenitor and Intermediate Cells. STEM CELLS. 2010;28:344–56. doi: 10.1002/stem.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiao J, Wang S, Qiao R, Vivanco I, Watson PA, Sawyers CL, et al. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67:6083–91. doi: 10.1158/0008-5472.CAN-06-4202. [DOI] [PubMed] [Google Scholar]
- 31.Buresh-Stiemke RA, Malinowski RL, Keil KP, Vezina CM, Oosterhof A, Van Kuppevelt TH, et al. Distinct expression patterns of Sulf1 and Hs6st1 spatially regulate heparan sulfate sulfation during prostate development. Dev Dyn. 2012;241:2005–13. doi: 10.1002/dvdy.23886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domenech M, Yu H, Warrick J, Badders NM, Meyvantsson I, Alexander CM, et al. Cellular observations enabled by microculture: paracrine signaling and population demographics. Integrative biology : quantitative biosciences from nano to macro. 2009;1:267–74. doi: 10.1039/b823059e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domenech M, Bjerregaard R, Bushman W, Beebe DJ. Hedgehog signaling in myofibroblasts directly promotes prostate tumor cell growth. Integrative biology : quantitative biosciences from nano to macro. 2012;4:142–52. doi: 10.1039/c1ib00104c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Lipinski R, Shaw A, Gipp J, Bushman W. Lack of demonstrable autocrine hedgehog signaling in human prostate cancer cell lines. J Urol. 2007;177:1179–85. doi: 10.1016/j.juro.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 35.Edwards HV, Christian F, Baillie GS. cAMP: novel concepts in compartmentalised signalling. Seminars in cell & developmental biology. 2012;23:181–90. doi: 10.1016/j.semcdb.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Sarwar M, Sandberg S, Abrahamsson PA, Persson JL. Protein kinase A (PKA) pathway is functionally linked to androgen receptor (AR) in the progression of prostate cancer. Urologic oncology. 2013 doi: 10.1016/j.urolonc.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Hammerschmidt M, Bitgood MJ, McMahon AP. Protein kinase A is a common negative regulator of Hedgehog signaling in the vertebrate embryo. Genes Dev. 1996;10:647–58. doi: 10.1101/gad.10.6.647. [DOI] [PubMed] [Google Scholar]
- 38.Wang B, Fallon JF, Beachy PA. Hedgehog-Regulated Processing of Gli3 Produces an Anterior/Posterior Repressor Gradient in the Developing Vertebrate Limb. Cell. 2000;100:423–34. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 39.Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, et al. Hedgehog Signaling Promotes Prostate Xenograft Tumor Growth. Endocrinology. 2004;145:3961–70. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- 40.Dong H, Zitt C, Auriga C, Hatzelmann A, Epstein PM. Inhibition of PDE3, PDE4 and PDE7 potentiates glucocorticoid-induced apoptosis and overcomes glucocorticoid resistance in CEM T leukemic cells. Biochem Pharmacol. 2010;79:321–9. doi: 10.1016/j.bcp.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Suggitt M, Bibby MC. 50 years of preclinical anticancer drug screening: empirical to target-driven approaches. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:971–81. [PubMed] [Google Scholar]
- 42.Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In vitro cellular & developmental biology Animal. 1995;31:14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- 43.Ahuja D, Saenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24:7729–45. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 44.MacKenzie SJ, Houslay MD. Action of rolipram on specific PDE4 cAMP phosphodiesterase isoforms and on the phosphorylation of cAMP-response-element-binding protein (CREB) and p38 mitogen-activated protein (MAP) kinase in U937 monocytic cells. The Biochemical journal. 2000;347:571–8. doi: 10.1042/0264-6021:3470571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang HH, Chen BY, Wu CY, Tsao ZJ, Chen YY, Chang CP, et al. Hedgehog overexpression leads to the formation of prostate cancer stem cells with metastatic property irrespective of androgen receptor expression in the mouse model. Journal of Biomedical Science. 2011;18:6. doi: 10.1186/1423-0127-18-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzelepi V, Karlou M, Wen S, Hoang A, Logothetis C, Troncoso P, et al. Expression of hedgehog pathway components in prostate carcinoma microenvironment: shifting the balance towards autocrine signalling. Histopathology. 2011;58:1037–47. doi: 10.1111/j.1365-2559.2011.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shigemura K, Huang WC, Li X, Zhau HE, Zhu G, Gotoh A, et al. Active sonic hedgehog signaling between androgen independent human prostate cancer cells and normal/benign but not cancer-associated prostate stromal cells. Prostate. 2011;71:1711–22. doi: 10.1002/pros.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. The New England journal of medicine. 2012;366:2171–9. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng YC, L CM, Zahid S, Wilson EL, Joyner AL. Sonic hedgehog signals to multiple prostate stromal stem cells that replenish distinct stromal subtypes during regeneration. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1315729110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugimura Y, Cunha GR, Donjacour AA. Morphological and histological study of castration-induced degeneration and androgen-induced regeneration in the mouse prostate. Biol Reprod. 1986;34:973–83. doi: 10.1095/biolreprod34.5.973. [DOI] [PubMed] [Google Scholar]