Abstract
Rationale
The mechanisms leading to an expanded neutrophil and monocyte supply after stroke are incompletely understood.
Objective
To test the hypothesis that transient middle cerebral artery occlusion (tMCAO) in mice leads to activation of hematopoietic bone marrow stem cells.
Methods and Results
Serial in vivo bioluminescence reporter gene imaging in mice with tMCAO revealed that bone marrow cell cycling peaked 4 days after stroke (p<0.05 versus pre tMCAO). FACS and cell cycle analysis showed activation of the entire hematopoietic tree, including myeloid progenitors. The cycling fraction of the most upstream hematopoietic stem cells increased from 3.34%±0.19 to 7.32±0.52 after tMCAO (p<0.05). In vivo microscopy corroborated proliferation of adoptively transferred hematopoietic progenitors in the bone marrow of mice with stroke. The hematopoietic system’s myeloid bias was reflected by increased expression of myeloid transcription factors, including PU.1 (p<0.05), and by a decline in lymphocyte precursors. In mice after tMCAO, tyrosine hydroxylase levels in sympathetic fibers and bone marrow noradrenaline levels rose (p<0.05, respectively), associated with a decrease of hematopoietic niche factors that promote stem cell quiescence. In mice with genetic deficiency of the β3 adrenergic receptor, hematopoietic stem cells did not enter the cell cycle in increased numbers after tMCAO (naive control, 3.23±0.22; tMCAO, 3.74±0.33, p=0.51).
Conclusions
Ischemic stroke activates hematopoietic stem cells via increased sympathetic tone, leading to a myeloid bias of hematopoiesis and higher bone marrow output of inflammatory Ly6Chigh monocytes and neutrophils.
Keywords: Bone marrow, stroke, hematopoietic stem cells, monocyte
INTRODUCTION
The majority of strokes result from thrombotic events leading to ischemic injury of the brain. This sterile injury to the brain triggers a profound reaction of the immune system. Microglia, which are the most numerous resident immune cells of the central nervous system, proliferate and undergo inflammatory activation. Importantly, brain ischemia also triggers a systemic immune response. While blood lymphocyte numbers decline, levels of circulating neutrophils and monocytes increase in stroke patients1, 2. These myeloid cells are recruited to the brain3 where they may contribute to the brain’s recovery but also to reperfusion injury. Thus, the systemic number of innate immune cells, which recent studies relate to outcomes in patients2, 4, 5, increases acutely after stroke. These increased levels of circulating cells may reflect demargination from tissue vascular beds or increased production. Here we tested whether increased cell production contributed to this observed phenomenon. Innate immune cells have a life span on the order of hours to a few days. The number of leukocytes in blood is limited and cell reserves in the marginal blood pool, the bone marrow and the spleen exhaust rapidly after ischemic injury. We therefore examined the source of increased innate immune cell numbers in the circulation and in the ischemic brain, and the signals that regulate leukocyte supply after stroke. We hypothesized that bone marrow hematopoietic stem cells, a source of neutrophils and monocytes in the steady state, increase activity after transient middle cerebral artery occlusion (tMCAO) in mice.
We report that tMCAO activates the hematopoietic system at its most upstream point. Shortly after brain injury, hematopoietic stem cells enter the cell cycle, giving rise to downstream myeloid progenitors and innate immune cells. Bone marrow hematopoiesis acquires a strong myeloid bias, with reduced frequency of lymphoid progenitor cells. Increased autonomic nervous system activity after stroke activates hematopoietic stem cells through modulation of the hematopoietic bone marrow niche environment, contributing to the leukocytosis observed in patients.
METHODS
A detailed method section is available online.
Animals and stroke procedure
Adult C57BL/6 and FVB/N mice (10–12 weeks old) were obtained from Jackson Laboratories and repTOP™ mitoIRE mice were purchased from Charles River Laboratories. Adrb3−/− mice (gift from P. Frenette) and Nestin-GFP reporter mice (gift from G. Enikolopov) were bred in our facilities. Experimental stroke was induced by a transient occlusion of the middle cerebral artery (tMCAO). The Subcommittee on Research Animal Care at Massachusetts General Hospital approved all procedures.
In vivo staining of bone marrow vasculature and bone lining cells
To visualize bone structures, mice were administered intravenously with OsteoSense® 750EX (4 nmol/mouse, PerkinElmer). For in vivo endothelial cells labeling, mice were given i.v. APC anti-mouse CD31 (MEC13.3), Alexa Fluor® 647 anti-mouse VE-Cadherin (BV13) and APC anti-mouse Sca-1 (D7, 2 μg/mouse in 100μl PBS).
Whole mount immunofluorescence staining of the sternum
Sterna were harvested and processed as described previously6, sectioned longitudinally and stained with rabbit anti-mouse tyrosine hydroxylase antibody (Millipore). Samples were incubated with secondary antibody and imaged using an Olympus IV100 microscope.
Confocal microscopy
For serial intravital microscopy of the calvarium, SLAM HSCs were FACS-sorted, labeled with DiD (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate, Molecular Probes) and injected i.v. into non-irradiated Nestin-GFP recipient mice. The GFP signal, while not specific for mesenchymal stem cells, guided revisiting similar regions of interest in serial intravital imaging. To highlight bone architecture, OsteoSense 750EX was administered (PerkinElmer). We used rhodamine-labelled Griffonia simplicifolia lectin (RL-1102, Vector Laboratories) to outline the vasculature. In vivo imaging was performed on days 1 and 5 after the adoptive cell transfer with a confocal microscope (IV100 Olympus). Z-stacks images for each location were acquired at 2μm steps. For visualization of LKS cells in sternum, Lin− c-Kit+ Sca-1+ cells were FACS sorted and labeled ex-vivo with two different fluorescent dyes including CellTracker™ CM-Dil and SP-DiOC18(3) (Molecular Probes) prior to adoptive transfer into recipient mice.
RESULTS
Bone marrow activity increases after stroke
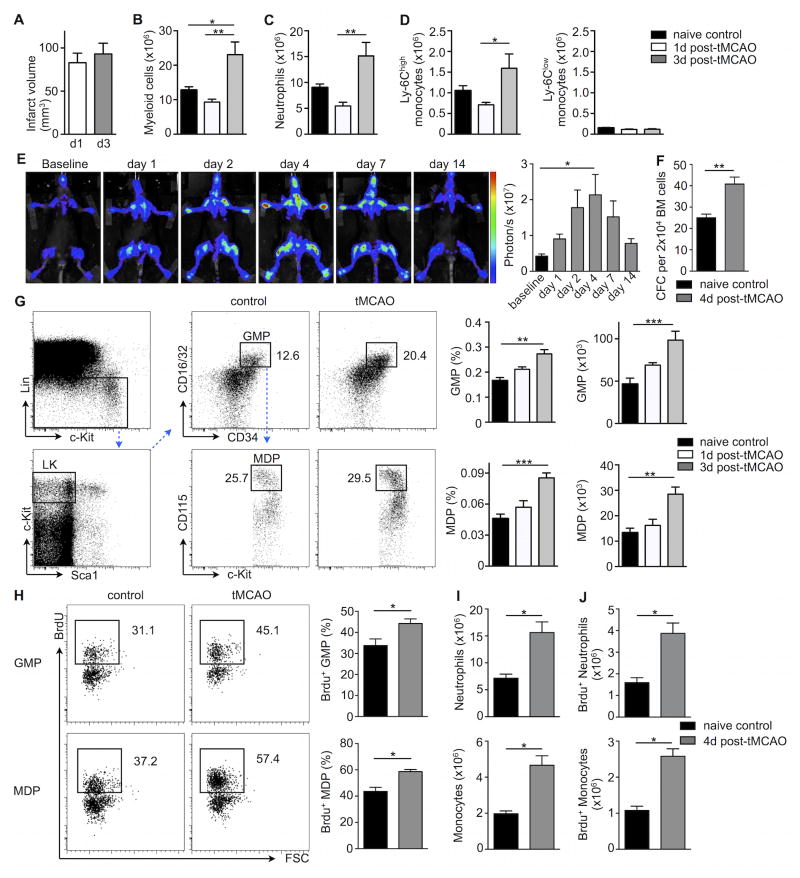
We used a transient middle cerebral artery occlusion model of experimental stroke (tMCAO) in mice to address the link between the bone marrow and blood monocytosis and neutrophilia seen after ischemic brain injury in patients7, 8 and rodents9–12. Because mature myeloid cells, which are recruited to the inflamed brain and contribute to stroke, originate in the bone marrow, we evaluated bone marrow myeloid cell subsets by flow cytometry following stroke induction. While infarct sizes were comparable on day 1 and day 3 after tMCAO (Figure 1A), the percentages and numbers of mature CD11b+ cells (Online Figure I and Figure 1B) including neutrophils (Figure 1C) and inflammatory Ly6Chigh monocytes (Figure 1D) significantly increased in the bone marrow when compared to naive control mice. In contrast, bone marrow Ly6Clow monocyte levels did not change. Bone marrow myeloid cell content increased somewhat, but remained significantly lower after a sham procedure that exposed mice to similar surgical trauma as tMCAO but did not induce brain ischemia (Online Figure IIA).
Figure 1. Stroke increases bone marrow progenitor activity.
A, Infarct size from TTC-stained brain sections on day 1 and day 3 after stroke induced by tMCAO in C57BL/6 mice (n= 4–6 mice per group). Flow cytometric-based enumeration of (B) CD11b+ myeloid cells, (C) neutrophils and (D) monocyte subsets per femur following tMCAO (n= 4–6 mice per group, one-way ANOVA). E, Representative bioluminescence images of MITO-Luc mice monitored one day before (baseline) and after tMCAO over a 2-week period. Quantification of luciferase activity in MITO-Luc mice following tMCAO at indicated time points is shown on the right (n= 4 mice per group, one-way ANOVA). F, Bone marrow (BM) colony-forming unit (CFU) assay on day 4 after tMCAO (n = 8 mice per group, Mann-Whitney test). G, Representative gating strategy and FACS dot plots for GMP (Lin− c-kit+ Sca-1− CD16/32+ CD34+) and MDP (Lin− c-kit+ Sca-1− CD16/32+ CD34+ CD115+) in the bone marrow on day 3 after tMCAO. Bar graphs display both frequencies in whole bone marrow and absolute numbers of cells per femur (n = 6–10 mice per group, Mann-Whitney test). H, On day 3 after tMCAO, mice were given BrdU i.p (1mg) and BrdU incorporation in myeloid progenitors was analyzed 24 hours later. Representative FACS dot plots show BrdU+ GMP (upper panels) and MDP (lower panels). Bar graphs on the right display percentages of BrdU+ GMP and MDP progenitors. I, Absolute numbers of neutrophil (upper panels) and monocytes per femur (lower panels). J, Analysis of BrdU incorporation in bone marrow neutrophils and monocytes following a single injection of BrdU (n= 4–6 mice per group, Mann-Whitney test). Mean ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001.
To investigate whether the bone marrow undergoes broad activation after experimental stroke, we took advantage of MITO-Luc mice13. These transgenic mice harbor a transgene in which luciferase gene expression is driven by the activity of a Nuclear Factor-Y-dependent cyclin B2 promoter (NF-Y). This enabled us to non-invasively monitor cell proliferation in the bone marrow of living mice before and after ischemic brain injury over a two-week period. As shown in Figure 1E, bone marrow luciferase activity increased rapidly after stroke, peaked on day 4 and returned to baseline levels by day 14. Sham surgery did not lead to a significant increase of bone marrow bioluminescence on day 4 (Online Figure IIB).
Stroke accelerates myelopoiesis
After stroke, myeloid cells, especially neutrophils and monocytes, increase their presence in circulation. These cells are also recruited to the ischemic brain. Encouraged by the observed increase of proliferation imaging signal, we therefore next focused on hematopoiesis’ contribution to increased systemic leukocyte numbers. Whole bone marrow cultures from animals with stroke gave rise to higher numbers of colonies after 7 days (Figure 1F), reflecting increased hematopoietic progenitor cell activity. To further explore the marrow’s response following tMCAO, we performed flow cytometry to examine whether the increased mature myeloid cell numbers arise from upstream myeloid progenitor’s activity. We observed a significant increase in both, relative frequencies and absolute numbers of Lin− c-Kit+ Sca-1− CD16/32+ CD34+ granulocyte macrophage progenitors (GMP) and Lin− c-Kit+ Sca-1− CD16/32+ CD34+ CD115+ macrophage and dendritic cell progenitors (MDP) after tMCAO (Fig. 1G). After sham surgery, a mild increase of GMP and MDP did not reach statistical significance when compared to naive controls. Their numbers remained significantly lower than what was observed after tMCAO (Online Figure IIC, D).
To determine whether myeloid progenitors increased proliferative activity in the context of stroke, mice were given i.p. injections of 5-bromo-2-deoxyuridine (BrdU) on day 3 after tMCAO. Twenty-four hours after a single dose of BrdU, the fraction of BrdU+ GMP and MDP expanded significantly in animals with stroke when compared to naive control mice (Figure 1H). To decipher whether downstream mature neutrophils and monocytes derive from the accelerated proliferation of myeloid progenitors, we next examined BrdU incorporation into these differentiated cells. Indeed, higher numbers of both neutrophils and monocytes (Figure 1I) had incorporated BrdU (Figure 1J) after stroke, indicating that they had recently emerged from cycling progenitors. Because each GMP or MDP can give rise to multiple neutrophils and monocytes, the increase in BrdU+ cells was more pronounced in differentiated leukocytes than in progenitors.
Stroke induces a myeloid bias of hematopoiesis
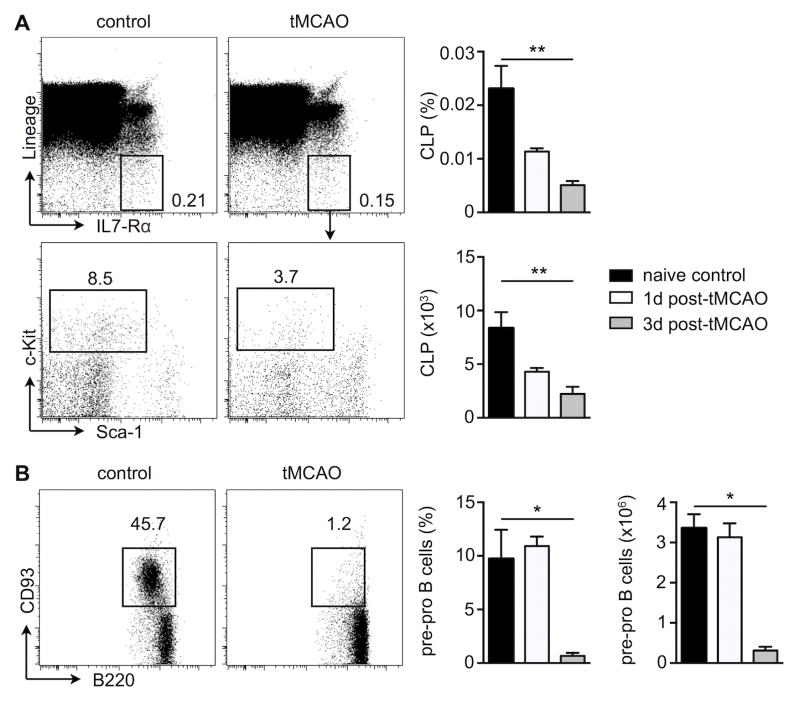
Leukocytosis after stroke is often associated with lymphopenia. We therefore also examined behavior of lymphoid committed progenitors in the bone marrow following stroke. In contrast to the expansion of myeloid progenitor cells, flow cytometric analyses of lymphoid progenitors revealed a drastic reduction in both, the relative frequency and absolute number of Lin− IL7Rα + c-Kitint Sca-1int common lymphoid progenitors (CLP), starting as early as day 1 after stroke induction (Figure 2A). Early immature B cells defined as Lin− B220int CD93+ were significantly diminished in the bone marrow on day 3 after tMCAO (Figure 2B). Altogether, the divergent activity changes of myeloid and lymphoid progenitors suggest that stroke may skew hematopoiesis toward the myeloid lineage.
Figure 2. Stroke decreases activity of lymphoid progenitors in the bone marrow.
A, Representative flow cytometry staining of CLP (Lin− IL7Rα+ c-kitint Sca-int) from bone marrow cells on day 3 after tMCAO. B, Representative staining of immature B cells (Lin− B220int CD93+) from bone marrow cell suspensions. Bar graphs show both frequencies of lymphoid progenitors in total bone marrow cells and total cell numbers per femur. n= 4–6 mice per group, one-way ANOVA. Mean ± s.e.m., *P < 0.05, **P < 0.01.
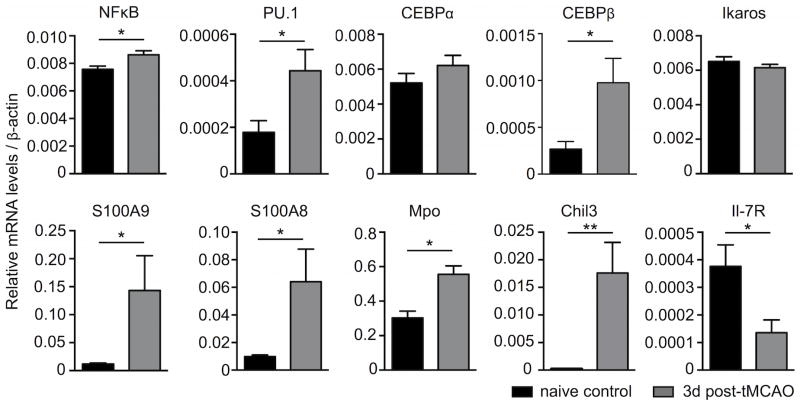
To further explore this hypothesis, we FACS-sorted LKS from mice 3 days after tMCAO. These cells occupy a position in the hematopoietic tree that is just upstream of the divergence of lymphopoiesis and myelopoiesis. Consistent with the activation and expansion of myeloid progenitors at the expense of lymphoid progenitors, LKS from mice with stroke expressed higher levels of NF-κB as well as transcription factors involved in myeloid differentiation such as PU.1 and CEBPβ14, 15. Furthermore, expression of several myeloid specific genes, including S100A9 and S100A8 alarmins, myeloperoxidase and chitinase 3-like-3 were also increased in LKS isolated from animals with stroke (Figure 3). In contrast, expression levels of mtg16, Mcsfr, c-myb and irf8 were not altered (Online Figure III). In agreement with the severe reduction of lymphoid progenitors and despite unchanged Ikaros expression levels, IL7Rα, which is expressed by lymphoid LKS, was significantly reduced after tMCAO, highlighting a blockade of lymphoid commitment (Figure 3).
Figure 3. Bone marrow LKS cells exhibit a myeloid bias after stroke.
Experimental stroke was induced or not in C57BL/6 mice by tMCAO and LKS from the bone marrow were sorted by FACS three days later. Changes in gene expression levels were evaluated by RT-qPCR. n = 4–5 mice per group, Mann-Whitney test. Mean ± s.e.m., *P < 0.05, **P < 0.01.
HSC are the upstream point of bone marrow activation after stroke
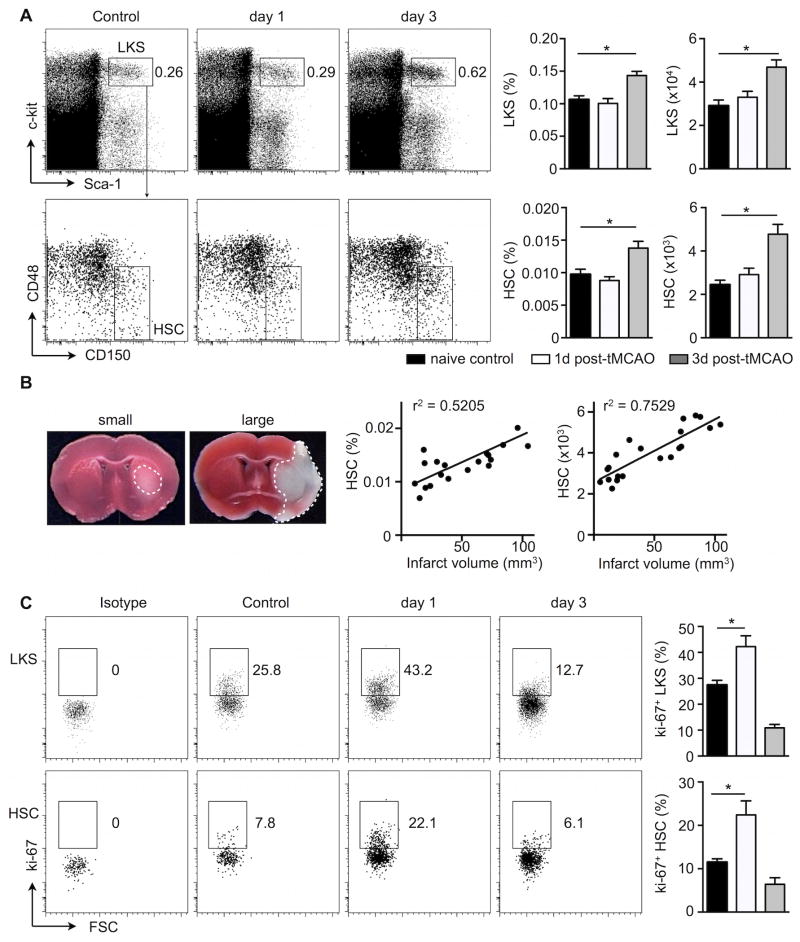
Since myeloid but not lymphoid progenitors increased activity after stroke, we next asked whether upstream HSPC, which give rise to these cells, also respond to ischemic brain injury. Both, relative percentages and absolute numbers of LKS (Lin− c-Kit+ Sca-1+) in the femur bone marrow were significantly elevated after stroke (Figure 4A). The frequency and absolute number of the most upstream SLAM HSC subpopulation (Lin− c-Kit+ Sca-1+ CD48− CD150+) increased in mice with tMCAO (Figure 4A, please see Online Figure IV for expanded gating strategy). Notably, the extent of the bone marrow HSC response correlated with infarct size (Figure 4B). Sham surgery did not result in significantly increased stem cell numbers (Online Figure IIE, F). The increase in numbers of hematopoietic stem cells after stroke was corroborated by higher percentages of both LKS and SLAM HSCs in the non G0 phase of the cell cycle 24 hours after stroke, as assessed by flow cytometry after ki-67 staining (Figure 4C). These data indicate that HSC enter the cell cycle, expand in numbers and give rise to downstream progeny after ischemic stroke.
Figure 4. The bone marrow response after stroke occurs at the most upstream hematopoietic stem cell level and correlates with injury size.
Bone marrow cell suspensions were stained for LKS and HSC on days 1 and 3 after tMCAO in C57BL/6 mice. A, Representative staining, frequencies and numbers of LKS (Lin− c-kit + Sca-1+; upper panels) and HSC (Lin− c-kit + Sca-1+ CD48 − CD150+; lower panels) per femur. B, Correlation between frequencies and numbers of SLAM HSC with infarct sizes was evaluated from different cohorts of mice on day 3 after tMCAO. C, FACS analysis of Ki-67+ LKS and HSC cells at indicated time points after experimental stroke. n= 6 mice per group, one-way ANOVA. Mean ± s.e.m., *P < 0.05.
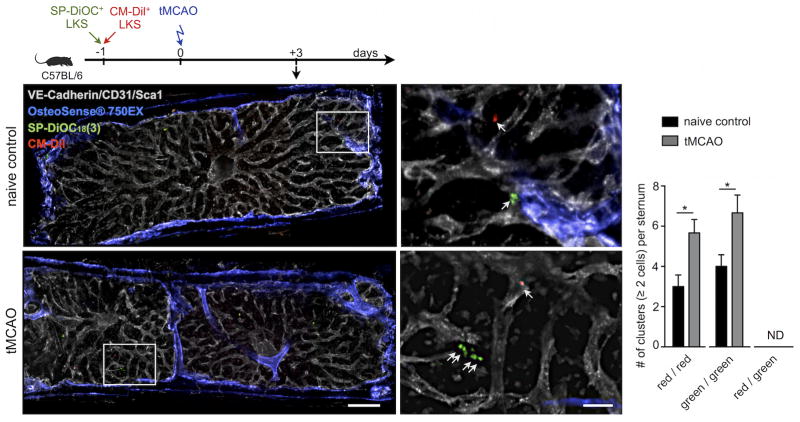
To directly visualize the proliferation of upstream stem and progenitor cells in the bone marrow after stroke, we performed ex vivo confocal microscopy on sternal bone marrow preparations following adoptive transfer of labeled LKS. One day before tMCAO, non-irradiated recipients received an intravenous mixture of FACS-sorted LKS that were labeled with two differently colored fluorescent membrane dyes (40,000 LKS per mouse; 1:1 cell ratio). Three days after stroke and four days after adoptive cell transfer, red CM-Dil+ and green SP-DiOC18(3)+ LKS were imaged in the sternal bone marrow, using naive control recipients without stroke as controls. The number of cell clusters, i.e. equal to or more than 2 cells in direct proximity, was increased in the marrow of mice with stroke (Figure 5). Notably, we never observed clusters containing a mix of red and green cells, indicating that proximate LKS likely proliferated after seeding the marrow.
Figure 5. Confocal imaging of LKS progenitor expansion in bone marrow of mice with stroke.
Mice were injected intravenously with a mixture of FACS-sorted Lin− c-kit + Sca-1+ labeled ex-vivo with red CM-Dil and green SP-DiOC18(3) fluorescent dyes prior to stroke induction. Imaging of whole mount sternal bone marrow preparations was performed three days after tMCAO. Fluorescent signal from the bisphosphonate imaging agent Osteosense-750 is depicted in blue and outlines bone. Vascular endothelial cells were stained by intravenous injection of fluorescently labeled antibodies targeting CD31, Ve-Cadh and Sca1. Scale bar represents 200 μm (low magnification) and 50μm (high magnification). The bar graph on the right displays the number of either green (SP-DiOC18) or red (CM-Dil) clusters with ≥2 cells per sternal preparation (n=3 mice per group). Neighboring cells of mixed color were not detected (ND). Mean ± s.e.m., *P < 0.05.
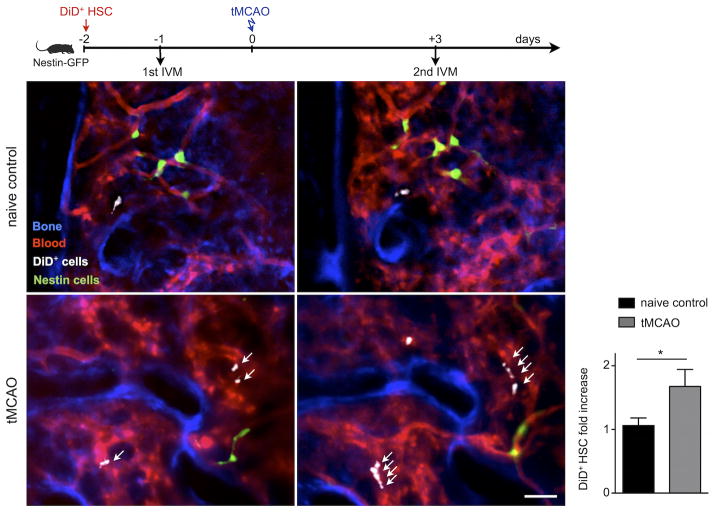
We next performed serial intravital microscopy of the skull bone marrow following intravenous adoptive transfer of 20,000 FACS-sorted DiD labeled SLAM HSCs, cells that are upstream of LKS, into Nestin-GFP recipient mice. The GFP signal, together with vascular dye, aided in relocation of the same bone marrow regions in the second imaging session. Calvaria were imaged one day before and again 3 days after stroke, enabling us to serially monitor individual HSCs. Similar to transplanted LKS, SLAM HSCs also increased in number 3 days after tMCAO (Figure 6). Collectively, these data indicate that ischemic injury of the brain triggers proliferation of the most upstream hematopoietic stem and progenitor cells, suggesting that the entire hematopoietic tree, but especially the myeloid lineage, increases proliferation and causes the blood leukocytosis observed in patients after stroke.
Figure 6. Serial intravital microscopy reports increased HSC expansion in the bone marrow of mice with stroke.
Mice with and without stroke were transplanted with 20, 000 FACS-sorted donor Lin− c-kit + Sca-1+ CD48 − CD150+ HSC labeled ex-vivo with DiD fluorescent dye. Intravital microscopy of the mouse calvarium was performed serially, one day before and again 3 days after tMCAO. Blue color represents the fluorescence signal produced by the bone imaging agent Osteosense-750; the fluorescence lectin signal stained blood vessels in red. DiD labeled hematopoietic stem cells (HSC) are shown in white. Scale bar represents 50 μm. Bar graph displays the fold increase of HSC per field of view between the two imaging sessions in both groups (n=3 mice per group). Mean ± s.e.m., *P < 0.05.
Stroke increases the sympathetic tone in bone marrow
HSC activity is regulated by the microenvironment of the bone marrow stem cell niche. Interestingly, autonomic tone, which is increased after stroke16–19 may influence the signaling of hematopoietic niche cells20, 21. Local sympathetic nerve fibers may release norepinephrine within the bone marrow to increase cell cycling, as observed in circadian rhythms or after MI20, 21 and in mice exposed to chronic psychosocial stress22. We therefore studied whether sympathetic tone alerts bone marrow cells after stroke.
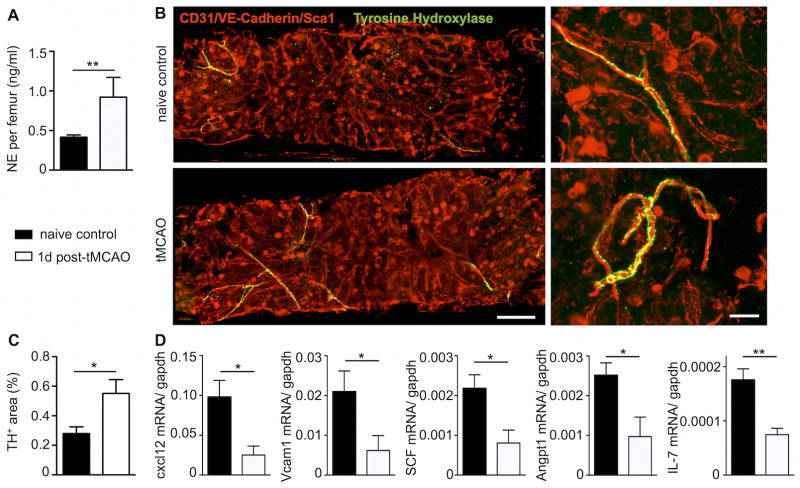
Norepinephrine content in the bone marrow of mice with tMCAO increased significantly (Figure 7A). In line with this finding, immunofluorescence staining for tyrosine hydroxylase, the rate limiting enzyme in norepinephrine synthesis, was more pronounced in whole mount sternal preparations one day after cerebral ischemic injury. The increased staining pattern followed the typical distribution of sympathetic nerve fibers along bone marrow arterioles (Figure 7B–C). Norepinephrine indirectly regulates HSPC migration and proliferation by modulating stromal cell expression of several factors, including Cxcl1220, which retains HSC in position and promotes a quiescent HSC state. In whole bone marrow, Cxcl12, VCAM-1, stem cell factor, angiopoietin-1 and IL-7 mRNA fell significantly 24 hours after tMCAO (Figure 7D), indicating that after stroke the molecular interaction between stromal niche cells and hematopoietic cells is markedly altered.
Figure 7. Stroke increases the sympathetic nervous activity in the bone marrow.
A, One day following tMCAO, bone marrow norepinephrine content was measured by ELISA. B, Whole mount immunofluorescence staining of tyrosine hydroxylase rich nerve fibers of the sternal BM on day 1 after tMCAO. C, Bar graph shows quantification of tyrosine hydroxylase positive area per field of view. D, Quantitative real-time PCR of HSC retention/maintenance related genes in the bone marrow on day 1 after tMCAO. n= 4–6 mice per group, Mann-Whitney test. Mean ± s.e.m., *P < 0.05.
Bone marrow β3-adrenergic receptor signaling activates HSC but not LKS after stroke
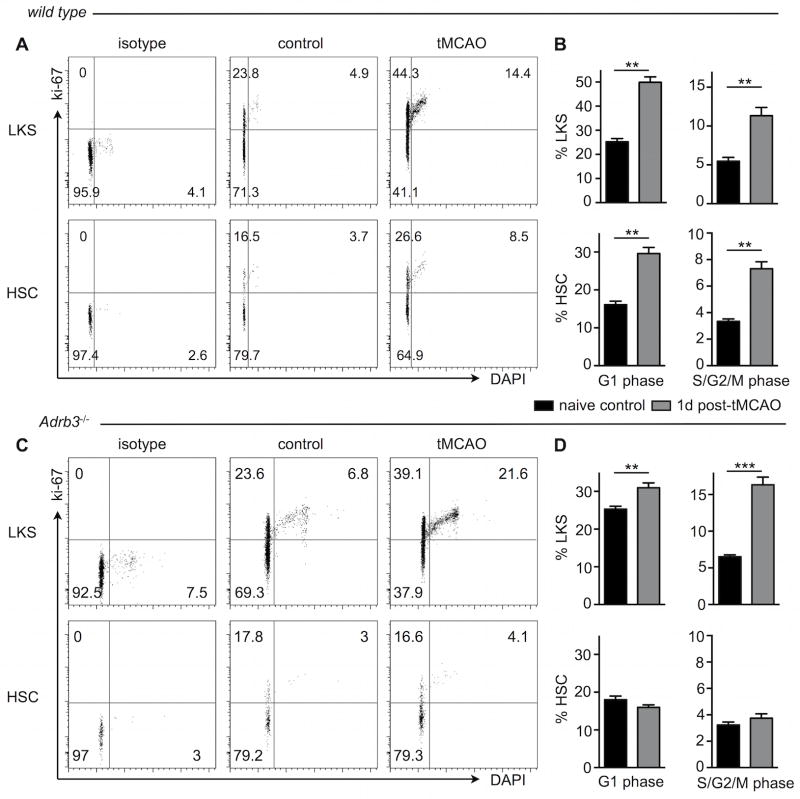
Bone marrow norepinephrine acts through β3-adrenergic receptors expressed by mesenchymal stromal cells, triggering downregulation of above maintenance factors20. We attempted to measure expression of the β3-adrenergic receptor in HSC and LKS isolated by flow sorting, however, in contrast to robust expression of this receptor by niche cells22, β3-adrenergic receptor mRNA was not detectable in HSPC. To determine the role of β3-adrenergic signaling in bone marrow niche cells after stroke, we conducted flow cytometric cell cycle analyses in Adrb3−/− mice after tMCAO. Since HSC activation correlates with stroke size (Figure 4 B), only mice with large stroke volumes (defined as > 35 mm3) were used for this analysis (wild type, 71.2±21.2; Adrb3−/−, 68.5±10.3 mm3). In wild type mice, LKS and HSC increased frequencies in both G1 and S/G2/M cell cycle phases one day after tMCAO (Figure 8A, B). In contrast to wild type mice, in which the number of cycling HSC rose to 7.32% ± 0.52% after tMCAO, HSC in mice with genetic deficiency for the β3 adrenergic receptor did not increase cycling (3.74% ± 0.33%, p<0.01 versus wild type tMCAO), suggesting that the sympathetic nervous system regulates HSC activation through this receptor. Downstream LKS increased cell cycle entry after stroke in Adrb3−/− mice (Figure 8C, D). 24 hours after tMCAO, infarct size was comparable in Adrb3−/− and FVB/N wild type control mice (48.0±8.8 versus 42.9±14.6, p=0.23, n = 8–14). The loss of body weight after stroke was comparable in both cohorts (Adrb3−/− 2.6±0.2g, FVB/N 2.6±1.1g, p=0.49).
Figure 8. Adrenergic signaling regulates hematopoietic stem cell activation after stroke via the β3 adrenergic receptor.
tMCAO was induced in wild type (A,B) and Adrb3−/− mice (C,D) and cell cycle was analyzed in LKS progenitors and hematopoietic stem cells (HSC) one day later. A,C Representative FACS dot plots of cell cycle staining in LKS (upper panels) and HSC (lower panels). B, D, Bar graphs show flow cytometric analysis of LKS and HSC in both G1 and S/G2/M phases of the cell cycle. In wild type mice, HSC and LKS entered the active cell cycle phases, whereas Adrb3−/− mice showed a lack of HSC activation after stroke. n= 4–8 mice per group, Mann-Whitney test. Mean ± s.e.m., **P < 0.01 versus naive controls of same genotype.
Altogether, these data are consistent with the notion that after ischemic stroke, sympathetic nervous signaling acts on hematopoietic niche cells, which then alter the bone marrow microenvironment to push increased numbers of HSC into the cell cycle.
DISCUSSION
In contrast to a maturing knowledge on local inflammation in the brain and changes in circulating immune cells9, 11, 12, 23, remarkably little is known about how the bone marrow, the primary site of hematopoiesis, reacts to ischemic stroke24, 25. In the current study, we report that tMCAO in mice activates hematopoietic stem cells and downstream hematopoietic progenitors, leading to an increased output of inflammatory monocytes and neutrophils while the number of lymphocyte progenitors declined. We identify enhanced bone marrow adrenergic signaling through the β3 adrenoreceptor on niche cells as a mechanism of hematopoietic stem cell activation. As a result, niche cells altered expression of cytokines and retention factors that regulate hematopoiesis.
Cells residing in the hematopoietic niche regulate leukocyte production through a number of soluble signals and adhesion molecules. These cells include mesenchymal stem cells, perivascular cells, endothelial cells, macrophages and osteoblasts26, 27. Integration of cell-cell communication informs hematopoietic stem cells whether they should remain quiescent, proliferate, differentiate or migrate. Quiescent hematopoietic stem cells and cycling downstream progenitors may reside in different bone marrow locations28. Some reports indicate that the most upstream, quiescent hematopoietic stem cells reside close to the endosteum next to arterioles ensheathed with nestin-expressing mesenchymal stem cells, whereas more downstream precursors may locate in proximity to sinusoids29. Several key niche components, including Cxcl12 (also known as SDF-1), are provided by multiple cell types altering the localization and quiescence of HSC30. During circadian oscillation of bone marrow activity20, after myocardial infarction21, and in mice exposed to chronic psychosocial stress22, the sympathetic nervous tone changes, reflected by increased bone marrow levels of norepinephrine. This neurotransmitter binds β3 receptors on niche cells, which leads to downregulation of maintenance factors in the niche20. Reduction of the quiescence signal Cxcl12 increases hematopoietic stem cell activity26, 27, in parallel to what we observed in mice with stroke. Thus, it appears that several stimuli, including circadian rhythms, ischemic injury and chronic psychosocial stress converge on a similar neuro-immunological pathway.
Cxcl12 and IL7, whose expression fell after stroke, are also essential for the development of lymphoid progenitors such as early B cell precursors and CLPs31, 32. As stem cells, lymphoid and myeloid precursors in the bone marrow may not share the same micro-environmental niches for their respective maintenance and development, it is likely that stroke has broad but differential effects on multiple hematopoietic niches.
Genetic deficiency of β3 adrenoreceptor abolished the increase in HSC cycling after tMCAO; however, downstream LKS were still activated in Adrb3−/− mice. This interesting divergence suggests that specific regulation of progenitor cell classes may occur, possibly via differences in their location. Increased sympathetic signaling may primarily affect mesenchymal cells that are located next to arterioles in quiescent niches29, and therefore reach hematopoietic stem cells residing there. After activated hematopoietic stem cells translocate to positions closer to sinusoids, they may be exposed to different, potentially soluble signals, e.g. circulating danger associated molecular patterns33. For instance, hematopoiesis increases in response to toll-like receptor ligands34 or interferons35, 36 in the setting of infection. In the context of stroke, break down of the blood-brain barrier allows systemic distribution of circulating messengers including inflammatory cytokines and alarmins liberated from ischemic and necrotic brain cells, which could likewise alert the hematopoietic system37–40. How local or circulating stimuli, including danger signals, chemokines, survival factors and pro-inflammatory cytokines may differentially impact on distinct progenitor cell subsets (i.e. myeloid versus lymphoid) and/or their respective niches in the bone marrow after stroke will be the focus of future studies. Experiments with cell-specific deletion of the β3-adrenergic receptor should further refine the cellular identity of involved niche cells.
While activation and proliferation of microglia dominate the early cellular response to stroke, break down of the blood-brain barrier also enables robust recruitment of circulating immune cells, including neutrophils and monocytes3, 23, 41, 42. These cells are centrally involved in wound healing, including the healing of ischemic wounds. After myocardial infarction, monocytes are essential for tissue repair but their oversupply is detrimental for healing and leads to heart failure43. Macrophage depletion studies suggest comparable mechanisms for brain ischemia, as lack of these cells leads to hemorrhagic conversion of ischemic stroke44. On the other end of the spectrum, the level of inflammatory monocyte subtypes in blood correlates with worse outcome in stroke patients2. Thus, there may be a hypothetical “sweet spot” of immune cell activity after stroke, in which salutary functions of resident microglia and recruited myeloid cells support resolution of inflammation and regeneration of the injured brain.
Thirty percent of acute stroke patients acquire infections, especially pneumonia45. While infections frequently occur after any injury, and stroke-related sequela such as paralysis may also contribute, the high incidence of post-stroke infections is accompanied by lymphocyte apoptosis. Some authors postulated the existence of a peripheral immunosuppression, reporting an impressive atrophy of the thymus and the spleen after stroke that lasted for several days thereafter, a phenotype attributed to lymphocyte apoptosis9, 16. Our data indicate that hematopoiesis increased and that the bone marrow accelerated production of innate immune cells, pointing to an important exception from the peripheral immunosuppression after stroke. The lymphoid lineage suppression we observed in the bone marrow of mice with stroke may contribute to the previously reported blood lymphopenia9, 16.
The very leukocytes that are supplied to the ischemic brain and circulate in increasing numbers after stroke are also a driving force behind complications of atherosclerosis. Monocytes give rise to plaque macrophages and foam cells, which become apoptotic and form necrotic cores. Macrophage-derived proteases digest the protective fibrous cap43. Such a destabilized plaque (e.g., within the carotid artery) may rupture and lead to ischemia of the brain. We recently described that experimental myocardial infarction accelerates hematopoiesis and atherosclerosis21, possibly explaining the high secondary event rates observed in patients with a first infarct. Likewise, stroke may increase the supply of neutrophils and monocytes to remote atherosclerotic plaque, thus promoting stroke reoccurrence.
In ischemic heart disease, blood leukocytosis is associated with worse outcome. There are limited data for stroke patients, possibly reflecting that leukocytes and their subsets have dual roles, including support of recovery and healing. In addition, stroke pathophysiology is heterogeneous, and patients often suffer from infections, which can influence leukocyte levels. In 1999, the Stockholm study46 reported that when controlled for initial stroke severity, multivariate regression analysis did not show association of leukocytosis to outcome. This study included patients with hemorrhagic stroke, and CT identified infarction in 60% of the population. A more recent study, which only included patients with ischemic stroke, found an association of leukocytosis with early poor outcome5. Another report correlated increased CD14high CD16− monocyte levels with worse 90-day outcome and higher mortality in patients after adjustment for age and symptoms on admission2. Many preclinical studies imply that anti-inflammatory intervention may improve stroke recovery23. However, translation of these studies into clinical therapy has proven difficult, arguing that better understanding of systemic leukocyte subset fate in the setting of stroke is warranted33.
Our study reports that increased signaling of the sympathetic nervous system activates hematopoietic stem cell activity in the bone marrow, increasing the output of neutrophils and inflammatory Ly-6Chigh monocytes after stroke. At the present time, it is unclear whether the bone marrow’s response to stroke represents a therapeutic target to improve stroke recovery and to prevent secondary stroke. Preclinical47–49 and some early clinical52, 53 data suggest that beta blocker use may be beneficial after stroke. Observed effects may or may not be related to bone marrow activity, as the affinity of most clinical beta blockers for the β3 adrenoreceptor is low. Beta blockers lower blood pressure, which likely also influences outcome and reoccurrence of stroke. In addition, bone marrow-derived immune cells may support stroke recovery. Hence, while targeting overproduction of inflammatory immune cells is worthwhile in cardiovascular disease52, there is a compelling need to gather additional data on precisely when and how to therapeutically intervene after stroke.
Supplementary Material
Novelty and Significance.
What Is Known?
Microglia and recruited blood leukocytes contribute to the innate immune response after stroke.
Ischemic stroke leads to monocytosis and neutrophilia, inflammatory immune cells that migrate to atherosclerotic lesions as well as ischemic tissue.
What New Information Does This Article Contribute?
The bone marrow provides increased numbers of monocytes and neutrophils after stroke through increased myelopoiesis, while lymphoid progenitors are less active.
Indirect sympathetic signaling to bone marrow niche cells leads to activation of most upstream hematopoietic stem cells.
Stroke increases bone marrow noradrenaline levels that alter the hematopoietic niche by signaling through β3 adrenergic receptors.
Neutrophils and monocytes are recruited from the blood stream into the acutely ischemic brain where they, together with locally activated microglia, mount the response to ischemic stroke. While it is known that systemic numbers of these inflammatory myeloid cells expand after stroke, their source was incompletely understood. Here we show, using flow cytometry and confocal microscopy of the bone marrow, that ischemic stroke activates the entire hematopoietic tree. Bioluminescence imaging revealed that proliferative bone marrow activity peaks on day 4 after ischemia. A myeloid bias of hematopoiesis accelerates production of innate immune cells while bone marrow lymphopoiesis is suppressed. After stroke, bone marrow levels of noradrenaline increase, associated with reduced Cxcl12 levels. In mice with genetic lack of β3 adrenergic receptors, the activation of hematopoietic stem cells is inhibited. These data show that increased bone marrow production of leukocytes contributes to the acute immune system activation after stroke.
Acknowledgments
The authors thank members of the Mouse Imaging Program at the Center for Systems Biology, including Benoit Tricot, Greg Wojtkiewicz and Peter Waterman for help with imaging, Michael Waring and Adam Chicoine (Ragon Institute Imaging core) as well as Danny Cao and Jie Zhao, M.D., Ph.D (Wellman Center for Photomedicine) for assistance with cell sorting. We thank P. Frenette (Albert Einstein College of Medicine) and B. Lowell (Beth Israel Deaconess Medical Center) for providing Adrb3−/− mice and G. Enikolopov (Cold Spring Harbor Laboratory) for providing nestin-GFP reporter mice.
SOURCES OF FUNDING
This work was funded in part by grants from the National Institutes of Health (R01-NS084863, R01-HL117829, R01-HL096576, HHSN268201000044C), the MGH Research Scholar Award, the American Heart Association (13POST16580004), the German Research Foundation (HE6382/1-1 and SA1668/2-1), and the Deutsche Herzstiftung (S/05/12).
Nonstandard Abbreviations and Acronyms
- Adrb3
β3 adrenoreceptor
- BrdU
5-bromo-2-deoxyuridine
- tMCAO
transient middle cerebral artery occlusion, a model of experimental stroke in mice
- HSPC
hematopoietic stem and progenitor cells
- HSC
hematopoietic stem cells
- LKS
Lin− c-Kit+ Sca-1+ hematopoietic stem and progenitor cell
- GMP
granulocyte macrophage progenitor
- MDP
macrophage and dendritic cell progenitor
- CLP
common lymphoid progenitor
- GFP
green fluorescent protein
Footnotes
DISCLOSURES
None.
References
- 1.Kaito M, Araya S, Gondo Y, Fujita M, Minato N, Nakanishi M, Matsui M. Relevance of distinct monocyte subsets to clinical course of ischemic stroke patients. PLoS One. 2013;8:e69409. doi: 10.1371/journal.pone.0069409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urra X, Villamor N, Amaro S, Gomez-Choco M, Obach V, Oleaga L, Planas AM, Chamorro A. Monocyte subtypes predict clinical course and prognosis in human stroke. J Cereb Blood Flow Metab. 2009;29:994–1002. doi: 10.1038/jcbfm.2009.25. [DOI] [PubMed] [Google Scholar]
- 3.Garcia JH, Liu KF, Yoshida Y, Lian J, Chen S, del Zoppo GJ. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat) Am J Pathol. 1994;144:188–199. [PMC free article] [PubMed] [Google Scholar]
- 4.Boehme AK, Kumar AD, Lyerly MJ, Gillette MA, Siegler JE, Albright KC, Beasley TM, Martin-Schild S. Persistent Leukocytosis-Is this a Persistent Problem for Patients with Acute Ischemic Stroke? J Stroke Cerebrovasc Dis. 2014;23:1939–1943. doi: 10.1016/j.jstrokecerebrovasdis.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nardi K, Milia P, Eusebi P, Paciaroni M, Caso V, Agnelli G. Admission leukocytosis in acute cerebral ischemia: influence on early outcome. J Stroke Cerebrovasc Dis. 2012;21:819–824. doi: 10.1016/j.jstrokecerebrovasdis.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Takaku T, Malide D, Chen J, Calado RT, Kajigaya S, Young NS. Hematopoiesis in 3 dimensions: human and murine bone marrow architecture visualized by confocal microscopy. Blood. 2010;116:e41–e55. doi: 10.1182/blood-2010-02-268466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emsley HC, Smith CJ, Gavin CM, Georgiou RF, Vail A, Barberan EM, Hallenbeck JM, del Zoppo GJ, Rothwell NJ, Tyrrell PJ, Hopkins SJ. An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis. J Neuroimmunol. 2003;139:93–101. doi: 10.1016/s0165-5728(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 8.Vogelgesang A, Grunwald U, Langner S, Jack R, Broker BM, Kessler C, Dressel A. Analysis of lymphocyte subsets in patients with stroke and their influence on infection after stroke. Stroke. 2008;39:237–241. doi: 10.1161/STROKEAHA.107.493635. [DOI] [PubMed] [Google Scholar]
- 9.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 10.Liesz A, Hagmann S, Zschoche C, Adamek J, Zhou W, Sun L, Hug A, Zorn M, Dalpke A, Nawroth P, Veltkamp R. The spectrum of systemic immune alterations after murine focal ischemia: immunodepression versus immunomodulation. Stroke. 2009;40:2849–2858. doi: 10.1161/STROKEAHA.109.549618. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 12.Stevens SL, Bao J, Hollis J, Lessov NS, Clark WM, Stenzel-Poore MP. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 2002;932:110–119. doi: 10.1016/s0006-8993(02)02292-8. [DOI] [PubMed] [Google Scholar]
- 13.Goeman F, Manni I, Artuso S, Ramachandran B, Toietta G, Bossi G, Rando G, Cencioni C, Germoni S, Straino S, Capogrossi MC, Bacchetti S, Maggi A, Sacchi A, Ciana P, Piaggio G. Molecular imaging of nuclear factor-Y transcriptional activity maps proliferation sites in live animals. Mol Biol Cell. 2012;23:1467–1474. doi: 10.1091/mbc.E12-01-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laiosa CV, Stadtfeld M, Graf T. Determinants of lymphoid-myeloid lineage diversification. Annu Rev Immunol. 2006;24:705–738. doi: 10.1146/annurev.immunol.24.021605.090742. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki H, Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity. 2007;26:726–740. doi: 10.1016/j.immuni.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harms H, Reimnitz P, Bohner G, Werich T, Klingebiel R, Meisel C, Meisel A. Influence of stroke localization on autonomic activation, immunodepression, and post-stroke infection. Cerebrovasc Dis. 2011;32:552–560. doi: 10.1159/000331922. [DOI] [PubMed] [Google Scholar]
- 18.Chamorro A, Amaro S, Vargas M, Obach V, Cervera A, Gomez-Choco M, Torres F, Planas AM. Catecholamines, infection, and death in acute ischemic stroke. J Neurol Sci. 2007;252:29–35. doi: 10.1016/j.jns.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Yu L, Jiang C, Fu X, Liu X, Wang M, Ou C, Cui X, Zhou C, Wang J. Cerebral ischemia increases bone marrow CD4+CD25+FoxP3+ regulatory T cells in mice via signals from sympathetic nervous system. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.07.022. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 21.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nature Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denes A, McColl BW, Leow-Dyke SF, Chapman KZ, Humphreys NE, Grencis RK, Allan SM, Rothwell NJ. Experimental stroke-induced changes in the bone marrow reveal complex regulation of leukocyte responses. J Cereb Blood Flow Metab. 2011;31:1036–1050. doi: 10.1038/jcbfm.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courties G, Moskowitz MA, Nahrendorf M. The innate immune system after ischemic injury: lessons to be learned from the heart and brain. JAMA Neurol. 2014;71:233–236. doi: 10.1001/jamaneurol.2013.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208:421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egawa T, Kawabata K, Kawamoto H, Amada K, Okamoto R, Fujii N, Kishimoto T, Katsura Y, Nagasawa T. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15:323–334. doi: 10.1016/s1074-7613(01)00185-6. [DOI] [PubMed] [Google Scholar]
- 32.Dias S, Silva HJ, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201:971–979. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 37.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeda K, Malykhin A, Teague-Weber BN, Sun XH, Farris AD, Coggeshall KM. Interleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus-prone B6.Sle1.Yaa animals. Blood. 2009;113:4534–4540. doi: 10.1182/blood-2008-12-192559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265–278. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynaud D, Pietras E, Barry-Holson K, Mir A, Binnewies M, Jeanne M, Sala-Torra O, Radich JP, Passegue E. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell. 2011;20:661–673. doi: 10.1016/j.ccr.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, Shimada T, Mizuno Y, Urabe T. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003;117:531–539. doi: 10.1016/s0306-4522(02)00954-5. [DOI] [PubMed] [Google Scholar]
- 42.Li T, Pang S, Yu Y, Wu X, Guo J, Zhang S. Proliferation of parenchymal microglia is the main source of microgliosis after ischaemic stroke. Brain. 2013;136:3578–3588. doi: 10.1093/brain/awt287. [DOI] [PubMed] [Google Scholar]
- 43.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gliem M, Mausberg AK, Lee JI, Simiantonakis I, van Rooijen N, Hartung HP, Jander S. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann Neurol. 2012;71:743–752. doi: 10.1002/ana.23529. [DOI] [PubMed] [Google Scholar]
- 45.Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kammersgaard LP, Jorgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS. Leukocytosis in acute stroke: relation to initial stroke severity, infarct size, and outcome: the Copenhagen Stroke Study. J Stroke Cerebrovasc Dis. 1999;8:259–263. doi: 10.1016/s1052-3057(99)80076-7. [DOI] [PubMed] [Google Scholar]
- 47.Standefer M, Little JR. Improved neurological outcome in experimental focal cerebral ischemia treated with propranolol. Neurosurgery. 1986;18:136–140. doi: 10.1227/00006123-198602000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Savitz SI, Erhardt JA, Anthony JV, Gupta G, Li X, Barone FC, Rosenbaum DM. The novel beta-blocker, carvedilol, provides neuroprotection in transient focal stroke. J Cereb Blood Flow Metab. 2000;20:1197–1204. doi: 10.1097/00004647-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Han RQ, Ouyang YB, Xu L, Agrawal R, Patterson AJ, Giffard RG. Postischemic brain injury is attenuated in mice lacking the beta2-adrenergic receptor. Anesth Analg. 2009;108:280–287. doi: 10.1213/ane.0b013e318187ba6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laowattana S, Oppenheimer SM. Protective effects of beta-blockers in cerebrovascular disease. Neurology. 2007;68:509–514. doi: 10.1212/01.wnl.0000253186.23949.fd. [DOI] [PubMed] [Google Scholar]
- 51.Dziedzic T, Slowik A, Pera J, Szczudlik A. Beta-blockers reduce the risk of early death in ischemic stroke. J Neurol Sci. 2007;252:53–56. doi: 10.1016/j.jns.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Mulder WJ, Jaffer FA, Fayad ZA, Nahrendorf M. Imaging and nanomedicine in inflammatory atherosclerosis. Sci Transl Med. 2014;6:239sr1. doi: 10.1126/scitranslmed.3005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.