Abstract
Objective
To investigate oxidative stress as a mechanism of preterm birth in human subjects, we examined associations between urinary biomarkers of oxidative stress measured at multiple time points during pregnancy and preterm birth.
Study Design
This nested case-control study included 130 mothers who delivered preterm and 352 who delivered term who were originally recruited as part of an ongoing prospective birth cohort at Brigham and Women’s Hospital. Two biomarkers, including 8-hydroxydeoxyguanosine (8-OHdG) and 8-isoprostane were measured in urine samples collected at up to four time points (median 10, 18, 26, and 35 weeks) during gestation.
Results
Urinary concentrations of 8-isoprostane and 8-OHdG decreased and increased, respectively, as pregnancy progressed. Average levels of 8-isoprostane across pregnancy were associated with increased odds of spontaneous preterm birth (adjusted odds ratio [aOR]=6.25, 95% confidence interval [CI]=2.86, 13.7), and associations were strongest with levels measured later in pregnancy. Average levels of 8-OHdG were protective against overall preterm birth (aOR=0.19, 95%CI=0.10, 0.34), and there were no apparent differences in the protective effect in cases of spontaneous preterm birth compared to cases of placental origin. Odds ratios for overall preterm birth were more protective in association with urinary 8-OHdG concentrations measured early in pregnancy.
Conclusions
Maternal oxidative stress may be an important contributor to preterm birth, regardless of subtype and timing of exposure during pregnancy. The two biomarkers measured in the present study had opposite associations with preterm birth; an improved understanding of what each represents may help to more precisely identify important mechanisms in the pathway to preterm birth.
Keywords: Epidemiology, longitudinal, oxidative stress, preterm birth, repeated measures
Introduction
Preterm birth (PTB) is a leading cause of neonatal mortality and morbidity, and occurs in over 1 in 10 births in the US.1 Despite the significance of this public health problem, mechanisms for PTB are poorly understood.2 Many risk factors, such as maternal age, race/ethnicity, and tobacco use have been linked to PTB but underlying pathways for these relationships remain unclear. This may be attributable in part to the difficulties in examining PTB in a small animal model; PTB has been induced in rodents in some studies but generally requires gene knockouts or high doses of lipopolysaccharides injection, making translation of results to humans difficult.3, 4 Alternatively, screening of molecular biomarkers in humans may be helpful for identifying mechanisms contributing to PTB.
Inflammation and infection at the maternal-fetal interface are among the most well-established precursors to PTB, and justifiably much of the research on predictive biomarkers has focused on inflammatory cytokines and other indicators of inflammation.5 While predictive value of these biomarkers for use by clinicians is limited, these data provide in vivo evidence for a causative role of inflammation for some cases of PTB.5
Oxidative stress, defined as an imbalance between antioxidant capacity and reactive oxygen species (ROS) generation, has received less attention in the study of PTB, but may play a role through multiple pathways. A number of biomarkers exist to measure oxidative stress, and each is formed through a unique mechanism and has the potential for differing downstream physiologic effects. In the present study we measured two biomarkers in maternal urine samples collected from up to four time points during pregnancy, 8-isoprostane and 8-hydroxydeoxyguanosine (8-OHdG). 8-isoprostane is a useful biomarker of oxidative stress in humans because of its stability, sensitivity to oxidant injury, and specificity to arachidonic acid peroxidation by ROS.6 In addition to use in studies of adverse pregnancy outcomes, levels are commonly used as markers of oxidative damage in the study of cardiovascular disease.7 8-OHdG, an oxidized nucleoside released upon repair of damaged DNA, is also utilized commonly as a marker of oxidative stress.8 We examined changes in these markers longitudinally across pregnancy as well as their relationship with overall prematurity and subtypes with homogenous etiologies (spontaneous PTB and PTB of placental origin).
Materials and Methods
Study population
Subjects for this nested case-control study were selected from a longitudinal birth cohort designed to identify predictors of preeclampsia in pregnant women who delivered at Brigham and Women’s Hospital in Boston, MA, between 2006 and 2008.9 Participants were recruited early in pregnancy (median 10 weeks gestation), completed demographic and medical history questionnaires and provided informed consent at enrollment. Gestational age was assessed by last menstrual period with verification by first trimester ultrasound. Participants additionally provided urine samples from up to a total of four visits across gestation (median 10, 18, 26, and 35 weeks gestation). At delivery, detailed birth outcome and infant data was recorded. From this parent population we selected all 130 women who delivered live singleton infants preterm, or prior to 37 weeks completed gestation, as well as 352 random controls.9 This study was approved by institutional review boards at the University of Michigan and Brigham and Women’s Hospital.
Women who delivered preterm were divided into subgroups. Women who presented at delivery with spontaneous preterm labor or preterm premature rupture of the membranes (pPROM) were combined into a single group, as previous research shows that women with these delivery precursors show similar patterns of placental inflammation.10 These births were considered spontaneous preterm (N=56). A second category included women whose preterm deliveries were determined to be a result of preeclampsia or intrauterine growth restriction (IUGR), as these groups can be combined based on a similar etiology of abnormal placentation.10 These births were considered placental preterm for analysis (N=35). Assignment of pregnancy outcomes was based on the criteria of the American College of Obstetricians and Gynecologists and standard clinical practice. The remaining PTBs (N=39) did not fall into either etiology-based subset (e.g., followed repeated C-section or other maternal/fetal complications not listed above) and were not examined separately due to the lack of a hypothesized shared mechanism. Nevertheless, they were included in the primary analysis because unknown mechanisms may link oxidative stress to PTB overall and this was an exploratory analysis.
Oxidative stress biomarker analysis
Urine samples (N=1678 samples, N=482 women) were stored at −80° C after collection until the time oxidative stress biomarkers were measured. Both 8-OHdG and total 8-isoprostane were measured by Cayman Chemical (Ann Arbor, MI). For total 8-isoprostane, urine samples were hydrolyzed to deconjugate 8-isoprostane esterified to phospholipids and were passed through affinity columns for purification. Eluted samples were dried and resuspended in a buffer before measurement with enzyme immunoassay (EIA). The lower limit of detection was 3.9 pg/mL. For 8-OHdG, samples were diluted directly into buffer without purification. Concentrations were also measured using EIA with a detection limit of 10.3 pg/mL. Levels of biomarkers below the limit of detection (LOD) were replaced with the LOD divided by the square root of 2.11
To account for urine dilution, specific gravity was measured in urine samples using a digital handheld refractometer (Atago Co., Ltd., Tokyo, Japan). For examining biomarker distributions and variability, concentrations were corrected for specific gravity using the following formula: OSc=OS[(1.015–1)/(SG-1)].12 OSc represents the corrected biomarker concentration; OS is the uncorrected urinary concentration; 1.015 is the median specific gravity in all samples; and SG is the specific gravity of the sample. For regression analyses uncorrected concentrations were modeled and specific gravity was included as a covariate. Extremely concentrated (specific gravity >1.04) samples were excluded from all analyses (N=4). Distributions of concentrations for both raw and corrected biomarkers were log-normal and ln-transformed for data analysis.
Statistical analysis
Analysis was performed using R version 3.0.2. Differences in biomarker levels by visit were tested using linear mixed models with random intercepts only to adjust for intra-individual correlation, with biomarker regressed on visit of sample collection. To depict non-linear trends in levels across gestation, generalized additive mixed models (GAMM; mgcv package in R)13 were created with biomarker regressed on a smooth term for gestational age at urine sample collection, also with random intercepts only. Predicted values from GAMM models were plotted to show average trends over time. As an additional measure of variability in biomarker concentrations we calculated intraclass correlation coefficients (ICC), which represent the ratio of within to between individual variability.14
Associations between oxidative stress markers and PTB were examined for overall PTB and also for the subtypes defined above. Odds ratios were calculated using a geometric average biomarker concentration for each subject from levels measured at visits 1–3. Visit 4 concentrations were excluded from the average because the proportion of cases with a measure at this time point was low, as many had already delivered (100% of cases had samples available at visit 1; 91% at visit 2; 86% at visit 3; 51% at visit 4). Crude models were adjusted for specific gravity and full models included covariates associated with oxidative stress biomarkers in bivariate analysis that have been linked to PTB in previous studies. Covariates that were considered included maternal age, race/ethnicity, education level, health insurance provider, body mass index (BMI) at visit 1, use of tobacco or alcohol during pregnancy, parity and gender of infant, and use of assisted reproductive technology.
Additionally, individual logistic regression models examining urinary oxidative stress biomarkers at each visit in relation to PTB were constructed to investigate whether oxidative stress at a particular time point was more predictive of preterm delivery. These models were created using the same covariates included in average models.
Results
Characteristics of controls, cases, and spontaneous and placental cases separately are presented in Table 1. Overall mothers were median 32.7 years of age at the first study visit and were predominantly white (58.5%), well educated (38.8% with a college education or higher), and had private (79.9%) rather than public health insurance.9 Few mothers used tobacco (6.4%) or alcohol (4.1%) during pregnancy. Most women were underweight to normal weight at visit 1 (BMI <25 kg/m2, 51.9%).9 Gestational age at delivery ranged from 23.4 to 36.9 weeks for mothers who delivered preterm and from 37 to 42.7 weeks for mothers who delivered term.
Table 1.
Distributions of demographic characteristics in study population: N (%).
| Characteristic | Term | Preterm | Spontaneous preterm | Placental preterm | |
|---|---|---|---|---|---|
| Race/ethnicity (missing = 0) | White | 207 (58.8) | 75 (57.7) | 30 (53.6) | 20 (57.1) |
| African American | 55 (15.6) | 22 (16.9) | 8 (14.3) | 8 (22.9) | |
| Other | 90 (25.6) | 33 (25.4) | 18 (32.1) | 7 (20.0) | |
| Education (missing = 11) | High school | 47 (13.7) | 21 (16.3) | 7 (12.5) | 10 (28.6) |
| Technical school | 52 (15.2) | 25 (19.4) | 12 (21.4) | 5 (14.3) | |
| Junior college/some college | 101 (29.5) | 38 (29.5) | 16 (28.6) | 12 (34.3) | |
| College graduate | 142 (41.5) | 45 (34.9) | 21 (37.5) | 8 (22.9) | |
| Health insurance (missing = 12) | Private/HMO/self-pay | 277 (81.0) | 108 (84.4) | 48 (85.7) | 26 (78.8) |
| Medicaid/SSI/MassHealth | 65 (19.0) | 20 (15.6) | 8 (14.3) | 7 (21.2) | |
| BMI (missing = 4) | <25 kg/m2 | 188 (54.0) | 62 (47.7) | 30 (53.6) | 8 (22.9)a |
| 25–30 kg/m2 | 94 (27.0) | 32 (24.6) | 15 (26.8) | 9 (25.7)a | |
| ≥30 kg/m2 | 66 (19.0) | 36 (27.7) | 11 (19.6) | 18 (51.4)a | |
| Tobacco use (missing =6) | Yes | 20 (5.8) | 11 (8.5) | 4 (7.2) | 7 (20.0)a |
| No | 326 (94.2) | 119 (91.5) | 52 (92.8) | 28 (80.0)a | |
| Alcohol use (missing = 10) | Yes | 19 (5.5) | 1 (0.8)a | 0 (0) | 0 (0) |
| No | 326 (94.5) | 126 (99.2)a | 54 (100) | 35 (100) | |
| Parity (missing = 0) | Nulliparous | 160 (45.5) | 55 (42.3) | 24 (42.9) | 20 (57.1) |
| Parous | 192 (54.5) | 75 (57.7) | 32 (57.1) | 15 (42.9) | |
| Gender (missing = 0) | Male | 158 (44.9) | 56 (43.1) | 27 (48.2) | 16 (45.7) |
| Female | 194 (55.1) | 74 (56.9) | 29 (51.8) | 19 (54.3) | |
| Use of ART (missing = 0) | Yes | 33 (9.4) | 12 (9.2) | 4 (7.1) | 4 (11.4) |
| No | 319 (90.6) | 118 (90.8) | 42 (92.9) | 31 (88.6) | |
Abbreviations: BMI, body mass index; HMO, health maintenance organization; SSI, supplemental security income; ART, assisted reproductive technology.
P <0.05 for χ2 test comparing distributions in overall, spontaneous, or placental preterm vs. term groups.
Specific gravity corrected urinary concentrations of 8-isoprostane were higher in African American women (geometric mean=274 pg/mL) and women of other race/ethnicity (geometric mean=210 pg/mL) compared to white women (geometric mean=165 pg/mL). 8-isoprostane concentrations were also significantly higher in women with lower education levels, public health insurance, high BMI (≥30 kg/m2), and in women who smoked during pregnancy. Maternal age at visit 1 was inversely correlated with 8-isoprostane average (Spearman R=−0.23, p<0.01). For 8-OHdG, the only significant difference observed with categorical covariates was that women with private health insurance had lower levels (123 ng/mL) compared to women with public health insurance (146 ng/mL). As with 8-isoprostane, 8-OHdG average was inversely correlated with maternal age (Spearman R=−0.13, p<0.01).
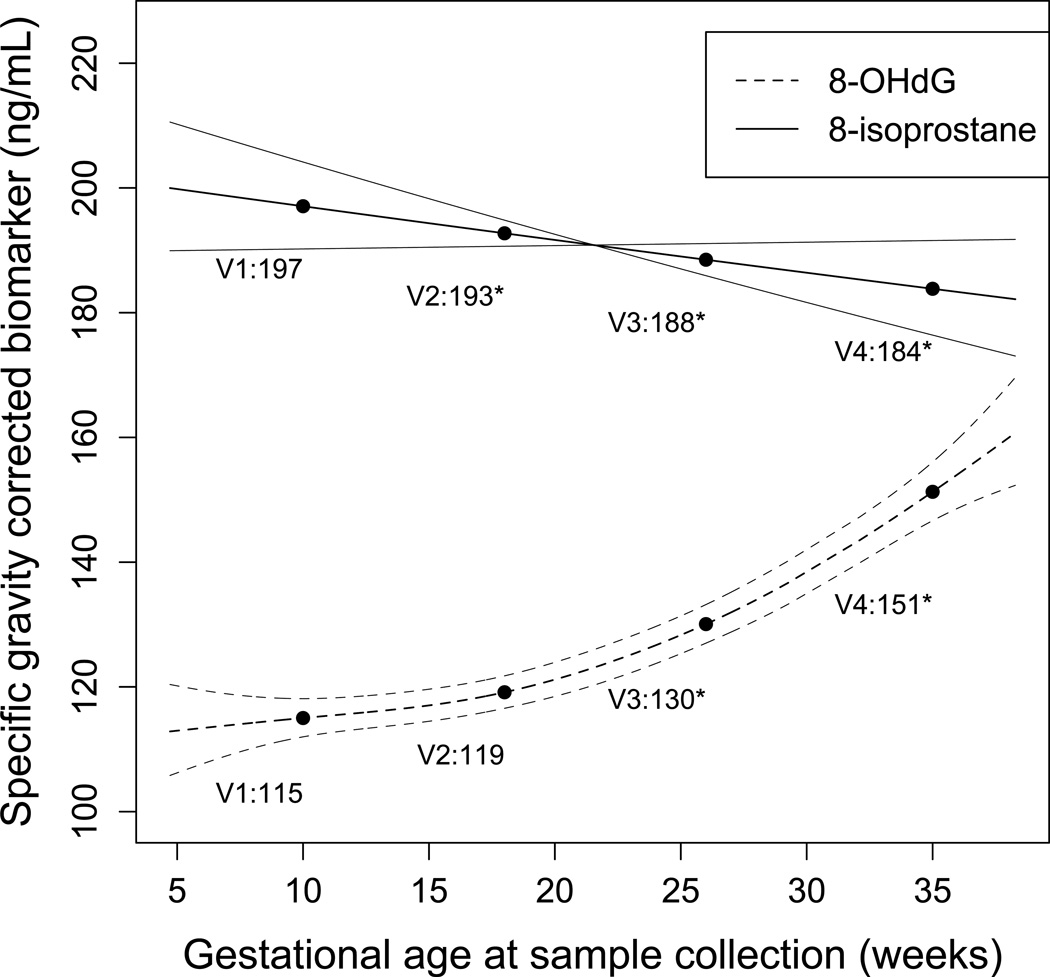
Predicted values of specific gravity corrected 8-OHdG and 8-isoprostane from GAMM are presented in Figure 1. Levels of each biomarker at the median gestational age for each visit, extracted from GAMM models, are plotted for reference. For 8-isoprostane, levels decreased slightly and linearly (indicated by crossing confidence intervals) across pregnancy, and levels at visits 2, 3, and 4 were significantly lower than at visit 1. For 8-OHdG, levels increased in a quadratic form as gestation progressed, and levels at visit 3 and 4 were significantly higher than levels at visit 1. No differences in trends were observed in cases compared to controls.
Figure 1. Oxidative stress biomarker concentrations across pregnancy.
Predicted values (95% confidence intervals) of specific gravity-corrected urinary oxidative stress biomarker concentrations by gestational age at sample collection from generalized additive models adjusted for random intercepts only. Biomarker concentrations at visits 1 (V1; median 10 weeks gestation), 2 (V2; median 15 weeks gestation), 3 (V3; median 26 weeks gestation), and 4 (V4; median 35 weeks gestation) plotted for reference. *Denotes significant difference (p<0.05) in urinary biomarker concentration for the respective visit compared to visit 1, tested using linear mixed model with random intercepts only. Abbreviations: 8-OHdG, 8-hydroxydeoxyguanosine.
Variability in concentrations was also captured using ICCs. An ICC ranges from 0 to 1, with 0 indicating no reproducibility in measures and 1 indicating perfect reproducibility.14 ICCs for both urinary oxidative stress biomarkers were good (between 0.40 and 0.75), but 8-OHdG was less reliable over time (ICC=0.32, 95% confidence interval [CI]=0.27, 0.38) compared to 8- isoprostane (ICC=0.60, 95% CI=0.56, 0.64).14, 15 Correlations between biomarkers were weak but statistically significant at each study visit (Spearman R=0.10–0.20, p<0.05).
In logistic regression models of geometric average biomarker concentrations, 8-isoprostane was significantly associated with increased odds of overall PTB in crude models adjusted only for urinary specific gravity and in full models additionally adjusted for maternal age, race/ethnicity, education level, health insurance provider, and pre-pregnancy BMI. Tobacco use did not alter effect estimates. Adjusted odds ratios (aOR) of PTB in association with an interquartile range increase in urinary oxidative stress biomarker concentration are presented in Table 2. The association between 8-isoprostane and overall PTB was driven by associations with spontaneous PTB; analysis by subtypes showed large odds ratios for spontaneous PTB (aOR=6.25, 95%CI=2.86, 13.7) and null associations for placental PTB (aOR=0.94, 95%CI=0.52, 1.70). Cross-sectional logistic regression models by study visit (Table 3) showed that the odds for spontaneous PTB were lower early in pregnancy compared to subsequent visits.
Table 2.
Odds ratios (95% confidence intervals) for preterm birth in association with interquartile range increase in geometric average (visits 1–3) urinary oxidative stress biomarkers.
| Overall preterm birth | ||||||
| Model 1a | Model 2b | |||||
| Biomarker | N (cases, controls) | OR (95% CI) | p | N (cases, controls) | OR (95% CI) | p |
| 8-OHdG | 129, 349 | 0.19 (0.11, 0.34) | <0.001 | 126, 331 | 0.19 (0.10, 0.34) | <0.001 |
| 8-Isoprostane | 129, 349 | 2.17 (1.48, 3.20) | <0.001 | 126, 331 | 2.22 (1.47, 3.36) | <0.001 |
| Spontaneous preterm birth | ||||||
| Model 1a | Model 2b | |||||
| Biomarker | N (cases, controls) | OR (95% CI) | p | N (cases, controls) | OR (95% CI) | p value |
| 8-OHdG | 56, 349 | 0.21 (0.10, 0.42) | <0.001 | 56, 331 | 0.18 (0.09, 0.40) | <0.001 |
| 8-Isoprostane | 56, 349 | 4.25 (2.21, 8.15) | <0.001 | 56, 331 | 6.25 (2.86, 13.7) | <0.001 |
| Placental preterm birth | ||||||
| Model 1a | Model 2b | |||||
| Biomarker | N (cases, controls) | OR (95% CI) | p | N (cases, controls) | OR (95% CI) | p value |
| 8-OHdG | 35, 349 | 0.17 (0.07, 0.41) | <0.001 | 33, 331 | 0.11 (0.04, 0.32) | <0.001 |
| 8-Isoprostane | 35, 349 | 1.45 (0.79, 2.66) | 0.24 | 33, 331 | 0.94 (0.52, 1.70) | 0.84 |
Abbreviations: 8-OHdG, 8-hydroxydeoxyguanosine; OR, odds ratio.
Model 1 adjusted for urinary specific gravity only.
Model 2 adjusted for urinary specific gravity, maternal age, race/ethnicity, education level, health insurance provider, and pre-pregnancy body mass index (BMI).
Table 3.
Odds ratios (95% confidence intervals) for preterm birth in association with interquartile range increase in urinary oxidative stress biomarkers by study visit.
| Overall preterm birth | ||||||
| 8-OHdG | 8-Isoprostane | |||||
| N (cases, controls) | OR (95% CI) | p value | N (cases, controls) | OR (95% CI) | p value | |
| Visit 1 | 123, 326 | 0.25 (0.14, 0.46) | <0.01 | 123, 326 | 1.72 (1.18, 2.51) | 0.01 |
| Visit 2 | 114, 289 | 0.21 (0.10, 0.42) | <0.01 | 114, 289 | 2.33 (1.44, 3.77) | <0.01 |
| Visit 3 | 107, 282 | 0.44 (0.24, 0.81) | <0.01 | 107, 282 | 2.05 (1.31, 3.19) | <0.01 |
| Visit 4 | 59, 294 | 0.45 (0.23, 0.90) | 0.02 | 59, 294 | 1.76 (1.08, 2.88) | 0.02 |
| Spontaneous preterm birth | ||||||
| 8-OHdG | 8-Isoprostane | |||||
| N (cases, controls) | OR (95% CI) | p value | N (cases, controls) | OR (95% CI) | p value | |
| Visit 1 | 54, 326 | 0.26 (0.12, 0.56) | <0.01 | 54, 326 | 2.72 (1.46, 5.06) | <0.01 |
| Visit 2 | 52, 289 | 0.30 (0.13, 0.71) | <0.01 | 52, 289 | 7.10 (2.80, 18.0) | <0.01 |
| Visit 3 | 47, 282 | 0.33 (0.14, 0.74) | <0.01 | 47, 282 | 4.45 (1.96, 10.1) | <0.01 |
| Visit 4 | 24, 294 | 0.75 (0.28, 2.01) | 0.57 | 24, 294 | 5.27 (1.82, 15.3) | <0.01 |
| Placental preterm birth | ||||||
| 8-OHdG | 8-Isoprostane | |||||
| N (cases, controls) | OR (95% CI) | p value | N (cases, controls) | OR (95% CI) | p value | |
| Visit 1 | 33, 326 | 0.21 (0.07, 0.58) | <0.01 | 33, 326 | 1.02 (0.62, 1.69) | 0.93 |
| Visit 2 | 29, 289 | 0.12 (0.04, 0.40) | <0.01 | 29, 289 | 1.19 (0.60, 2.34) | 0.62 |
| Visit 3 | 30, 282 | 0.40 (0.17, 0.94) | 0.04 | 30, 282 | 0.79 (0.44, 1.43) | 0.44 |
| Visit 4 | 12, 294 | 0.19 (0.04, 0.85) | 0.03 | 12, 294 | 0.59 (0.29, 1.19) | 0.14 |
Abbreviations: 8-OHdG, 8-hydroxydeoxyguanosine; OR, odds ratio. Models adjusted for urinary specific gravity, maternal age, race/ethnicity, education level, health insurance provider, and pre-pregnancy body mass index (BMI).
Average 8-OHdG was strongly associated with reduced odds of overall PTB (aOR=0.19, 95%CI=0.10, 0.34), and the relationship was similar in cases of spontaneous (aOR=0.18, 95%CI=0.09, 0.40) and placental (aOR=0.11, 95%CI=0.04, 0.32) PTB (Table 2). As with 8-isoprostane, effect estimates were similar in models adjusted for specific gravity only and in models with all covariates. When odds ratios were calculated for each visit, we observed more protective odds ratios for spontaneous PTB when 8-OHdG levels were measured early in pregnancy. For placental PTB, the most protective odds ratio was in association with levels measured at visit 2 (median 18 weeks gestation).
Comment
There are plausible mechanisms for a role of oxidative stress in the pathway to PTB. ROS could a) act as a precursor to inflammatory responses that may prematurely initiate parturition processes;16 b) damage collagen in the cervical stroma or fetal membranes resulting in pPROM;17 or c) cause apoptosis of the syncytiotrophoblast early in pregnancy, impairing spiral arteriole invasion of the myometrial wall and resulting in dysfunctional placentation.18, 19 Some prior evidence supports a relationship between oxidative stress and PTB in studies measuring maternal biomarkers.20–23
One prospective study (N=508) examined 8-isoprostane levels in urine collected once between 5–16 weeks gestation and observed a slight but significant trend for decreased gestational duration with increasing quintiles of 8-isoprostane.22 Another study (N=503) measured 8-isoprostane in maternal plasma collected once between 24–26 weeks gestation and observed higher levels in mothers who developed preeclampsia or had small for gestational age infants.20 Neither group observed an association with PTB, though the number of cases in each study was small (N=48 and 37, respectively). Other studies have examined 8-isoprostane levels in amniotic fluid and found higher levels in women with term PROM24 and pPROM.21 Finally, two studies examined differences in maternal serum levels of malondialdehyde (MDA), another marker of lipid peroxidation, in mothers who delivered preterm compared to term. One case-control study observed higher levels of MDA in mothers who delivered preterm (N=30) vs. term (N=30), but in another cohort study (N=200) no differences were observed.25, 26 Importantly, in both studies, blood samples were taken immediately prior to or following delivery.
Our results provide strong evidence for an association between urinary 8-isoprostane levels and spontaneous PTB in a large nested case-control study examining urinary concentrations at multiple time points during pregnancy. We also examined urinary excretion patterns during pregnancy which have not been previously explored. Urinary concentrations decreased slightly across pregnancy but generally showed good intra-individual reproducibility, suggesting that future studies examining this marker may only need to do so at one time point. However, by measuring levels at multiple time points, we were able to illustrate that associations with spontaneous PTB were strongest when 8-isoprostane was measured at visits 2–4 compared to visit 1. This supports the hypothesis that oxidative stress levels later in pregnancy may cause PTB by weakening tissue integrity or initiating events leading to spontaneous preterm labor.
The aforementioned study in which 8-isoprostane was measured in urine also examined 8-OHdG in urine samples and found slight but significant associations with decreased gestational length and birth weight, but not with PTB.22 A previous smaller study by the same group (N=18 cases of low birth weight, growth restriction, or preterm; N=34 controls) reported significantly higher 8-OHdG concentrations measured in urine from the third trimester in cases compared to controls;23 however, those results were not specific to PTB. In another study, where 8-OHdG was measured in urine collected once between 24–26 weeks gestation, concentrations were higher in women who went on to deliver a low birth weight infant, but no association was observed with PTB.20 Finally, one study which measured 8-OHdG in umbilical cord serum found elevated levels in samples from babies born both preterm and with low birth weight.27
Unexpectedly, we observed that women with higher urinary concentrations of 8-OHdG had reduced odds of PTB. This was true for both spontaneous and placental PTB, and odds ratios were most protective for spontaneous PTB at visit 1 (median 10 weeks gestation) and for placental PTB at visit 2 (median 18 weeks gestation). We hypothesized that 8-OHdG, like 8-isoprostane, would be associated with increased odds of PTB because 8-OHdG is also utilized commonly as a marker of oxidative stress generated upon repair of oxidative DNA damage.8 Despite the common understanding that these markers both represent oxidative stress, levels of the two markers were lowly correlated with one another in this and a previous study,22 indicating that they are truly representing different physiologic processes.
One explanation for the protective effect of 8-OHdG is that it is associated with unmeasured confounders in this population that strongly reduce the risk of PTB. For example, 8-OHdG levels increase with exercise28 and ferritin levels.22 Current literature shows that associations between these factors and PTB is generally null;29, 30 however, previous studies are not definitive and these or other prenatal factors could be confounders that reduce the risk of PTB. An alternative hypothesis hinges on the fact that 8-OHdG not only tracks oxidative DNA damage but also the successful execution of the excision repair process.8 It is possible that in mothers where this process is impaired there is increased risk of PTB. Under this scenario, 8-OHdG is not a marker of oxidative stress but instead the mother’s ability to repair oxidative damage. This hypothesis has been suggested in another study in which women who delivered infants with birth defects had lower urinary 8-OHdG levels compared to mothers with normal infants.22 This possibility deserves exploration in future research.
Consistent with prior studies, we observed that 8-OHdG levels were moderately variable within-woman across gestation, and that levels were higher in later compared to early pregnancy. Another study that examined urinary 8-OHdG concentrations at 6–8 weeks, 15–20 weeks, and 26–30 weeks gestation also observed that levels were elevated later in pregnancy.31 A third study measuring levels at 20 and 30 weeks gestation and also at delivery showed that levels were higher in urine collected at delivery compared to earlier in pregnancy, although these results could have been complicated by laboring processes.32
Our study had several limitations. The measurement of 8-isoprostane via EIA; liquid chromatography-mass spectrometry (LC/MS) may be preferable because of its improved specificity for the isomer. However, the correlation between levels measured with Cayman Chemical EIA and LC/MS is strong, specificity is improved in EIA following affinity purification which was used in this study, and other isoprostane isomers may also be produced by ROS.33 Also, LC/MS methods would have been cost-prohibitive; we would have been unable to analyze samples from as many subjects and time points, which was a major strength of our study. Urinary 8-OHdG analysis via EIA is preferred to LC/MS and correlation between results from the two methods is generally strong.8 Also, the study population was drawn from a prospective cohort of pregnant women delivering at a major tertiary care center in the Boston area. When extending results to other populations subject characteristics should be taken into account; these associations may not be consistent in women who are at high risk of having a PTB.
There were many advantages to our study design as well. We measured 8-isoprostane and 8-OHdG in maternal urine samples from repeated time points, which allowed us to create stable averages of maternal oxidative stress levels across pregnancy to investigate associations with PTB. With these repeated measures we were also able to examine variability in levels across pregnancy and associations with PTB at individual exposure windows when vulnerability could differ. Additionally, we measured urinary rather than plasma concentrations of 8-isoprostane, which are preferred because plasma may be susceptible to auto-oxidation during storage, while urine samples, with low lipid concentrations, are not.34 Lastly, this represents one of the largest studies to date to examine oxidative stress markers in relation to PTB, and included by far the largest number of preterm cases.
In conclusion, we observed that increased 8-isoprostane levels were associated with increased odds of spontaneous but not placental PTB, and odds ratios were highest in association with levels measured later in pregnancy. Conversely, we found that 8-OHdG levels were significantly associated with decreased odds of both spontaneous and placental preterm subtypes, particularly for measures taken early in pregnancy. These results were unexpected, but could provide valuable information about heretofore unidentified factors that are preventative of PTB. While the present study was not designed to identify predictive markers of PTB for the purposes of screening or prevention, these results may further the understanding of the role of oxidative stress in PTB. Better characterization of this relationship could lead to future treatment and prevention strategies.
Acknowledgements
We thank Dr. Elizabeth Hurst and colleagues of Cayman Chemical in Ann Arbor, Michigan, USA, for analysis of urinary oxidative stress biomarkers.
Financial support: Funding for this project was provided by the National Institute of Environmental Health Sciences, National Institutes of Health (R01ES018872, P42ES017198, P01ES022844, and P30ES017885).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors report no conflicts of interest.
References
- 1.Martin JA. Centers for Disease C, Prevention. Preterm births - United States, 2007. MMWR Surveill Summ. 2011;60(Suppl):78–79. [PubMed] [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cha J, Bartos A, Egashira M, et al. Combinatory approaches prevent preterm birth profoundly exacerbated by gene-environment interactions. J Clin Invest. 2013;123:4063–4075. doi: 10.1172/JCI70098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaga N, Katsuki Y, Obata M, Shibutani Y. Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am J Obstet Gynecol. 1996;174:754–759. doi: 10.1016/s0002-9378(96)70460-x. [DOI] [PubMed] [Google Scholar]
- 5.Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol. 2010;116:393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- 6.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 7.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 8.Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168:61–67. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168:980–989. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hornung RW, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 12.Meeker JD, Hu H, Cantonwine DE, et al. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environmental health perspectives. 2009;117:1587–1592. doi: 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. ournal of the Royal Statistical Society (B) 2011;73:3–36. [Google Scholar]
- 14.Rosner B. Fundamentals of biostatistics. Boston, MA: Brooks/Cole; Number of pages; [Google Scholar]
- 15.Ferguson KK, McElrath TF, Chen Y, Mukherjee B, Meeker JD. Urinary phthalate metabolites are associated with increased oxidative stress biomarkers in pregnant women. (submitted). [Google Scholar]
- 16.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reproductive sciences. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 17.Woods JR., Jr Reactive oxygen species and preterm premature rupture of membranes-a review. Placenta. 2001;22(Suppl A):S38–S44. doi: 10.1053/plac.2001.0638. [DOI] [PubMed] [Google Scholar]
- 18.Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30(Suppl A):S43–S48. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heazell AE, Moll SJ, Jones CJ, Baker PN, Crocker IP. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta. 2007;28(Suppl A):S33–S40. doi: 10.1016/j.placenta.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh TT, Chen SF, Lo LM, Li MJ, Yeh YL, Hung TH. The association between maternal oxidative stress at mid-gestation and subsequent pregnancy complications. Reprod Sci. 2012;19:505–512. doi: 10.1177/1933719111426601. [DOI] [PubMed] [Google Scholar]
- 21.Longini M, Perrone S, Vezzosi P, et al. Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clin Biochem. 2007;40:793–797. doi: 10.1016/j.clinbiochem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Peter Stein T, Scholl TO, Schluter MD, et al. Oxidative stress early in pregnancy and pregnancy outcome. Free Radic Res. 2008;42:841–848. doi: 10.1080/10715760802510069. [DOI] [PubMed] [Google Scholar]
- 23.Scholl TO, Stein TP. Oxidant damage to DNA and pregnancy outcome. J Matern Fetal Med. 2001;10:182–185. doi: 10.1080/714904323. [DOI] [PubMed] [Google Scholar]
- 24.Kwiatkowski S, Torbe A, Dolegowska B, et al. Isoprostanes 8-iPF2alpha-III: risk markers of premature rupture of fetal membranes? Biomarkers. 2009;14:406–413. doi: 10.1080/13547500903045583. [DOI] [PubMed] [Google Scholar]
- 25.Pathak R, Suke SG, Ahmed T, et al. Organochlorine pesticide residue levels and oxidative stress in preterm delivery cases. Hum Exp Toxicol. 2010;29:351–358. doi: 10.1177/0748233710363334. [DOI] [PubMed] [Google Scholar]
- 26.Weber D, Stuetz W, Bernhard W, et al. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur J Clin Nutr. 2013 doi: 10.1038/ejcn.2013.263. [DOI] [PubMed] [Google Scholar]
- 27.Negi R, Pande D, Kumar A, Khanna RS, Khanna HD. In vivo oxidative DNA damage and lipid peroxidation as a biomarker of oxidative stress in preterm low-birthweight infants. J Trop Pediatr. 2012;58:326–328. doi: 10.1093/tropej/fmr078. [DOI] [PubMed] [Google Scholar]
- 28.Okamura K, Doi T, Hamada K, et al. Effect of repeated exercise on urinary 8-hydroxydeoxyguanosine excretion in humans. Free Radic Res. 1997;26:507–514. doi: 10.3109/10715769709097821. [DOI] [PubMed] [Google Scholar]
- 29.Scholl TO. Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr. 2005;81:1218S–1222S. doi: 10.1093/ajcn/81.5.1218. [DOI] [PubMed] [Google Scholar]
- 30.Weissgerber TL, Wolfe LA, Davies GA, Mottola MF. Exercise in the prevention and treatment of maternal-fetal disease: a review of the literature. Appl Physiol Nutr Metab. 2006;31:661–674. doi: 10.1139/h06-060. [DOI] [PubMed] [Google Scholar]
- 31.Hung TH, Lo LM, Chiu TH, et al. A longitudinal study of oxidative stress and antioxidant status in women with uncomplicated pregnancies throughout gestation. Reprod Sci. 2010;17:401–409. doi: 10.1177/1933719109359704. [DOI] [PubMed] [Google Scholar]
- 32.Shoji H, Franke C, Campoy C, Rivero M, Demmelmair H, Koletzko B. Effect of docosahexaenoic acid and eicosapentaenoic acid supplementation on oxidative stress levels during pregnancy. Free Radic Res. 2006;40:379–384. doi: 10.1080/10715760500539147. [DOI] [PubMed] [Google Scholar]
- 33.Smith KA, Shepherd J, Wakil A, Kilpatrick ES. A comparison of methods for the measurement of 8-isoPGF(2alpha): a marker of oxidative stress. Ann Clin Biochem. 2011;48:147–154. doi: 10.1258/acb.2010.010151. [DOI] [PubMed] [Google Scholar]
- 34.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a noncyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]