Abstract
The prevalence of both domestic violence (DV) and HIV among Kenyan women is known to be high, but the relationship between them is unknown. Nationally representative cross-sectional data from married and formerly married (MFM) women responding to the Kenya Demographic and Health Survey 2008/2009 were analyzed adjusting for complex survey design. Multivariable logistic regressions were used to assess the covariate-adjusted associations between HIV serostatus and any reported DV as well as four constituent DV measures: physical, emotional, sexual, and aggravated bodily harm, adjusting for co-variates entered into each model using a forward stepwise selection process. Co-variates of a priori interest included those representing marriage history, risky sexual behavior, substance use, perceived HIV risk, and socio-demographic characteristics. The prevalence of HIV among MFM women was 10.7% (any DV: 13.1%, no DV: 8.6%); overall prevalence of DV was 43.4%. Among all DV measures, only physical DV was associated with HIV (11.9%; adjusted odds ratio: 2.01, p < 0.05). Efforts by the government and women's groups to monitor and improve policies to reduce DV, such as the Sexual Offences Act of 2006, are urgently needed to curb HIV, as are policies that seek to provide DV counseling and treatment to MFM women.
Keywords: Domestic violence, Epidemiology, HIV/AIDS, Kenya, Women
In Kenya, as in much of Sub-Saharan Africa (SSA), women are disproportionately affected by both HIV (UNAIDS, 2012) and domestic violence (Goo & Harlow, 2012; Jewkes, 2002; Kishor & Johnson, 2004; Koenig, 2003; Wanyoni & Lumumba, 2010). Among Kenyans aged 15-49 years, 8% of women compared to 4% of men report HIV infection (KNBS & ICF Macro, 2010), and higher prevalence of domestic violence among Kenyan women has been previously reported (Abuya, Onsomu, Moore, et al., 2012; Fonck, Leye, Kidula, et al., 2005; Goo & Harlow, 2012; Kishor & Johnson, 2004; Wanyoni & Lumumba, 2010). Despite the high prevalence of both DV and HIV among Kenyan women, the relationship between DV and HIV remains unclear. Although several investigators have observed an association between DV and HIV (Dude, 2011; Jewkes, Dunkle, Nduna, et al., 2010; Shi, Kouyoumdjian, and Dushoff , 2013; Silverman, Decker, Saggurti, et al., 2008), in the largest study on this topic—which incorporated data from 10 developing countries including Kenya —an association was not observed (Harling, Msisha, and Subramanian, 2010). Yet all of these studies are subject to important methodology and context limitations which may in part explain the discrepant findings. In the current study, we have addressed many of the limitations found in the existing literature in order to more accurately assess the relationship between DV and HIV infection among Kenyan women. An accurate understanding of the relationship between DV and HIV is paramount to the development of interventions to address these deeply rooted societal problems, which take a particularly heavy toll among women in Kenya and women throughout SSA.
Intimate Partner Violence (IPV), which includes DV, is the most common form of gender-based violence (García-Moreno, Jansen, Ellsberg, et al., 2006). It is defined as “the range of sexually, psychologically and physically coercive acts used against adult and adolescent women by current or former male intimate partners” (WHO, 1997, p. 5). Experts estimate that in African countries, 25-48% of women will suffer abuse at one point in their lives (Goo & Harlow, 2012; Jewkes, 2002; Kishor & Johnson, 2004; Koenig, 2003; Wanyoni & Lumumba, 2010). Its prevalence in Kenya is established (Abuya et al., 2012; Fonck et al., 2005; Goo & Harlow, 2012; Kishor & Johnson, 2004; Wanyoni & Lumumba, 2010), and Abuya et al. (2012) showed that physical (42%) and sexual (14%) violence toward Kenyan women fell in the middle range of multi-country estimates reported by the World Health Organization, 14-61% and 6-59%, respectively (WHO, 2005). Emotional violence is also rampant (Abuya et al., 2012; Fonck et al., 2005; Goo & Harlow, 2012; Kimuna, 2008).
Physical and sexual violence, including sexual assault within marriage, increase transmission of the virus as tears and lacerations to the vaginal canal enable its invasion of the vaginal epithelia (García-Moreno & Watts, 2000; Kishor et al., 2004; van der Straten, 1998; Wittenberg, Joshi, Thomas, & McCloskey, 2007). Socially, the threat of IPV often impedes open communication regarding disease risk. Women refrain from discussing their husband's risky behaviors, such as extramarital partners or frequenting sex workers (Lary, Maman, Katebalila, McCauley, & Mbwambo, 2004; Lasee & Becker, 1997; Karamagi, Tumwine, Tylleskar, & Heggenhougen, 2006), and avoid disclosing their own HIV serostatus in fear of accusations of infidelity, abandonment, discrimination, physical and emotional violence, and disruption of family relationships (Antelman, Smith Fawzi, Kaaya, et al. 2001; Gaillard et al., 2002; Medley, García-Moreno, McGill, & Maman, 2004).
Women reporting physical and emotional IPV also reported impaired emotional and social functioning, including depression, helplessness, resignation, and isolation from friends, family, and religious groups (Dietz, Gazmararian, Goodwin, et al., 1997; Wittenberg et al., 2007). Further, IPV has been shown to affect a woman's participation in household decision making, including decisions about her own health; for example, whether to seek skilled healthcare (Antelman, Smith Fawzi, Kaaya, et al. 2001; Dietz, Gazmararian, Goodwin et al., 1997; Dunkle, 2004; Fonck et al., 2005; Gaillard et al., 2002; Goo & Harlow, 2012; Izugbara & Ngilangwa, 2010; Malhotra, Schuler, & Boender, 2002; Maman et al., 2002; Medley et al., 2004; WHO, 2005).
IPV is also associated with increased HIV risk in women because men who abuse their wives exhibit other risky behaviors, including drug abuse and alcohol misuse (Gielen, McDonnell, O'Campo, 2002; Karamagi et al., 2006; Zablotska, 2009), multiple sexual partners (Martin, Kilgallen, Tsui, et al., 1999; Onsomu, Kimani, Abuya, et al., 2013), and lack of condom use (Gielen et al., 2002; Karamagi et al., 2006). Patriarchal cultural pressures that encourage men toward early sexual initiation and multiple sexual partners prior to marriage are also associated with increased incidence of infection (Abuya et al., 2012; Dunkle, 2006; Lary et al., 2004; Silverman et al., 2008). These factors are exacerbated by dominant and controlling men who manipulate their partners (Wang & Rowley, 2007; Wingood & DiClemente, 1998) and increase women's risk of contracting HIV (Decker et al., 2008; Dude, 2011; Silverman et al., 2008).
One of the nine priority areas in the UNAIDS Outcome Framework for 2009-2011 (2009) is to end violence against girls and women, especially because it increases their susceptibility to HIV infection (Andersson et al., 2008; Campbell et al., 2008; García-Moreno & Watts, 2000; Martin & Curtis, 2004; WHO, 2004). Although prevalence varies, many countries acknowledge the association between violence and HIV susceptibility among women. For instance, in eastern and southern Africa, IPV is associated with high risk of HIV infection (Abuya et al., 2012; Dunkle et al., 2004; Fonck et al., 2005; Jewkes, Levin, & Penn-Kekana, 2003; Jewkes, Dunkle, et al., 2010; Karamagi et al., 2006; Kiarie et al., 2006; Lary et al. 2004; Maman et al., 2002; van der Straten et al., 1998).
Additionally, qualitative studies have highlighted the links between HIV/AIDS, gender inequities, and DV as an outcome of the patriarchal nature of African societies and notions of masculinity that emphasize male strength and toughness and perpetuate control of women (Coovadia et al., 2009; Go et al., 2003; Jewkes, Dunkle, et al., 2010). Such norms have led some women to accept and tolerate male dominance to the extent of rationalizing IPV (Izugbara & Ngilangwe, 2010; Lawoko, 2008). For example, researchers found that traditional practices in some rural Kenyan communities could predispose women to higher risk of physical violence (Abuya et al., 2012). The prevalence of violence impedes women's ability to negotiate for safe sex, which often results in low condom use (Abuya et al., 2012; Andersson et al., 2008; Campbell et al., 2008; García-Moreno & Watts, 2000; Go et al., 2003; Karamagi et al., 2006; WHO, 2004).
Although research has shown that women are at greater risk of HIV infection, particularly in areas where HIV infection is high, prevention messages largely continue to focus on HIV testing, male condom use (Go et al., 2003), treatment of sexually transmitted diseases (STDs), and, most recently, male circumcision and antiretroviral treatment. Notably, interventions have not focused on gender-specific problems nor benefited vulnerable women (Christofides et al., 2010; Wawer et al., 2009).
From the foregoing arguments, research continues to show that gender-based violence, usually an outcome of male dominance, results in high-risk sexual behavior (Dunkle et al., 2004; Gilbert et al., 2000; Jewkes et al., 2003; Jewkes, Dunkle, Nduna, et al., 2006; Wingood & DiClemente, 1998; Zablotska et al., 2009). Women who experience violence in highly unequal relationships have greater chances of contracting HIV (Decker et al., 2008; Dude, 2011; Jewkes & Morrell, 2010; Karamagi et al., 2006; Silverman et al., 2008). Nonetheless, scholars examining HIV and IPV among women in ten developing countries, including Kenya, found no association (Harling et al., 2010). In the current study, we provide further evidence about the association between DV and HIV serostatus among married and formerly married women in Kenya and improve on previous estimates by controlling for possible confounders.
Methods
Data source
Our cross-sectional study used a population-based national sample, the 2008/2009 Kenya Demographic and Health Survey (KDHS-2008/09), with data collected between November 2008 and February 2009. This survey was the second to collect information on HIV serostatus, following the KDHS-2003 (Central Bureau of Statistics; CBS, 2004). Data were limited to a subsample of women aged 15-49 from a merged dataset that considered those who were married (n=5,041) or formerly married (n=863); of these women, 4,906 (83.1%) responded to questions about DV. Among these married and formerly married women, 2,669 of them agreed to be tested for HIV; among them 442 did not respond to DV questions and were excluded from the final analyses. The total sample of 2,227 (83.4%) were tested for HIV and responded to DV questions, which allowed us to estimate the association between DV and HIV serostatus. Study data were weighted to account for a clustering effect to eliminate over- and underestimation in the standard errors (StataCorp, 2013).
Survey measures
HIV serostatus
National Public Health Laboratory Services personnel were involved in the collection of dried blood spot (DBS) samples, voluntary counseling and testing, and laboratory testing for HIV. All positive samples and a random selection of negative samples (10%) were subjected to further testing at the HIV laboratory of the Kenya Medical Research Institute (KEMRI) using the same procedure. Further analysis by polymerase chain reaction (PCR) of the deoxyribonucleic acid (DNA) in the same laboratory on 30 discrepant samples were conducted. See KNBS & ICF Macro (2010, pp. 9-10) for a complete description of the HIV procedures and testing. All DBS testing was done in early June 2009.
Domestic violence
Evaluation of DV among married and formerly married women was based on a modified Conflict Tactics Scale (CTS) used in the KDHS-2008/09, which has proven effective in measuring DV across cultures (Strauss, 1990, cited in KNBS & ICF Macro, 2010). Questions were asked to evaluate abuse and coded as no, “0”, or yes, “1”. Common factor analysis was used to group and identify patterns from the various questions while maintaining the needed information with minimal loss.
The factors that mostly explained/measured certain themes based on rotated factor loadings were retained, and named based on the overall theme represented by their constituent items; these themes were named and used for analyses as the study exposures. Dichotomous variables generated from the retained factors explained most of the total variance (40-62%) for each of the four themes identified: (a) physical violence [Push you, shake you, or throw something at you?; Slap you?; Twist your arm or pull your hair?; Punch you with his fist or with something that could hurt you?; and Kick you or drag you or beat you up?]:(b) emotional violence [Say or do something to humiliate you in front of others?; Threaten to hurt or harm you or someone close to you?; and Insult you or make you feel bad about yourself?], (c) sexual violence [Physically forced you to have sexual intercourse even when you did not want to?; and Force you to perform any sexual acts you did not want to?]: and (d) violence with aggravated bodily harm (AGBH) [Try to choke you or burn you on purpose?; and Threaten to attack you with a knife, gun, or any other weapon?]. A fifth theme was generated from all of the four variables and named “all forms of violence.” All themes were coded as “0” if respondents indicated that they did not experience violence, and “1” if they did. Weights and correlations between each variable (factor loading) were determined at <0.3 (UCLA IDRE, n.d.).
Socio-demographic factors
The survey captured several partner, personal, social, and demographic characteristics, and since they could have an effect on or explain the association between DV and HIV serostatus, we controlled for them in the final logistic multivariable models. They include: (a) age, measured in five-year increments, and ranging from 15-49 years, considered reproductive age; (b) risky sexual behavior, a variable generated from three questions: were you given or did you receive money/gifts for sex in the past 12 months?; how many individuals have you had sex with other than your husband in the last 12 months?; and was a condom used in the last intercourse?; (c) number of lifetime sexual partners; (d) whether husband consumes alcohol; (e) presence of a sexually transmitted disease, a variable constructed from two questions: have you had a genital sore/ulcer? and genital discharge in the last 12 months?; (f) number of co-wives, coded as no other co-wife or two or more co-wives; (g) education; (h) religion; (i) wealth index, a variable generated from the household's ownership of consumer goods, dwelling characteristics, drinking water source, and toilet facilities, among other socioeconomic characteristics (Gwatkin et al., 2000, cited in KNBS & ICF Macro, 2010); (j) residence; (k) age at first marriage; (l) occupation; (m) health insurance; and (n) perceived risk of acquiring HIV.
Data analysis
The survey responses and HIV test result datasets were merged. All descriptive, bivariate, univariate, and multivariable data analyses were conducted using Stata/SE 13.1 with a “svyset” command, taking into consideration the weights, strata, cluster, and single unit to attain linearized standard errors. Hence, we accounted for nonindependence within the primary sampling unit and survey nonresponse. Bivariate analyses were used to estimate the prevalence of HIV serostatus. Univariate logistic regression analyses were used to estimate the association between the main outcome measure (HIV serostatus) and independent variables. Multivariate logistic regression analyses were conducted by including variables identified through the forward stepwise regression method and manual inclusion. Univariate (unadjusted) and multivariable (adjusted) analyses are reported using odds ratios (ORs) and 95% confidence intervals (CIs), with study significance set at a two tailed p-value of < 0.05.
Institutional Review Board approval
The current study involved secondary data analysis of the KDHS-2008/2009. Administration of the survey involved multiple organizations, including the Kenya National Bureau of Statistics (KNBS), the MEASURE Demographic and Health Survey (DHS) program at ICF Macro, the United States Agency for International Development (USAID), among others. For the HIV test, the blood specimen collection and analysis protocol was developed by the DHS program, with revisions completed by KEMRI and the Kenya National AIDS Control Council (NACC). It was reviewed and approved by KEMRI Scientific and Ethical Review Committee (KNBS & ICF Macro, 2010). Further human subject review and study oversight were granted by the Winston-Salem State University Institutional Review Board (IRB) under exempt status.
Results
Descriptive statistics
The study included both married and formerly married women aged 15-49 (n=2,227). Overall, HIV prevalence among those who responded to DV questions was 10.67%, which differed between those who were currently married (7.03%), formerly married (34.19%), and both (married and formerly married) women (10.67%). Prevalence of any DV was 44%, 42%, and 43% among these groups of women respectively. Figure 1 reports the various forms of DV among married and formerly married women; ranging from a low of 4% for married women experiencing violence with AGBH to a high of 32% for formerly married women experiencing physical violence. Overall, physical, emotional, sexual, and violence with AGBH were slightly higher among formerly married women with the exception of all forms of violence (see Table 1 and Figure 1).
Figure 1. Percentage of domestic violence among married and formerly married women agreeing to be tested for HIV in Kenya, KDHS 2008/2009.
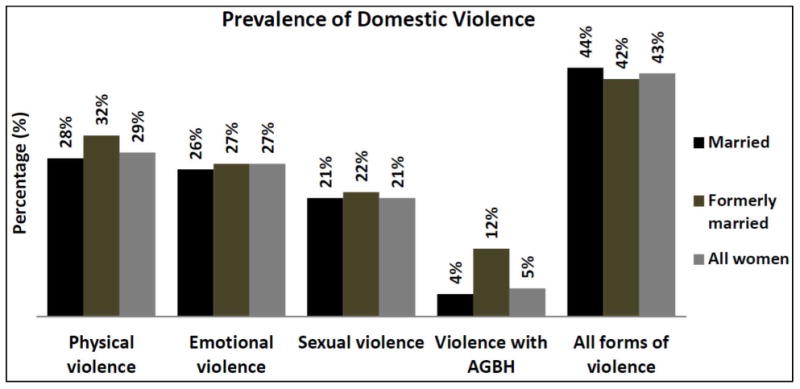
AGBH: aggravated bodily harm
Table 1. Study population characteristics, KDHS 2008/2009.
| Married | Formerly married | Married and formerly married | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Study Characteristics | n | % | n | % | n | % |
| HIV serostatus (n=2,227) | ||||||
| Negative | 1789 | 93 | 203 | 66 | 1992 | 89 |
| Positive§ | 146 | 7 | 89 | 34 | 235 | 11 |
| Physical violence (n=2,224) | ||||||
| No | 1370 | 72 | 186 | 68 | 1556 | 71 |
| Yes | 563 | 28 | 105 | 32 | 668 | 29 |
| Emotional violence (n=2,226) | ||||||
| No | 1442 | 74 | 201 | 73 | 1643 | 73 |
| Yes | 493 | 26 | 90 | 27 | 583 | 27 |
| Sexual violence (n=2,225) | ||||||
| No | 1567 | 79 | 219 | 78 | 1786 | 79 |
| Yes | 366 | 21 | 73 | 22 | 439 | 21 |
| Violence with AGBHβ (n=2,224) | ||||||
| No | 1861 | 96 | 253 | 88 | 2114 | 95 |
| Yes | 72 | 4 | 38 | 12 | 110 | 5 |
| All forms of violence (n=2,227) | ||||||
| No | 1106 | 56 | 153 | 58 | 1259 | 57 |
| Yes | 829 | 44 | 139 | 42 | 968 | 43 |
| Risky sexual behavior (n=2,224) | ||||||
| No | 1825 | 95 | 152 | 51 | 1977 | 89 |
| Yes | 107 | 5 | 140 | 49 | 247 | 11 |
| Number of lifetime sexual partners (n=2,221) | ||||||
| One | 901 | 43 | 61 | 22 | 962 | 40 |
| Two | 566 | 32 | 84 | 28 | 650 | 31 |
| Three | 279 | 16 | 75 | 27 | 354 | 17 |
| Four or more | 183 | 9 | 72 | 23 | 255 | 11 |
| Husband consumes alcohol (n=2,224) | ||||||
| No | 1290 | 65 | 134 | 42 | 1424 | 62 |
| Yes | 643 | 35 | 157 | 58 | 800 | 38 |
| Sexually transmitted diseases (n=2,226) | ||||||
| No STDs | 1799 | 94 | 267 | 92 | 2066 | 94 |
| STDs Present | 135 | 6 | 25 | 8 | 160 | 6 |
| Number of co-wives (n=1,934) | ||||||
| No other wife | 1645 | 88 | - | - | 1645 | 88 |
| Two or more | 289 | 12 | - | - | 289 | 12 |
| Education (n=2,227) | ||||||
| Less than primary/none | 348 | 11 | 48 | 11 | 396 | 11 |
| Primary | 1059 | 59 | 171 | 63 | 1230 | 60 |
| Secondary | 393 | 24 | 58 | 22 | 451 | 23 |
| Higher/college/graduate | 135 | 6 | 15 | 4 | 150 | 6 |
| Age (n=2,227) | ||||||
| 15-19 | 93 | 3 | 3 | 1 | 96 | 3 |
| 20-24 | 427 | 21 | 41 | 14 | 468 | 20 |
| 25-29 | 435 | 26 | 42 | 16 | 477 | 24 |
| 30-34 | 361 | 19 | 60 | 19 | 421 | 19 |
| 35-39 | 266 | 13 | 48 | 12 | 314 | 13 |
| 40-44 | 184 | 10 | 44 | 20 | 228 | 11 |
| 45-49 | 169 | 9 | 54 | 19 | 223 | 10 |
| Religion (n=2,226) | ||||||
| Protestant | 1140 | 66 | 171 | 67 | 1311 | 67 |
| Roman catholic | 380 | 22 | 76 | 24 | 456 | 22 |
| Muslim | 343 | 8 | 37 | 7 | 380 | 8 |
| Other religions | 71 | 3 | 8 | 2 | 79 | 3 |
| Wealth index (n=2,227) | ||||||
| Poorest | 448 | 19 | 64 | 20 | 512 | 19 |
| Poorer | 311 | 17 | 59 | 19 | 370 | 17 |
| Middle | 317 | 17 | 49 | 18 | 366 | 18 |
| Richer | 362 | 20 | 45 | 18 | 407 | 20 |
| Richest | 497 | 26 | 75 | 25 | 572 | 26 |
| Residence (n=2,227) | ||||||
| Urban | 519 | 23 | 80 | 25 | 599 | 24 |
| Rural | 1416 | 77 | 212 | 75 | 1628 | 76 |
| Age at first marriage (n=2,227) | ||||||
| ≥ 25 | 196 | 9 | 31 | 15 | 227 | 10 |
| 20-24 | 562 | 31 | 82 | 27 | 644 | 30 |
| 15-19 | 973 | 50 | 146 | 47 | 1119 | 50 |
| ≤14 | 204 | 10 | 33 | 11 | 237 | 10 |
| Occupation (n=2,222) | ||||||
| Not working | 754 | 34 | 57 | 18 | 811 | 32 |
| Teaching and professional | 381 | 19 | 63 | 22 | 444 | 20 |
| Agriculture-self employed | 478 | 29 | 77 | 29 | 555 | 29 |
| Sales | 124 | 7 | 24 | 9 | 148 | 7 |
| Other occupations | 194 | 10 | 70 | 22 | 264 | 12 |
| Health insurance (n=2,226) | ||||||
| No | 1800 | 92 | 284 | 97 | 2084 | 92 |
| Yes | 134 | 8 | 8 | 3 | 142 | 8 |
| Perceived risk of acquiring HIV (n=2,214) | ||||||
| No risk at all | 159 | 8 | 26 | 7 | 185 | 8 |
| Small risk | 705 | 33 | 106 | 40 | 811 | 34 |
| Moderate risk | 725 | 41 | 93 | 31 | 818 | 40 |
| Great risk | 336 | 18 | 64 | 21 | 400 | 18 |
Exact HIV prevalence: married women (7.03%), formerly married women (34.19%), and married and formerly married women (10.67%)
AGBH: aggravated bodily harm
Bivariate analysis
Of married women who experienced physical violence, 11.9% tested positive for HIV compared to 5.8% (F1, 378 = 14.24, p < 0.001) among those who did not experience physical violence. Also, 11.2% of married women who experienced sexual violence tested positive for HIV compared to 6.7% (F1, 378 = 3.83, p < 0.05) among those who did not experience sexual violence. Overall, 10.6% of married women experiencing all forms of violence tested positive for HIV compared to 5.2% (F1, 378 = 6.22, p < 0.05) among those who did not. For formerly married women, 20.5% who reported previous sexual violence tested positive for HIV compared to 33.8% (F1, 190 = 5.01, p < 0.05) of those who did not experience sexual violence. For married and formerly married women who experienced physical violence, 14.8% tested positive for HIV compared to 8.7% (F1, 379 = 5.76, p < 0.05) among those who did not (see Table 2).
Table 2. Bivariate analysis, number and percentage of the association between HIV serostatus and domestic violence, KDHS 2008/2009.
| Married women | ||||
|---|---|---|---|---|
|
|
||||
| Negative (n) | Positive (n) | HIV Prevalence | p-value | |
| Physical violence | n=1,933 | |||
| No | 1291 | 79 | 5.8 | X2 (n=1933, df=378) = 14.25*** |
| Yes | 496 | 67 | 11.9 | |
| Emotional violence | n=1,935 | |||
| No | 1347 | 95 | 6.6 | X2 (n=1935, df=378) = 0.88NS |
| Yes | 442 | 51 | 10.3 | |
| Sexual violence | n=1,933 | |||
| No | 1462 | 105 | 6.7 | X2 (n=1933, df=378) = 3.83* |
| Yes | 325 | 41 | 11.2 | |
| Violence with AGBHβ | n=1,933 | |||
| No | 1724 | 137 | 7.4 | X2 (n=1933, df=378) = 1.53NS |
| Yes | 63 | 9 | 12.5 | |
| All forms of violence | n=1,935 | |||
| No | 1048 | 58 | 5.2 | X2 (n=1935, df=378) = 6.22* |
| Yes | 741 | 88 | 10.6 | |
| Formerly married women | ||||
|---|---|---|---|---|
| Physical violence | n=291 | |||
| No | 130 | 56 | 30.1 | X2 (n=291, df=189) = 0.4NS |
| Yes | 73 | 32 | 30.5 | |
| Emotional violence | n=291 | |||
| No | 138 | 63 | 31.3 | X2 (n=291, df=189) = 0.87NS |
| Yes | 65 | 25 | 27.7 | |
| Sexual violence | n=292 | |||
| No | 145 | 74 | 33.8 | X2 (n=292, df=190) = 5.01* |
| Yes | 58 | 15 | 20.5 | |
| Violence with AGBHβ | n=291 | |||
| No | 171 | 82 | 33.4 | X2 (n=291, df=189) = 1.92NS |
| Yes | 32 | 6 | 15.4 | |
| All forms of violence | n=292 | |||
| No | 103 | 50 | 32.7 | X2 (n=292, df=190) = 1.97NS |
| Yes | 100 | 39 | 28.1 | |
| Married and formerly married women | ||||
|---|---|---|---|---|
| Physical violence | n=2,224 | |||
| No | 1421 | 135 | 8.7 | X2 (n=2224, df=379) = 5.76* |
| Yes | 569 | 99 | 14.8 | |
| Emotional violence | n=2,226 | |||
| No | 1485 | 158 | 9.6 | X2 (n=2226, df=379) = 0.02NS |
| Yes | 507 | 76 | 13 | |
| Sexual violence | n=2,225 | |||
| No | 1607 | 179 | 10 | X2 (n=2225, df=379) = 0.19NS |
| Yes | 383 | 56 | 12.8 | |
| Violence with AGBHβ | n=2,224 | |||
| No | 1895 | 219 | 10.4 | X2 (n=2224, df=379) = 1.04NS |
| Yes | 95 | 15 | 13.6 | |
| All forms of violence | n=2,227 | |||
| No | 1151 | 108 | 8.6 | X2 (n=2227, df=379) = 0.68NS |
| Yes | 841 | 127 | 13.1 | |
p < 0.05;
p < 0.01;
p < 0.001;
not significant
AGBH: aggravated bodily harm
Certain characteristics entered into the final model through stepwise forward multivariate analysis and manual selection method were associated with HIV serostatus. The following were not associated; age at first marriage, husband's alcohol consumption, and education - selected by stepwise forward multivariate method, and health insurance status, wealth index, and age, which were selected manually and added back in the final model. These variables have been identified as relevant in previous studies of DV and HIV serostatus (Decker, Miller, Kapur, et al., 2008; Decker, Seage, Hemenway, et al., 2009; Dude, 2011; Gielen et al., 2002; Jewkes, Dunkle, Nduna, et al., 2006; Jewkes, Dunkle, Nduna, et al., 2010; Maman et al., 2002; Shi et al., 2013; Silverman et al., 2008).
Association between HIV serostatus and domestic violence
Unadjusted results
Table 3 presents unadjusted (crude) odds ratios (ORs) in a univariate logistic regression model of the association between DV and HIV serostatus. Among married women, the OR for HIV infection was higher among those who experienced physical violence compared to those who did not 2.42 (p < 0.001). The ORs were also higher among married women who experienced sexual violence and all forms of violence compared to those who did not 1.66 (p < 0.05) and 1.83 (p < 0.01) respectively. Among formerly married women, the OR for HIV infection among those who experienced sexual violence compared to those who did not was 0.42 (p < 0.05).
Table 3. Unadjusted odds ratios and 95% confidence intervals of HIV serostatus and domestic violence among married and formerly married women in Kenya, in a univariate logistic regression model, KDHS-2008/2009.
| Married women | Formerly married women | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| HIV Serostatus | ORs | 95% CI | ORs | 95% CI | ||
| Physical abuse | n=1,933 | n=291 | ||||
| No | 1 Ref | 1 Ref | ||||
| Yes | 2.42*** | 1.51 | 3.87 | 0.78 NS | 0.36 | 1.69 |
| Emotional abuse | n=1,935 | n=291 | ||||
| No | 1 Ref | 1 Ref | ||||
| Yes | 1.24 NS | 0.79 | 1.95 | 0.69 NS | 0.31 | 1.52 |
| Sexual abuse | n=1,933 | n=292 | ||||
| No | 1 Ref | 1 Ref | ||||
| Yes | 1.66* | 0.99 | 2.77 | 0.42* | 0.19 | 0.91 |
| Violence with AGBHβ | n=1,933 | n=291 | ||||
| No | 1 Ref | 1 Ref | ||||
| Yes | 1.68 NS | 0.73 | 3.88 | 0.46 NS | 0.15 | 1.42 |
| All forms of violence | n=1,935 | n=292 | ||||
| No | 1 Ref | 1 Ref | ||||
| Yes | 1.83** | 1.13 | 2.96 | 0.6 NS | 0.29 | 1.24 |
p < 0.05;
p < 0.01;
p < 0.001;
not significant
1 Ref: Reference Category | ORs: Odds Ratios | CIs: Confidence Intervals
AGBH: aggravated bodily harm
Among married women, the OR for HIV infection among those with risky sexual behavior compared to those without was 0.25 (p < 0.001). Married women reporting two, three, and four or more lifetime sexual partners compared to those who reported only one lifetime sexual partner had increased ORs for HIV infection: 1.77 (p < 0.05), 1.73 (p < 0.001), and 1.48 (p < 0.001), respectively. Similarly, among married women, the OR for HIV infection among those whose husbands had two or more other wives compared to those who were the only wife was 2.8 (p < 0.001).
Among married women aged 20-24, 35-39, and 45-49 compared to those aged 15-19, the ORs for HIV infection were 0.28 (p < 0.01), 0.76 (p < 0.05), and 0.79 (p < 0.01), respectively. Among formerly married women aged between age groups 20-24 and 45-49 compared to those aged 15-19, the ORs for HIV infection were higher with more than 8.12 (p < 0.001). Among married Muslims, the OR for HIV infection compared to those affiliated with Protestant religions was 0.65 (p < 0.05). The OR for HIV infection was 0.39 (p < 0.01) among formerly married Muslims. Among married women, the OR for HIV infection among those who perceived themselves to have a small risk of acquiring HIV compared to those who perceived themselves to have no risk at all was 0.42 (p < 0.05).
Adjusted results
After controlling for socio-demographic, partner, and personal characteristics, statistically significant associations were observed between HIV serostatus and DV (see Table 4). Among married women, the OR for HIV infection was higher among those who experienced physical violence compared to those who did not 2.01 (p < 0.05). In modeling the association between physical violence and HIV serostatus among married women, other covariates that had statistically associations with HIV serostatus were risky sexual behaviors, number lifetime sexual partners (three and four or more), number of co-wives, age, religion (Muslim), and age at first marriage (≤14 years).
Table 4. Adjusted odds ratios and 95% confidence intervals of HIV serostatus and domestic violence among married women in Kenya, in a multivariable logistic regression model, KDHS-2008/2009.
| Physical violence§ | Emotional violence§ | Sexual violence§ | Violence with AGBHβ,§ | All forms of violence§ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| HIV Serostatus | ORs | 95% CI | ORs | 95% CI | ORs | 95% CI | ORs | 95% CI | ORs | 95% CI | |||||
| Domestic violence | |||||||||||||||
| No | 1 Ref | 1 Ref | 1 Ref | 1 Ref | 1 Ref | ||||||||||
| Yes | 2.01* | 1.12 | 3.6 | 0.93NS | 0.55 | 1.58 | 1.14NS | 0.63 | 2.07 | 1.15NS | 0.41 | 3.23 | 1.37NS | 0.78 | 2.4 |
| Risky sexual behavior | |||||||||||||||
| No | 1 Ref | 1 Ref | 1 Ref | 1 Ref | 1 Ref | ||||||||||
| Yes | 0.2*** | 0.1 | 0.41 | 0.21*** | 0.1 | 0.42 | 0.21*** | 0.1 | 0.42 | 0.21*** | 0.1 | 0.42 | 0.21*** | 0.1 | 0.43 |
| Number of lifetime sexual partners | |||||||||||||||
| One | 1 Ref | 1 Ref | 1 Ref | 1 Ref | 1 Ref | ||||||||||
| Two | 1.86NS | 0.99 | 3.51 | 1.94* | 1.04 | 3.61 | 1.9* | 1 | 3.61 | 1.93* | 1.04 | 3.59 | 1.87NS | 0.99 | 3.52 |
| Three | 1.56* | 1.06 | 2.28 | 1.64** | 1.14 | 2.35 | 1.62** | 1.13 | 2.34 | 1.63** | 1.14 | 2.35 | 1.59* | 1.09 | 2.31 |
| Four or more | 1.49** | 1.13 | 1.98 | 1.52** | 1.16 | 1.99 | 1.5** | 1.14 | 1.98 | 1.52** | 1.16 | 1.98 | 1.49** | 1.13 | 1.96 |
| Number of co-wives | |||||||||||||||
| No other wife | 1 Ref | 1 Ref | 1 Ref | 1 Ref | 1 Ref | ||||||||||
| Two or more | 2.6*** | 1.46 | 4.61 | 2.68*** | 1.53 | 4.69 | 2.68*** | 1.53 | 4.69 | 2.67*** | 1.52 | 4.69 | 2.67*** | 1.52 | 4.68 |
| Age | |||||||||||||||
| 15-19 | 1 Ref | 1 Ref | 1 Ref | 1 Ref | 1 Ref | ||||||||||
| 20-24 | 0.18** | 0.06 | 0.53 | 0.18** | 0.06 | 0.54 | 0.18** | 0.06 | 0.54 | 0.18** | 0.06 | 0.53 | 0.18** | 0.06 | 0.52 |
| 25-29 | 0.57* | 0.35 | 0.93 | 0.59* | 0.37 | 0.93 | 0.58* | 0.37 | 0.93 | 0.58* | 0.37 | 0.93 | 0.58* | 0.36 | 0.93 |
| 30-34 | 0.58** | 0.4 | 0.83 | 0.59** | 0.41 | 0.84 | 0.59** | 0.41 | 0.84 | 0.59** | 0.41 | 0.84 | 0.58** | 0.41 | 0.83 |
| 35-39 | 0.62*** | 0.47 | 0.83 | 0.63*** | 0.48 | 0.83 | 0.63*** | 0.48 | 0.83 | 0.63*** | 0.48 | 0.83 | 0.63*** | 0.48 | 0.83 |
| 40-44 | 0.7** | 0.54 | 0.89 | 0.7** | 0.55 | 0.89 | 0.7** | 0.55 | 0.88 | 0.7** | 0.55 | 0.88 | 0.69** | 0.54 | 0.88 |
| 45-49 | 0.68*** | 0.55 | 0.86 | 0.69*** | 0.55 | 0.86 | 0.69*** | 0.55 | 0.86 | 0.69*** | 0.55 | 0.86 | 0.68*** | 0.55 | 0.85 |
| Religion | |||||||||||||||
| Protestant | 1 Ref | 1 Ref | 1 Ref | 1 Ref | 1 Ref | ||||||||||
| Roman catholic | 0.73NS | 0.39 | 1.37 | 0.73NS | 0.39 | 1.36 | 0.73NS | 0.39 | 1.36 | 0.73NS | 0.39 | 1.36 | 0.72NS | 0.39 | 1.35 |
| Muslim | 0.56* | 0.34 | 0.91 | 0.52* | 0.32 | 0.86 | 0.53* | 0.32 | 0.87 | 0.52* | 0.32 | 0.86 | 0.53* | 0.32 | 0.87 |
| Other religions | 0.7 NS | 0.37 | 1.34 | 0.7NS | 0.38 | 1.28 | 0.69NS | 0.37 | 1.28 | 0.69NS | 0.37 | 1.27 | 0.69NS | 0.37 | 1.29 |
| Age at first marriage | |||||||||||||||
| ≥ 25 | 1 Ref | 1 Ref | 1 Ref | 1 Ref | 1 Ref | ||||||||||
| 20-24 | 1.6NS | 0.6 | 4.28 | 1.71NS | 0.62 | 4.68 | 1.67NS | 0.61 | 4.56 | 1.69NS | 0.62 | 4.67 | 1.61NS | 0.59 | 4.4 |
| 15-19 | 1.09NS | 0.63 | 1.87 | 1.18NS | 0.68 | 2.06 | 1.16NS | 0.67 | 2.04 | 1.17NS | 0.67 | 2.05 | 1.13NS | 0.65 | 1.96 |
| ≤14 | 1.72** | 1.13 | 2.61 | 1.81** | 1.17 | 2.82 | 1.79** | 1.16 | 2.77 | 1.81** | 1.16 | 2.81 | 1.76** | 1.15 | 2.69 |
n=1,927;
p < 0.05;
p < 0.01;
p < 0.001;
not significant
1 Ref: Reference Category | ORs: Odds Ratios | CIs: Confidence Intervals
Other variables controlled for but were non-significant: husband alcohol consumption, sexually transmitted diseases, education, wealth index, residence, occupation, health insurance, and perceived risk of acquiring HIV
AGBH: aggravated bodily harm
Note: Formerly married women did not respond to the question, does your husband/partner have other wives or does he live with other women as if married?
Of the married women in the study experiencing all forms of violence (44%), the OR for HIV infection among those with risky sexual behaviors compared to those without was 0.21 (p < 0.001). Among married women who experienced physical violence, the OR for HIV infection was higher among those who had two or more co-wives compared to those without co-wives was 2.6 (p < 0.001). Among married women experiencing various types of violence, those who indicated that they had previously had two, three, or four or more lifetime sexual partners had higher ORs for HIV infection ranging from 1.49 (p < 0.01) to 1.93 (p < 0.05) compared to those who reported only one lifetime sexual partner.
Among married women who experienced physical violence, the ORs for HIV infection among those aged 20-24 (21%) and 45-49 (10%) compared to those aged 15-19 was 0.18 (p < 0.01) and 0.68 (p < 0.001) respectively. Furthermore, among women experiencing physical violence, higher OR for HIV infection among those who were aged ≤14 (10%) at first marriage compared to those who were aged ≥25 was 1.72 (p < 0.01). Among women experiencing all forms of violence, the OR for HIV infection was 1.76 (p < 0.001). Among married Muslims (8%), the ORs for HIV infection among those experiencing various types of abuse compared to those affiliated to Protestants religions ranged between 0.52 to 0.56 (p < 0.05).
Discussion
Researchers sought to evaluate the association between domestic violence and HIV serostatus among married and formerly married women in Kenya. This objective was motivated by contradictory findings in the literature on the association between IPV and HIV/AIDS in different contexts, including Kenya (Harling et al., 2010); and methodological issues (Shi et al., 2013), that demanded re-examination. Establishing the pathways by which violence may both be a marker for and directly facilitate HIV infection among women should inform prevention and treatment strategies (Abuya et al., 2012; Decker, Miller, Kapur, et al., 2008; Dunkle, 2004; Fonck et al., 2005; Goo & Harlow, 2012; Jewkes et al., 2003; Maman et al., 2002; Martin & Curtis, 2004; Silverman et al., 2008; van der Straten, 1998). Compared to other studies that examined the association between DV and HIV infection (Decker, Miller, Kapur, et al., 2008; Decker, Seage, Hemenway, et al., 2009; Dude, 2011; Gielen et al., 2002; Jewkes, Dunkle, et al., 2006; Jewkes, Dunkle, et al., 2010; Maman et al., 2002; Shi et al., 2013; Silverman et al., 2008; van der Straten et al., 1998), our study controlled for most of the identified confounders.
Overall, we found HIV prevalence among those who responded to DV questions to be 10.67%, this was higher than the 8% reported for women aged 15-49 by the KDHS 2008/2009 (KNBS & ICF Macro, 2010). The prevalence of DV was 44%, 42%, and 43% among married and formerly married women and women who have been both, respectively, which confirms the findings of Abuya et al. (2012); Fonck et al. (2005); Goo and Harlow (2012); Kishor et al. (2004); Wanyoni and Lumumba (2010), who established the continued prevalence of IPV in Kenya. The finding that 42% of formerly married women have experience DV is consistent with a study by Abuya et al. (2012) that established the prevalence of physical violence at 42% based on the 2003 KDHS. Given that our study used the 2008/2009 KDHS, we see no significant change in the prevalence of physical violence against formerly married women in Kenya. It falls within the middle range of the 14-61% reported in a 2005 WHO multi-country study on women's health and DV.
We found that married women who experienced physical violence had 2.01 (p < 0.05) times the odds of testing positive for HIV compared to those who did not experience physical violence. This finding corroborates the work of scholars in the last decade who have reported associations between partner violence and high risk of HIV infection in eastern and southern Africa (Abuya et al., 2012; Dunkle et al., 2004; Fonck et al., 2005; Jewkes et al., 2003; Jewkes, Dunkle, et al., 2010; Karamagi et al., 2006; Kiarie et al., 2006; Lary et al 2004; Maman et al., 2002; van der Straten et al., 1998). Therefore, the need to implement the UNAIDS Outcome Framework for 2009-2011 priority to end violence against girls and women is urgent (UNAIDS, 2009). Emotional violence, sexual violence, violence with AGBH, and all forms of violence were not observed to have any significant association with HIV serostatus. However, except for emotional violence, they had higher ORs of HIV infection compared to those who did not experience any DV. Another explanation for lack of significance for other forms of DV can be due to the small sample size as observed from the confidence intervals.
Aside from DV, other factors that increased the likelihood of testing positive for HIV among married women included number of lifetime sexual partners, number of co-wives, and age at first marriage. These factors, including risky sexual behaviors and age, have been associated with increased risk of infection, not only among women, but also among men, who then spread the disease to their female partners (Martin, Kilgallen, Tsui, et al., 1999). However, our study found that married women aged 20-49 who experienced physical violence had less risk (OR: 0.18 to 0.7) of testing positive for HIV compared to their counterparts aged 15-19. With regard to age at first marriage, the study found that women who were aged ≤14 when first married and experienced physical violence had 1.72 (p < 0.01) times the odds of testing positive for HIV compared to those aged ≥25. Overall, these findings implicate marriage as a risk factor for contracting HIV and age at first marriage with contracting the virus later in life.
The patriarchal nature of African society is known to support a notion of masculinity that perpetuates control of women by their male partners (Coovadia et al., 2009). In many African societies, including Kenya, women are expected to accept and to tolerate male dominance to the extent of rationalizing severe forms, such as domestic violence (Izugbara & Ngilangwa, 2010; Lawoko, 2008). Furthermore, age differences in sexual partnerships have long been associated with increased risk of intergenerational HIV transmission (Gregson, Nyamukapa, Garnett, et al., 2002), especially between older men and younger women (Longfield, Glick, Waithaka, et al., 2004), in part, because older men have had time to acquire sexually transmitted infections from other partners (Kelly, Gray, Sewankambo, et al., 2003). Younger women are also likely to be economically dependent on their often older partners and unlikely to leave a sexually risky or abusive relationship (Luke, 2003). Younger wives experiencing DV are unlikely to ask their husbands about their HIV status and other previous or current sexual partners, leaving them more vulnerable to infection (Sa & Larsen, 2007).
Our study may be limited by the survey on which it is based. Since our measurements of the variables are restricted to one time point, we could not assess the relationship between DV and HIV serostatus over time. Our findings are also limited to Kenya and cannot be generalized to other SSA countries. While the instrument was found sensitive to cultural differences and effective in measuring DV (Strauss, 1990, cited in KNBS & ICF Macro, 2010), self-response might have led to underreporting. Since our data was limited to women who responded to DV questions and agreed to be tested for HIV; a large percentage of women who respond to DV questions were not considered in the final analyses. This raises questions about differences between women who experienced DV and tested for HIV vs. those who experienced DV and were not tested for HIV.
Cultural expectations and norms in the context of most African countries, including Kenya, could have led some respondents to think that some forms of violence are acceptable and not “actual domestic violence.” Furthermore, the survey did not capture timeline for violence, which could have caused recall bias, leading to nondifferential misclassification of the exposure (DV) and possibly biasing the findings toward the null (Birkett, 1992; Dosemeci, Wacholder, & Lubin, 1990). However, these married and formerly married women would probably identify most of these forms as DV and respond appropriately, reducing the possibility of nondifferential misclassification. Potential recall bias by HIV serostatus could have been possible among women who were aware of being HIV positive at the time of the survey. These women, especially those who were formerly married may have been more likely to report a history of violence than women who had not tested HIV positive previously. This could explain the high HIV prevalence rates for various types of DV among formerly married women that ranged between 15.4 - 30.5%.
Overall, our findings have significant policy implications for women's health outcomes in Kenya. They call for increasing the level of awareness, protection, and subsequent empowerment of women. Articles 3, 7, and 10 (Domestic Violence and Sexual Violence) of the Sexual Offenses Act (No. 3 of 2006, rev.2007) address domestic violence (Kenya Law Reports and the Government of Kenya, 2009). The adoption of effective and concrete measures to combat domestic and sexual violence against women, sensitization of society as a whole on these issues, prosecution of perpetrators, and provision of assistance and protection to victims are some of the measures proposed.
The importance of women's protection and empowerment is premised on consistent findings from prior studies and the current study, which show that violence, particularly physical abuse, makes women susceptible to HIV infection and other sexually transmitted diseases (Abuya et al., 2012; Decker, Miller, Kapur, et al., 2008; Dunkle, 2004; Fonck et al., 2005; Jewkes et al., 2003; Maman et al., 2002; Martin & Curtis, 2004; Silverman et al., 2008; van der Straten, 1998). Our study argues strongly for the immediate implementation of the proposals in the Sexual Offences Act of 2006.
In Kenya, services for victims of gender-based violence have been provided mainly by non-state actors. The Kenyan government should be more involved and develop nationwide emergency shelters to provide accommodation, medical care, and counseling services for victims of gender-based violence (Federation of Women Lawyers; FIDA, 2011). They are essential because many women are economically and emotionally dependent on their abusers (FIDA, 2011; Luke, 2003). Our study supports this notion and reiterates the need to implement the UNAIDS (2009) critical priority to end violence against girls and women, especially to protect young women from early marriage, which we found increases their risk for testing positive for HIV later in life compared to those who married when they were aged ≥25. One plausible explanation is that such young women are more likely to be economically dependent on their older partners, which prevents them from leaving what are often sexually risky and abusive relationships.
Additionally, both men and women in marital relationships should be sensitized about ways to protect themselves against HIV infection. In particular, younger women who experience domestic violence and are afraid to ask their husbands about their previous or current sexual partners and HIV status are more vulnerable to infection (Sa & Larsen, 2007). Also, economic empowerment and micro-financing programs for women in countries like Kenya will go a long way toward making women less dependent on men and more able to make informed choices when faced with circumstances that threaten their sexual and personal health.
This study adds to the body of literature associating IPV and HIV serostatus by critically examining numerous risk factors. Women responding to questions about IPV were categorized as married and formerly married to provide finer distinction on life status and a more nuanced analysis; however, for formerly married women, the sample (n=292) was not sufficient to allow multiple logistic regression analysis. A major innovation of our study was the examination of different components of DV (physical, emotional, sexual, violence with AGBH, and all forms of violence) which could contribute to the literature on intimate partner violence. Our study did associate physical violence and HIV serostatus among married women. Because of the high prevalence of HIV (all forms of violence) among formerly married women (28.1%); which could be explained by the fact that their husbands might have died from HIV, prospective studies should be conducted to identify the silent factors of domestic violence that were not captured by the national survey among this vulnerable group. Such study findings can be used to develop interventions targeting formerly married women. These high HIV prevalence for various DVs; physical (30.5%), emotional (27.7%), sexual (20.5%), violence with AGBH (15.4%), and all forms of violence (28.1%) have never been reported before.
By virtue of their life status, formerly married women who are HIV positive may experience triple jeopardy: the disease, alienation, and stigma. Emotionally, these women need support to achieve better health outcomes. Studies show that women who report IPV also report impaired emotional and social functioning, including depression, helplessness, resignation, and isolation from friends, family, and religious groups (Dietz, 1997; Wittenberg, 2007). Broad community-based initiatives to deal with the underlying gender norms and social attitudes about HIV/AIDS and DV against women must accompany individually focused initiatives to create a safer and more comfortable environment for women. Given the prevalence of HIV among married and formerly married women experiencing IPV, initiatives that support them toward healthy lifestyles should be encouraged through policies that enable tailored counseling and medication services.
Only an end to violence against women can promote their physical, social, and emotional integrity. Curbing IPV will enable them to make optimal decisions about their life and health, including safeguards against sexually transmitted diseases including HIV, so their contributions to society can flourish.
Acknowledgments
Statement on Funding: This study was supported by the UNC Center for AIDS Research, an NIH funded program (P30 A150410), Ronald I. Swanstrom, PhD, Principal Investigator).
Footnotes
Competing Interests: The authors declare no interest or influence by any organization towards the submission of this work.
Contributor Information
Dr Elijah O Onsomu, Email: oonsomu@gmail.com, Winston-Salem State University, Divison of Nursing, Winston-Salem, 27110 United States.
Dr Benta A Abuya, African Population and Health Research Center (APHRC), Education Research Program, Nairobi, Kenya.
Dr Irene N Okech, Department of Research and Policy, Alpharetta, 30009 United States; and Imbako Public Health, Nairobi, Kenya.
Dr David L Rosen, University of North Carolina at Chapel Hill, Gillings School of Public Health, Chapel Hill, United States.
Dr Vanessa Duren-Winfield, Winston-Salem State University, Healthcare Management, Winston-Salem, 27110 United States.
Miss Amber C Simmons, University of South Carolina, Arnold School of Public Health, Columbia, United States.
References
- Abuya BA, Onsomu EO, Moore D, Piper CN. Association between education and domestic violence among women being offered an HIV test in urban and rural areas in Kenya. Journal of Interpersonal Violence. 2012;27(10):2022–2038. doi: 10.1177/0886260511431437. [DOI] [PubMed] [Google Scholar]
- Andersson N, Cockcroft A, Shea B. Gender-based violence and HIV: Relevance for HIV prevention in hyperendemic countries of southern Africa. AIDS. 2008;22(Suppl 4):S73–86. doi: 10.1097/01.aids.0000341778.73038.86. [DOI] [PubMed] [Google Scholar]
- Antelman G, Smith Fawzi MC, Kaaya S, Mbwambo J, Msamanga GI, Hunter DJ, Fawzi WW. Predictors of HIV-1 serostatus disclosure: A prospective study among HIV-infected pregnant women in Dar es Salaam, Tanzania. AIDS. 2001;15(14):1865–1874. doi: 10.1097/00002030-200109280-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett NJ. Effect of nondifferential misclassification on estimates of odds ratios with multiple levels of exposure. American Journal of Epidemiology. 1992;136(3):356–362. doi: 10.1093/oxfordjournals.aje.a116500. [DOI] [PubMed] [Google Scholar]
- Campbell JC, Baty ML, Ghandour RM, Stockman JK, Francisco L, Wagman J. The intersection of intimate partner violence against women and HIV/AIDS: A review. International Journal of Injury Control and Safety Promotion. 2008;15(4):221–231. doi: 10.1080/17457300802423224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Central Bureau of Statistics [Kenya], Ministry of Health [Kenya], and ORC Macro. Kenya demographic and health survey 2003. Calverton, MD: Author; 2004. [Google Scholar]
- Christofides N, Jewkes R. Acceptability of universal screening for intimate partner violence in voluntary HIV testing and counseling services in South Africa and service implications. AIDS Care. 2010;22(3):279–285. doi: 10.1080/09540120903193617. [DOI] [PubMed] [Google Scholar]
- Coovadia H, Jewkes R, Barron P, Sanders D, McIntyre D. The health and health system of South Africa: historical roots of current public health challenges. Lancet. 2009;374(9692):817–834. doi: 10.1016/S0140-6736(09)60951-X. [DOI] [PubMed] [Google Scholar]
- Decker MR, Miller E, Kapur NA, Gupta J, Raj A, Silverman JG. Intimate partner violence and sexually transmitted disease symptoms in a national sample of married Bangladeshi women. International Journal of Gynaecology and Obstetrics. 2008;100(1):18–23. doi: 10.1016/j.ijgo.2007.06.045. [DOI] [PubMed] [Google Scholar]
- Decker MR, Seage GR, III, Hemenway D, Raj A, Saggurti N, Balaiah D, Silverman JG. Intimate partner violence functions as both a risk marker and risk factor for women’s HIV infection: Findings from Indian husband–wife dyads. Journal of Acquired Immune Deficiency Syndromes. 2009;51(5):593–600. doi: 10.1097/QAI.0b013e3181a255d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz PM, Gazmararian JA, Goodwin MM, Bruce FC, Johnson CH, Rochat RW. Delayed entry into prenatal care: Effect of physical violence. Obstetrics and Gynecology. 1997;90(2):221–224. doi: 10.1016/s0029-7844(97)00252-4. [DOI] [PubMed] [Google Scholar]
- Dosemeci M, Wacholder S, Lubin JH. Does nondifferential misclassification of exposure always bias a true effect toward the null value? American Journal of Epidemiology. 1990;132(4):746–748. doi: 10.1093/oxfordjournals.aje.a115716. [DOI] [PubMed] [Google Scholar]
- Dude AM. Spousal intimate partner violence is associated with HIV and other STIs among married Rwandan women. AIDS and Behavior. 2011;15(1):142–152. doi: 10.1007/s10461-009-9526-1. [DOI] [PubMed] [Google Scholar]
- Dunkle KL, Jewkes RK, Nduna M, Levin J, Jama N, Khuzwayo N, Duvvury N. Perpetration of partner violence and HIV risk behavior among young men in the rural Eastern Cape, South Africa. AIDS. 2006;20(16):2107–2114. doi: 10.1097/01.aids.0000247582.00826.52. [DOI] [PubMed] [Google Scholar]
- Dunkle KL, Jewkes RK, Brown HC, Gray GE, McIntyre JA, Harlow SD. Gender-based violence, relationship power, and risk of HIV infection in women attending antenatal clinics in South Africa. Lancet. 2004;363(9419):1415–1421. doi: 10.1016/S0140-6736(04)16098-4. [DOI] [PubMed] [Google Scholar]
- Federation of Women Lawyer's Kenya. Assessment of the Implementation of the Previous Concluding Observations on Kenya (CCPR/CO/83/KEN) at the time of the Review of the Third Periodic Report. Nairobi: FIDA; 2011. [Google Scholar]
- Fonck K, Leye E, Kidula N, Ndinya-Achola J, Temmerman M. Increased risk of HIV in women experiencing physical partner violence in Nairobi, Kenya. AIDS and Behavior. 2005;9(3):335–339. doi: 10.1007/s10461-005-9007-0. [DOI] [PubMed] [Google Scholar]
- Gaillard P, Melis R, Mwanyumba F, Claeys P, Muigai E, Mandaliya K, Temmerman M. Vulnerability of women in an African setting: Lessons for mother-to-child HIV transmission prevention programmes. AIDS. 2002;16(6):937–939. doi: 10.1097/00002030-200204120-00019. [DOI] [PubMed] [Google Scholar]
- García-Moreno C, Watts C. Violence against women: Its importance for HIV/AIDS. AIDS. 2000;14(Suppl 3):S253–265. [PubMed] [Google Scholar]
- García-Moreno C, Jansen HA, Ellsberg M, Heise L, Watts CH. Prevalence of intimate partner violence: Findings from the WHO multi-country study on women's health and domestic violence. Lancet. 2006;368(9543):1260–1269. doi: 10.1016/S0140-6736(06)69523-8. [DOI] [PubMed] [Google Scholar]
- Gielen AC, McDonnell KA, O'Campo PJ. Intimate partner violence, HIV status, and sexual risk reduction. AIDS and Behavior. 2002;6(2):107–116. [Google Scholar]
- Gilbert L, El-Bassel N, Schilling RF, Wada T, Bennet B. Partner violence and sexual HIV risk behaviors among women in methadone treatment. AIDS and Behavior. 2000;4(3):261–269. [Google Scholar]
- Go VF, Sethulakshmi CJ, Bentley ME, Sivaram S, Srikrishnan AK, Solomon S, Celentano DD. When HIV-prevention messages and gender norms clash: The impact of domestic violence on women's HIV risk in slums of Chennai, India. Aids and Behavior. 2003;7(3):263–271. doi: 10.1023/a:1025443719490. [DOI] [PubMed] [Google Scholar]
- Goo L, Harlow SD. Intimate Partner violence affects skilled attendance at most recent delivery among women in Kenya. Maternal and Child Health Journal. 2012;16(5):1131–1137. doi: 10.1007/s10995-011-0838-1. [DOI] [PubMed] [Google Scholar]
- Gregson S, Nyamukapa CA, Garnett GP, Mason PR, Zhuwau T, Carael M, Anderson RM. Sexual mixing patterns and sex-differentials in teenage exposure to HIV infection in rural Zimbabwe. Lancet. 2002;359(9321):1896–1903. doi: 10.1016/S0140-6736(02)08780-9. [DOI] [PubMed] [Google Scholar]
- Harling G, Msisha W, Subramanian SV. No association between HIV and intimate partner violence among women in 10 developing countries. PLoS ONE. 2010;5(12):e14257. doi: 10.1371/journal.pone.0014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izugbara CO, Ngilangwa DP. Women, poverty and adverse maternal outcomes in Nairobi, Kenya. BMC Womens Health. 2010;10:33. doi: 10.1186/1472-6874-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewkes R. Intimate partner violence: Causes and prevention. Lancet. 2002;359(9315):1423–1429. doi: 10.1016/S0140-6736(02)08357-5. [DOI] [PubMed] [Google Scholar]
- Jewkes R, Dunkle K, Nduna M, Levin J, Jama N, Khuzwayo N, Duvvury N. Factors associated with HIV sero-status in young rural South African women: Connections between intimate partner violence and HIV. International Journal of Epidemiology. 2006;35(6):1461–1468. doi: 10.1093/ije/dyl218. [DOI] [PubMed] [Google Scholar]
- Jewkes R, Morrell R. Gender and sexuality: Emerging perspectives from the heterosexual epidemic in South Africa and implications for HIV risk and prevention. Journal of the International AIDS Society. 2010;13:6. doi: 10.1186/1758-2652-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewkes RK, Dunkle K, Nduna M, Shai N. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: A cohort study. Lancet. 2010;376(9734):41–48. doi: 10.1016/S0140-6736(10)60548-X. [DOI] [PubMed] [Google Scholar]
- Jewkes RK, Levin JB, Penn-Kekana LA. Gender inequalities, intimate partner violence and HIV preventive practices: Findings of a South African cross-sectional study. Social Science Medicine. 2003;56(1):125–134. doi: 10.1016/s0277-9536(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Karamagi CA, Tumwine JK, Tylleskar T, Heggenhougen K. Intimate partner violence against women in eastern Uganda: Implications for HIV prevention. BMC Public Health. 2006;6:284. doi: 10.1186/1471-2458-6-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RJ, Gray RH, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, Wawer MJ. Age differences in sexual partners and risk of HIV-1 infection in rural Uganda. Journal of the Acquired Immune Deficiency Syndrome. 2003;32(4):446–451. doi: 10.1097/00126334-200304010-00016. [DOI] [PubMed] [Google Scholar]
- Kenya Law Reports and the Government of Kenya. The Sexual Offences Act (Number 3 of 2006) Nairobi: The National Council for Law Reporting with the Authority of the Attorney General; 2009. Retrieved from http://www.kenyalaw.org/family/statutes/download.php?file=Sexual%20Offences%20Act.pdf. [Google Scholar]
- Kenya National Bureau of Statistics (KNBS) and ICF Macro. Kenya Demographic and Health Survey 2008-09. Calverton, Maryland: KNBS and ICF Macro; 2010. [Google Scholar]
- Kiarie JN, Farquhar C, Richardson BA, Kabura MN, John FN, Nduati RW, John-Stewart GC. Domestic violence and prevention of mother-to-child transmission of HIV-1. AIDS. 2006;20(13):1763–1769. doi: 10.1097/01.aids.0000242823.51754.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimuna SR, Djamba YK. Gender based violence: Correlates of physical and sexual wife abuse in Kenya. Journal of Family Violence. 2008;23(5):333–342. [Google Scholar]
- Kishor S, Johnson K. Profiling Domestic Violence – A Multi-Country Study. Calverton, Maryland: ORC Macro; 2004. [Google Scholar]
- Koenig MA, Lutalo T, Zhao F, Nalugoda F, Wabwire-Mangen F, Kiwanuka N, Gray R. Intimate partner violence in rural Uganda: Evidence from a community-based study. Bulletin of World Health Organisation. 2003;81(1):53–60. [PMC free article] [PubMed] [Google Scholar]
- Lary H, Maman S, Katebalila M, McCauley A, Mbwambo J. Exploring the association between HIV and violence: Young people's experiences with infidelity, violence and forced sex in Dar es Salaam, Tanzania. International Family Planning Perspectives. 2004;30(4):200–206. doi: 10.1363/3020004. [DOI] [PubMed] [Google Scholar]
- Lawoko S. Predictors of attitudes toward intimate partner violence: A comparative study of men in Zambia and Kenya. Journal of Interpersonal Violence. 2008;23(8):1056–1074. doi: 10.1177/0886260507313972. [DOI] [PubMed] [Google Scholar]
- Lasee A, Becker S. Husband-wife communication about family planning and contraceptive use in Kenya. International Family Planning Perspectives. 1997;23(1):15–20. [Google Scholar]
- Longfield K, Glick A, Waithaka M, Berman J. Relationships between older men and younger women: Implications for STIs/HIV in Kenya. Studies in Family Planning. 2004;35(2):125–134. doi: 10.1111/j.1728-4465.2004.00014.x. [DOI] [PubMed] [Google Scholar]
- Luke N. Age and economic asymmetries in the sexual relationships of adolescent girls in sub-Saharan Africa. Studies in Family Planning. 2003;34(2):67–86. doi: 10.1111/j.1728-4465.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Schuler SR, Boender C. Measuring Women's Empowerment as a variable in international development. World Bank, Gender and Development Group; Washington DC: 2002. [Google Scholar]
- Maman S, Mbwambo JK, Hogan NM, Kilonzo GP, Campbell JC, Weiss E, Sweat MD. HIV-positive women report more lifetime partner violence: Findings from a voluntary counseling and testing clinic in Dar es Salaam, Tanzania. American Journal of Public Health. 2002;92(8):1331–1337. doi: 10.2105/ajph.92.8.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SL, Kilgallen B, Tsui AO, Maitra K, Singh KK, Kupper LL. Sexual behaviors and reproductive health outcomes: Associations with wife abuse in India. JAMA. 1999;282(20):1967–1972. doi: 10.1001/jama.282.20.1967. [DOI] [PubMed] [Google Scholar]
- Martin SL, Curtis S. Gender-based violence and HIV/AIDS: Recognizing links and acting on evidence. Lancet. 2004;363(9419):1410–1411. doi: 10.1016/S0140-6736(04)16133-3. [DOI] [PubMed] [Google Scholar]
- Medley A, García-Moreno C, McGill S, Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: Implications for prevention of mother-to-child transmission programmes. Bulletin of the World Health Organization. 2004;82(4):299–307. [PMC free article] [PubMed] [Google Scholar]
- Onsomu EO, Kimani JK, Abuya BA, Arif AA, Moore D, Duren-Winfield V, Harwell G. Delaying sexual debut as a strategy for reducing HIV epidemic in Kenya. African Journal of Reproductive Health. 2013;17(2):46–57. [PubMed] [Google Scholar]
- Sa Z, Larsen U. Gender inequality increases women's risk of HIV infection in Moshi, Tanzania. Journal of Biosocial Science. 2007;40(4):505–525. doi: 10.1017/S002193200700257X. [DOI] [PubMed] [Google Scholar]
- Shi CF, Kouyoumdjian FG, Dushoff J. Intimate partner violence is associated with HIV infection in women in Kenya: A cross-sectional analysis. BMC Public Health. 2013;13:512. doi: 10.1186/1471-2458-13-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JG, Decker MR, Saggurti N, Balaiah D, Raj A. Intimate partner violence and HIV infection among married Indian women. JAMA. 2008;300(6):703–710. doi: 10.1001/jama.300.6.703. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- UCLA Institute for Digital Research and Education. Stata Annotated Output: Factor analysis. n.d. Retrieved from http://www.ats.ucla.edu/stat/stata/output/fa_output.htm.
- UNAIDS. Joint action for results: UNAIDS outcome framework, 2009-2011. 2009 UN Doc. UNAIDS/09.13E / JC1713E. Retrieved from http://www.unaids.org/en/media/unaids/contentassets/dataimport/pub/basedocument/2010/jc1713_joint_action_en.pdf.
- UNAIDS. Regional Fact Sheet 2012: Sub-Saharan Africa. 2012 Retrieved from http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/2012_FS_regional_ssa_en.pdf.
- van der Straten A, King R, Grinstead O, Vittinghoff E, Serufilira A, Allen S. Sexual coercion, physical violence, and HIV infection among women in steady relationships in Kigali, Rwanda. AIDS and Behavior. 1998;2(1):61–73. [Google Scholar]
- Wang SH, Rowley W. Rape: How women, the community and the health sector respond. Geneva: Sexual Violence Research Initiative and World Health Organization; 2007. Retrieved from http://www.svri.org/rape.pdf. [Google Scholar]
- Wawer MJ, Makumbi F, Kigozi G, Serwadda D, Watya S, Nalugoda F, Gray RH. Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: A randomised controlled trial. Lancet. 2009;374(9685):229–37. doi: 10.1016/S0140-6736(09)60998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanyoni M, Lumumba V. Gender-Based Violence Kenya Demographic and Health Survey 2008-09. Calverton, Maryland: KNBS and ICF Macro; 2010. [Google Scholar]
- WHO. Violence against women: A priority health issue. World Health Organization; Geneva: 1997. WHO/FRH/WHD/97.8. [Google Scholar]
- WHO. Violence against women and HIV/AIDS: Critical intersections (Intimate Partner Violence and HIV/AIDS) Geneva: World Health Organization; 2004. [Google Scholar]
- WHO. Multi-country study on women's health and Intimate Partner violence against women: Summary report of initial results on prevalence, health outcomes and women's responses. Geneva: World Health Organization; 2005. [Google Scholar]
- Wingood GM, DiClemente RJ. Rape among African American women: Sexual, psychological, and social correlates predisposing survivors to risk of STD/HIV. Journal of Women's Health. 1998;7(1):77–84. doi: 10.1089/jwh.1998.7.77. [DOI] [PubMed] [Google Scholar]
- Wittenberg E, Joshi M, Thomas KA, McCloskey LA. Measuring the effect of intimate partner violence on health-related quality of life: A qualitative focus group study. Health and Quality of Life Outcomes. 2007;5:67. doi: 10.1186/1477-7525-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotska IB, Gray RH, Koenig MA, Serwadda D, Nalugoda F, Kigozi G, Wawer M. Alcohol use, intimate partner violence, sexual coercion and HIV among women aged 15–24 in Rakai, Uganda. AIDS and Behavior. 2009;13(2):225–233. doi: 10.1007/s10461-007-9333-5. [DOI] [PubMed] [Google Scholar]


