Abstract
The tumor suppressor p53 (TP53) has a well-studied role in triggering cell cycle checkpoint in response to DNA damage. Previous studies have suggested that functional p53 enhances chemosensitivity. In contrast, data are presented to show that p53 can be required for cell survival following DNA damage due to activation of reversible cell cycle checkpoints. The cellular outcome to DNA damage is determined by the duration and extent of the stimulus in a p53-dependent manner. In response to transient or low levels of DNA damage, p53 triggers a reversible G2 arrest whereas a sustained p53-dependent cell cycle arrest and senescence follows prolonged or high levels of DNA damage. Regardless of the length of treatment, p53-null cells arrest in G2, but ultimately adapt and proceed into mitosis. Interestingly, they fail to undergo cytokinesis, become multinucleated, and then die from apoptosis. Upon transient treatment with DNA damaging agents, wild-type p53 cells reversibly arrest and repair the damage, whereas p53-null cells fail to do so and die. These data indicate that p53 can promote cell survival by inducing reversible cell cycle arrest, thereby allowing for DNA repair. Thus, transient treatments may exploit differences between wild-type p53 and p53-null cells.
Keywords: p53, cell cycle, checkpoint, DNA damage, apoptosis
Introduction
The tumor suppressor p53 is a sequence-specific transcription factor induced in response to a variety of cellular stresses, including DNA damage. It is commonly mutated in human cancer (1, 2). Depending on cellular conditions, p53 can mediate either growth arrest or apoptosis. The relevance of the growth arrest response to tumor suppression has recently been highlighted by studies using a tumor-derived mutant p53 defective for apoptosis but competent for growth arrest (3). In this study, mice expressing this mutant were more resistant to tumor formation than p53-null mice, and tumors that did form had a low frequency of aneuploidy. HCT116 colorectal carcinoma cells treated with DNA damage for up to 96 hours were shown to arrest with a 4N DNA content when expressing p53; however, HCT116 cells with a targeted deletion of p53 arrested transiently with a 4N DNA content before undergoing cell death (4). Several p53 targets have been implicated in the growth arrest response with the best-characterized being p21CIP1 (5, 6), which inhibits the function of several cyclin-dependent kinase (cdk) complexes. Cells that are null for p21 show impaired arrest in G1 and cells lacking p53 do not induce significant levels of p21 (6–9).
Several studies have implicated the p53 pathway in the regulation of the G2 to M transition (10). Both Cdc2 (Cdk1) and the associated cyclin B can be transcriptionally repressed by p53 (11, 12), preventing entry into mitosis. Similarly, the dual-specificity phosphatase Cdc25C is down-regulated by p53 in response to DNA damage, preventing the activation of Cdc2 (13). Additionally, the p53 target gene GADD45 has been shown to arrest cells in early mitosis (14). p53 is not required for the mitotic spindle checkpoint, but following adaptation to prolonged mitotic arrest with nocodazole, p53 and p21 are required for arrest in a G1 tetraploid state with elevated cyclin E and hypophosphorylated Rb levels (15). Cells deficient for p21 undergo endoreduplication following microtubule disruption, and this is prevented by restoration of p21 expression in these cells (16). Following prolonged G2 arrest induced by DNA damage, it has been reported that cells adapt and progress to the subsequent G1 phase with a 4N DNA content, and undergo a cell cycle arrest involving p53 and Rb (17–19). This step may reflect a crucial juncture in the formation of tumors, as tetraploidy often precedes aneuploidy during carcinogenesis (20).
In this report, the role of p53 in both short- and long-term responses to DNA damage is examined. Cells with ablated p53 levels were more sensitive to DNA damage-induced cell death than isogenic cell lines expressing p53. The data presented herein provide evidence that p53 is required for sustained cell cycle arrest following prolonged DNA damage and for recovery after brief exposure to genotoxic drugs. These studies suggest that transient treatments can exploit differences between wild-type p53 and p53 null cells. Although p53 status has been suggested as a clinical predictor of chemotherapeutic efficacy, studies to date have not always supported this. It is proposed that p53 promotes cell survival following DNA damage by inducing a reversible cell cycle arrest, allowing for DNA repair.
Materials and Methods
Cells and transfections
HCT116 and HCT116 p53 -/- cells were a kind gift of B. Vogelstein. U2OS cells were purchased from ATCC. U2OS cells were co-transfected with either control or p53 shRNA vectors and pBabe-Puro and puromycin-resistant colonies were picked and expanded. The plasmids encoded short hairpin RNA molecules directed against either a control sequence or against the p53 mRNA sequence, respectively. The p53 shRNA sequence was: 5′-GACTCCAGTGGTAATCTACttcaagagaGTAGATTACCACTGGAGTCTTTTT-3′, where bases in lowercase represent the hairpin loop formed upon annealing of the complimentary flanking sequences. For transient siRNA transfections, siRNA oligonucleotides were purchased from Dharmacon.
SDS polyacrylamide gel electrophoresis and immunoblotting
Cells were lysed in 50 mM HEPES, 150 mM NaCl, 1 mM MgCl2, 50 μg/ml aprotinin, 1 mM PMSF, 5 μg/ml leupeptin, and 1% Triton X-100. All primary antibodies were from Santa Cruz (p53 DO-1, p21 C-19, cyclin E HE-111, PARP H-250, cdc25C H6, cyclin A C19-G, Cyclin B1 GNS-1, cdc2 17, KU-70, and E1A) except for actin (Calbiochem Ab-1). Peroxidase-conjugated secondary antibodies against either mouse or rabbit IgG (Cappel) or goat IgM (Calbiochem) were detected using the enhanced ECL system (Amersham).
Flow cytometry
Cells were fixed with 70% ethanol, and stained with 20 μg/ml propidium iodide (PI) with 1 mg/ml RNase A. Cell cycle data was collected using a FACSCalibur flow cytometer and analyzed using Cell Quest software (Becton Dickinson). For Annexin-V/PI analysis, live cells were incubated with TACS™ annexin V reagent and propidium iodide, and then analyzed by flow cytometry. In phosphorylated histone H3 assays, cells were fixed in 0.5% formaldehyde in PBS, and permeabilized with cold 90% methanol. Cells were incubated with primary anti-phosphorylated serine 10-H3 (Cell Signaling) with 100 μg/ml RNAse A, followed by FITC-conjugated secondary antibody, then resuspended in PBS/propidium iodide, and analyzed by flow cytometry.
Mitotic Indices
Cells were analyzed at varying time points by light microscopy to quantitate the percentage of cells with a mitotic morphology.
Colony formation assays
Cells were seeded at 4 × 105 cells per well in 6 well culture dishes overnight, treated with drugs, and incubated for indicated periods of time. Media with fresh drug was replenished every four days, and then staining with Giemsa was used to visualize cells.
Senescent-associated β-galactosidase staining
Cells were seeded at 4 × 105 cells per well in 6 well culture dishes overnight, treated with drugs, and incubated for indicated periods of time. Cells were fixed with 2% formaldehyde and 0.2% glutaraldehyde and then stained solution with 150 mM NaCl, 2 mM MgCl2, 40 mM citric acid/sodium phosphate buffer, 5mM ferrocyanide, 5mM ferricyanide, and 1 mg/ml X-gal in DMF.
Immunofluorescence
Coverslips were placed in 100 mm tissue culture dishes and cells were seeded. Following the appropriate treatment, cover slips were transferred to 6 well dishes, one cover slip per well, for further processing. The remaining cells in the 100 mm dish were harvested and analyzed by flow cytometry. Coverslips were fixed with paraformaldehyde and sucrose in PBS, permeabilized with 70 mg/ml sucrose, 0.5% Triton X-100, and 3 mM MgCl2 in PBS, and then blocked with 3% BSA in PBS. Antibody against γ-H2AX (Upstate) were added followed by a secondary antibody (Alexa 594, Molecular Probes-Invitrogen). Coverslips were mounted on glass slides with 1X Anti-Fade DAPI solution in 50% glycerol. Fluorescence was captured with a E-700 epifluorescence photo-microscope (Nikon) through a SPOT digital camera (Diagnostics Instruments).
Results
Loss of p53 enhances tumor cell death in response to continuous chemotherapeutic treatment
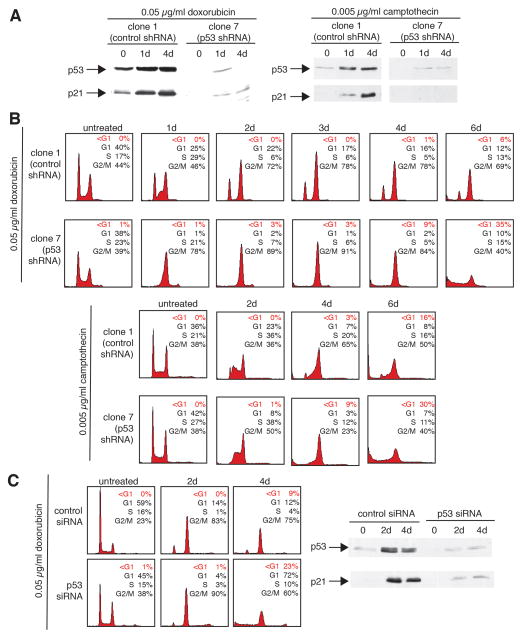
In order to investigate the role of p53 in the G2 response, U2OS cells stably expressing either control-shRNA or shRNA directed against p53 were generated. The response of U2OS-derived clones to sustained DNA damage was then examined using chemotherapeutic agents. DNA damage induced by doxorubicin leads to an increase of transcriptionally active p53 in a dose-dependent manner in U2OS cells (Supplemental and S1A). Treatment with doxorubicin (0.05 μg/ml) caused clone 1 (control shRNA-expressing) cells to arrest with a DNA content of 2N and 4N, and this arrest was maintained throughout the treatment period of 6 days (Fig. 1B). In contrast, clone 7 (p53 shRNA-expressing) U2OS cells arrested exclusively with a 4N DNA content, and this was not maintained, as cells underwent death at later time points as evidenced by the appearance of a broad hypodiploid peak (Fig. 1B). Immunoblotting indicates that clone 1 cells induced p53 and its target p21 following doxorubicin treatment but no such induction was observed in clone 7 (Fig. 1A, left). Similar results were observed when DNA damage was induced with camptothecin (0.005 μg/ml; Fig. 1A, right and 1B). Clone 1 cells maintained a cell cycle arrest in response to camptothecin treatment (Fig. 1B). In contrast, cells expressing shRNA to p53 only transiently arrested, undergoing subsequent cell death. As expected, only clone 1 cells showed increases in p53 and p21 following camptothecin treatment (Fig. 1A).
Figure 1.
Loss of p53 enhances tumor cell death in response to continuous chemotherapeutic treatment. A, Stable expression of an shRNA directed against p53 abrogates the increase in p53 expression upon treatment with chemotherapeutic agents. The U2OS derivatives, clone 1 (expressing control shRNA) or clone 7 (expressing p53 shRNA) were untreated or treated with either doxorubicin-treated (0.05 μg/ml) or camptothecin (0.005 μg/ml) for 1–4 days and then subjected to immunoblot analysis for p53 and p21 expression. B, Cells stably expressing an shRNA directed against p53 accumulate with a hypodiploid DNA content in response to treatment with chemotherapeutic agents. Clone 1 (control shRNA) or clone 7 (p53 shRNA) cells were untreated or treated with either doxorubicin (0.05 μg/ml) or camptothecin (0.005μg/ml) for 1–6 days and cell cycle profiles were then analyzed by flow cytometry. The percentage of cells in G1, S, and G2/M, or with a hypodiploid DNA content (<G1) are indicated in the upper right hand corner of each histogram. C, Transient ablation of p53 expression using an siRNA approach results in cells that accumulate with a hypodiploid DNA content in response to treatment with chemotherapeutic agents. Parental U2OS cells were transfected with oligonucleotides encoding either a control siRNA or siRNA directed against p53 and then either untreated or treated with 0.05 μg/ml doxorubicin for 2–4 days and cell cycle profiles were then analyzed by flow cytometry. The percentage of cells in each phase of the cell cycle or with a DNA content of less than 2N (<G1) are indicated in the upper right hand corner of each histogram. Cell extracts were prepared from corresponding cells and analyzed by immunoblotting for p53 and p21 levels.
To rule out that these effects are clonal in nature, two U2OS-derived control shRNA and two p53-shRNA clones were treated with doxorubicin and compared (Supplemental Fig. S1B and S1C). Both control clones induced p53 and p21 and maintained a cell cycle arrest. In contrast, both p53-shRNA clones were impaired in p53 and p21 induction and underwent a transient cell cycle arrest in G2, followed by eventual cell death.
The above results were validated using a transient approach (Fig. 1C). Transfection of an siRNA oligonucleotide targeting p53 mRNA led to efficient abrogation of both basal p53 expression and p53 induction upon treatment with 0.05 μg/ml doxorubicin (Fig. 1C, right). Transient transfection of p53-siRNA also prevented accumulation of cells in G1 following doxorubicin treatment, with the appearance of a hypodiploid peak (Fig. 1C, left). These data indicate that p53 ablation restricts the ability of cells to arrest in G1 or to maintain a 4N cell cycle arrest following sustained DNA damage, leading to cell death.
These data suggest that p53 status determines the cellular response to DNA damage. To investigate this further, the response of a panel of cancer cell lines to doxorubicin treatment was assayed (Supplemental Fig. S2). Four p53 wild-type cell lines (HCT116, U2OS, HT-1080, and G-361) maintained a 2N and 4N cell cycle arrest following doxorubicin exposure, with only a small subset of cells becoming hypodiploid by 96 hours. In contrast, three p53-null cell lines (HCT116 p53 -/-, Saos2, and H1299) responded to doxorubicin with a transient 4N cell cycle arrest followed by cell death (Supplemental Fig. S2). Thus, p53 status in multiple cancer cell lines consistently correlates with cellular response to DNA damage.
The mechanism of cell death in p53-ablated tumor cells is apoptosis
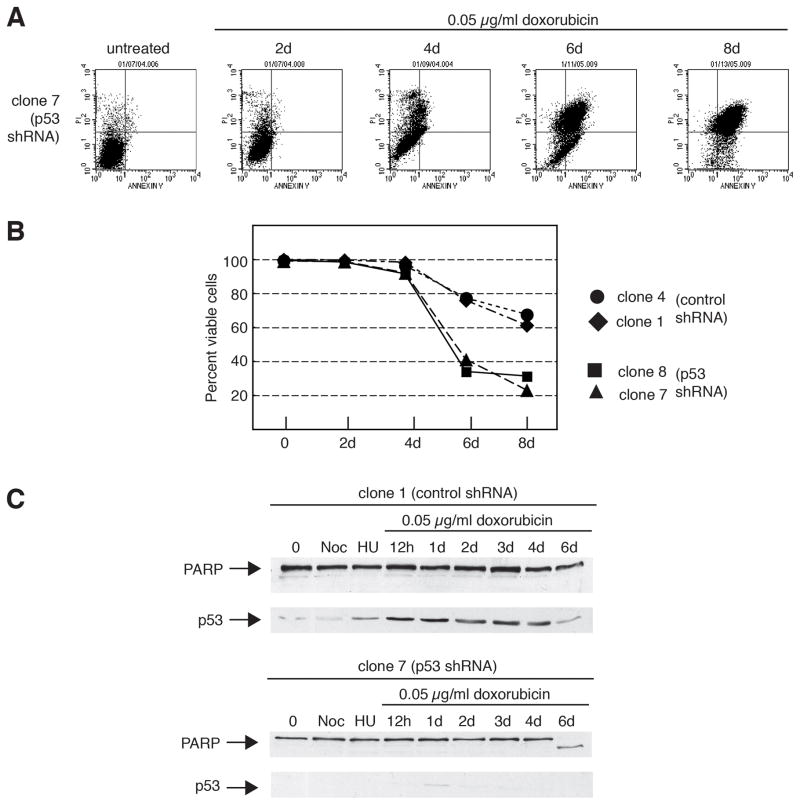
To determine the basis for p53-ablated cell death, doxorubicin-treated cells were analyzed by annexin V-propidium iodide (PI) staining (Fig. 2A and 2B) and immunoblotting (Fig. 2C and Supplemental Fig. S1C). Cells expressing p53-shRNA showed significant staining for annexin-V (Fig 2A). To rule out clonal effects, multiple clones were investigated and similar results were observed (Fig. 2B). Two p53-ablated clones showed increased viability following prolonged DNA damage. In contrast, two control clones underwent substantially less cell death. In addition, cell extracts from doxorubicin-treated, p53-ablated cells, but not those from control cells, demonstrated significant cleavage of the caspase target, PARP (Fig. 2C and Supplemental Fig. S1C), a finding consistent with apoptosis.
Figure 2.
The mechanism of cell death in p53-ablated tumor cells is apoptosis. A: Cells stably expressing an shRNA directed against p53 show positive cell-surface staining with annexin V in response to treatment with a chemotherapeutic agent. U2OS derivative, clone 7 (p53-shRNA) cells were treated with 0.05 μg/ml doxorubicin for 2–8 days. Live cells were double-stained for annexin-V positivity and propidium iodide, and then analyzed by flow cytometry. Apoptotic cells are present within the upper left quadrant of the dot plots (positive for annexin-V but not propidium iodide). B: Cells stably expressing an shRNA directed against p53 show reduced viability as determined by double-labeling with annexin V and propidium iodide in response to treatment with a chemotherapeutic agent. Clones 1 and 4 (expressing control shRNA) or clones 7 and 8 (expressing p53 shRNA) were either untreated or treated with 0.05 μg/ml doxorubicin from 2 to 8 days, and then subjected to flow cytometric analysis for viable cells as in A. C: The caspase substrate PARP is cleaved in cells stably expressing an shRNA directed against p53 in response to treatment with a chemotherapeutic agent. Clones 1 (expressing control shRNA) or clone 7 (expressing p53 shRNA) were either untreated, or treated with 0.4 μg/ml nocodazole (Noc) or 2 mM hydroxyurea (HU) for 1 day, or 0.05 μg/ml doxorubicin for 12 hours to 6 days and then subjected to immunoblot analysis for PARP and p53 expression.
p53-ablated tumor cells enter mitosis, but fail to undergo cytokinesis in response to continuous chemotherapeutic treatment
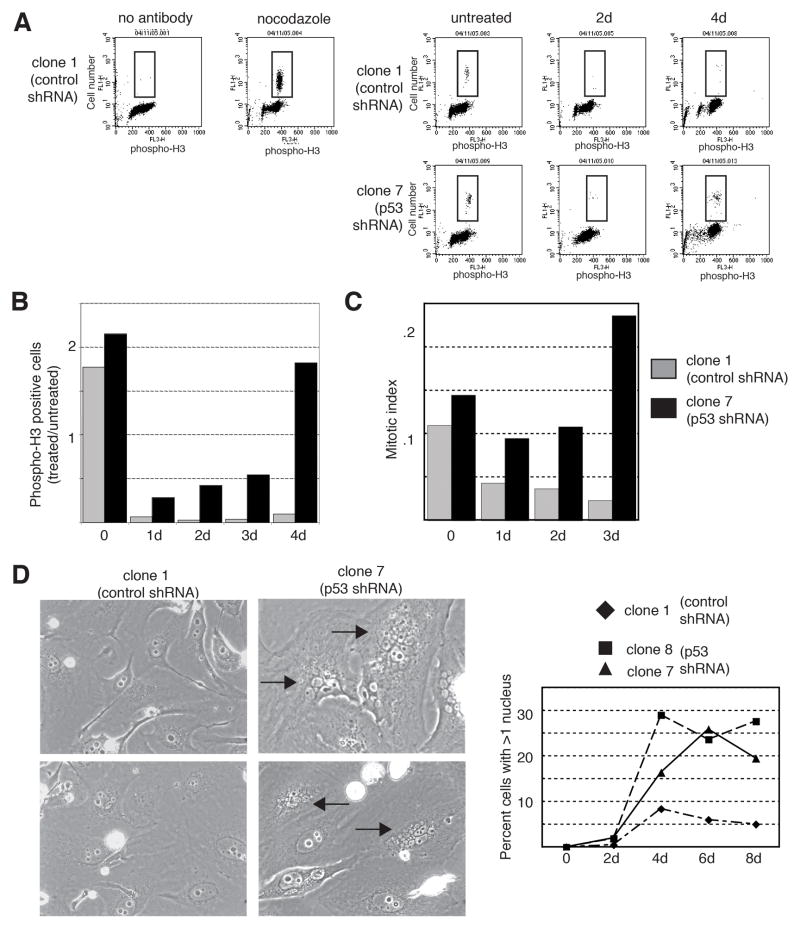
These data demonstrate that cells with ablated p53 levels transiently arrest in G2 and then undergo apoptotic cell death following DNA damage. It is unclear whether these cells undergo apoptosis directly from G2 or if they resume cycling en route to the induction of apoptosis. The phosphorylation of histone H3 on serine 10 serves as a useful marker for mitotic entry (21). Control cells showed a marked reduction in phospho-H3 positivity following drug treatment, indicating that following prolonged DNA damage they do not enter mitosis. In contrast, p53-ablated cells showed a transient decrease in histone H3 phosphorylation, but eventually attained levels of phosphorylation similar to those in untreated cells by four days of treatment (Fig. 3A and 3B). In contrast to cells expressing wild-type p53, those with ablated p53 expression arrest in G2 only transiently and subsequently enter mitosis after sustained DNA damage (Fig. 3A and 3B). Consistent with these data is the observation that, in contrast to control cells, a high proportion of p53-ablated cells treated with doxorubicin had a rounded mitotic morphology on light microscopy (Fig. 3C). Additionally, p53-ablated cells that enter mitosis appear to have a defect in cytokinesis, as there is a notable increase in cells with multiple micronuclei (Fig. 3D and Supplemental Fig. S3). Whereas multinucleation is a rare event in control cells, as many as 30% of p53-ablated cells contained more than two nuclei following prolonged drug treatment (Fig. 3D, right). The presence of nuclear membranes surrounding the micronuclei and positive staining with DAPI (data not shown) suggests that these cells have exited mitosis without completing cytokinesis.
Figure 3.
p53-ablated tumor cells enter mitosis, but fail to undergo cytokinesis in response to continuous chemotherapeutic treatment. A–B: Cells stably expressing an shRNA directed against p53 show enhanced staining for the mitotic marker, phosphorylated histone H3, in response to treatment with a chemotherapeutic agent. The U2OS derivatives, clone 1 (expressing control shRNA) or clone 7 (expressing p53 shRNA) were either untreated or treated with 0.05 μg/ml doxorubicin for 2 or 4 days followed by double-staining for phosphorylated histone H3 and DNA content (propidium iodide), and analysis by flow cytometry. The no antibody sample was not incubated with primary antibody to phosphorylated histone H3 and the nocodazole-treated (0.4 μg/ml for 1 day) sample was a positive control for mitotic cells. Results are plotted as the ratio of treated cells positive for phosphorylated histone H3 versus untreated cells positive for histone H3 phosphorylation. C: Cells stably expressing an shRNA directed against p53 show enhanced entry into mitosis in response to treatment with a chemotherapeutic agent. Clone 1 (expressing control shRNA) or clone 7 (expressing p53 shRNA) cells were either untreated or treated with 0.05 μg/ml doxorubicin for 1–3 days. The mitotic index was then determined based on morphologic changes associated with mitosis (cell rounding and chromatin condensation). D: Cells stably expressing an shRNA directed against p53 become multi-nucleated in response to treatment with a chemotherapeutic agent. Clone 1 (expressing control shRNA) or clone 7 and 8 (expressing p53 shRNA) cells were either untreated or treated with 0.05 μg/ml doxorubicin for 2–8 days, examined by phase contrast microscopy, and the number of multinucleated cells was quantitated. Representative fields of clone 1 or clone 7 cells at 6 days are shown. Arrows indicate multinucleated cells.
p53-expressing tumor cells circumvent mitosis, express markers consistent with a G1-like state, and become senescent in response to continuous chemotherapeutic treatment
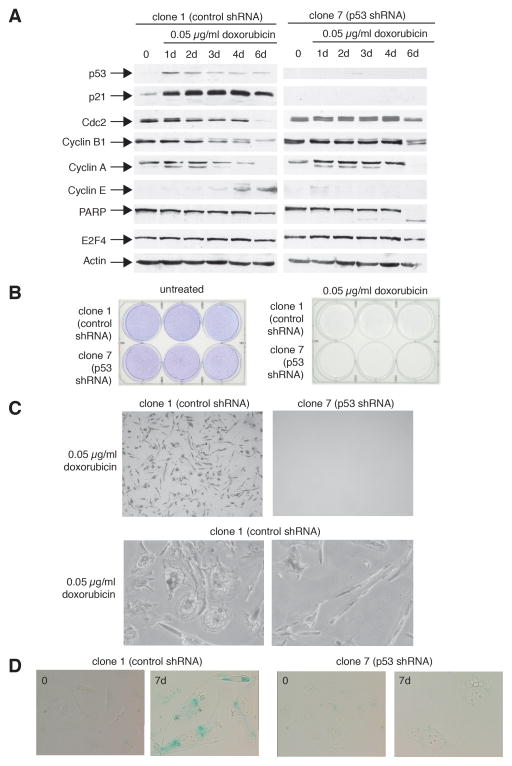
Control cells expressing wild-type p53 maintain a 4N cell cycle arrest following prolonged doxorubicin treatment. In order to determine if U2OS cells adapt to G2 arrest and enter a subsequent G1 phase, cells were treated with 0.05 μg/ml doxorubicin and analyzed for expression of the G1 marker cyclin E by immunoblotting (Fig. 4A). In these experiments, control cells showed a time-dependent accumulation of cyclin E protein. Cyclin E levels reached those seen when cells are arrested at the G1/S border with hydroxyurea (Supplemental Fig. S4). In contrast, p53-ablated cells did not show evidence of cyclin E accumulation following sustained DNA damage (Fig. 4A). Results were verified in a second p53 shRNA-expressing U2OS clone (Supplemental Fig. S4). Therefore, it appears that p53 plays both a role in restricting entry into mitosis as well as in arresting 4N cells in a G1-like state.
Figure 4.
p53-expressing tumor cells circumvent mitosis, express markers consistent with a G1-like state, and become senescent in response to continuous chemotherapeutic treatment. A: p53-expressing cells show a decrease in the G2 markers, Cdc2, Cyclin B1, and Cyclin A, and an increase in the G1 marker, cyclin E, in response to treatment with a chemotherapeutic agent. The U2OS derivatives, clone 1 (expressing control shRNA) or clone 7 (expressing p53 shRNA) were either untreated or treated with 0.05 μg/ml doxorubicin for the indicated times. Cell extracts were analyzed by immunoblotting for levels of the indicated proteins. Actin levels serve as a protein loading control. B: Both p53-expressing and p53-null cells fail to proliferate in response to continuous treatment with a chemotherapeutic agent. Clone 1 (expressing control shRNA) or clone 7 (expressing p53 shRNA) cells were either untreated or treated with 0.05 μg/ml doxorubicin for three weeks and then stained with Giemsa. C: p53-expressing cells persist but do not proliferate with two distinct morphologies in response to continuous treatment with a chemotherapeutic agent. Cells treated as in B were examined by phase contrast microscopy. D: p53-expressing cells contain enhanced senescent-associated β-galactosidase activity in response to treatment with a chemotherapeutic agent. Clone 1 (expressing control shRNA) or clone 7 (expressing p53 shRNA) were either untreated or treated with 0.05 μg/ml doxorubicin for 7 days. Cells were then stained for senescence-associated β-galactosidase activity.
In order to examine this in more detail, extracts from doxorubicin-treated cells were analyzed for expression of Cyclin B1, Cdc2, and Cyclin A, the levels of which are up-regulated in G2 and mitosis. In control cells, following extended treatment with doxorubicin, there was a decrease in all three proteins and an increase in cyclin E at later time points (Fig. 4A, left panel). Interestingly, p53-ablated cells retained high levels of both Cdc2 and Cyclin B1 and did not accumulate cyclin E (Fig. 4B, right panel). In these cells, a decrease in Cdc2 and Cyclin B1 levels was observed at time points when PARP cleavage was seen, suggesting that this may be related to ongoing cell death. An alternative explanation is that Cdc2 and Cyclin B1 levels decline after the cells have passed through mitosis. Although p130 and E2F4 have been implicated in p53-mediated cdc2 repression (22), no change in either protein was observed in control cells containing normal p53 levels (Fig. 4A, left panel and data not shown).
In order to investigate the long-term outcome of sustained exposure to chemotherapeutic agents, clone 1 and clone 7 cells were treated with doxorubicin for 3 weeks and proliferation was compared to untreated cells by Giemsa staining (Fig. 4B) and light microscopy (Fig. 4C). In the absence of DNA damage, both clone 1 and clone 7 cells grew to confluency (Fig. 4B, left). In contrast, neither cell type proliferated in the continued presence of doxorubicin (Fig. 4B, right). Closer observation of doxorubicin-treated cells microscopically demonstrates that, although they do not proliferate, clone 1 cells persist throughout the duration of treatment (Fig. 4C, top left). Higher power magnification of these cells reveals two predominating morphologies. One group of cells has a flattened, “fried egg” appearance, resembling the appearance of senescent cells (Fig. 4C, bottom left), and the other group has an elongated, spindle-like morphology (Fig. 4C, bottom right). Microscopic examination of doxorubicin-treated clone 7 cells fails to reveal any remaining cells at 3 weeks (Fig. 4C, top right), suggesting that all cells have undergone cell death by apoptosis.
In order to investigate the possibility that the clone 1 cells with the “fried egg” morphology represent senescent cells, senescent-associated β-galactosidase (β-gal) staining was performed on cells following no treatment or continuous exposure to doxorubicin (0.05 μg/ml) for 7 days (Fig. 4D). In contrast to untreated clone 1 cells, those undergoing doxorubicin treatment exhibited a high degree of β-gal staining at 7 days. No β-gal positivity was observed in clone 7 cells before or after doxorubicin exposure. Taken together, these data indicate that cells expressing p53 respond to prolonged DNA damage by stably arresting with a 4N DNA content, expressing cell cycle markers consistent with G1, and become senescent.
p53-expressing tumor cells recover from short-term chemotherapeutic treatment whereas p53- ablated tumor cells do not
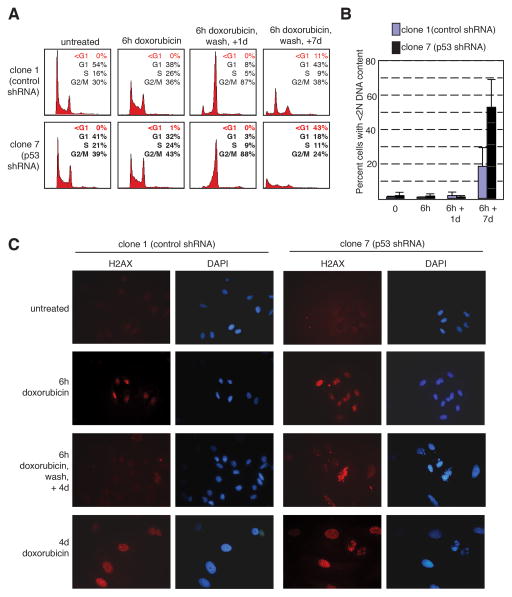
The above experiments addressed the role of p53 in the response to continuous exposure to chemotherapeutic drugs. In order to investigate the role of p53 in the cellular response to transient DNA damage, the U2OS-derived shRNA clones were pulsed with 0.05 μg/ml doxorubicin for 6 hours followed by drug wash-out and analyzed by flow cytometry (Fig. 5A and 5B). After 6 hours of doxorubicin treatment, clone 1 and clone 7 cells had similar cell cycle profiles, and one day following wash-out of drug, both cell types were cell cycle arrested. However, following an observation period of seven days, the p53-replete control cells resumed cycling and had a cell cycle profile resembling untreated cells. In contrast, the majority of p53-ablated cells had a hypodiploid DNA content, consistent with apoptosis. The percentage of hypodiploid cells at each time point is summarized in Fig. 5B. The presence of micronuclei following transient exposure to doxorubicin was also analyzed (Supplemental Fig. S5). Following treatment with 0.05 μg/ml doxorubicin for 6 hours followed by drug wash-out, p53-ablated clone 7 cells were observed to contain multiple nuclei at high rates by two days after treatment, and this phenomenon was observed throughout the observation period. In contrast, multinucleation was a rare event in p53-expressing clone 1 cells.
Figure 5.
p53-expressing cells recover from short-term chemotherapeutic treatment whereas p53-ablated cells do not. A–B: The U2OS derivatives clone 1 (expressing control shRNA) or clone 7 (expressing p53 shRNA) were untreated or treated with 0.05 μg/ml doxorubicin for 6 hours, then washed, and allowed to grow in complete medium without drug for 1 or 7 days. DNA content was assayed by propidium iodide staining. Representative histograms from a single experiment are shown (A). Percent of cells with less than 2N DNA content was quantitated. The average of three independent experiments was averaged and is shown as a bar graph (B). C: Both p53-expressing and p53-null cells show activation of the DNA damage checkpoint in response to short-term treatment with a chemotherapeutic agent. Clone 1 (expressing control shRNA) or clone 7 (expressing p53 shRNA) cells were either untreated, treated with 0.05 μg/ml doxorubicin for 6 hours or 4 days, or treated with doxorubicin for 6 hours, washed, and then incubated in the absence of drug for 4 days. Cells were then subjected to immunofluorescence staining for γ-H2AX (left panels, red). Nuclei were counterstained with DAPI (right panels, blue). D: p53-expressing cells recover from a 6 hour treatment with a chemotherapeutic agent, but not after a 2 day treatment. Clone 1 (expressing control shRNA) or clone 7 (expressing p53 shRNA) cells were treated with 0.05 μg/ml doxorubicin for 6 hours or 2 days, then washed, and allowed to grow without drug for 2–7 days. Cells were then double-stained for phosphorylated histone H3 and DNA content (propidium iodide), and analyzed by flow cytometry. Results are plotted as the ratio of treated cells positive for phosphorylated histone H3 compared to untreated cells positive for histone H3 phosphorylation.
The cell cycle profiles obtained in the doxorubicin wash-out experiments suggest that cells expressing p53, but not p53-ablated cells, respond to brief exposure to chemotherapeutic drugs by transiently arresting and then resuming cell cycling. In order to address the possibility that these cells are repairing doxorubicin-induced DNA damage, the presence of DNA damage was assayed by γ-H2AX immunoflourescence following drug wash-out (Fig. 5C). Both control and p53-ablated cells showed a strong signal following treatment with 0.05 μg/ml doxorubicin for 6 hours or four days. After four days following wash-out of doxorubicin, p53-ablated tumor cells exhibited a high degree of γ-H2AX positivity whereas control cells had levels similar to untreated cells.
Reversibility of the p53-dependent response is determined by the length of chemotherapeutic treatment and correlates with specific changes in target gene expression
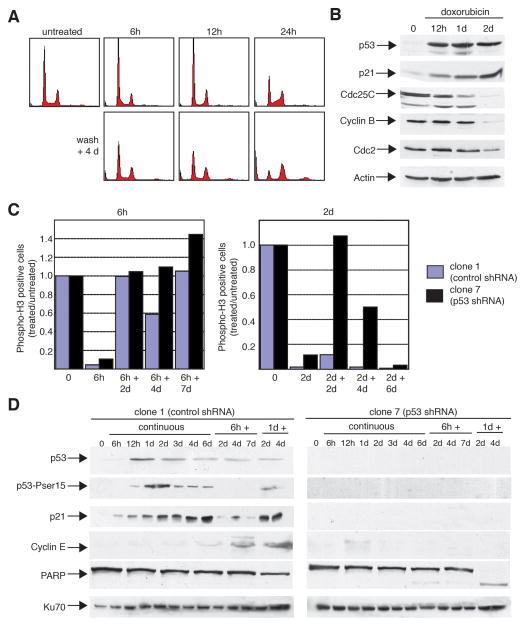
The ability of cells containing p53, but not p53-ablated cells, to recover following DNA damage implies that p53 has a role in a reversible cell cycle arrest. In order to examine this in more detail, U2OS-derived shRNA cells expressing wild-type p53 were treated with a short pulse of doxorubicin for 6 to 24 hours followed by wash-out of drug for 4 days (Fig. 6A). Both control and p53-ablated cells underwent cell cycle arrest by 6 hours as seen by a decrease in phospho-histone H3 positivity (Fig. 6C). Incorporation of bromo-deoxyuridine was unaffected at 6 hours, consistent with the idea that these cells are arresting prior to entry into mitosis (rather than at the G1/S border) (Supplemental Fig. S6). However, only cells expressing p53 showed recovery by four days after removal of drug with a cell cycle profile similar to that of untreated cells (Fig. 6A). p53-ablated cells become multinucleated at higher rates than control cells following removal of drug and do not regain a normal cell cycle distribution (Supplemental Fig. S5). Additionally, there appears to be a temporal effect, as control cells exposed to doxorubicin for 24 hours remained cell cycle arrested four days following drug removal (Fig. 6A). Following more prolonged drug treatment and wash-out, p53-ablated cells enter mitosis (Fig. 6C, right panel) and undergo extensive cell death (data not shown). Whereas p53-expressing control cells continued to restrict mitotic entry as long as 6 days after sustaining DNA damage for 48 hours, p53-ablated cells entered mitosis shortly following removal of doxorubicin. These data argue that, in the absence of p53, DNA damage leads to mitotic entry even if the genotoxic stimulus is removed. Accordingly, after either 4 days of constant treatment (Fig. 3), or 2 days after a 48 hour pulse of doxorubicin (Fig. 6C, right panel), p53-ablated cells adapt to G2 arrest and progress into mitosis.
Figure 6.
Reversibility of the p53-dependent response is determined by the length of chemotherapeutic treatment and correlates with specific changes in target gene expression. A: p53-expressing cells recover from a 6–12 hour treatment with a chemotherapeutic agent, but not from a 24 hour treatment. The U2OS derivative clone 1 (expressing control shRNA) cells were untreated or treated with 0.05 μg/ml doxorubicin for 6, 12, or 24 hours, cells were then washed, and allowed to grow in complete medium without drug for 4 days. DNA content was assayed by propidium iodide staining. B: In p53-expressing cells, p21 is upregulated early, followed by downregulation of Cdc25C, and then Cyclin B and Cdc2 in response to treatment with a chemotherapeutic agent. Parental U2OS cells were either untreated, or treated with 0.05 μg/ml doxorubicin for the indicated times. Cell extracts were analyzed by immunoblotting for levels of p53, p21, Cdc25C, Cyclin B, and Cdc2 protein levels. Actin levels serve as a loading control. C: p53-expressing cells recover from a 6 hour treatment with a chemotherapeutic agent, but not after a 2 day treatment. Clone 1 (expressing control shRNA) or clone 7 (expressing p53 shRNA) cells were treated with 0.05 μg/ml doxorubicin for 6 hours or 2 days, then washed, and allowed to grow without drug for 2–7 days. Cells were then double-stained for phosphorylated histone H3 and DNA content (propidium iodide), and analyzed by flow cytometry. Results are plotted as the ratio of treated cells positive for phosphorylated histone H3 compared to untreated cells positive for histone H3 phosphorylation. D: Clone 1 (expressing control shRNA) or clone 7 (expressing p53 shRNA) cells were either untreated, treated with 0.05 μg/ml doxorubicin continuously for 6 hours to 6 days, or treated with doxorubicin for either 6 hours or 1 day, washed, and incubated in the absence of drug for 2–7 days. Cell extracts were analyzed by immunoblotting for levels of p53, Ser15-phosphorylated p53, p21, cyclin E, and PARP protein levels. Ku70 levels serve as a loading control.
Analysis of cell cycle markers by immunoblotting following transient DNA damage provides further evidence for the dual roles of p53 in the G2 checkpoint (Fig. 6B and 6D). Treatment of parental U2OS cells expressing wild-type p53 with doxorubicin (0.05 μg/ml) leads to the time-dependent accumulation of p53 and p21 with a reciprocal down-regulation of Cyclin B1, Cdc2, and Cdc25C at later time points (Fig. 6B). In doxorubicin-treated clone 1 (p53 shRNA-expressing) cells, evidence of phosphorylation on Ser15 of p53 appears by 12 hours, and this correlates with both p21 induction (Fig. 6D) and repression of the dual-specificity phosphatase cdc25C (data not shown). In contrast, no increase in p53 or p21 is observed in clone 7 cells (Fig. 6D). Additionally, cyclin E accumulates selectively in clone 1 cells in a time-dependent manner after prolonged exposure to doxorubicin. This result, in conjunction with the concomitant decrease in cyclin B1 and Cdc2 levels suggests that p53-expressing cells transition from a G2 arrest to a G1-like state. Similar findings are observed after wash-out of a 24 hour pulse of doxorubicin (Fig. 6D, lanes marked 1d+). In this situation, p53 levels remain high and cyclin E protein accumulates. In contrast, treatment for a shorter length of time (6 hours) followed by drug washout leads to only a transient increase in p53 protein levels with little Ser15 phosphorylation detected. Strikingly, cyclin E levels do not accumulate, p21 levels recede (Fig. 6B), and Cdc2, cyclin B1, and cdc25C levels remain largely unchanged (data not shown). This suggests that transient changes, such as p53 protein accumulation and p21 induction, occur in response to short exposure to genotoxic stress, and that other, more permanent changes in gene expression occur only following longer exposures. In contrast, clone 7 cells are unable to down-regulate cyclin B1 and Cdc2 following sustained DNA damage (Fig. 4A). Coupled with the phosphorylated histone H3 data (Fig. 3 and Fig. 6C), these findings and the fact that PARP is cleaved at long exposures argue that p53-ablated cells inappropriately enter mitosis and undergo apoptosis without accumulating in G1. Furthermore, the presence of PARP cleavage at the four day time point after drug washout (Fig. 6D, 6 and 24 hour treatments) suggests that cells not expressing p53 are impaired in their ability to maintain a cell cycle arrest or to recover from transient DNA damage. Taken together, these data indicate that p53 plays a role in both a reversible cell cycle arrest and a sustained restriction of cell cycle progression in response to different durations of DNA damage.
p53 status determines tumor cell survival in response to transient chemotherapeutic treatment
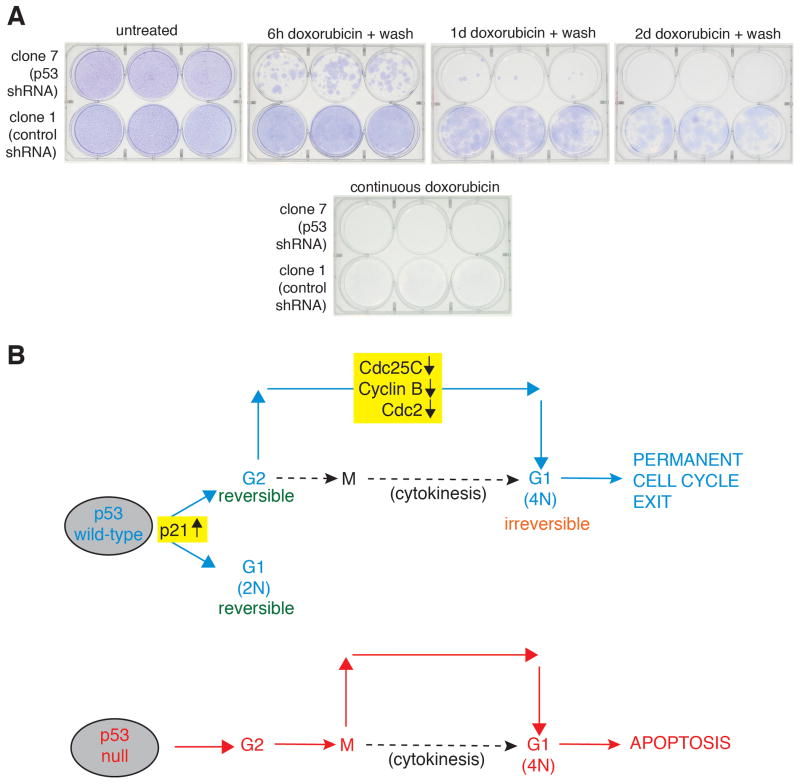
The data reported thus far indicates that cells with ablated p53 levels lack crucial checkpoint activity and are subject to a higher degree of cell death following sustained DNA damage than are cells with normal p53 levels. To examine the role p53 plays in the transient DNA damage response over longer periods of time, colony formation assays were performed (Fig. 7A). Untreated control and p53-ablated cells grew to confluency over the 30 day experimental period, and neither cell type proliferated following continuous doxorubicin (0.05 μg/ml) exposure for 30 days, consistent with earlier results (Fig. 7A). U2OS-derived cells expressing control shRNA achieved confluency following a 6 hour exposure to doxorubicin with subsequent wash-out, and achieved a high density following a one day drug exposure. Additionally, the control cells showed evidence of proliferation following a two-day drug treatment followed by wash-out. However, p53 shRNA-expressing U2OS-derived cells were impaired in their ability to proliferate following a 6 hour exposure to doxorubicin and only rare colonies appeared following a one day drug treatment. It is unclear whether the colonies which formed in the clone 7 cells represent populations of cells which no longer express the p53-shRNA plasmid or rather expansion of a small number of cells surviving the transitory genotoxic insult. Nevertheless, these data indicate that cells expressing p53 are able to recover from transient DNA damage and resume proliferation. Furthermore, these data suggest that an optimal exposure time or dosage can be ascertained to exploit the differential responses observed in p53-replete and –depleted cells in response to chemotherapeutic agents.
Figure 7.
p53 status determines tumor cell survival in response to transient chemotherapeutic treatment. A: p53 status determines tumor cell survival in response to transient chemotherapeutic treatment. Clone 1 (expressing control shRNA) or clone 7 (expressing p53 shRNA) cells were either untreated or treated with 0.05 μg/ml doxorubicin for 6 hours, 1 day, or 2 days, cells were then washed, allowed to grow in complete medium without drug for 30 days, and then stained with GiemsaCells were also treated continuously for 30 days without any washing. B: The tumor suppressor p53 plays multiple roles at the G2 checkpoint following DNA damage. There is both a reversible p53-dependent G2 arrest following transient DNA damage and a sustained p53-dependent cell cycle arrest following prolonged DNA damage. Wild-type p53 prevents mitotic entry and leads to arrest in a 4N G1 state. Cells lacking p53 transiently arrest in G2, adapt to this checkpoint, and enter mitosis. However, they fail to undergo cytokinesis and die from apoptosis in the G1 phase.
Discussion
The role of p53 in mediating the G2 DNA damage response is summarized in the model in Fig. 7B. p53 wild-type cells encountering low levels of DNA damage engage a reversible cell cycle arrest in G1 and G2 during which recovery is mediated. This involves both p53-independent (Chk1 pathway) and p53-dependent pathways. Upon more sustained or more profound damage, changes in gene expression take place, such as the down-regulation of Cdc25C, cyclin B1, and Cdc2, preventing cells from entering mitosis. These cells subsequently enter G1 with a 4N DNA content and remain presence of tetraploidy and/or DNA damage is not advantageous, and the cells become senescent. Cells lacking p53 arrest in G2 following DNA damage, but fail to down-regulate target genes and progress into mitosis. This may be due to the continuous accumulation of cyclin B1 and Cdc2 protein as the cells remain arrested in G2. In the absence of p53-dependent transcriptional repression of cyclin B1 and cdc2, levels of these gene products may accumulate past a critical threshold required for entry into mitosis. However, cells do not complete mitosis due to a failure of cytokinesis and become multinucleated and undergo apoptosis. One possible explanation for the failure to divide may be that topoisomerase II plays a role in many phases of the cell cycle, including the regulation of several processes vital to mitosis (23). Secondly, it is likely that DNA damaged mitotic chromosomes fail to complete mitosis properly, leading to mitotic exit and reformation of nuclei in a multipolar fashion.
These results highlight the importance of p53 in both short- and long-term responses to DNA damage. At early times, the presence of p53 leads to induction of the Cdk inhibitor p21, arresting cells in G1. Cells lacking p53 fail to arrest with a 2N DNA content, solely arresting in G2. Furthermore, the 4N arrest induced in p53-ablated cells is transitory, and cells proceed to cell death. Over longer periods of time, p53 protects cells from DNA damage-induced killing, and allows affected cells to repair cellular damage and recover (Fig. 5), or to permanently arrest. In contrast, p53-ablated cells receiving as little as a 6 hour pulse of doxorubicin were unable to recover from the damage and were eliminated by cell death even after drug wash-out. Therefore, under well-defined cellular circumstances, these studies demonstrate that p53 can mediate a reversible cell cycle arrest in response to DNA damage with doxorubicin.
These data are consistent with those of Bunz et al. and established the mechanism of cell death in the p53-ablated cells as apoptosis. Although the system utilized by Bunz et al. studies cells with a complete genetic disruption of the p53 locus, the system described herein offers several advantages. The control and p53 shRNA-expressing clones underwent parallel selection and therefore are truly isogenic in nature. Secondly, HCT116 cells are known to be defective for DNA repair and might not be informative for use in the DNA damage recovery assays. Additionally, these studies include molecular analyses to describe the nature of the 4N peak observed in prior studies.
Doxorubicin treatment of HeLa cells in which p53 expression was restored by targeting E6 with siRNA led to enhanced cell death by apoptosis, whereas treatment with other chemotherapeutics led to increased survival (24). In contrast, the data reported herein demonstrate that ablation of p53 in U2OS cells impairs cell cycle arrest, DNA repair, and cell survival in response to DNA damage with doxorubicin. This discrepancy may be explained by the epithelial nature of HeLa cells, although Supplemental figure S2 demonstrates that several epithelial-derived p53-replete and p53-null tumor cell lines behave similarly to U2OS in response to doxorubicin treatment. The data reported in the current study show consistent results for doxorubicin and to a lesser extent camptothecin treatment in a variety of cell types. It remains possible that p53 status may impact cells treated with other DNA damaging agents differently and that the cellular decisions influenced by p53 are cell type- and/or cell context-dependent.
Upon sustained DNA damage, p53 wild-type U2OS cells remained stably arrested with a 4N DNA content, moving from G2 to a tetraploid G1 state, which cannot be distinguished by flow cytometric analysis. A large proportion of cells expressing p53 underwent a transition from G2 to G1 despite retaining a 4N DNA content following extended exposure to doxorubicin or at long time points after drug wash-out. This change was evidenced by diminished levels of Cdc2 and cyclins A and B and elevated cyclin E expression (Fig. 4A). Interestingly, wild-type p53 cells expressed senescent-associated β-galactosidase following prolonged exposure to doxorubicin (Fig. 4D), consistent with the idea that these cells are irreversibly arrested and senescent. It is therefore likely that the role of p53 in response to a constant level of DNA damage is to prevent G2 arrested cells from entering mitosis, thereby avoiding gross aneuploidy, and causing permanent cell cycle arrest. Additionally, p53 accomplishes this switch by inhibiting the action of cyclin B/Cdc2, likely, at least partly, through the down-regulation of Cdc25C (13). Without p53, Cdc25C levels remain intact, allowing it to dephosphorylate and activate Cdc2, leading to mitotic progression. Recent work by Xue et al. demonstrated that restoring p53 expression in liver carcinomas in mice results in senescence and tumor regression in vivo, suggesting that p53-dependent induction of senescence in cancer cells is physiologically relevant (25). Indeed, studies examining the responsiveness of mouse mammary tumors with defined p53 status in vivo to treatment with doxorubicin are consistent with these findings (27). Jackson et al. demonstrated that wild-type p53 mediated cell cycle effects prevents a p53-independent apoptotic response. This is consistent with findings using clinical samples in which mutant p53-expressing breast cancers have a better response to genotoxic chemotherapy (28).
Previous studies have suggested that p53 does not play a role in initiating the observed G2 arrest following DNA damage, but does influence the maintenance of cell cycle arrest via target gene induction (7, 26). Consistent with these observations, p53-ablated (Fig. 1A) or –null (Supplemental Fig. S2) cells are able to arrest with a 4N DNA content and escape this arrest. However, cells expressing p53 were able to repair DNA damage accrued during a short pulse of doxorubicin and resumed cycling within four days of drug washout (Fig. 5 and 6), indicating that p53 does play a key role in recovery from DNA damage suffered in G2.
These data highlight the importance of p53 in mediating a reversible checkpoint in response to DNA damage. The presence of p53 is necessary for DNA-damaged cells to arrest, repair the damage, and re-enter the cell cycle. Cells lacking p53 function are unable to maintain a cell cycle arrest or to repair damaged DNA following doxorubicin treatment and undergo apoptosis. The pivotal role p53 plays in the DNA damage response may therefore prove to be an important factor in the clinical response of tumors to DNA-damaging therapies.
Supplementary Material
Implications.
Although p53 status has been suggested as a clinical predictor of chemotherapeutic efficacy, studies to date have not always supported this. This study demonstrates that p53 is still an important determinant of cell fate in response to chemotherapy, under the appropriate treatment conditions
Acknowledgments
The excellent technical assistance of Wen-jun Liu was especially appreciated. The advice and support of Matthew O’Connell, Zhen-Qiang Pan, Stuart Aaronson, Luciana Giono, and Shohreh Varmeh-Ziaie are gratefully acknowledged.
Grant Support
NIH grants R01 CA125741 (J.J. Manfredi) and T32 CA78207 (D.J. Lukin)
Footnotes
Conflicts of interest: None.
References
- 1.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 3.Liu G, Parant JM, Lang G, et al. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat Genet. 2004;36:63–8. doi: 10.1038/ng1282. [DOI] [PubMed] [Google Scholar]
- 4.Bunz F, Hwang PM, Torrance C, et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–9. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.el-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 6.Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–90. [PubMed] [Google Scholar]
- 7.Bunz F, Dutriaux A, Lengauer C, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 8.Polyak K, Waldman T, He TC, Kinzler KW, Vogelstein B. Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev. 1996;10:1945–52. doi: 10.1101/gad.10.15.1945. [DOI] [PubMed] [Google Scholar]
- 9.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–84. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 10.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–15. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 11.Taylor WR, DePrimo SE, Agarwal A, et al. Mechanisms of G2 arrest in response to overexpression of p53. Mol Biol Cell. 1999;10:3607–22. doi: 10.1091/mbc.10.11.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Innocente SA, Abrahamson JL, Cogswell JP, Lee JM. p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci U S A. 1999;96:2147–52. doi: 10.1073/pnas.96.5.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Clair S, Giono L, Varmeh-Ziaie S, et al. DNA damage-induced downregulation of Cdc25C is mediated by p53 via two independent mechanisms: one involves direct binding to the cdc25C promoter. Mol Cell. 2004;16:725–36. doi: 10.1016/j.molcel.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Wang XW, Zhan Q, Coursen JD, et al. GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci U S A. 1999;96:3706–11. doi: 10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanni JS, Jacks T. Characterization of the p53-dependent postmitotic checkpoint following spindle disruption. Mol Cell Biol. 1998;18:1055–64. doi: 10.1128/mcb.18.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart ZA, Leach SD, Pietenpol JA. p21(Waf1/Cip1) inhibition of cyclin E/Cdk2 activity prevents endoreduplication after mitotic spindle disruption. Mol Cell Biol. 1999;19:205–15. doi: 10.1128/mcb.19.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreassen PR, Lacroix FB, Lohez OD, Margolis RL. Neither p21WAF1 nor 14-3-3sigma prevents G2 progression to mitotic catastrophe in human colon carcinoma cells after DNA damage, but p21WAF1 induces stable G1 arrest in resulting tetraploid cells. Cancer Res. 2001;61:7660–8. [PubMed] [Google Scholar]
- 18.Andreassen PR, Lohez OD, Lacroix FB, Margolis RL. Tetraploid state induces p53-dependent arrest of nontransformed mammalian cells in G1. Mol Biol Cell. 2001;12:1315–28. doi: 10.1091/mbc.12.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borel F, Lohez OD, Lacroix FB, Margolis RL. Multiple centrosomes arise from tetraploidy checkpoint failure and mitotic centrosome clusters in p53 and RB pocket protein-compromised cells. Proc Natl Acad Sci U S A. 2002;99:9819–24. doi: 10.1073/pnas.152205299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shackney SE, Smith CA, Miller BW, et al. Model for the genetic evolution of human solid tumors. Cancer Res. 1989;49:3344–54. [PubMed] [Google Scholar]
- 21.Nowak SJ, Corces VG. Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation. Trends Genet. 2004;20:214–20. doi: 10.1016/j.tig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Taylor WR, Schonthal AH, Galante J, Stark GR. p130/E2F4 binds to and represses the cdc2 promoter in response to p53. J Biol Chem. 2001;276:1998–2006. doi: 10.1074/jbc.M005101200. [DOI] [PubMed] [Google Scholar]
- 23.Porter AC, Farr CJ. Topoisomerase II: untangling its contribution at the centromere. Chromosome Res. 2004;12:569–83. doi: 10.1023/B:CHRO.0000036608.91085.d1. [DOI] [PubMed] [Google Scholar]
- 24.Koivusalo R, Krausz E, Helenius H, Hietanen S. Chemotherapy compounds in cervical cancer cells primed by reconstitution of p53 function after short interfering RNA-mediated degradation of human papillomavirus 18 E6 mRNA: opposite effect of siRNA in combination with different drugs. Molecular pharmacology. 2005;68:372–82. doi: 10.1124/mol.105.011189. [DOI] [PubMed] [Google Scholar]
- 25.Xue W, Zender L, Miething C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan TA, Hermeking H, Lengauer C, Kinzler KW, Vogelstein B. 14-3-3Sigma is required to prevent mitotic catastrophe after DNA damage. Nature. 1999;401:616–20. doi: 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- 27.Jackson JG, Pant V, Li Q, Chang L, Quintás-Cardama A, Garza D, et al. p53-mediated senescence impairs the apoptotic response to chemotherapy and clinical outcome in breast cancer. Cancer Cell. 2012;21:793–806. doi: 10.1016/j.ccr.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertheau P, Lehmann-Che J, Varna M, Dumay A, Poirot B, Porcher R, et al. p53 in breast cancer subtypes and new insights into response to chemotherapy. Breast. 2013;22 (Suppl 2):S27–9. doi: 10.1016/j.breast.2013.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.