Abstract
Ovarian cancer is a lethal disease with the majority of diagnosed women having distant metastases. Interestingly, while Notch3 overexpression has been correlated with poor survival in epithelial ovarian cancer (EOC) little is known about its mechanism of action. Data show that Notch3 specifically promotes anoikis resistance. In addition, data indicate a positive role for focal adhesion kinase (FAK) as well as downstream signaling kinases such as AKT and ERK 1/2 in promoting anchorage-independent growth. Mechanistically, both mRNA transcript and protein levels of type IV collagen (COL4A2) are reduced when Notch3 levels are decreased and exogenous collagen IV supplementation reverses the anoikis sensitivity. Reduction of COL4A2 expression by RNAI-mediated knockdown induces cell death. Finally, elevated Notch3 expression levels correlate with higher COL4A2 expression in human ovarian tumor specimens.
Keywords: Notch3, Epithelial Ovarian Cancer, col4α2, anoikis resistance, Bim
Introduction
EOC is the most lethal gynecologic malignancy. In 2011, the National Cancer Institute estimated that 21,990 women would be diagnosed with ovarian cancer and 15,460 women would die from it. Approximately 70% of women diagnosed with the disease will have metastases beyond the regional lymph nodes. Once the cancer has spread from lymph nodes, the five-year survival rate drops from 72% to 27% 1. Current research focuses on developing new targets to improve overall survival as well as quality of life. The Notch signaling pathway has lately come into focus as a potential target for ovarian cancer treatments 2. Notch3 overexpression in human ovarian tumor samples correlates with shorter progression free survival as well as shorter overall survival 3. Moreover, in vitro data shows that Notch3 signaling protects cells from apoptosis 4,5. Expression of Notch3 can be oncogenic or tumor suppressive depending on the cellular context, indicating a complex role for this isoform and a strong need for further study to better understand its mechanistic role in tumor progression 6,7.
The process of metastasis in ovarian cancer requires that cells develop the ability to survive while non-adherent in the peritoneum. Given the position of the primary tumor in the peritoneal cavity, EOC cells metastasize by breaking off and using the flow of peritoneal fluid to seed the abdomen. Anoikis is a process by which normal cells undergo a specific form of apoptosis following detachment from the extracellular matrix (ECM). Normal cells that remain in contact with the ECM cross-talk with the microenvironment, promoting pro-survival signaling. Such signaling is often integrated via FAK, a protein complex that connects interior and exterior cellular signaling. Lack of ECM contact disrupts these pro-survival signals, resulting in a specialized form of apoptosis termed anoikis. Ovarian cancer cells must develop anoikis resistance in order to successfully survive in non-adherent conditions. Several mechanisms of anoikis resistance are known, including the overexpression of ECM proteins. These overexpressed ECM components coat the cell surface and allow them to “carry their own soil” in a non-adherent environment, thus maintaining downstream pro-survival signals. Classic pro-survival proteins like Akt and Erk 1/2 have been shown to cross-talk downstream of FAK to promote non-adherent cell survival 8,9. Integrin and EGFR signaling have also been implicated in regulating anoikis resistance 10.
While anoikis resistance has been studied in many cancer types, it not well understood in the context of EOC. Proteins such as GLI1, c-Met and HTRA1 have been implicated in anoikis resistance in EOC 9,11,12. Recently, Notch3 over-expression has been implicated in preventing apoptosis in EOC cells 6. Here we provide evidence that elevated Notch3 levels promote anoikis resistance in EOC cells through the excess expression of the type IV alpha 2 collagen col4a2 gene. Elevated Notch3 levels correlate with pro-survival signaling via FAK and activated Akt and Erk 1/2 to repress the pro-apoptotic protein Bim. When Notch3 and Col4a2 are reduced in ovarian cancer cells, exogenous treatment with Collagen IV is sufficient to restore cell survival signaling. Moreover, we uncovered a highly positive correlation between Notch3 and Col4a2 mRNA levels in human ovarian metastases compared to levels in primary ovarian tumors. Together, these data illuminate a Notch3-Col4a2 circuit promoting ovarian cancer cells’ ability to resist anoikis and progress to metastases in vitro and in patients.
Materials and Methods
Cell lines and reagents
Human ovarian cancer cell lines ES2, Hey, OVCAR-8 and SKOV-3 were a gift from Dr. Alexander Brodsky (Brown University). IGROV-1, OVCAR-3 and OVCAR-4 were obtained from the National Cancer Institute (NCI). A2780 cells were purchased from Sigma (93112519). Fallopian tube secretory cells (FTSEC240) were generously provided by the Drapkin lab at the Dana Farber Cancer Institute and maintained as described 13. All cells were maintained at 37°C in 5% CO2. ES2, Hey, OVCAR-8 and SKOV-3 cell lines were maintained in DMEM (Sigma) in 1% penicillin/streptomycin and 10% heat inactivated fetal bovine serum. IGROV-1, OVCAR-3 and OVCAR-4 cell lines were maintained in RPMI-1640 (HyClone), 1% penicillin streptomycin and 10% heat inactivated fetal bovine serum (Fisher Scientific). Z-VAD-FMK was purchased from RnD Systems. Y11 was purchased from Tocris (4498). Antibodies are detailed in Supplementary Table 1. siRNA and transfection reagents were purchased from Thermoscientific. On-TargetPlus Non-targeting control (D-001810-01-05), On-TargetPlus SmartPool Notch3 (011093) and On-TargetPlus SmartPool COL4A2 (003645), DharmaFECT-1 (T-2001-01). Human collagens type I and IV were purchased from Millipore (CC050 and CC076 respectively).
Cell line screen
Cells from all eight lines were collected and screened for Notch3 expression levels by qPCR and Western blotting as described previously 14. Primer sequences are detailed in Supplementary Table 2. Cells were transfected according to manufacturer’s protocol with DharmaFECT-1. All cells were collected 48h post-transfection unless otherwise noted.
Cell viability assays
Cells were plated to 50% confluence, transfected with siRNA 24h after plating and harvested 48h post-transfection by standard trypsinization protocol and stained for viability with trypan blue as described previously 15. Z-VAD-FMK was used at 50μM and cells were treated concurrently with siRNA for 48h. Cells used in adherence assays were transfected 24 hours prior to trypsinization and re-plated in adherent (A) or non-adherent (NA) dishes. Y11 was used at 10μM and cells were treated after re-plating on to A or NA dishes. LY294002 was used at 20μM and cells were treated 2h prior to collection.
Collagen rescue assay
Twenty-four hours post-transfection, siControl or siNotch3 cells were plated on dishes pre-coated with PBS, collagen type I or type IV at 6μg/cm2. Then, 24h post-plating, cells were collected for counting. Col4α2 collagen rescue experiments were conducted similarly.
Primary and metastatic human tumor samples
Pearson correlations and their significance were calculated in Microsoft Excel. Primary and metastatic tumor data are available at GSE30587. All TCGA data were downloaded from the TCGA data portal using the published dataset freeze (http://www.nature.com/nature/journal/v474/n7353/full/nature10166.html). All TCGA data include primary ovarian tumors only. The Australian Oncology Group microarray data for ovarian tumors, GSE9891, was downloaded from GEO and processed using RMA. Q values were calculated in R.
Statistical Analysis
Two-way ANOVA and Tukey’s test were used and a p value of 0.05 was considered significant. All data is reported as means ± SD. Statistical analyses were performed using GraphPad software.
Results
Notch3 signaling promotes anoikis resistance in human ovarian cancer cell lines
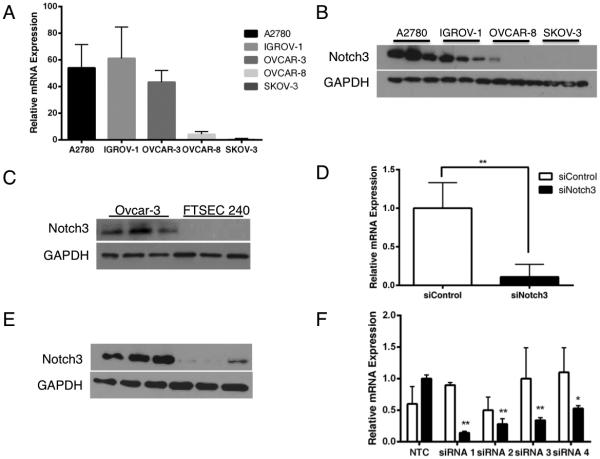
The Notch signaling pathway has emerged as a therapeutic target in many diverse types of cancer 16-18. In EOC, oncogenic Notch3 signaling has been shown to protect human ovarian cancer cells from apoptosis 6,19. We hypothesized that an elevated level of Notch3 was not only protecting these cells from classical cell death but that Notch3 was preventing a specific type of apoptosis: anoikis. To test this hypothesis, we screened five human epithelial ovarian cancer cell lines and an immortalized, non-transformed fallopian tube secretory cell line (FTSEC) for relative levels of Notch3 expression (Figure 1A, 1B and 1C) and established a reliable siRNA-mediated knockdown of Notch3 in a subset of these cells using a pool of four siRNAs (Figure 1D and E). The specificity and potency of individual siRNAs were tested by qPCR and found to be slightly less effective but equally specific to the siRNA pool (Figure 1F). We found that out of five cell lines examined, three had significantly increased levels of Notch3 at the mRNA and protein levels: A2780, OVCAR-3 and IGROV-1. In order to examine the cellular response to a reduction of Notch3 signaling, we used a trypan blue exclusion count of control (siControl) and of Notch3-knockdown (siNotch3) IGROV-1 cells at 48 hours post-transfection. We found that a reduction in Notch3 expression resulted in a significant increase in trypan blue positive/dead cells (Figure 2A). Additionally, concurrent treatment with the pan-caspase inhibitor Z-VAD-FMK resulted in a rescue of the cell population at 48 hours post-transfection (Figure 2B). To confirm that siNotch3 cells are undergoing apoptosis, we examined levels of cleaved PARP and saw that siNotch3 cells had elevated levels of cleaved PARP compared with siControl cells by immunoblot (Figure 2C). These data indicate that siNotch3 knockdown IGROV-1 cells were undergoing apoptosis, as had been previously reported in other cell lines 19.
Figure 1.
Human IGROV-1 ovarian cancer cells model the upregulation of Notch3 in human ovarian tumors. A2780, IGROV-1 and OVCAR-3 cells express significantly more Notch3 mRNA (A) and protein (B) than OVCAR-8 or SKOV-3 cells. OVCAR-3 cells express Notch3 whereas FTSEC 240 cells do not (C). Transfection with Notch3 siRNA efficiently reduces expression of the Notch3 receptor at the mRNA (D) and protein (E) levels (p < 0.01). (F) Individual Notch3 siRNA strands significantly reduce Notch3 mRNA expression (* p <0.05 and ** p < 0.01).
Figure 2.
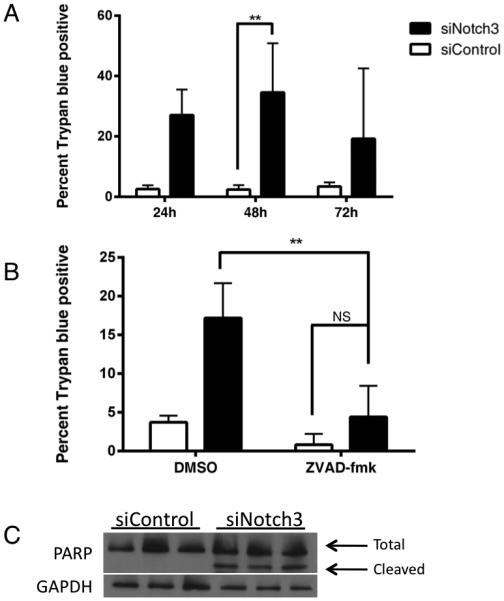
Notch 3 is required for survival of IGROV-1 cells. (A) Cell count analysis by trypan blue exclusion showed significantly fewer cells at 96h between 0h siCtrl-48h siCtrl and 0h siNotch3-48h siNotch3. (B) Treatment of siNotch3 IGROV-1 cells with the pan-caspase inhibitor Z-VAD-FMK showed a significant reduction of apoptotic cells compared with vehicle treated siNotch3 cells. There was a significant (p < 0.01) increase in trypan blue positive cells in siNotch3 DMSO treated cells as compared with siControl DMSO treated cells and a non-significant reduction in siControl cells treated with Z-VAD-FMK. These relationships are not shown for clarity of the figure. (C) siNotch3 cells express cleaved PARP whereas siControl cells do not.
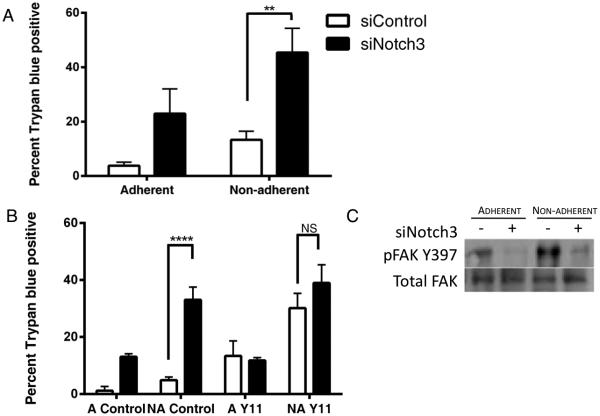
In order to determine if siNotch3 cells were undergoing anoikis, we compared their survival in adherent and non-adherent conditions. To this end, trypsinized IGROV-1 cells that had been treated with Notch3 siRNA for 24 hours were transferred to either adherent or ultra-low attachment plates. At 48 hours post-transfection, we used trypan blue exclusion to assess the extent of cell viability. We found that there was significantly more dead cells in the siNotch3 cells cultured in non-adherent conditions than in control cells in non-adherent wells or Notch3-knockdown cells in adherent conditions (Figure 3A). A non-significant increase in trypan blue positive cells was seen in non-adherent siControl cells as compared with adherent siControl cells. A small increase in cell death was expected and observed in non-adherent siControl cells following transfer to non-adherent conditions. To support the notion that these cells were undergoing anoikis and not just apoptosis, we used the FAK inhibitor Y11 with siControl and siNotch3 cells. This inhibitor prevents FAK from autophosphorylation at Y397 and activating downstream pro-survival pathways. Treatment with 10μM Y11 increased trypan blue positive cells in non-adherent siControl cells but had no significant impact on siNotch3 cells in either adherent or non-adherent conditions (Figure 3B).
Figure 3.
Notch3 promotes anoikis resistance. (A) Cells treated with control or Notch3 siRNA were incubated in adherent (A=Adherent) or non-adherent (NA= Non-adherent) conditions for 24, 48 and 72 hours and counted by trypan blue exclusion. siNotch3 cells plated in non-adherent conditions undergo significantly more apoptosis than their siControl counterparts. There was a significant increase (p < 0.01) in trypan blue positive cells in adherent siNotch3 samples as compared with siControl samples. There was no significant difference in siControl adherent and non-adherent samples. These relationships are not shown for clarity of the figure. (B) Pharmacological inhibition of focal adhesion kinase (FAK) activation by Y11 in control cells phenocopies the Notch3 knockdown and Y11 treatment did not significantly alter the number of apoptotic siNotch3 cells. Significantly more trypan blue positive cells were seen in non-adherent siNotch3 cells compared with siControl cells (p < 0.001). (C) Phosphorylated FAK (Y397) is decreased in siNotch3 compared with siControl cells in both adherent and non-adherent conditions.
We also observed that the levels of phosphorylated FAK at Y397 were reduced in the siNotch3 cells as compared with siControl (Figure 3C). One of the hallmarks of anoikis is the upregulation of the pro-apoptotic protein Bim 19. Analysis by immunoblot showed that Bim was enriched in siNotch3 knockdown cells compared with siControl cells (Figure 4A). Plating siNotch3 cells in non-adherent conditions further increased Bim expression and induced the expression of the L isoform of Bim in addition to the EL isoform. An increase in Bim in control cells in non-adherent conditions was expected due to the increase in cell death shown in Figure 3A. Interestingly, mRNA levels of Bim were not significantly altered (Figure 4C). This result agrees with published data suggesting a post-transcriptional mechanism of Bim suppression 20. Additionally, levels of other pro-apoptosis proteins Bad, Bax and Bak were not significantly altered between siControl and siNotch3 cells. Reduction of Notch3 expression did not significantly impact expression of the pro-survival proteins Bcl-2, Bcl-XL or Mcl-1. Together, these data indicate that siNotch3 cells are undergoing intrinsic, Bim-mediated anoikis and that elevated levels of Notch3 may promote anoikis-resistance.
Figure 4.
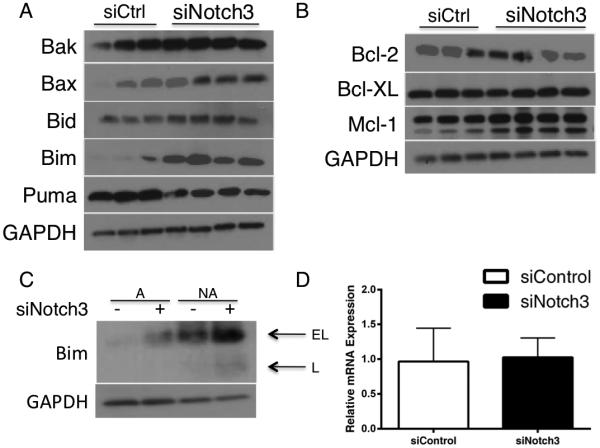
Bim expression and activation correlates with reduced Notch3 expression and increased anoikis. (A) IGROV-1 cells treated with Notch3 siRNA have increased Bim EL expression compared with control counterparts. (B) siNotch3 cells in non-adherent conditions express more Bim than siNotch3 cells in adherent conditions or siCtrl cells in adherent or non-adherent conditions. (C) Bim mRNA expression does not significantly change with Notch3 reduction.
Notch3 promotes anoikis resistance via activated Akt and Erk
The focal adhesion complex promotes cell survival through the activation, modification and regulation of many proteins, including the PI3K/Akt and MAPK/Erk pathways. Cross-talk between Akt and Erk has been shown to promote anoikis resistance in other cancer types 11,20. We examined total and activated levels of Akt and Erk 1/2 in siControl and siNotch3 cells by Western blot and found that loss of Notch3 decreased phosphorylated levels of both these proteins (Figure 5A). Comparing levels of phosphorylated Akt and Erk 1/2 between adherent and non-adherent Notch3-knockdown cells showed a reduction in their expression in non-adherent samples. Interestingly, when treated with the PI3K inhibitor LY294002, these cells showed a decrease in both phospho-Akt and phospho-Erk 1/2 (Figure 5B) suggesting cross-talk between these pathways during Notch3-mediated anoikis resistance.
Figure 5.
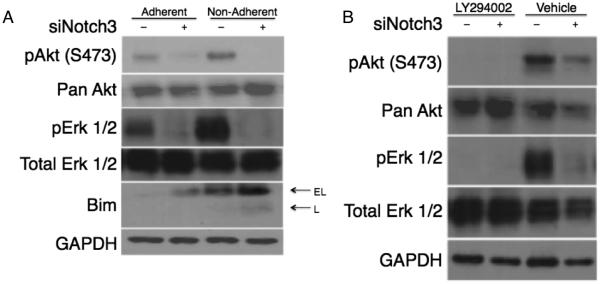
Notch3 signaling promotes Akt and Erk regulation of Bim. Notch3 signaling induces phosphorylation of Akt Ser473 and Erk 1/2 in both adherent and non-adherent conditions. (A) The reduction of pAkt and pErk 1/2 result in an increase in Bim expression. Inhibition of PI3K is sufficient to reduce both Akt and Erk 1/2 activation. (B) Treatment of siCtrl and siNotch3 cells with the PI3K inhibitor LY294002 (50μM) for 1h in adherent conditions is sufficient to inhibit phosphorylation of Erk 1/2 comparable to vehicle treated siNotch3 cells.
Notch3 signaling promotes the expression of the type IV alpha 2 collagen gene
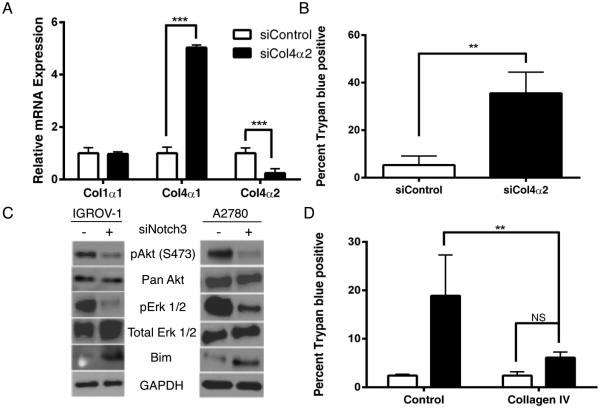
One of the mechanisms by which cancer cells can induce anoikis resistance is via the overexpression of ECM proteins 21. We hypothesized that the anoikis resistance we observed in human ovarian cancer cells may be due to the cells coating themselves in ECM, activating integrins and inducing pro-survival signaling in the absence of adherence to the basement membrane. Collagen type IV is a significant component of basement membranes. To determine if collagen was being aberrantly expressed, we assessed levels of collagen alpha chains by quantitative RT-PCR. We found that mRNA levels of col4α2 were significantly decreased in siNotch3 cells compared with siControl in both IGROV-1 and OVCAR-3 cell lines (Figure 6A and B). Reduction in Notch1 levels did not alter levels of col4α2 expression (Supplementary Figure 1). As the promoters of col4α2 and col4α1 are in a head-to-head conformation, we examined expression levels of col4α1 as well and saw a non-significant trend towards decreased expression of col4α1 in IGROV-1 siNotch3 cells. However, in OVCAR-3 siNotch3 cells, the decrease in both col4α2 and col4α1 was statistically significant. No change was observed in col1α1 expression. Analysis by western blot showed that levels of collagen type IVα2 protein were reduced in siNotch3 IGROV-1 cells (Figure 6C). These data suggest that Notch3 promotes the proper expression of col4α2, which may promote anoikis resistance.
Figure 6.
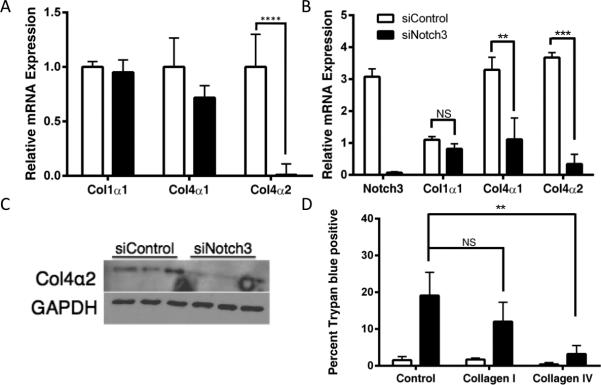
Notch3 is required for proper expression of col4α2. Both IGROV-1 and OVCAR-3 siNotch3 cells express significantly less col4α2 mRNA (A and B) than their control counterparts (** p = < 0.01, *** = p < 0.005, **** = p < 0.001). Col4α2 mRNA expression is not significantly altered in siNotch1 cells. (C) Protein-level expression of the col4α2 chain is reduced in IGROV-1 siNotch3 cells as compared with siControl cell by immunoblot. (D) Exogenous collagen IV rescues Notch3 knockdown cells (** = p < 0.01, *** = p < 0.005). IGROV-1 cells 24h post-transfection with Notch3 siRNA show significantly less cell death when plated on exogenous human collagen type IV for 24h than siNotch3 cells plated in human collagen Type I or control wells (p < 0.01).
Given the key role type IV collagens have in the basement membrane and our finding that col4α2 expression was selectively reduced in siNotch3 cells, we aimed to determine whether siNotch3 cells could be rescued by the addition of exogenous type IV collagen. To that end, we coated plates with PBS, type I collagen or type IV collagen, on which we seeded cells that had been transfected with non-targeting control siRNA or Notch3 siRNA for 24 hours. We then determined the extent of cell death by trypan blue exclusion 24 hours post-plating and found that type IV but not type I collagen significantly improved cell survival in siNotch3 samples (Figure 6D). These data indicate that increased expression of Type IV collagen may promote anoikis resistance.
In order to more definitively determine the role of col4α2 in the promotion of anoikis resistance in ovarian cancer, we transfected IGROV-1 cells with col4α2 siRNA. We used qPCR to measure the expression of col4α2 at the mRNA level and observed a decrease of col4α2 and a concurrent increase in col4α1 expression (Figure 7A). Assessment of cell death by trypan blue exclusion revealed a significant increase in cell death among the siCol4α2 samples (Figure 7B). Analysis of Bim, phospho-Akt and phospho-Erk 1/2 in siCol4α2 samples phenocopied siNotch3 cells with a reduction in pAkt, pErk 1/2 and concomitant increase in Bim in both the IGROV-1 and A2780 cell lines (Figure 7C). Similarly, siCol4α2 cells were rescued via plating on exogenous type IV collagen, as were the siNotch3 cells (Figure 7D). A similar reduction in phosphorylated Akt and Erk 1/2 were seen in A2780 siCol4α2 cells. These experimental data indicate that the Notch3-Col4α2 circuit promotes anoikis resistance in human ovarian cancer cells.
Figure 7.
Collagen type IVα2 is critical to ovarian cancer cell survival. (A) Reduction in Col4α2 expression induces cell death. IGROV-1 cells transfected with Col4α2 siRNA have significantly more cell death and fewer total cells compared with their control counterparts (p < 0.005). (B) Type IV collagen is sufficient to rescue siCol4α2 cells from cell death (p < 0.01). (C) siCol4α2 show a reduction in phosphorylated Akt and Erk 1/2 as well as an increase in Bim. Shown are representative samples of 3 biological replicates. (D) siCol4α2 cells plated on exogenous collagen type IV have significantly fewer dead cells and more total cells than siCol4α2 cells plated in control wells (p < 0.01).
Increased notch3 expression correlates with increased col4α2 mRNA expression in human ovarian tumors and their metastases
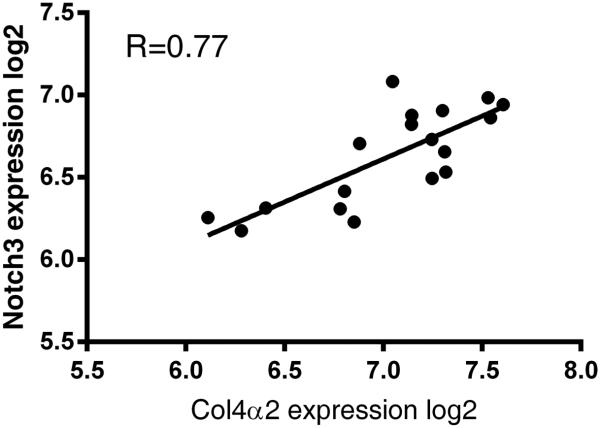
In order to determine if the relationship between notch3 and col4α2 observed in ovarian cancer cells is relevant to tumors derived from epithelial ovarian cancer patients, we examined the correlation of mRNA expression using multiple datasets. Strikingly, the expression of Notch3 and col4α2 are significantly correlated in a small but matching set of nine primary tumors and their corresponding metastatic tumors (Figure 8). This relationship is also observed in large tumor datasets from TCGA (Supplemental Table 3). Together, these observations are consistent with the positive regulation of notch3 over-expression with col4α2 expression in human ovarian tumors and metastases. Together, the clinical and experimental data suggest that targeting the Notch3-col4α2 circuit may have beneficial effects in controlling the development or spread of EOC.
Figure 8.
Notch3 overexpression correlates with increased col4α2 expression in metastatic versus primary human ovarian tumors. Pearson correlation of 0.77 indicates a strong correlation between notch3 expression and col4α2 expression (p < 0.000143)
Discussion
Like many advanced cancers, metastatic epithelial ovarian cancer remains difficult to treat. Standard therapies result in remission but rarely a cure. Survival data suggest that preventing or reversing anoikis resistance could assist in preventing tumor cell spread and increasing survival. The results of recent studies show that aberrant Notch3 signaling promotes survival in ovarian cancer cells 19,22. We confirmed these results and demonstrated that not only does Notch3 prevent apoptosis, it prevents a very specific form of apoptosis: anoikis. We demonstrated that Notch3 promotes anoikis resistance by promoting the expression of col4α2 and therefore the completion of the type IV collagen trimer–a key component of basement membrane. The increased expression of type IV collagen may allow advanced ovarian cancer cells to deceive proteins like integrins, which are charged with detection of ECM contact, into maintaining pro-survival signals even when no true ECM contact is at hand. Via these linker proteins, the activation of FAK and the activation of key pro-survival cytosolic proteins like Akt and Erk 1/2, the cell is able to repress Bim, which is responsible for the initiation of anoikis.
Indeed, this reduction in collagen IV expression is so important to cell survival that growth on exogenous collagen type IV is all that is required to rescue dying siNotch3 ovarian cancer cells. Additionally, reduction in col4α2 expression induces cell death similarly to the reduction in Notch3 and prevents phosphorylation of Akt and Erk 1/2 while promoting the expression of Bim. This demonstrates a reliance of EOC cells on these external signals to maintain viability when non-adherent. Our data also show that mRNA expression of Bim is not altered in siNotch3 cells, indicating a post-translational modification of the protein. Activated Akt and Erk 1/2 have been shown to phosphorylate Bim, targeting it for ubiquitination 23. This is consistent with the results we have seen at the mRNA and protein levels.
Notch3 overexpression has been demonstrated in vitro to prevent apoptosis, induce chemoresistance and alter the cell cycle 5,24. Altered copy number of Notch3 is associated with aggressive tumor behavior and a poor prognosis 2,5. Our results indicate that Notch3 promotes cross-talk between the ECM and cytosolic proteins, playing a significant role in disease progression. Not only can the ECM provide pro-survival signals but also the collagen matrix can alter the cell cycle and induce proliferation changes 25, which may account for some of the cell cycle alterations observed in previous studies. The ECM and collagen are increasingly understood to be important mediators of EOC progression. Transcriptional differences in collagen gene expression have been associated with poor overall survival in metastatic serous ovarian cancer and type IV collagen expression has been shown to be higher in metastatic EOC tumors than in the primary tumor 26,27. These data, combined with our results, indicate that more work is required on the interactions between the cell surface, the ECM and EOC disease progression.
Additionally, while the majority of studies in human samples have been performed using high-grade serous tumors, some data suggests that endometrioid-type tumors may also develop deleterious Notch3 amplifications in recurrent tumors 19. The three cell lines used in our studies have been shown to be of different subtypes. While OVCAR-3 cells are likely to be of the high-grade serous (HGSOC) subtype, the A2780 are not 28. IGROV-1 cells are considered “hypermutated” and may have developed new adaptations although the original tumor was determined to be mainly endometrioid with some characteristics of HGSOC and undifferentiated foci 29.
While the effect of Notch3 overexpression is increasingly studied in EOC, anoikis resistance remains relatively unexamined. However, we feel that exploring this concept in the context of EOC is critical to the development of better drug targets for ovarian cancer patients. Indeed, Taddei et al. described anoikis resistance as an “emerging hallmark” of cancer in a 2012 review 21. Due to the position of a primary ovarian tumor within the peritoneum, EOC cells can break off from the main tumor and travel via the peritoneal fluid to seed the abdominal cavity. Thus, metastasis in EOC does not require intravasation, survival in the bloodstream and extravasation; therefore the development of anoikis resistance may be a major requirement for dissemination. Unlike many other cancers, spread from the vasculature does not impact patient survival 30. In EOC, downregulation of Htra1 was shown to promote anoikis resistance as was the suppression of Bcl-XL 9,31. Stress has also been shown to activate FAK and protect ovarian cancer cells from anoikis by hormonal regulation 32. The over expression of TrkB or c-Met has also been implicated in the development or maintenance of anoikis resistance 11,33. We have seen that that the expression of type IV collagen is the key to anoikis resistance in three divergent models of EOC: IGROV-1, OVCAR-3 and A2780 cells. The stimulation of cell survival by the expression of the type IV collagen chain may activate different cytosolic pro-survival pathways, depending on the EOC subtype, stage of disease and mutations acquired.
We did not directly examine binding of the Notch3/CSL complex to the col4α2 promoter although this is a logical next step. Given the dramatic changes in expression of col4α2 mRNA following reduction of Notch3 expression, col4α2 could be a direct target of Notch3 signaling. However, the Notch pathways are notoriously complex and further analysis by ChIP or ChIP-seq is required to better understand the regulation of col4α2 by Notch3. It was also interesting to see that a reduction in Notch1 did not significantly alter the expression of col4α2. Due to the specificity of Notch signaling, it is not completely surprising that the different receptors would have unique downstream targets and impacts. This difference only highlights the targeted nature of Notch signaling and the importance of understanding the impact of each isoform. In that vein, it could be possible for expression levels of Notch3 to impact signaling, that amount of Notch3 expressed could correlate with different cellular outcomes.
Chen et al. showed in a ChIP-on-chip analysis of Notch3 targets in the OVCAR-3 cell line, two integrins were shown to be significantly enriched: ITGA2B and ITGAE 24. These could be noteworthy alterations in expression as both are involved in adhesion of leukocytes and have the RGD peptide sequences required to bind collagens 34. Though the binding of CD41 and CD103 to type IV collagen is not well characterized, it can be speculated that expression of these integrin alpha chains allowed the EOC cell to fine-tune the pro-survival signaling through FAK to promote survival in a specific set of environmental conditions.
Long-term survival for patients with advanced ovarian cancer is at best uncertain. Novel therapies are required to make significant gains in five-year survival rates in these populations. Targeting the Notch3-specific pathway and its downstream effects has been shown to be promising– indeed there are clinical trials underway using Notch3 inhibitors in combination with standard chemotherapeutics. One of the main goals of these Notch3 inhibitors is the “re-sensitization” of tumors that have developed resistance to mainstream chemotherapeutics. We hope these mechanistic data will provide novel targets and strategies for future clinical trials and enhance the responsiveness of tumors to treatment.
Implications: These data highlight type IV collagen as a novel therapeutic target for metastatic epithelial ovarian cancer.
Acknowledgements
The authors would like to thank the members of the Freiman lab for critical input throughout these studies and especially thank Jennifer Ribeiro, Kathryn Grive and Kimberly Seymour for helpful suggestions on the manuscript. We would like to thank Kim Boekelheide and Maggie Vantangoli of Brown University for the generous gift of the PARP antibody. The authors would like to thank Ronny Drapkin and Alison Karst of the Dana Farber Cancer Institute for the gift of the FTSEC cell line. We thank the Pathobiology Graduate Program and the Division of Biology and Medicine at Brown who generously funded this research. We acknowledge the support of the following awards, ACS Research Scholar Grant (DMC-117629) and NIH R01 (HD065445) to RNF; NIH COBRE Center for Cancer Signaling Networks (RR031153); IMSD (2R25GM083270)
Financial support: NIH R01 HD065445 and ACS Research Scholar Grant DMC-117629 to RNF, NIH COBRE Center for Cancer Signaling Networks RR031153 and Initiative to Maximize Student Diversity R25GM083270 to Brown University.
Footnotes
Conflicts of interest: none
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. SEER Cancer Statistics Review 1975-2010 National Cancer Institute. Natl. Cancer Inst. 2013;1:1992–2010. [Google Scholar]
- 2.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JT, Shih I-M, Wang T-L. Identification of Pbx1, a potential oncogene, as a Notch3 target gene in ovarian cancer. Cancer Res. 2008;68:8852–60. doi: 10.1158/0008-5472.CAN-08-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi JH, Park JT, Davidson B, Morin PJ, Shih LM, Wang TL. Jagged-1 and Notch3 juxtacrine loop regulates ovarian tumor growth and adhesion. Cancer Res. 2008;68:5716–23. doi: 10.1158/0008-5472.CAN-08-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JT, Li M, Nakayama K, Mao TL, Davidson B, Zhang Z, et al. Notch3 gene amplification in ovarian cancer. Cancer Res. 2006;66:6312–8. doi: 10.1158/0008-5472.CAN-05-3610. [DOI] [PubMed] [Google Scholar]
- 6.Park JT, Chen X, Tropè CG, Davidson B, Shih IeM, Wang TL. Notch3 overexpression is related to the recurrence of ovarian cancer and confers resistance to carboplatin. Am. J. Pathol. 2010;177:1087–94. doi: 10.2353/ajpath.2010.100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui H, Kong Y, Xu M, Zhang H. Notch3 functions as a tumor suppressor by controlling cellular senescence. Cancer Res. 2013;73:3451–9. doi: 10.1158/0008-5472.CAN-12-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y, Gierut J, Wang Z, Miao J, Asara JM, Tyner AL. Protein tyrosine kinase 6 protects cells from anoikis by directly phosphorylating focal adhesion kinase and activating AKT. Oncogene. 2013;32:4304–12. doi: 10.1038/onc.2012.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He X1, Ota T, Liu P, Su C, Chien J, Shridhar V. Downregulation of HtrA1 promotes resistance to anoikis and peritoneal dissemination of ovarian cancer cells. Cancer Res. 2010;70:3109–3118. doi: 10.1158/0008-5472.CAN-09-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reginato MJ1, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat. Cell Biol. 2003;5:733–40. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 11.Tang MK, Zhou HY, Yam JW, Wong AS. c-Met overexpression contributes to the acquired apoptotic resistance of nonadherent ovarian cancer cells through a cross talk mediated by phosphatidylinositol. Neoplasia. 2010;12:128–138. doi: 10.1593/neo.91438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandala PK, Srivastava SK. Diindolylmethane-mediated Gli1 protein suppression induces anoikis in ovarian cancer cells in vitro and blocks tumor formation ability in vivo. J. Biol. Chem. 2012;287:28745–54. doi: 10.1074/jbc.M112.351379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karst AM, Levanon K, Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc. Natl. Acad. Sci. U. S. A. 2011;108:7547–7552. doi: 10.1073/pnas.1017300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wardell JR, Hodgkinson KM, Binder AK, Seymour KA, Korach KS, Vanderhyden BC, et al. Estrogen responsiveness of the TFIID subunit TAF4B in the normal mouse ovary and in ovarian tumors. Biol. Reprod. 2013;89:116. doi: 10.1095/biolreprod.113.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strober W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001;3 doi: 10.1002/0471142735.ima03bs21. Appendix 3B. [DOI] [PubMed] [Google Scholar]
- 16.Gramantieri L, Giovannini C, Lanzi A, Chieco P, Ravaioli M, Venturi A, et al. Aberrant Notch3 and Notch4 expression in human hepatocellular carcinoma. Liver Int. 2007;27:997–1007. doi: 10.1111/j.1478-3231.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- 17.Ban J, Bennani-Baiti IM, Kauer M, Schaefer KL, Poremba C, Jug G, et al. EWS-FLI1 suppresses NOTCH-activated p53 in Ewing’s sarcoma. Cancer Res. 2008;68:7100–9. doi: 10.1158/0008-5472.CAN-07-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006;66:1517–25. doi: 10.1158/0008-5472.CAN-05-3054. [DOI] [PubMed] [Google Scholar]
- 19.Iwakiri D, Minamitani T, Samanta M. Epstein-Barr virus latent membrane protein 2A contributes to anoikis resistance through ERK activation. J. Virol. 2013;87:8227–34. doi: 10.1128/JVI.01089-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007;4:e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taddei M, Giannoni E. Anoikis: an emerging hallmark in health and diseases. J. Pathol. 2012;226:380–393. doi: 10.1002/path.3000. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Samadi AK, Roby KF, Timmermann B, Cohen MS. Inhibition of cell growth and induction of apoptosis in ovarian carcinoma cell lines CaOV3 and SKOV3 by natural withanolide Withaferin A. Gynecol. Oncol. 2012;124:606–12. doi: 10.1016/j.ygyno.2011.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins N, Reginato M. G1/S Cell Cycle Arrest Provides Anoikis Resistance through Erk-Mediated Bim Suppression. Mol. Cell. Biol. 2005;25:5282. doi: 10.1128/MCB.25.12.5282-5291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Thiaville MM, Chen L, Stoeck A, Xuan J, Gao M, et al. Defining NOTCH3 target genes in ovarian cancer. Cancer Res. 2012;72:2294–2303. doi: 10.1158/0008-5472.CAN-11-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koohestani F, Braundmeier AG, Mahdian A, Seo J, Bi J, Nowak RA. Extracellular Matrix Collagen Alters Cell Proliferation and Cell Cycle Progression of Human Uterine Leiomyoma Smooth Muscle Cells. PLoS One. 2013;21 doi: 10.1371/journal.pone.0075844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bar JK, Grelewski P, Popiela A, Noga L, Rabczyñski J. Type IV collagen and CD44v6 expression in benign, malignant primary and metastatic ovarian tumors: correlation with Ki-67 and p53 immunoreactivity. Gynecol. Oncol. 2004;95:23–31. doi: 10.1016/j.ygyno.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 27.Cheon DJ, Tong Y, Sim MS, Dering J, Berel D, Cui X, et al. A collagen-remodeling gene signature regulated by TGFβ signaling is associated with metastasis and poor survival in serous ovarian cancer. Clin. Cancer Res. 2013;20:711–23. doi: 10.1158/1078-0432.CCR-13-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bénard J, Da Silva J, De Blois MC, Boyer P, Duvillard P, Chiric E, et al. Characterization of a Human Ovarian Adenocarcinoma Line , IGROV1 , in Tissue Culture and in Nude Mice. Cancer Res. 1985;45:4970–4979. [PubMed] [Google Scholar]
- 30.Cormio G, Rossi C, Cazzolla A, Resta L, Loverro G, Greco P, et al. Distant metastases in ovarian carcinoma. Int. J. Gynecol. Cancer. 2003;13:125–9. doi: 10.1046/j.1525-1438.2003.13054.x. [DOI] [PubMed] [Google Scholar]
- 31.Frankel A, Rosen K, Filmus J, Kerbel RS. Induction of Anoikis and Suppression of Human Ovarian Tumor Growth in Vivo by Down-Regulation of Bcl-XL. Cancer Res. 2001;16:4837–4841. [PubMed] [Google Scholar]
- 32.Sood AK, Armaiz-Pena GN, Halder J, Nick AM, Stone RL, Hu W, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J. Clin. Invest. 2010;120:1515–1523. doi: 10.1172/JCI40802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu X, Liu L, Cai B, He Y, Wan X. Suppression of anoikis by the neurotrophic receptor TrkB in human ovarian cancer. Cancer Sci. 2008;99:543–52. doi: 10.1111/j.1349-7006.2007.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takada Y, Hemler ME. The primary structure of the VLA-2/collagen receptor alpha 2 subunit (platelet GPIa): homology to other integrins and the presence of a possible collagen-binding domain. J. Cell Biol. 1989;109:397–407. doi: 10.1083/jcb.109.1.397. [DOI] [PMC free article] [PubMed] [Google Scholar]