Abstract
Objective
High altitude has been implicated in a variety of adverse pregnancy outcomes including preeclampsia and stillbirth. Smaller studies show conflicting data on the association between high altitude and preterm birth (PTB). The objective of this study was to assess the association between altitude and PTB.
Study Design
A retrospective cohort study was performed using data from the Perinatal Information System which includes deliveries from 43 hospitals in Peru from 2000–2010. Altitude was classified into: low (0–1999m), moderate (2000–2900m), and high (3000–4340m). The primary outcome was PTB (delivery <37 weeks). Secondary outcomes were cesarean delivery and small for gestational age (SGA). Deliveries <23 weeks are not included in the database. Chi-square analyses were performed to compare categorical variables and logistic regression was used to calculate odds ratios and control for confounders. Clustering by hospital was accounted for using generalized estimating equations.
Results
550,166 women were included (68% low, 15% moderate, 17% high altitude). The overall PTB rate was 5.9% with no difference in PTB rate among the 3 altitudes (5.6, 6.2, 6.8%, p=0.13). There was a significant difference in cesarean rates (28.0, 26.6, 20.6%, p<0.001) with a 34% decreased risk at high vs. low altitude adjusted for confounders (aOR 0.66 [0.51–0.85]). There was a difference in SGA (3.3, 3.6, 5.0%, p=0.02) with a 51% increased risk at high vs. low altitude adjusted for confounders (aOR 1.49 [1.14–1.93]).
Conclusions
High altitude is not associated with PTB. At high altitude, the cesarean rate was reduced and SGA rate was increased.
Keywords: Cesarean delivery, high altitude, Peru, preterm birth, small for gestational age
Introduction
Preterm birth (PTB) is a leading cause of perinatal morbidity and mortality worldwide (1–4). The reality of neonatal morbidity and health care costs associated with PTB are well known and are a major public health concern both nationwide and worldwide. In developing countries, PTB is a known contributor to neonatal mortality. In 2000, the United Nations held a summit to create goals for improving the world’s health and poverty. The fourth Millennium Development Goal is to reduce the child mortality rate by two-thirds from 1990 to 2015 (5). Among child mortality, neonatal deaths are a major contributor, with a large proportion of neonatal deaths being from complications of prematurity.
In developing countries, where there are fewer resources, fewer hospitals, and fewer healthcare providers trained in caring for a preterm infant, the neonatal mortality rate (NMR) remains incredibly high. In Latin America, the PTB rate is estimated to be 6% (5) with an NMR of 15 per 1000 live births, which is 3 times as high as the United States (1,2). These rates are even higher in the country of Peru where the PTB rate ranges from 6–18% and the NMR is 20 per 1000 live births, with large variations depending upon geographical location (2–4).
There are many identifiable risk factors for PTB (7–14); however, to date, there is conflicting data on the association between high altitude and PTB (15–17). Over 140 million people live at high altitude in North, Central and South America, East Africa, and Asia (18). Peru is a country of more than 30 million people. The country is divided into three regions: the coastal area at sea level, the Andean region at high altitudes and the Amazonian jungle region at low altitude. Approximately 9 million people live at moderate or high altitude in the Andean regions (3–4). High altitude has been associated with decreased uterine artery blood flow, increased uteroplacental resistance, alterations in the expression of placental factors, chronic hypoxia, and changes in vascularity and has been implicated in a variety of adverse pregnancy outcomes including intrauterine growth restriction, low birth weight infants, intrauterine fetal demise, and preeclampsia (15,19–24). Some of these same physiologic changes have been observed and linked specifically with PTB (26,27). Previous studies that have evaluated the impact of high altitude on PTB have not been designed nor powered to specifically evaluate the PTB outcome (15–17). Therefore, the objective of this study is to evaluate the association between PTB and high altitude in Peruvian pregnant women.
Materials and Methods
A retrospective cohort study was performed using data from the Perinatal Information System (PIS) database which includes deliveries from 43 urban, public hospitals belonging to the Ministry of Health in Peru. Institutional Review Board approval was obtained from the University of Pennsylvania and the Universidad Peruana Cayetano Heredia prior to this study.
The PIS database was developed by the Latin American Centre for Perinatology/Women and Reproductive Health (CLAP/SMR) in Uruguay and has become a well established national Peruvian database where individual hospitals report information on all obstetric patients. The database includes maternal demographic information, medical history, and labor and delivery information from the 43 hospitals included. The altitude of these hospitals ranges from 29 meters to 4340 meters above sea level. Data were collected from 2000–2010. The hospitals included health centers, community hospitals, and tertiary care referral hospitals. A quality assessment of the PIS database has been previously performed (23). During this assessment, Gonzales et al. validated the database in 3 ways: (1) computer checks to reduce the risk of typing errors, (2) computer review of records for missing or aberrant data, and (3) review of a random sample of records to compare with other sources of information. For the record review, records were randomly sampled and compared with other sources of information (i.e. a birth registry in a delivery room or records of neonatal services provided along with a review of the clinical record) to assure reliability of the information in the database.
Data are grouped according to altitude of residency: low altitude (0–1999 m), moderate altitude (2000–2999 m), and high altitude (3000–4340 m). The unexposed patients are those living at low altitude and include 22 hospitals. The exposed patients are those living at moderate altitude (8 hospitals) and high altitude (13 hospitals). The altitude at the hospital site of delivery was used to define the altitude of residency. Almost all women deliver in close proximity to their place of residency thereby making the hospital site altitude an acceptable way to classify this exposure (15).
Our primary outcome was preterm birth (PTB) which was defined by delivery <37 weeks gestation and included both spontaneous PTB (sPTB) and medically indicated PTB. Our secondary outcomes were PTB <34 weeks gestation, spontaneous PTB (sPTB) <37 weeks, preeclampsia/eclampsia, small for gestational age (SGA), stillbirth, Apgar <6 at 5 minutes, and cesarean delivery rate. SGA was defined as birth weight below the 10th percentile for gestational age using the Latin American Center for Perinatology (CLAP) standard. The birth weights for Peru are similar to those derived by CLAP (27). Stillbirth was defined as birth of a fetus ≥ 22 weeks with no signs of life after birth.
Gestational age of delivery was determined by last menstrual period or ultrasound and confirmed by physical exam. Those that did not have two forms of gestational age and those with incongruent data (for example, listed a gestational age of 24 weeks and birth weight of 4000kg) were not included in the analysis (0.5% of cases) because of possible inaccuracies when sites inputted that data. Deliveries <23 weeks are not included in the database. Multiple gestations and women with fetal anomalies were excluded from the analysis.
Analysis occurred in two stages. First, we compared demographic and outcome data among the three groups. Next, we used bivariate comparisons to assess for potential confounders or risk factors for both the exposure, altitude, and the outcome, PTB. We included risk factors in our multivariable model that had an association at a significance level of p<0.1. We then created our multivariable model and used a backwards stepwise elimination strategy to obtain a parsimonious model. Confounders retained in the model were those that had an impact on the effect size of >15%. The confounders included in the final model were maternal age, parity, maternal weight, education, and type of hospital (health centers, community hospitals, and academic hospitals) and year (2000–2010). Clustering by hospital was accounted for in all analyses using generalized estimating equations (28) to account for non-independent data within each hospital.
Data analyses were performed using STATA 12.0 for Windows (STATA Corporation, College Station, TX) logistic procedure including vce(cluster) option to estimate the robust variances. Statistical significance was set at p<0.05. A post-hoc power analysis was performed which showed greater than 80% power to detect an odds ratio of 1.16 for the association between altitude and PTB. To be conservative with this calculation, we assumed 1 year’s worth of data. A preterm birth rate of 5.6% in the low altitude group was used and a ratio of 4:1 was used given 4 times as many patients in the low altitude compared to high altitude. The actual power is > 80% given 10 years of data.
Results
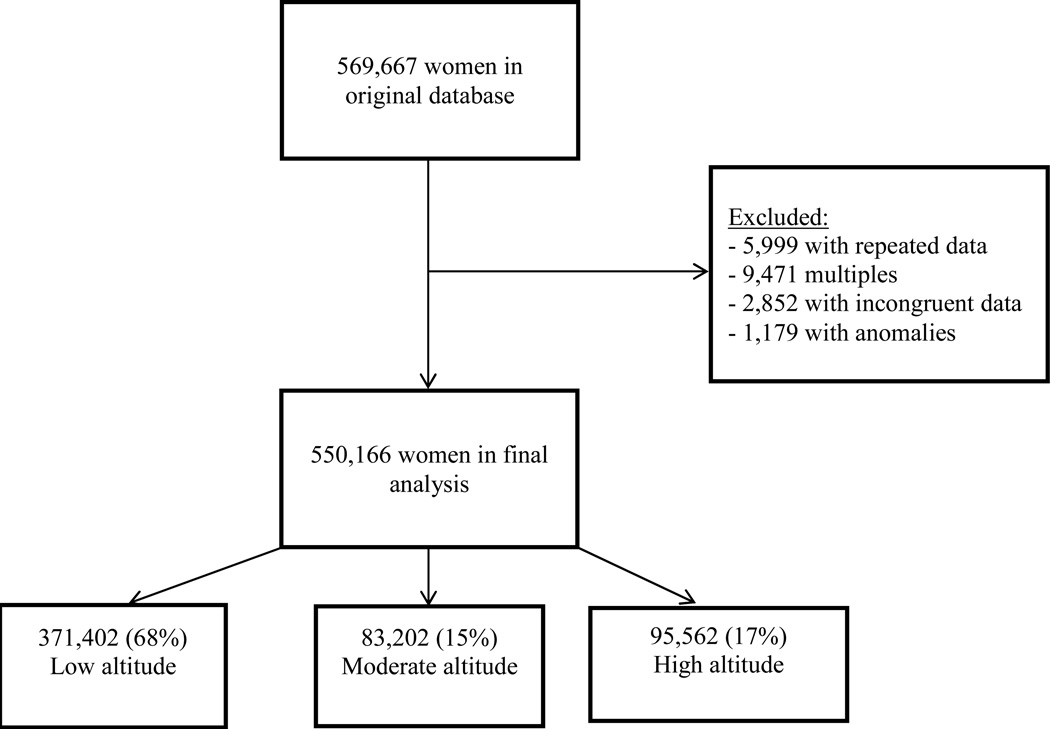
There were 569,667 patients in the database and 550,166 were included in our analysis. Of those, 68% (n=371,402) resided at low altitude, 15% (n=83,202) at moderate altitude, and 17% (95,562) at high altitude. The average number of patients per hospital was 11,960 and ranged from 440 to 55,775. Table 1 lists the maternal demographics for low, moderate, and high altitude. Maternal age, maternal weight, level of education, and type of hospital were significantly different among groups.
Table 1.
Maternal demographics at low, moderate, high altitude
| Low altitude n=371,402 (68%) (22 hospitals) |
Moderate altitude n=83,202 (15%) (8 hospitals) |
High altitude n=95,562 (17%) (13 hospitals) |
p-valuea | |
|---|---|---|---|---|
| Maternal age (years)b | 25.3 (6.6) | 25.9 (6.8) | 25.7 (6.6) | 0.04 |
| Maternal age category | ||||
| <18 years | 10 | 9 | 8 | 0.0002 |
| 18–35 years | 79 | 78 | 80 | |
| ≥35 years | 11 | 13 | 12 | |
| Parity b | 1.7 (1.4) | 1.9 (1.7) | 1.9 (1.7) | 0.09 |
| Prenatal care visits | ||||
| None | 20 | 21 | 21 | 0.71 |
| 1–5 visits | 32 | 35 | 36 | |
| >5 visits | 47 | 44 | 43 | |
| Marital Status | ||||
| Married | 18 | 20 | 18 | |
| Living together | 67 | 61 | 65 | 0.13 |
| Single | 14 | 16 | 15 | |
| Other | 1 | 3 | 1 | |
| Education | ||||
| Primary | 17 | 24 | 18 | 0.02 |
| Secondary | 67 | 53 | 56 | |
| Advanced with university | 11 | 15 | 17 | |
| Technical school | 5 | 8 | 9 | |
| Maternal weight at delivery (kg) b | 56.0 (9.6) | 55.4 (8.7) | 54.3 (7.9) | 0.03 |
| Type of labor: | ||||
| Spontaneous | 81 | 82 | 85 | 0.14 |
| Induction | 4 | 3 | 3 | |
| Scheduled cesarean | 15 | 15 | 12 | |
| Level of hospital | ||||
| Community hospital | 9 | 7 | 11 | 0.002 |
| Health center | <1 | 1 | 2 | |
| Tertiary care referral hospital | 91 | 92 | 87 | |
| Gestational age of delivery (weeks) b | 38.8 (1.9) | 38.9 (2.1) | 38.7 (2.1) | 0.46 |
| Year | ||||
| 2000 | 79 | 10 | 11 | <0.001 |
| 2001 | 72 | 14 | 15 | |
| 2002 | 77 | 10 | 13 | |
| 2003 | 76 | 11 | 13 | |
| 2004 | 71 | 15 | 14 | |
| 2005 | 65 | 18 | 18 | |
| 2006 | 66 | 13 | 21 | |
| 2007 | 64 | 15 | 21 | |
| 2008 | 58 | 21 | 22 | |
| 2009 | 61 | 18 | 21 | |
| 2010 | 74 | 17 | 9 | |
Data are presented as percentages unless otherwise indicated. Some percentages do not add up to 100% as each percentage point was rounded up to a whole number.
Tests of significance were adjusted for clustering by hospital using generalized estimating equations.
Mean (±SD)
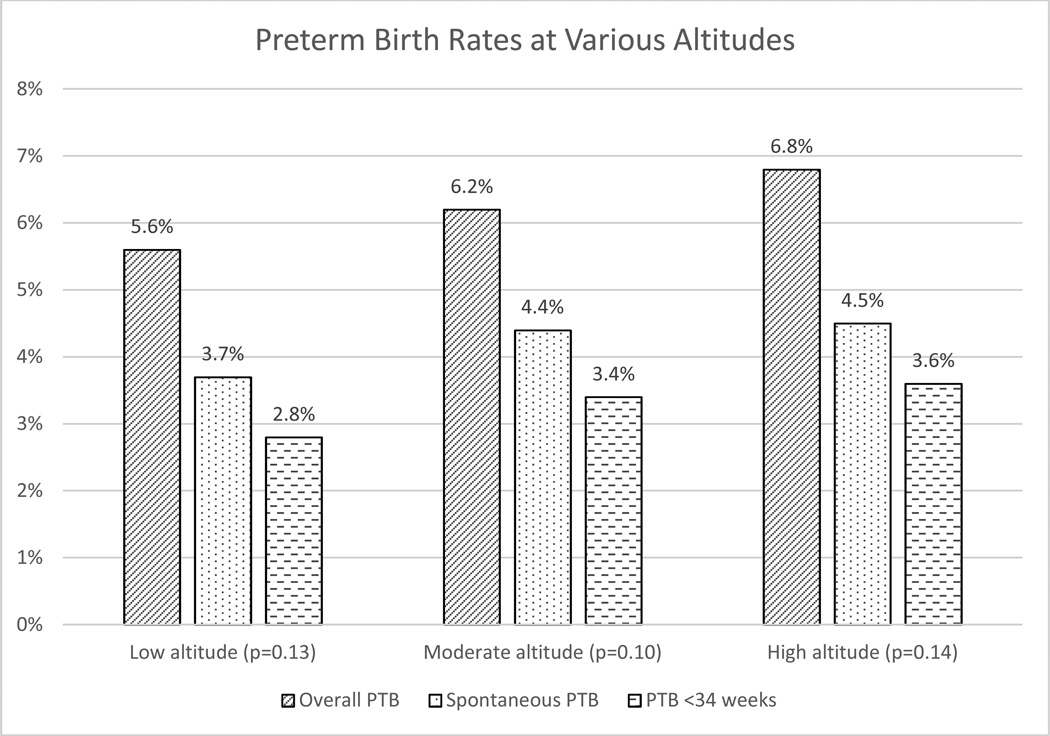
Table 2 displays the primary and second outcomes among the 3 altitudes. The overall PTB rate, our primary outcome, was 5.9% with no difference in the rate of PTB among the 3 altitudes (5.6, 6.2, 6.8%, p=0.13). While there was no association between altitude and PTB, pairwise comparisons show a modest elevated risk of PTB when comparing high altitude to low altitude (OR 1.23 [1.01–1.50]). The values for the odds ratios for the entire multivariable model, after adjusting for confounders, can be found in Table 3. Similarly, there was no difference in the sPTB rate (p=0.10) or PTB rate <34 weeks gestation (p=0.14) among groups.
Table 2.
Primary and secondary outcomes at low, moderate, high altitude
| Low altitude n=371,402 (68%) (22 hospitals) |
Moderate altitude n=83,202 (15%) (8 hospitals) |
High altitude n=95,562 (17%) (13 hospitals) |
p-valuea | |
|---|---|---|---|---|
| Overall PTB | 5.6 | 6.2 | 6.8 | 0.13 |
| Spontaneous PTB | 3.7 | 4.4 | 4.5 | 0.10 |
| PTB <34 weeks | 2.8 | 3.4 | 3.6 | 0.14 |
| Preeclampsia | 4.6 | 2.8 | 2.9 | 0.09 |
| SGA | 3.3 | 3.6 | 5.0 | 0.02 |
| Birthweight (g)b | 3218 (575) | 3159 (569) | 3041 (532) | <0.001 |
| Stillbirth | 1.3 | 1.5 | 1.7 | 0.11 |
| Apgar <6 at 5 min | 0.8 | 1.1 | 1.3 | 0.004 |
| Cesarean section | 28.0 | 26.6 | 20.6 | <0.001 |
Data are presented as percentages unless otherwise indicated
Tests of significance were adjusted for clustering by hospital using generalized estimating equations.
Mean (±SD)
PTB: preterm birth, SGA: small for gestational age
Table 3.
Adjusted odds ratios and 95% Confidence intervals, aOR [CI], for preterm birth, cesarean delivery, and small for gestational age including output for the confounders.
| Variable | Preterm birth | Cesarean delivery | Small for gestational age |
|---|---|---|---|
| Low altitude | 1.00 | 1.00 | 1.00 |
| Moderate altitude | 1.06 [0.78–1.41] | 0.86 [0.69–1.08] | 1.01 [0.70–1.46] |
| High altitude | 1.21 [1.00–1.47] | 0.62 [0.47–0.81] a | 1.50 [1.16–1.95] a |
| Maternal age < 18 | 1.00 | 1.00 | 1.00 |
| Maternal age 18–35 | 0.79 [0.74–0.84] a | 1.05 [1.00–1.10] a | 0.83 [0.75–0.91] a |
| Maternal age ≥35 | 1.01 [0.94–1.08] | 1.46 [1.33–1.59] a | 0.98 [0.89–1.08] |
| Community hospital | 1.00 | 1.00 | 1.00 |
| Health center | 1.44 [1.13–1.83] a | 2.54 [1.62–3.97] a | 0.81 [0.60–1.10] |
| Tertiary care referral hospital | 1.24 [1.00–1.54] a | 1.77 [1.26–2.49] a | 0.81 [0.59–1.12] |
| Primary education | 1.00 | 1.00 | 1.00 |
| Secondary education | 0.82 [0.77–0.88] a | 1.02 [0.94–1.12] | 0.80 [0.75–0.85] a |
| University | 0.80 [0.73–0.88] a | 1.27 [1.10–1.45] a | 0.69 [0.64–0.75] a |
| Technical school | 0.79 [0.73–0.85] a | 1.48 [1.28–1.71] a | 0.71 [0.65–0.77] a |
| Multiparous | 1.03 [0.97–1.08] | 0.78 [0.73–0.82] a | 1.00 [0.93–1.07] |
| Delivery year: 2000 | 1.00 | 1.00 | 1.00 |
| Delivery year: 2001 | 0.97 [0.88–1.07] | 0.96 [0.79–1.17] | 1.06 [0.89–1.23] |
| Delivery year: 2002 | 1.02 [0.89–1.16] | 0.96 [0.75–1.12] | 1.10 [0.92–1.32] |
| Delivery year: 2003 | 0.97 [0.85–1.11] | 0.93 [0.76–1.14] | 1.16 [0.94–1.44] |
| Delivery year: 2004 | 1.05 [0.93–1.18] | 0.99 [0.82–1.19] | 1.11 [0.91–1.36] |
| Delivery year: 2005 | 1.02 [0.90–1.16] | 1.08 [0.91–1.28] | 1.10 [0.89–1.33] |
| Delivery year: 2006 | 1.04 [0.92–1.17] | 1.19 [1.00–1.41] a | 1.08 [0.88–1.32] |
| Delivery year: 2007 | 0.99 [0.85–1.15] | 1.24 [1.03–1.47] a | 1.05 [0.87–1.26] |
| Delivery year: 2008 | 1.04 [0.88–1.23] | 1.35 [1.15–1.59] a | 1.12 [0.91–1.36] |
| Delivery year: 2009 | 1.02 [0.86–1.21] | 1.38 [1.15–1.65] a | 1.04 [0.84–1.28] |
| Delivery year: 2010 | 1.10 [0.92–1.32] | 1.36 [1.04–1.76] a | 1.10 [0.83–1.44] |
p-value<0.05 for joint Wald test for the association between all categories of the covariate and specified outcome.
When evaluating secondary outcomes, there was a decreased rate of cesarean delivery with increasing altitude. Specifically, there was a 35% decreased odds of a cesarean delivery at high altitude as compared to women at low altitude (OR 0.65 [0.50–0.83], p=0.001).This remained after adjusting for confounders, which can be seen in Table 3. There was no statistically significant difference in cesarean risk at moderate altitude as compared to low altitude (OR 0.90 [0.69–1.18], p=0.5). As seen in Table 3, the risk of cesarean increased after 2006, p<0.001.
As noted in Table 2, the average birth weight decreased and the percentage of SGA infants increased as altitude increased. There was a 56% increased odds of an SGA infant at high altitude as compared to low altitude (OR 1.56 [1.51–1.62], p=0.006) which remained when adjusted for confounders (Table 3). There was no statistical difference in the rate of preeclampsia between the groups although there was a trend to lower levels in high altitude pregnancies.
Comment
To our knowledge, this is one of the first studies to specifically evaluate the risk of preterm birth at varying altitudes. We found an overall PTB rate of 5.9% with no increased risk in overall or spontaneous PTB at moderate or high altitude when compared to those at low altitude. This PTB rate is consistent with the overall PTB in Latin America, estimated to be 6% based on information from the WHO (3). The United States has a notoriously high PTB rate, estimated at 11.7% (29–31). The higher rates of PTB in the United States may be partly explained by the larger African American population in the US compared to Peru as well as the larger number of medically indicated PTB rate in the United States as well. We did; however, find a 35% decreased risk of cesarean at high altitude and a 56% increased risk of SGA at high altitude.
Smaller studies have been conflicting as to whether or not altitude was associated with an increased risk of PTB. The first study to suggest an association between high altitude and PTB was the small landmark study by Lichty et al. evaluating the impact of high altitude on births in Colorado (16). Jensen et al. conducted a large review of all deliveries in the state of Colorado from 1989–1991 and found no difference in the rates of PTB for those at high altitude (17); however, this study was specifically designed to evaluate birth weight as an outcome and not PTB. Gonzales et al. looked at the PTB rates at low and high altitude among 6 public hospitals (15). While that study suggested a possible association between PTB and high altitude, the analysis targeted only a small portion of hospitals included in the database. Additionally, altitude was evaluated as a dichotomous exposure in their study. Our study is the largest study with the primary objective to answer the specific question of whether or not PTB and high altitude were associated. Most importantly, we were powered to actually see a difference so the likelihood of a Type 2 error is lower in this study compared to the smaller studies that are currently in the literature. Our finding of an increased risk of SGA supports multiple prior studies suggesting this risk (15,20–24).
While we did not find a difference in the rates of preeclampsia, we were not powered to evaluate this outcome. While the diagnosis and documentation of preeclampsia could be a contributing factor to why our study did not find these results, PTB and gestational age of delivery could be a surrogate marker for preterm preeclampsia. The cesarean delivery rate at varying altitudes is not well described in the literature and therefore it is difficult to compare the lower rates at high altitude that we found to other studies. Given that the rate of induction of labor is similar at each altitude, the decreased risk of cesarean cannot be attributed to induction. A greater proportion of patients delivered at non-tertiary referral hospitals among the women at higher altitude. It is possible that these hospitals are not as well equipped to perform cesarean deliveries in a timely fashion. Therefore, a delay in cesarean delivery as well as the potential unrecognized need for cesarean may be an explanation for the decreased percentage. The increased risk of a low Apgar score at 5 minutes supports this theory; although the number of stillbirths was the same among all the groups suggesting that the rate of intrapartum fetal death was not increased. With an increase in the rate of SGA at high altitude, another possible explanation for a decreased CD risk may be a decreased rate of cephalopelvic disproportion given the smaller neonates at high altitude. We therefore restricted our analysis of CD risk at high altitude to non-SGA infants to evaluate whether or not this impacted the results. We found the CD risk was unchanged at high altitude when SGA infants were excluded (aOR 0.61 [0.46–0.80], p<0.001). While unable to be answered by this study, this finding of a decreased risk of cesarean at high altitude is striking and warrants further investigation.
There are many strengths to our study. It is a large database that includes nationwide deliveries across Peru. The inclusion of a variety of hospital types and women with different demographic backgrounds allows for generalizability to other populations. Additionally, the prior validation of the database (15) gives us confidence that the data are reliable and that our conclusions are valid.
The limitations of our study are as follows. As many as 28% of deliveries in Peru occur at home (4) which can therefore underestimate the PTB and stillbirth rate, especially at higher altitudes where home births occur more frequently. Additionally, we do not have the indication for cesarean and therefore, with our current database, we are unable to further explore possible explanations for the lower rate of cesareans at high altitude. Another limitation is the inability to control specifically for ancestry, ethnicity, and socioeconomic status (SES). It is believed that high altitude ancestry is protective against complications of pregnancy (18, 23, 32); however, antiquity is not able to be assessed from our database. There are close to 77 ethnicities in Peru and the PIS databse does not account for all of the different ethnicities. While there was no specific variable for SES, this study was performed with data from the PIS database. This database only includes urban, public hospitals and therefore all of the women included in the database are from lower SES. Women with higher SES attend hospitals from the Social Security (middle class) or private clinics (high-middle, high class). Additionally, we attempted to account for potential variations in SES by including variables such as the number of prenatal visits and education level in our model.
While populations residing at high altitude have less developed infrastructures for healthcare and communication, it is reassuring and encouraging to find that high altitude is not associated with an increased risk of preterm birth. While the PTB rate was not different, future studies should focus on evaluating the NMR at various altitudes. Despite a similar percentage of preterm deliveries, fewer resources and differences in healthcare utilization at high altitude may place these preterm infants at a higher risk for mortality than those born at low altitude. If this is found to be true, those at high risk for preterm delivery can be targeted to ensure a location of delivery that is capable of appropriate resuscitation of a preterm infant.
Figure 1.
Flow diagram of patients included in the analysis
Figure 2.
Preterm birth rates at various altitudes
PTB: Preterm birth
Acknowledgments
None
Sources of funding: This study was funded in part by University of Pennsylvania Global Health Partnership Program with the Universidad Peruana Cayetano Heredia, Perelman School of Medicine at the University of Pennsylvania Pilot Grant Award and a career development award in Women’s Reproductive Health Research: K12-HD001265-14.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Disclosures for author Mary D. Sammel ScD are enclosed. Of note, she is a statistical consultant for the American Journal of Obstetrics and Gynecology
Disclaimer: None
Presentations: Presented as a poster at the annual meeting of the Society of Maternal Fetal Medicine in New Orleans, Louisiana on February 6th, 2014.
References
- 1.Hill K, Choi Y. Neonatal mortality in the developing world. Demographic Research. 2006;14:429–452. [Google Scholar]
- 2. [Access December 1, 2013]; http://whqlibdoc.who.int/publications/2006/9241563206_eng.pdf. [Google Scholar]
- 3.WHO. Making Pregnancy Safe. Peru_Country Profile. 2005 [Google Scholar]
- 4. [Access December 1, 2013]; http://www.who.int/maternal_child_adolescent/events/2008/mdg5/countries/final_cp_peru_1 9_09_08.pdf. [Google Scholar]
- 5. [Access December 1, 2013]; http://www.un.org/millenniumgoals/. [Google Scholar]
- 6.Villar J, Valladares E, Wojdyla, et al. Caesarean delivery rates and pregnancy outcomes: the 2005 WHO global survey on maternal and perinatal health in Latin America. Lancet. 2006;367:1819–1829. doi: 10.1016/S0140-6736(06)68704-7. [DOI] [PubMed] [Google Scholar]
- 7.Bloom SL, Yost NP, McIntire DD, Leveno KJ. Recurrence of preterm birth in singleton and twin pregnancies. Obstet Gynecol. 2001;98(3):379–385. doi: 10.1016/s0029-7844(01)01466-1. [DOI] [PubMed] [Google Scholar]
- 8.Esplin MS, O'Brien E, Fraser A, et al. Estimating Recurrence of Spontaneous Preterm Delivery. Obstet Gynecol. 2008;112(3):516–523. doi: 10.1097/AOG.0b013e318184181a. [DOI] [PubMed] [Google Scholar]
- 9.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334:567. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 10.Zhu BP, Rolfs RT, Nangle BE, Horan JM. Effect of the interval between pregnancies on perinatal outcomes. N Engl J Med. 1999;340(8):589–594. doi: 10.1056/NEJM199902253400801. [DOI] [PubMed] [Google Scholar]
- 11.Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295(15):1809–1823. doi: 10.1001/jama.295.15.1809. [DOI] [PubMed] [Google Scholar]
- 12.Blackmore-Prince C, Kieke B, Jr, Kugaraj KA, et al. Racial differences in the patterns of singleton preterm delivery in the 1988 National Maternal and Infant Health Survey. Matern Child Health J. 1999;3(4):189–197. doi: 10.1023/a:1022373205005. [DOI] [PubMed] [Google Scholar]
- 13.Knottnerus JA, Delgado LR, Knipschild PG, Essed GG, Smits F. Haematologic parameters and pregnancy outcome. A prospective cohort study in the third trimester. J Clin Epidemiol. 1990;43:461–466. doi: 10.1016/0895-4356(90)90134-b. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen K. Is there a causal relationship between iron deficiency or iron-deficiency anemia and weight at birth, length of gestation and perinatal mortality? J Nutr. 2001;131:590S–601S. doi: 10.1093/jn/131.2.590S. [DOI] [PubMed] [Google Scholar]
- 15.Gonzales GF, Steenland K, Tapia V. Maternal hemoglobin level and fetal outcome at low and high altitudes. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1477–R1475. doi: 10.1152/ajpregu.00275.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichty JA, Ting RY, Bruns PD, Dyar E. Studies of babies born at high altitude. AMA J Dis Child. 1957;93(6):666–669. doi: 10.1001/archpedi.1957.02060040668009. [DOI] [PubMed] [Google Scholar]
- 17.Jensen GM, Moore LG. The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am J Public Health. 1997;87(6):1003–1007. doi: 10.2105/ajph.87.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore Lorna G. Human Genetic Adaptation to High Altitude. High Altitude Medicine & Biology. 2001;2(2):257–279. doi: 10.1089/152702901750265341. [DOI] [PubMed] [Google Scholar]
- 19.Zamudio S, Wu Y, Ietta F, et al. Human placental hypoxia-inducible factor-1α expression correlates with clinical outcomes in chronic hypoxia in vivo. Am J of Path. 2007;170(6):2171–2179. doi: 10.2353/ajpath.2007.061185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julian CG, Wilson MJ, Lopez M, et al. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1564–R1575. doi: 10.1152/ajpregu.90945.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unger A, Weiser JK, McCullough RE, Keefer A, Grindlay Moore L. Altitude, low birth weight, and infant mortality in Colorado. JAMA. 1988;259:3427–3432. [PubMed] [Google Scholar]
- 22.Zamudio S. The placenta at high altitude. High Alt Med Biol. 2003;4:171–191. doi: 10.1089/152702903322022785. [DOI] [PubMed] [Google Scholar]
- 23.Gonzales GF, Tapia V, Carrillo CE. Stillbirth rates in Peruvian populations at high altitude. Int J Gynecol Obstet. 2008;100:221–227. doi: 10.1016/j.ijgo.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Zamudio S. High-altitude hypoxia and preeclampsia. Front Biosci. 2007;12:2967–2977. doi: 10.2741/2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali KZ, Ali ME, Khalid MEM. High altitude and spontaneous preterm birth. Int J Gynaecol Obstet. 1996;54:11–15. doi: 10.1016/0020-7292(96)02673-2. [DOI] [PubMed] [Google Scholar]
- 26.Naeye RL. Pregnancy hypertension, placental evidence of low uteroplacental blood flow and spontaneous preterm delivery. Hum Pathol. 1989;20(5):441–444. doi: 10.1016/0046-8177(89)90008-7. [DOI] [PubMed] [Google Scholar]
- 27.Gonzales GF, Tapia V. Birth weight charts for gestational age in 63,620 healthy infants born in Peruvian public hospitals at low and at high altitude. Acta Paediatrica. 2009;98(3):454–458. doi: 10.1111/j.1651-2227.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- 28.Liang Kung-Yee, Zeger Scott. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 29. [Accessed August 29, 2013]; http://www.cdc.gov/nchs/data/databriefs/db39.pdf.
- 30. [Accessed August 29, 2013]; http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_05.pdf.
- 31.Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2010–2011. Pediatrics. 2013;131(3):548–558. doi: 10.1542/peds.2012-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzales GF. Peruvian contributions to the study on human reproduction at high altitude: from the chronicles of the Spanish conquest to the present. Respir Physiol Neurobiol. 2007;158(2–3):172–179. doi: 10.1016/j.resp.2007.03.015. [DOI] [PubMed] [Google Scholar]