Abstract
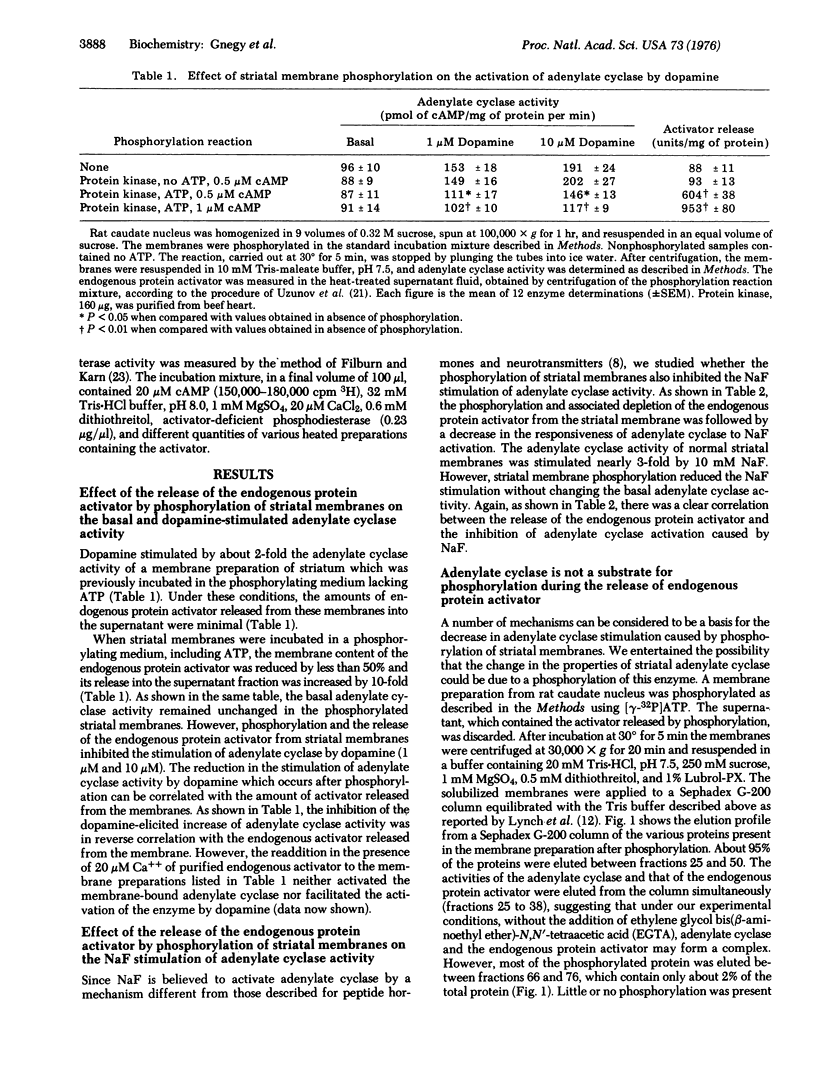
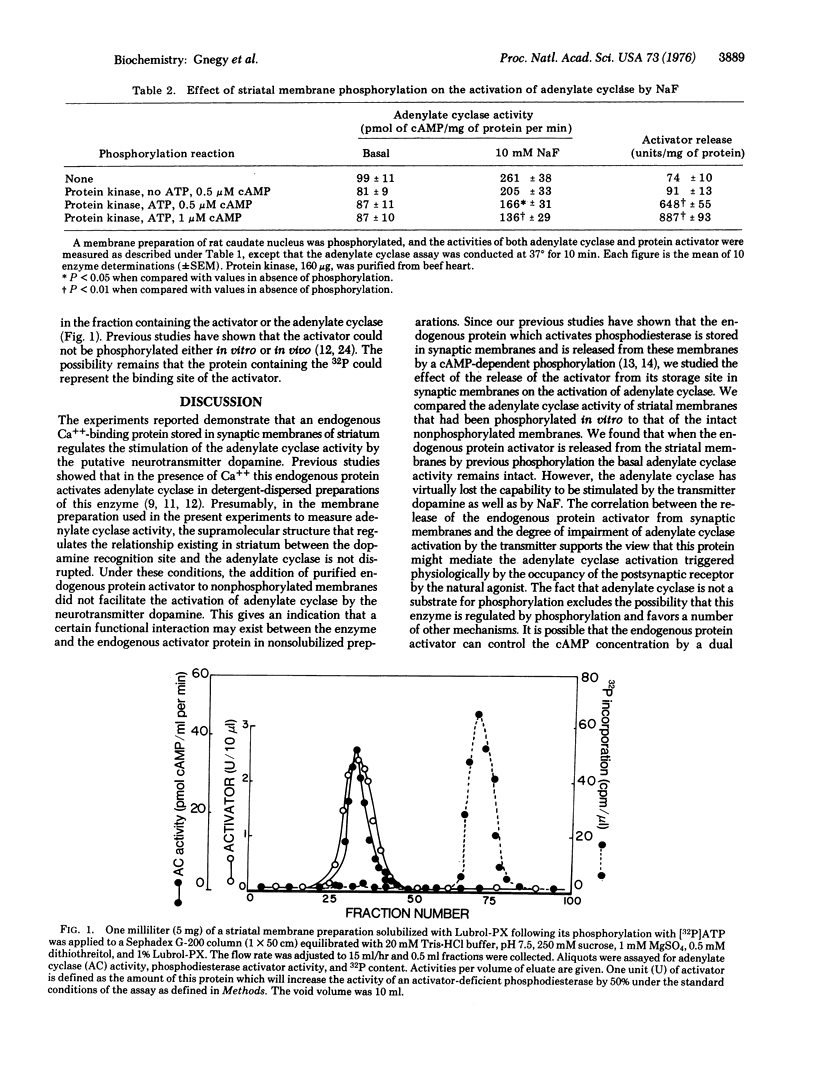
Membranes of rat caudate nucleus contain a dopamine-dependent adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] and a Ca++ binding protein that activates phosphodiesterase (3':5'-cyclic-AMP 5'-nucleotidohydrolase, EC 3.1.4.17). This activator can be released from the membranes by a phosphorylation with a 3':5' cAMP-dependent protein kinase (ATP-protein phosphotransferase, EC 2.7.1.37). Under the conditions of membrane phosphorylation and activator release, dopamine fails to activate striatal adenylate cyclase. The basal activity of this enzyme is not decreased by the release of the protein activator but the activation by NaF is reduced. Adenylate cyclase is not phosphorylated when the dopamine activation is blocked after the release of the activator, but other membrane proteins are phosphorylated. It is postulated that the endogenous protein stored in striatal membranes can regulate the intracellular concentration of cAMP by an activation of adenylate cyclase while stored in striatal membrane, and by an activation of phosphodiesterase when released into the cytosol after membrane phosphorylation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Pohl S. L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. II. Comparison between glucagon- and fluoride-stimulated activities. J Biol Chem. 1971 Mar 25;246(6):1857–1860. [PubMed] [Google Scholar]
- Brostrom C. O., Huang Y. C., Breckenridge B. M., Wolff D. J. Identification of a calcium-binding protein as a calcium-dependent regulator of brain adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):64–68. doi: 10.1073/pnas.72.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y., Bradham L. S., Lynch T. J., Lin Y. M., Tallant E. A. Protein activator of cyclic 3':5'-nucleotide phosphodiesterase of bovine or rat brain also activates its adenylate cyclase. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1055–1062. doi: 10.1016/0006-291x(75)90747-0. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Evidence for and properties of a protein activator. J Biol Chem. 1971 May 10;246(9):2859–2869. [PubMed] [Google Scholar]
- Clement-Cormier Y. C., Parrish R. G., Petzold G. L., Kerabian J. W., Greengard P. Characterization of a dopamine-sensitive adenylate cyclase in the rat caudate nucleus. J Neurochem. 1975 Aug;25(2):143–149. doi: 10.1111/j.1471-4159.1975.tb12241.x. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Jacobs S., Bennett V. Activation of adenylate cyclase by phosphoramidate and phosphonate analogs of GTP: possible role of covalent enzyme-substrate intermediates in the mechanism of hormonal activation. Proc Natl Acad Sci U S A. 1975 May;72(5):1739–1743. doi: 10.1073/pnas.72.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filburn C. R., Karn J. An isotopic assay of cyclic 3',5'-nucleotide phosphodiesterase with aluminum oxide columns. Anal Biochem. 1973 Apr;52(2):505–516. doi: 10.1016/0003-2697(73)90055-9. [DOI] [PubMed] [Google Scholar]
- Gnegy M. E., Costa E., Uzunov P. Regulation of transsynaptically elicited increase of 3':5'-cyclic AMP by endogenous phosphodiesterase activator. Proc Natl Acad Sci U S A. 1976 Feb;73(2):352–355. doi: 10.1073/pnas.73.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A., Zivkovic B., Pfeiffer R., Costa E. Involvement of 3',5'-cyclic adenosine monophosphate in the increase of tyrosine hydroxylase activity elicited by cold exposure. Naunyn Schmiedebergs Arch Pharmacol. 1973;278(2):195–206. doi: 10.1007/BF00500650. [DOI] [PubMed] [Google Scholar]
- KLAINER L. M., CHI Y. M., FREIDBERG S. L., RALL T. W., SUTHERLAND E. W. Adenyl cyclase. IV. The effects of neurohormones on the formation of adenosine 3',5'-phosphate by preparations from brain and other tissues. J Biol Chem. 1962 Apr;237:1239–1243. [PubMed] [Google Scholar]
- Kebabian J. W., Petzold G. L., Greengard P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the "dopamine receptor". Proc Natl Acad Sci U S A. 1972 Aug;69(8):2145–2149. doi: 10.1073/pnas.69.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin M. C., Salomon Y., Rendell M., Rodbell M. The hepatic adenylate cyclase system. II. Substrate binding and utilization and the effects of magnesium ion and pH. J Biol Chem. 1975 Jun 10;250(11):4246–4252. [PubMed] [Google Scholar]
- Londos C., Salomon Y., Lin M. C., Harwood J. P., Schramm M., Wolff J., Rodbell M. 5'-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch T. J., Tallant E. A., Cheung W. Y. Ca++-dependent formation of brain adenylate cyclase-protein activator complex. Biochem Biophys Res Commun. 1976 Jan 26;68(2):616–625. doi: 10.1016/0006-291x(76)91190-6. [DOI] [PubMed] [Google Scholar]
- Mishra R. K., Gardner E. L., Katzman R., Makman M. H. Enhancement of dopamine-stimulated adenylate cyclase activity in rat caudate after lesions in substantia nigra: evidence for denervation supersensitivity. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3883–3887. doi: 10.1073/pnas.71.10.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell M., Salomon Y., Lin M. C., Rodbell M., Berman M. The hepatic adenylate cyclase system. III. A mathematical model for the steady state kinetics of catalysis and nucleotide regulation. J Biol Chem. 1975 Jun 10;250(11):4253–4260. [PubMed] [Google Scholar]
- Rodbell M. On the mechanism of activation of fat cell adenylate cyclase by guanine nucleotides. An explanation for the biphasic inhibitory and stimulatory effects of the nucleotides and the role of hormones. J Biol Chem. 1975 Aug 10;250(15):5826–5834. [PubMed] [Google Scholar]
- Uzunov P., Gnegy M., Lehne R., Revuelta A., Costa E. A neurobiological role for a protein activator of cyclic nucleotide phosphodiesterase. Adv Biochem Psychopharmacol. 1976;15:283–301. [PubMed] [Google Scholar]
- Uzunov P., Lehne R., Revuelta A. V., Gnegy M. E., Costa E. A kinetic analysis of the cyclic nucleotide phosphodiesterase regulation by the endogenous protein activator. A study of rat brain and frog sympathetic chain. Biochim Biophys Acta. 1976 Feb 13;422(2):326–334. doi: 10.1016/0005-2744(76)90144-3. [DOI] [PubMed] [Google Scholar]
- Uzunov P., Weiss B. Separation of multiple molecular forms of cyclic adenosine-3',5'-monophosphate phosphodiesterase in rat cerebellum by polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1972 Sep 19;284(1):220–226. doi: 10.1016/0005-2744(72)90060-5. [DOI] [PubMed] [Google Scholar]
- de Haën C. Adenylate cyclase. A new kinetic analysis of the effects of hormones and fluoride ion. J Biol Chem. 1974 May 10;249(9):2756–2762. [PubMed] [Google Scholar]


