Abstract
Aim
Few epidemiological studies have investigated the association between circulating concentrations of the active vitamin D metabolite 1,25(OH)2D and metabolic syndrome. We sought to determine whether blood levels of 1,25(OH)2D are associated with metabolic syndrome and its individual components, including waist circumference, triglycerides, blood pressure, and glucose, and high-density lipoprotein. We also investigated these associations for the more abundant precursor vitamin D metabolite, 25(OH)D.
Methods
Participants from two completed clinical trials of colorectal neoplasia with available metabolic syndrome data and blood samples for measurement of 1,25(OH)2D (n=1048) and 25(OH)D (n=2096) were included. Cross-sectional analyses of the association between concentrations of 1,25(OH)2D, 25(OH)D, metabolic syndrome, and its components were conducted.
Results
A statistically significant inverse association was observed for circulating concentrations of 1,25(OH)2D and metabolic syndrome, with adjusted ORs (95% CIs) of 0.73 (0.52–1.04) and 0.52 (0.36–0.75) for the second and third tertiles of 1,25(OH)2D, respectively (p-trend <0.001). Significant inverse relationships were also observed between 1,25(OH)2D and high triglycerides (p-trend <0.001), and low high-density lipoprotein (p-trend <0.001). For 25(OH)D concentrations, significant inverse associations were found for metabolic syndrome (p-trend <0.01), high waist circumference (p-trend<0.04) and triglyceride levels (p-trend <0.01). Participants with 25(OH)D ≥ 30 ng/ml and in the highest tertile of 1,25(OH)2D demonstrated significantly lower odds of metabolic syndrome, with an OR (95% CI) of 0.38 (0.19–0.75) compared to those in the lowest category for both metabolites.
Conclusion
These results provide new evidence that the relatively rarely-studied active hormonal form of vitamin D, 1,25(OH)2D, is associated with metabolic syndrome and its components, and confirm prior findings for 25(OH)D. The finding that 1,25(OH)2D is related to high-density lipoprotein, while 25(OH)D is not, suggests that there may be an independent mechanism of action for 1,25(OH)2D in relation to metabolic dysregulation.
Keywords: Vitamin D; 25(OH)D; 1, 25(OH)2D; metabolic syndrome
1. Introduction
Metabolic syndrome (MetS) is an aggregation of characteristics including high waist circumference, blood pressure, and concentrations of triglycerides and fasting glucose, and low circulating levels of high-density lipoprotein (HDL). The presence of MetS is associated with multiple adverse health outcomes, including diabetes, cardiovascular disease, and cancer[1–3]. It is estimated that the prevalence of MetS among adults in the United States is 22.9%, though this figure varies by characteristics such as age, sex, and race/ethnicity[4]. While recent work suggests that the use of therapeutics is reducing the occurrence of MetS among some groups[4], identification of risk factors for this syndrome and its components remains critical.
Insufficient vitamin D status has been proposed to be a potential contributor to MetS. Vitamin D is a seco-steroid hormone that can be endogenously synthesized via UV exposure, or consumed in the diet. The circulating vitamin D metabolite most commonly investigated in epidemiological studies is 25-hydroxycholecalciferol [25(OH)D], which encompasses both endogenous synthesis and dietary intake[5]. However, enzymatic hydroxylation of 25(OH)D at the 1-carbon position is necessary to produce the active vitamin D metabolite, 1,25-dihydroxycholecalciferol [1,25(OH)2D]. This form is a potent hormone that binds to the vitamin D receptor (VDR) and mediates transcriptional regulation of multiple genes[6]. Compared to 25(OH)D, circulating concentrations of 1,25(OH)2D are maintained within a narrow range because of the key role of this metabolite in regulating calcium levels, which are also tightly controlled [6]. However, in recent work by our group, we observed statistically significant associations between circulating concentrations of 1,25(OH)2D and colorectal neoplasia[7], suggesting that there may be sufficient variation in this vitamin D metabolite to be used as a marker of health outcomes.
Several epidemiological studies have investigated the association between circulating concentrations of 25(OH)D and MetS and/or its components, with some showing a relationship[8–22], and others not[23–26]. However, to date, only one report has investigated the relationship between circulating concentrations of 1,25(OH)2D and MetS[27], and comparatively few studies have presented results stratified by sex[15,26]. Considering these important gaps, the goal of the present study was to ascertain whether levels of 1,25(OH)2D and 25(OH)D, independently and in combination, are related to MetS and its individual components. Sex-stratified analyses were also a primary objective, in order to assess whether there were differences in the associations between men and women.
2. Material and Methods
2.1 Subjects
Participant data from two completed randomized clinical trials, the Wheat Bran Fiber (WBF) Trial[28] and the Ursodeoxycholic Acid (UDCA) Trial[29], which have been described in detail elsewhere[28] [29], were employed for the present study. Briefly, the WBF trial was a double-blind, randomized, placebo-controlled trial conducted at the University of Arizona and designed to compare the effect of a high-fiber vs. a low-fiber cereal supplement on adenoma recurrence. Participants were individuals who had undergone colonoscopy and had one or more adenomas removed. A total of 1310 participants completed the study by undergoing one or more colonoscopies after randomization [28], and no effect of the intervention was observed for adenoma recurrence [28].
Similar to the WBF trial, the UDCA study was a randomized, double-blind, placebo-controlled trial. The objective of the study was to compare the effect of UDCA on adenoma recurrence. A total of 1192 participants completed the study, and no effect of UDCA on adenoma recurrence was observed in the primary analysis[29]. Both the WBF and UDCA studies were approved by the University of Arizona Human Subjects Committee and local hospital committees. Written informed consent was obtained from each participant prior to study enrollment. For the present study, data from all participants who completed the WBF and UDCA trials and who had an available blood sample for analysis of 1,25(OH)2D (n=1048) and 25(OH)D (n=2096), as well as data for components of MetS, were included.
2.2 Data Collection
Baseline information regarding general participant characteristics, diet, and medical history were collected from all participants in the WBF and UDCA trials with self-administered questionnaires. Dietary data were obtained with the Arizona Food Frequency Questionnaire (AFFQ), which was modified from the food frequency section of the National Cancer Institute’s Health Habits and History Questionnaire[30]. The AFFQ is a 113-item, semi-quantitative, scannable instrument, with study participants asked to report their usual intake of foods for the prior year[31]. A scale of seven categories ranging from >3 times/day to rarely/never was employed for most items; for beverages and commonly-consumed foods the scale ranged from >6 times/day to rarely/never[31]. Portion sizes of small, medium, or large were also recorded for each food item[31]. Total intake of nutrients was calculated by multiplying the frequency of each item’s consumption by the nutrient composition for each portion size[31].
In order to ascertain baseline waist circumference, study participants were asked to measure their waist three times at the smallest circumference of their natural waist and record each measurement to the nearest 1/16 of an inch. Upon study entry, all participants had their blood pressure taken by a study nurse, and fasting blood samples were drawn. Samples were collected in heparin-containing tubes and were sent for standard clinical analyses, which included fasting glucose, triglycerides, and high density lipoprotein (HDL).
2.3 Definitions of metabolic syndrome and components
Components of MetS were defined for the primary analysis using the modified definitions set by the National Cholesterol Education Program Adult Treatment Panel III (ATP III)[1,32]. These were: waist circumference >102cm (40 inches) for men and >88cm (35 inches) for women; triglycerides ≥150mg/dL, HDL cholesterol <40mg/dL for men and <50mg/dL for women; blood pressure ≥130/≥ 85mmHg; and fasting glucose ≥100mg/dL. In turn, study participants were classified as having MetS if they had ≥ 3 of these risk factors. We also conducted the analysis of MetS using the criteria of the International Diabetes Federation (IDF). The IDF definition employs the same cutpoints for the individual components of MetS; however, individuals are classified as having MetS if they exhibit central adiposity in addition to ≥ 2 of the criteria listed above[33].
2.4 Analysis of vitamin D metabolite levels
Measurement of both 25(OH)D and 1,25(OH)2D levels was performed at Heartland Assays (Ames, IA). Concentrations of total 25(OH)D were assessed with a competitive chemiluminescence immunoassay[34]. The coefficient of variation was less than 7.0% for 25(OH)D analyses. For measurement of 1,25(OH)2D, a 125I-based radioimmunoassay was employed, as has been described in detail previously[35]. The coefficient of variation was 11.5% for 1,25(OH)2D analysis. The laboratory utilized several QA/QC measures, including a pooled serum sample analyzed with batches of study samples to monitor analytical precision and identify possible laboratory shifts over time, as well as testing duplicates in different batches. All analyses were conducted in a blinded fashion.
Tertiles of 1,25(OH)2D were calculated based on the population distribution, with mean 1,25(OH)2D concentrations of 22.6 ± 4.5 pg/ml for the first tertile, 33.2 ± 2.8 pg/ml for the second tertile, and 46.6 ± 8.0 pg/ml for the third tertile. Participants were further classified by circulating concentrations of 25(OH)D into one of three categories: deficient (< 20 ng/mL), inadequate (20 to <30 ng/mL), and adequate (≥30 ng/mL)[36–39]. In addition, a classification system for vitamin D metabolite profiles was established that included data for 1,25(OH)2D and 25(OH)D. Participants were categorized as having a low vitamin D profile if they were in the lowest tertile of 1,25(OH)2D and exhibited deficient levels of 25(OH)D (n=118); they were categorized as having a high vitamin D profile if they were in the highest tertile for 1,25(OH)2D concentrations and had adequate levels of 25(OH)D (n=165).
2.5 Statistical Analysis
Summary statistics for baseline characteristics by presence or absence of MetS were calculated with means and standard deviations for the continuous variables and frequencies and percentages for the categorical variables. Unconditional logistic regression modeling was used to evaluate the associations between vitamin D metabolite concentrations, components of MetS, and MetS. Variables assessed for potential confounding were age, body mass index (BMI), waist-to-hip ratio (WHR), season of blood draw, sex, race, family history of colorectal cancer, current smoking, history of previous polyps, physical activity, and aspirin use; dietary and supplemental intake of vitamin D, supplement use, fat, fiber, folate, magnesium, and calcium; supplement use; and energy intake. If a variable changed the point estimate by 10% or greater, it was included in the final multivariate logistic regression analyses. P-for-trend was computed by treating the categories of the vitamin D metabolite concentrations as a continuous variable. All analyses were conducted using the Stata statistical software package [version 9.0, Stata Corporation, College Station, TX].
3. Results
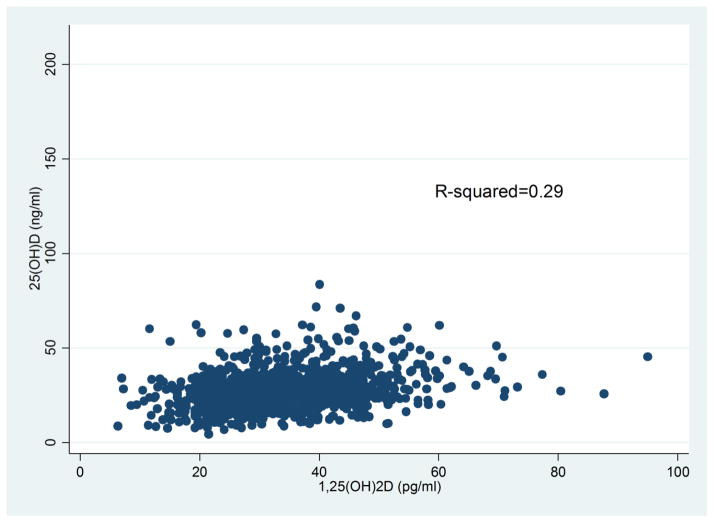
As shown in Table 1, men and women with and without MetS were similar in age, race/ethnicity, and education. For dietary factors, reported intake of energy, carbohydrates, fiber, fat, calcium, and alcohol was similar for those with and without MetS. Among men, dietary intake of vitamin D was somewhat lower for those without MetS as compared to those with MetS; while for women, the opposite was observed. No differences in intake of vitamin D from supplements were observed. The distribution for current smoking was similar for men with and without MetS; while for women, there were more current smokers among those without MetS. BMI and waist-to-hip ratio were higher among those with MetS for both men and women, as was the prevalence of self-reported diabetes. No significant differences in MetS by season of blood draw were observed for either men or women, although concentrations of 25(OH)D varied from a low of 25.9 ng/ml during Spring to 30.4 ng/ml in the Fall (data not shown). Levels of 1,25(OH)2D did not vary by season (data not shown) As shown in Figure 1, a weak positive correlation between circulating concentrations of 1,25(OH)2D and 25(OH)D was observed, with an r2=0.29.
Table 1.
Characteristics of study participants with and without metabolic syndrome (n=2096).
| Metabolic Syndrome Men (1439) |
Metabolic Syndrome Women (658) |
|||
|---|---|---|---|---|
| No (819) | Yes (620) | No (429) | Yes (229) | |
| Demographics | ||||
| Age, y | 65.9 ± 9.0 | 65.9 ± 8.3 | 64.8 ± 9.4 | 66.5 ± 7.7 |
| White (ethnicity/race) | 774 (95.1) | 572 (94.1) | 405 (95.5) | 212 (93.4) |
| Education, y | 14.0 ± 2.5 | 13.9 ± 2.3 | 13.3 ± 2.2 | 13.0 ± 2.1 |
| Dietary Intake | ||||
| Energy, kcal/d | 2096.9 ± 755.5 | 2217.3 ± 794.0 | 1564.9 ± 591.0 | 1581.9 ± 624.5 |
| Protein, g/d | 77.0 ± 28.3 | 83.3 ± 30.7 | 59.0 ± 21.8 | 62.6 ± 28.5 |
| Carbohydrates, g/d | 284.1 ± 114.2 | 303.3 ± 120.8 | 226.5 ± 95.0 | 222.4 ± 95.2 |
| Total dietary fiber, g/d | 22.7 ± 10.2 | 24.3 ± 11.3 | 19.2 ± 9.0 | 19.7 ± 10.4 |
| Total fat, g/d | 70.4 ± 31.3 | 74.1 ± 32.6 | 49.8 ± 24.8 | 52.5 ± 25.4 |
| Calcium, mg/day | 975.7 ± 451.4 | 1056.4 ± 457.7 | 852.9 ± 418.1 | 881.8 ± 482.8 |
| Vitamin D (diet), IU/d | 126.8 ± 87.8 | 141.5 ± 93.7 | 124.3 ± 111.2 | 111.4 ± 87.6 |
| Vitamin D (supp), IU/d | 232.5 ± 248.5 | 238.5 ± 244.5 | 283.6 ± 275.9 | 249.4 ± 239.4 |
| Alcohol, g/d | 10.5 ± 16.1 | 8.9 ± 15.9 | 3.4 ± 5.9 | 2.5 ± 4.9 |
| Non-dietary Factors | ||||
| Current smoking (yes)2 | 103 (12.5) | 79 (12.8) | 74 (17.3) | 24 (10.5) |
| Body Mass Index | 26.5 ± 3.4 | 30.4 ± 4.1 | 25.3 4.3 | 30.8 ± 5.3 |
| Waist-to-Hip Ratio | 0.94 ± 0.05 | 0.97 ± 0.05 | 0.79 0.08 | 0.86 ± 0.07 |
| Diabetes (self-reported) | 45 (5.5) | 105 (17.0) | 8 (1.9) | 42 (18.3) |
| Season of blood draw | ||||
| Fall | 194 (23.7) | 148 (23.9) | 90 (21.0) | 55 (24.0) |
| Winter | 197 (24.1) | 169 (27.3) | 111 (25.9) | 72 (31.4) |
| Spring | 229 (28.0) | 158 (25.5) | 131 (30.5) | 54 (23.6) |
| Summer | 199 (24.3) | 145 (23.4) | 97 (22.6) | 48 (21.0) |
Count and percentage for categorical variables; mean ± standard deviation for continuous variables.
Smoked cigarettes in the past month
Figure 1.
Relationship between circulating concentrations of 25(OH)D and 1,25(OH)2D.
Table 2 presents the results for the association between 1,25(OH)2D, components of MetS, and MetS. In the total study population, statistically significant inverse relationships were observed for 1,25(OH)2D and waist circumference; however, after adjustment for BMI, this association was attenuated. In contrast, even after adjustment for BMI or WHR, statistically significant inverse relationships were observed between 1,25(OH)2D and high triglycerides and low HDL concentrations. After adjustment for age, race/ethnicity, supplemental calcium intake, sex, and BMI, 1,25(OH)2D was also significantly inversely related to odds of MetS using the ATP-III criteria, with ORs (95% CIs) of 0.73 (0.52–1.04) and 0.52 (0.36–0.75) for the second and third tertiles of 1,25(OH)2D as compared to the lowest tertile, respectively (p-trend <0.001). These findings were confirmed when using the IDF definitions of MetS. The results were similar for men in sex-stratified analyses; however, among women only low HDL was significantly associated with concentrations of 1,25(OH)2D after adjustment for confounding. After log transformation of non-normally distributed data, linear regression models were also conducted for the associations between continuous variables for 1,25(OH)2D and each component of MetS (data not shown). The β-coefficients (p-values) for each were: −0.24 (<0.001) for waist circumference; −0.01 (<0.001) for triglycerides; 0.09 (<0.001) for HDL; and −0.04 (p<0.01) for glucose.
Table 2.
Crude and adjusted ORs (95% CIs) for components of Metabolic Syndrome and Metabolic Syndrome, by tertile of 1,25(OH)2D concentration.
| Tertile of 1,25(OH)2D Concentration (mean ± sd, pg/ml) | ||||
|---|---|---|---|---|
|
| ||||
| 1 22.6 ± 4.5 |
2 33.2 ± 2.8 |
3 46.6 ± 8.0 |
p-trend | |
| Total population | ||||
| High Waist Circumference1(n, %) | 176 (50.1) | 140 (40.1) | 113 (32.5) | |
| Crude OR (95% CI) | 1.00 | 0.67 (0.49–0.90) | 0.48 (0.35–0.65) | <0.001 |
| Adjusted OR (95% CI)2 | 1.00 | 0.63 (0.47–0.86) | 0.45 (0.33–0.62) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.78 (0.48– 1.27) | 0.75 (0.46–1.22) | 0.24 |
| Triglycerides ≥150 mg/dL (n, %) | 183 (52.1) | 137 (39.3) | 119 (34.2) | |
| Crude OR (95% CI) | 1.00 | 0.59 (0.44–0.80) | 0.48 (0.35–0.65) | <0.001 |
| Adjusted OR (95% CI)2 | 1.00 | 0.57 (0.42–0.78) | 0.45 (0.33–0.62) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.61 (0.45–0.84) | 0.53 (0.38–0.73) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.62 (0.46–0.85) | 0.49 (0.35–0.67) | <0.001 |
| Low HDL5 (n, %) | 133 (37.9) | 109 (31.2) | 82 (23.6) | |
| Crude OR (95% CI) | 1.00 | 0.74 (0.54–1.02) | 0.51 (0.36–0.70) | <0.001 |
| Adjusted OR (95% CI)2 | 1.00 | 0.72 (0.52–0.98) | 0.49 (0.35–0.69) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.75 (0.54–1.05) | 0.56 (0.40–0.80) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.76 (0.55–1.05) | 0.51 (0.36–0.71) | <0.001 |
| Blood Pressure ≥130/≥85 mmHg (n, %) | 205 (58.4) | 201 (57.6) | 182 (52.3) | |
| Crude OR (95% CI) | 1.00 | 0.97 (0.72–1.31) | 0.78 (0.58–1.05) | 0.10 |
| Adjusted OR (95% CI)2 | 1.00 | 1.01 (0.74– 1.38) | 0.88 (0.65–1.21) | 0.43 |
| Adjusted OR (95% CI)3 | 1.00 | 1.09 (0.79–1.50) | 1.01 (0.73–1.40) | 0.35 |
| Adjusted OR (95% CI)4 | 1.00 | 1.06 (0.77–1.45) | 0.90 (0.66–1.24) | 0.55 |
| Fasting glucose ≥100mg/dL (n, %) | 191 (54.4) | 191 (54.7) | 176 (50.6) | |
| Crude OR (95% CI) | 1.00 | 1.01 (0.75–1.36) | 0.86 (0.64–1.15) | 0.31 |
| Adjusted OR (95% CI)2 | 1.00 | 0.96 (0.70–1.32) | 0.81 (0.59–1.11) | 0.18 |
| Adjusted OR (95% CI)3 | 1.00 | 1.04 (0.75–1.44) | 0.99 (0.71–1.37) | 0.17 |
| Adjusted OR (95% CI)4 | 1.00 | 1.05 (0.76–1.44) | 0.87 (0.63–1.20) | 0.42 |
| Metabolic Syndrome (n, %)6 | 180 (51.3) | 148 (42.4) | 108 (31.0) | |
| Crude OR (95% CI) | 1.00 | 0.70 (0.52–0.94) | 0.43 (0.31–0.58) | <0.001 |
| Adjusted OR (95% CI)2 | 1.00 | 0.66 (0.48–0.90) | 0.39 (0.28–0.54) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.73 (0.52–1.04) | 0.52 (0.36–0.75) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.68 (0.49–0.93) | 0.40 (0.29–0.56) | <0.001 |
| Metabolic Syndrome (n, %)7,8 | 140 (60.9) | 105 (49.5) | 73 (35.3) | |
| Crude OR (95% CI) | 1.00 | 0.88 (0.43–0.92) | 0.35 (0.28–0.52) | <0.001 |
| Adjusted OR (95% CI)2 | 1.00 | 0.57 (0.38–0.85) | 0.30 (0.20–0.46) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.48 (0.24–0.96) | 0.53 (0.26–1.08) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.74 (0.45–1.22) | 0.37 (0.22–0.63) | <0.001 |
| Men | ||||
| High Waist Circumference1 (n, %) | 124 (56.9) | 105 (42.3) | 79 (32.8) | |
| Crude OR (95% CI) | 1.00 | 0.56 (0.39–0.80) | 0.37 (0.25–0.54) | <0.001 |
| Adjusted OR (95% CI)2 | 1.00 | 0.55 (0.37–0.80) | 0.35 (0.24–0.52) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.96 (0.52–1.77) | 0.76 (0.41–1.42) | 0.39 |
| Triglycerides ≥150 mg/dL (n, %) | 119 (54.6) | 92 (37.1) | 82 (34.0) | |
| Crude OR (95% CI) | 1.00 | 0.49 (0.34–0.71) | 0.43 (0.29–0.63) | <0.001 |
| Adjusted OR (95% CI)2 | 1.00 | 0.46 (0.31–0.67) | 0.39 (0.26–0.57) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.53 (0.36–0.78) | 0.48 (0.32–0.73) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.47 (0.32–0.70) | 0.44 (0.29–0.65) | <0.001 |
| Low HDL5 (n, %) | 85 (39.0) | 85 (34.3) | 58 (24.1) | |
| Crude OR (95% CI) | 1.00 | 0.82 (0.56–1.19) | 0.50 (0.33–0.74) | <0.001 |
| Adjusted OR (95% CI)2 | 1.00 | 0.80 (0.54–1.17) | 0.48 (0.32–0.73) | 0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.88 (0.59–1.32) | 0.58 (0.38–0.90) | <0.05 |
| Adjusted OR (95% CI)4 | 1.00 | 0.82 (0.55–1.21) | 0.51 (0.34–0.74) | <0.05 |
| Blood Pressure ≥130/≥85 mmHg (n, %) | 137 (62.8) | 141 (56.9) | 133 (55.2) | |
| Crude OR (95% CI) | 1.00 | 0.78 (0.54–1.13) | 0.73 (0.50–1.06) | 0.10 |
| Adjusted OR (95% CI)2 | 1.00 | 0.84 (0.58– 1.24) | 0.84 (0.57–1.23) | 0.37 |
| Adjusted OR (95% CI)3 | 1.00 | 0.93 (0.63–1.38) | 1.01 (0.67–1.52) | 0.94 |
| Adjusted OR (95% CI)4 | 1.00 | 0.85 (0.58–1.25) | 0.87 (0.58–1.29) | 0.53 |
| Fasting glucose ≥100mg/dL (n, %) | 141 (64.7) | 157 (63.3) | 141 (58.5) | |
| Crude OR (95% CI) | 1.00 | 0.94 (0.64–1.38) | 0.77 (0.53–1.12) | 0.17 |
| Adjusted OR (95% CI)2 | 1.00 | 1.00 (0.68–1.47) | 0.78 (0.53–1.14) | 0.19 |
| Adjusted OR (95% CI)3 | 1.00 | 1.11 (0.74–1.66) | 0.95 (0.63–1.44) | 0.79 |
| Adjusted OR (95% CI)4 | 1.00 | 1.04 (0.70–1.54) | 0.85 (0.57–1.26) | 0.60 |
| Metabolic Syndrome (n, %)6 | 130 (59.6) | 112 (45.2) | 79 (32.8) | |
| Crude OR (95% CI) | 1.00 | 0.56 (0.39–0.81) | 0.33 (0.23–0.48) | <0.001 |
| Adjusted OR (95% CI)2 | 1.00 | 0.55 (0.37–0.80) | 0.30 (0.20–0.45) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.68 (0.44–1.05) | 0.45 (0.28–0.70) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.57 (0.38–0.85) | 0.35 (0.23–0.52) | <0.001 |
| Metabolic Syndrome (n, %)7 | 101 (74.8) | 78 (55.3) | 53 (38.1) | |
| Crude OR (95% CI) | 1.00 | 0.42 (0.25–0.69) | 0.21 (0.12–0.35) | <0.001 |
| Adjusted OR (95% CI)2 | 1.00 | 0.43 (0.25–0.72) | 0.21 (0.12–0.35) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.48 (0.18–1.23) | 0.58 (0.22–1.51) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.45 (0.24–0.86) | 0.24 (0.12–0.45) | <0.001 |
| Women | ||||
| High Waist Circumference1 (n, %) | 52 (39.1) | 35 (34.7) | 34 (31.8) | |
| Crude OR (95% CI) | 1.00 | 0.83 (0.48–1.41) | 0.73 (0.42–1.24) | 0.24 |
| Adjusted OR (95% CI)2 | 1.00 | 0.80 (0.46–1.38) | 0.73 (0.42–1.25) | 0.24 |
| Adjusted OR (95% CI)3 | 1.00 | 0.54 (0.22– 1.31) | 0.90 (0.40–2.03) | 0.73 |
| Triglycerides ≥150 mg/dL (n, %) | 64 (48.1) | 45 (44.6) | 37 (34.6) | |
| Crude OR (95% CI) | 1.00 | 0.87 (0.52–1.46) | 0.57 (0.34–0.96) | <0.05 |
| Adjusted OR (95% CI)2 | 1.00 | 0.85 (0.50–1.43) | 0.57 (0.33–0.97) | 0.04 |
| Adjusted OR (95% CI)3 | 1.00 | 0.84 (0.48–1.46) | 0.60 (0.34–1.05) | 0.08 |
| Adjusted OR (95% CI)4 | 1.00 | 1.08 (0.62–1.88) | 0.57 (0.33–0.98) | 0.06 |
| Low HDL5 (n, %) | 48 (36.1) | 24 (23.8) | 24 (22.4) | |
| Crude OR (95% CI) | 1.00 | 0.55 (0.31–0.98) | 0.51 (0.29–0.91) | <0.05 |
| Adjusted OR (95% CI)2 | 1.00 | 0.55 (0.31–0.99) | 0.51 (0.29–0.91) | 0.02 |
| Adjusted OR (95% CI)3 | 1.00 | 0.54 (0.30–1.00) | 0.55 (0.30–1.00) | <0.05 |
| Adjusted OR (95% CI)4 | 1.00 | 0.66 (0.36–1.21) | 0.49 (0.27–0.89) | <0.05 |
| Blood Pressure ≥130/≥85 mmHg (n, %) | 68 (51.1) | 60 (59.4) | 49 (45.8) | |
| Crude OR (95% CI) | 1.00 | 1.40 (0.83–2.36) | 0.81 (0.48–1.34) | 0.48 |
| Adjusted OR (95% CI)2 | 1.00 | 1.47 (0.85–2.55) | 0.93 (0.54–1.58) | 0.85 |
| Adjusted OR (95% CI)3 | 1.00 | 1.66 (0.94–2.93) | 0.98 (0.56–1.72) | 0.94 |
| Adjusted OR (95% CI)4 | 1.00 | 1.80 (1.01–3.19) | 0.92 (0.53–1.59) | 0.87 |
| Fasting glucose ≥100mg/dL (n, %) | 50 (37.6) | 34 (33.7) | 35 (32.7) | |
| Crude OR (95% CI) | 1.00 | 0.84 (0.49–1.45) | 0.81 (0.47–1.38) | 0.42 |
| Adjusted OR (95% CI)2 | 1.00 | 0.86 (0.50–1.49) | 0.87 (0.51–1.50) | 0.60 |
| Adjusted OR (95% CI)3 | 1.00 | 0.83 (0.46–1.51) | 1.00 (0.56–1.79) | 0.95 |
| Adjusted OR (95% CI)4 | 1.00 | 1.06 (0.60–1.88) | 0.90 (0.51–1.57) | 0.73 |
| Metabolic Syndrome (n, %)6 | 50 (37.6) | 36 (35.6) | 29 (27.1) | |
| Crude OR (95% CI) | 1.00 | 0.92 (0.54–1.57) | 0.62 (0.36–1.07) | 0.09 |
| Adjusted OR (95% CI)2 | 1.00 | 0.93 (0.54–1.61) | 0.63 (0.36–1.11) | 0.12 |
| Adjusted OR (95% CI)3 | 1.00 | 0.87 (0.46–1.62) | 0.75 (0.40–1.41) | 0.37 |
| Adjusted OR (95% CI)4 | 1.00 | 1.44 (0.78–2.66) | 0.63 (0.34–1.17) | 0.20 |
| Metabolic Syndrome (n, %)7 | 39 (41.1) | 27 (38.0) | 20 (29.4) | |
| Crude OR (95% CI) | 1.00 | 0.88 (0.47–1.65) | 0.60 (0.31–1.16) | 0.14 |
| Adjusted OR (95% CI)2 | 1.00 | 0.89 (0.46–1.70) | 0.59 (0.30–1.17) | 0.72 |
| Adjusted OR (95% CI)3 | 1.00 | 0.46 (0.15–1.39) | 0.54 (0.17–1.64) | 0.17 |
| Adjusted OR (95% CI)4 | 1.00 | 1.98 (0.82–4.70) | 0.87 (0.34–2.24) | 0.13 |
High waist circumference classification for Men >40 inches; for Women >35 inches.
Model adjusted for age, race/ethnicity, supplemental calcium; also adjusted for sex in analyses that included the total population.
Model adjusted for age, race/ethnicity, supplemental calcium, and BMI; also adjusted for sex in analyses that included the total population.
Model adjusted for age, race/ethnicity, supplemental calcium, and WHR; also adjusted for sex in analyses that included the total population.
Low HDL cholesterol classification for Men <40mg/dL; for Women <50mg/dL.
Metabolic syndrome defined by modified ATP-III criteria.
Metabolic syndrome defined by IDF criteria.
P-interaction by sex for metabolic syndrome=0.20.
Crude and adjusted odds ratios for the association between circulating concentrations of 25(OH)D, components of MetS, and MetS are presented in Table 3. In the total study population and after adjustment for confounding variables except for BMI, statistically significant inverse relationships were observed for 25(OH)D and high waist circumference, triglycerides, low HDL, and fasting glucose, but not for high blood pressure. Concentrations of 25(OH)D were also significantly associated with MetS, with ORs (95% CIs) of 0.65 (0.51–0.83) and 0.39 (0.30–0.51) for participants who had inadequate and adequate 25(OH)D concentrations, respectively, compared to those who were deficient (p-trend <0.001). These results were replicated when applying the IDF criteria of MetS. When BMI was added to the models, results were generally attenuated, with significant associations observed for waist circumference and triglycerides. Addition of WHR to the model had less marked effects than BMI, with significant relationships remaining for triglycerides, HDL, and glucose. For MetS, and after adjustment for BMI, there remained a statistically significant association, with ORs (95% CIs) of 0.78 (0.58–1.04) and 0.59 (0.43–0.80) for those classified as having inadequate and adequate 25(OH)D concentrations, respectively, compared to those who were deficient (p-trend <0.01). Results were similar with the addition of WHR to the model. In sex-stratified analyses, the findings for men were similar to that of the total population. Among women, only low HDL was significantly associated with concentrations of 25(OH)D, after adjustment for BMI. After log transformation of non-normally distributed data, linear regression models were also conducted for the associations between continuous variables for 25(OH)D and each component of MetS (data not shown). The β-coefficients (p-values) for each were: −0.09 (<0.05) for waist circumference; −0.02 (<0.001) for triglycerides; −0.009 (0.56) for HDL; and −0.02 (p<0.05) for glucose.
Table 3.
Crude and adjusted ORs (95% CIs) for components of Metabolic Syndrome and Metabolic Syndrome, by vitamin D status as measured by 25(OH)D.
| Vitamin D Status1 | ||||
|---|---|---|---|---|
|
| ||||
| Deficient 25(OH)D < 20 ng/ml |
Inadequate 25(OH)D 20 to <30 ng/ml |
Adequate 25(OH)D ≥30 ng/ml |
p-trend | |
| Total population | ||||
| High Waist Circumference2 (n, %) | 202 (50.0) | 351 (42.7) | 231 (31.2) | |
| Crude OR (95% CI) | 1.00 | 0.74 (0.59–0.94) | 0.45 (0.35–0.58) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.66 (0.51–0.84) | 0.37 (0.29–0.49) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.87 (0.59–1.28) | 0.66 (0.44–1.00) | 0.04 |
| Triglycerides ≥150 mg/dL (n, %) | 203 (50.3) | 375 (45.6) | 269 (36.3) | |
| Crude OR (95% CI) | 1.00 | 0.83 (0.65–1.05) | 0.56 (0.44–0.72) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.81 (0.63–1.03) | 0.54 (0.42–0.71) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.90 (0.70–1.17) | 0.70 (0.53–0.92) | <0.01 |
| Adjusted OR (95% CI)5 | 1.00 | 0.87 (0.67–1.12) | 0.63 (0.48–0.82) | <0.001 |
| Low HDL6 (n, %) | 147 (36.4) | 249 (30.3) | 200 (27.0) | |
| Crude OR (95% CI) | 1.00 | 0.76 (0.59–0.98) | 0.65 (0.50–0.84) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.75 (0.58–0.97) | 0.62 (0.47–0.81) | 0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.84 (0.64–1.09) | 0.79 (0.59–1.05) | 0.12 |
| Adjusted OR (95% CI)5 | 1.00 | 0.80 (0.61–1.04) | 0.71 (0.53–0.94) | <0.05 |
| Blood Pressure ≥130/≥85 mmHg (n, %) | 233 (57.7) | 451 (54.8) | 406 (54.8) | |
| Crude OR (95% CI) | 1.00 | 0.89 (0.70–1.13) | 0.89 (0.70–1.14) | 0.41 |
| Adjusted OR (95% CI)3 | 1.00 | 0.82 (0.64–1.06) | 0.80 (0.61–1.03) | 0.11 |
| Adjusted OR (95% CI)4 | 1.00 | 0.92 (0.71–1.20) | 1.00 (0.76–1.31) | 0.87 |
| Adjusted OR (95% CI)5 | 1.00 | 0.86 (0.67–1.11) | 0.86 (0.66–1.12) | 0.35 |
| Fasting glucose ≥100mg/dL (n, %) | 225 (55.7) | 430 (52.3) | 392 (52.9) | |
| Crude OR (95% CI) | 1.00 | 0.87 (0.69–1.11) | 0.89 (0.70–1.14) | 0.46 |
| Adjusted OR (95% CI)3 | 1.00 | 0.71(0.55–0.92) | 0.66 (0.50–0.86) | <0.01 |
| Adjusted OR (95% CI)4 | 1.00 | 0.80 (0.61–1.04) | 0.86 (0.65–1.13) | 0.44 |
| Adjusted OR (95% CI)5 | 1.00 | 0.75 (0.58–0.96) | 0.73 (0.55–0.95) | 0.04 |
| Metabolic Syndrome (n, %)7 | 201 (49.8) | 349 (42.4) | 239 (32.3) | |
| Crude OR (95% CI) | 1.00 | 0.74 (0.59–0.94) | 0.48 (0.38–0.62) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.65 (0.51–0.83) | 0.39 (0.30–0.51) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.78 (0.58–1.04) | 0.59 (0.43–0.80) | <0.01 |
| Adjusted OR (95% CI)5 | 1.00 | 0.70 (0.54–0.92) | 0.47 (0.35–0.63) | <0.001 |
| Metabolic Syndrome (n, %)8 | 169 (59.1) | 253 (50.9) | 150 (37.2) | |
| Crude OR (95% CI) | 1.00 | 0.72 (0.53–0.96) | 0.41 (0.30–0.56) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.58 (0.42–0.79) | 0.29 (0.21–0.41) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.74 (0.43–1.28) | 0.56 (0.31–1.00) | <0.05 |
| Adjusted OR (95% CI)5 | 1.00 | 0.63 (0.42–0.96) | 0.41 (0.26–0.63) | <0.001 |
| Men | ||||
| High Waist Circumference2 (n, %) | 98 (53.3) | 263 (47.1) | 196 (32.5) | |
| Crude OR (95% CI) | 1.00 | 0.78 (0.56–1.09) | 0.42 (0.30–0.59) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.74 (0.53–1.04) | 0.40 (0.28–0.56) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.98 (0.57–1.68) | 0.64 (0.38–1.10) | 0.03 |
| Triglycerides ≥150 mg/dL (n, %) | 98 (53.3) | 261 (46.7) | 215 (35.7) | |
| Crude OR (95% CI) | 1.00 | 0.77 (0.55–1.08) | 0.49 (0.35–0.68) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.77 (0.55–1.08) | 0.49 (0.35–0.68) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.83 (0.58–1.18) | 0.59 (0.41–0.84) | <0.001 |
| Adjusted OR (95% CI)5 | 1.00 | 0.83 (0.59–1.17) | 0.56 (0.39–0.79) | <0.001 |
| Low HDL6 (n, %) | 63 (34.2) | 181 (32.4) | 169 (28.0) | |
| Crude OR (95% CI) | 1.00 | 0.92 (0.65–1.31) | 0.75 (0.53–1.06) | 0.06 |
| Adjusted OR (95% CI)3 | 1.00 | 0.96 (0.67–1.37) | 0.77 (0.54–1.11) | 0.08 |
| Adjusted OR (95% CI)4 | 1.00 | 1.05 (0.73–1.53) | 0.97 (0.66–1.41) | 0.71 |
| Adjusted OR (95% CI)5 | 1.00 | 1.01 (0.70–1.46) | 0.88 (0.61–1.27) | 0.34 |
| Blood Pressure ≥130/≥85 mmHg (n, %) | 117 (63.6) | 315 (56.4) | 334 (55.4) | |
| Crude OR (95% CI) | 1.00 | 0.74 (0.52–1.04) | 0.71 (0.51–1.00) | 0.09 |
| Adjusted OR (95% CI)3 | 1.00 | 0.70 (0.49–1.00) | 0.67 (0.47– 0.96) | 0.06 |
| Adjusted OR (95% CI)4 | 1.00 | 0.75 (0.53–1.08) | 0.81 (0.56–1.16) | 0.49 |
| Adjusted OR (95% CI)5 | 1.00 | 0.72 (0.51–1.03) | 0.72 (0.51–1.03) | 0.16 |
| Fasting glucose ≥100mg/dL (n, %) | 122 (66.3) | 329 (58.9) | 352 (58.4) | |
| Crude OR (95% CI) | 1.00 | 0.73 (0.51–1.03) | 0.71 (0.50–1.01) | 0.11 |
| Adjusted OR (95% CI)3 | 1.00 | 0.75 (0.52–1.06) | 0.75(0.53–1.07) | 0.22 |
| Adjusted OR (95% CI)4 | 1.00 | 0.80 (0.56–1.16) | 0.93 (0.64–1.34) | 0.86 |
| Adjusted OR (95% CI)5 | 1.00 | 0.77 (0.54–1.10) | 0.81 (0.57–1.16) | 0.49 |
| Metabolic Syndrome (n, %)7 | 105 (57.1) | 262 (46.9) | 205 (34.0) | |
| Crude OR (95% CI) | 1.00 | 0.66 (0.47–0.93) | 0.39 (0.28–0.54) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.65 (0.46–0.92) | 0.38 (0.27–0.54) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.71 (0.48–1.06) | 0.52 (0.35–0.78) | <0.001 |
| Adjusted OR (95% CI)5 | 1.00 | 0.70 (0.49–1.00) | 0.45 (0.31–0.65) | <0.001 |
| Metabolic Syndrome (n, %)8 | 86 (69.4) | 195 (58.9) | 128 (40.0) | |
| Crude OR (95% CI) | 1.00 | 0.63 (0.41–0.98) | 0.29 (0.28–0.54) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.65 (0.46–0.92) | 0.38 (0.19–0.46) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.62 (0.39–0.96) | 0.28 (0.18–0.45) | <0.05 |
| Adjusted OR (95% CI)5 | 1.00 | 0.64 (0.37–1.11) | 0.39 (0.23–0.68) | <0.001 |
| Women | ||||
| High Waist Circumference2 (n, %) | 104 (47.3) | 88 (33.3) | 35 (25.4) | |
| Crude OR (95% CI) | 1.00 | 0.56 (0.39–0.81) | 0.38 (0.24–0.60) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.56 (0.39–0.81) | 0.39 (0.24–0.62) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.69(0.40–1.21) | 0.90 (0.45–1.80) | 0.58 |
| Triglycerides ≥150 mg/dL (n, %) | 105 (47.7) | 114 (43.2) | 54 (39.1) | |
| Crude OR (95% CI) | 1.00 | 0.83 (0.58–1.19) | 0.70 (0.46–1.08) | 0.11 |
| Adjusted OR (95% CI)3 | 1.00 | 0.82 (0.57–1.18) | 0.70 (0.45–1.09) | 0.11 |
| Adjusted OR (95% CI)4 | 1.00 | 0.96 (0.65–1.41) | 1.00 (0.63–1.60) | 0.96 |
| Adjusted OR (95% CI)5 | 1.00 | 0.91 (0.62–1.33) | 0.88 (0.55–1.39) | 0.55 |
| Low HDL6 (n, %) | 84 (38.2) | 68 (25.8) | 31 (22.5) | |
| Crude OR (95% CI) | 1.00 | 0.56 (0.38–0.83) | 0.47 (0.29–0.76) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.57 (0.38–0.84) | 0.47 (0.29–0.76) | 0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.64 (0.43–0.97) | 0.62 (0.37–1.03) | <0.05 |
| Adjusted OR (95% CI)5 | 1.00 | 0.63 (0.42–0.95) | 0.59 (0.35–0.98) | <0.05 |
| Blood Pressure ≥130/≥85 mmHg (n, %) | 116 (52.7) | 136 (51.5) | 72 (52.2) | |
| Crude OR (95% CI) | 1.00 | 0.95 (0.67–1.36) | 0.98 (0.64–1.50) | 0.89 |
| Adjusted OR (95% CI)3 | 1.00 | 0.96 (0.66–1.39) | 0.99 (0.64–1.55) | 0.951 |
| Adjusted OR (95% CI)4 | 1.00 | 1.11 (0.76–1.65) | 1.37 (0.86–2.19) | 0.19 |
| Adjusted OR (95% CI)5 | 1.00 | 1.02 (0.70–1.49) | 1.13 (0.71–1.78) | 0.63 |
| Fasting glucose ≥100mg/dL (n, %) | 103 (46.8) | 101 (38.3) | 40 (29.0) | |
| Crude OR (95% CI) | 1.00 | 0.70 (0.49–1.01) | 0.46 (0.29–0.73) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.72(0.50–1.04) | 0.48(0.30–0.75) | 0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.84 (0.57–1.25) | 0.68 (0.42–1.10) | 0.12 |
| Adjusted OR (95% CI)5 | 1.00 | 0.76 (0.52–1.10) | 0.53 (0.33–0.85) | <0.05 |
| Metabolic Syndrome (n, %)7 | 96 (43.6) | 87 (33.0) | 34 (24.6) | |
| Crude OR (95% CI) | 1.00 | 0.63 (0.44–0.92) | 0.42 (0.26–0.68) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.63 (0.44–0.92) | 0.43 (0.27–0.69) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.83 (0.54–1.28) | 0.76 (0.44–1.32) | 0.30 |
| Adjusted OR (95% CI)5 | 1.00 | 0.70 (0.46–1.06) | 0.55 (0.33–0.93) | <0.05 |
| Metabolic Syndrome (n, %)8.9 | 83 (51.2) | 58 (34.9) | 22 (27.6) | |
| Crude OR (95% CI) | 1.00 | 0.51 (0.33–0.80) | 0.34 (0.19–0.61) | <0.001 |
| Adjusted OR (95% CI)3 | 1.00 | 0.53 (0.34–0.83) | 0.38 (0.21–0.69) | <0.001 |
| Adjusted OR (95% CI)4 | 1.00 | 0.59 (0.27–1.26) | 0.79 (0.29–2.13) | 0.42 |
| Adjusted OR (95% CI)5 | 1.00 | 0.64 (0.33–1.24) | 0.55 (0.24–2.16) | 0.12 |
Vitamin D status as assessed using Endocrine Society criteria for classification of 25(OH)D concentrations.
High waist circumference classification for Men >40 inches; for Women >35 inches.
Model adjusted for age, race/ethnicity; also adjusted for sex in analyses that included the total population.
Model adjusted for age, race/ethnicity, and BMI; also adjusted for sex in analyses that included the total population.
Model adjusted for age, race/ethnicity, supplemental calcium, and WHR; also adjusted for sex in analyses that included the total population.
Low HDL cholesterol classification for Men <40mg/dL; for Women <50mg/dL.
Metabolic syndrome defined by modified ATP-III criteria.
Metabolic syndrome defined by IDF criteria.
P-interaction by sex for metabolic syndrome=0.16.
A composite vitamin D metabolite classification system was established to evaluate whether having high concentrations of both 1,25(OH)2D and 25(OH)D compared to low levels of both metabolites may have a greater magnitude of effect than high levels of each metabolite alone with respect to MetS and components of MetS. These results are presented in Table 4. In the total population, and after adjustment for potentially confounding variables except BMI statistically significant inverse associations were shown between vitamin D metabolite profile and all of the MetS components. When models were further adjusted for BMI, the association between vitamin D metabolite profile and triglycerides and HDL remained significant, while the associations with other components were attenuated. A high vitamin D metabolite profile was also significantly inversely associated with MetS using the ATP-III criteria, with an adjusted OR (95% CI) of 0.38 (0.19–0.75) compared to those with a low vitamin D profile; results were similar when applying the IDF criteria for MetS. In sex-stratified analyses, and after adjustment for potential confounders including BMI, among men a high vitamin D profile was significantly associated with triglyceride levels and high blood pressure, as well as with MetS. In contrast, among women, a high vitamin D profile was only significantly related to low HDL levels.
Table 4.
Crude and adjusted ORs (95% CIs) for components of Metabolic Syndrome and Metabolic Syndrome, by overall vitamin D metabolite profile1.
| Vitamin D profile | ||
|---|---|---|
|
| ||
| Low | High | |
| Total population | ||
| High Waist Circumference (n, %)2 | 69 (58.5) | 45 (27.3) |
| Crude OR (95% CI) | 1.00 | 0.27 (0.16–0.44) |
| Adjusted OR (95% CI)3 | 1.00 | 0.23 (0.13–0.42) |
| Adjusted OR (95% CI)4 | 1.00 | 0.48 (0.18–1.27) |
| Triglycerides ≥150 mg/dL (n, %) | 65 (55.1) | 51 (30.9) |
| Crude OR (95% CI) | 1.00 | 0.36 (0.22–0.60) |
| Adjusted OR (95% CI)3 | 1.00 | 0.29 (0.16–0.51) |
| Adjusted OR (95% CI)4 | 1.00 | 0.38 (0.21–0.70) |
| Adjusted OR (95% CI)5 | 1.00 | 0.37 (0.20–0.68) |
| Low HDL6 (n, %) | 43 (36.4) | 34 (20.6) |
| Crude OR (95% CI) | 1.00 | 0.45 (0.27–0.77) |
| Adjusted OR (95% CI)3 | 1.00 | 0.40 (0.22–0.74) |
| Adjusted OR (95% CI)4 | 1.00 | 0.51 (0.27–0.98) |
| Adjusted OR (95% CI)5 | 1.00 | 0.51 (0.27–0.98) |
| Blood Pressure ≥130/≥85 mmHg (n, %) | 71 (60.2) | 81 (49.1) |
| Crude OR (95% CI) | 1.00 | 0.64 (0.40–1.03) |
| Adjusted OR (95% CI)3 | 1.00 | 0.47 (0.26–0.84) |
| Adjusted OR (95% CI)4 | 1.00 | 0.66 (0.35–1.23) |
| Adjusted OR (95% CI)5 | 1.00 | 0.52 (0.28–0.94) |
| Fasting glucose ≥100mg/dL (n, %) | 63 (53.4) | 84 (50.9) |
| Crude OR (95% CI) | 1.00 | 0.90 (0.56–1.45) |
| Adjusted OR (95% CI)3 | 1.00 | 0.50 (0.27–0.90) |
| Adjusted OR (95% CI)4 | 1.00 | 0.71 (0.37–1.36) |
| Adjusted OR (95% CI)5 | 1.00 | 0.63 (0.34–1.19) |
| Metabolic Syndrome (n, %)6 | 63 (53.4) | 46 (27.9) |
| Crude OR (95% CI) | 1.00 | 0.34 (0.21–0.55) |
| Adjusted OR (95% CI)3 | 1.00 | 0.22 (0.12–0.42) |
| Adjusted OR (95% CI)4 | 1.00 | 0.38 (0.19–0.75) |
| Adjusted OR (95% CI)5 | 1.00 | 0.31 (0.16–0.60) |
| Metabolic Syndrome (n, %)7 | 58 (63.0) | 27 (27.8) |
| Crude OR (95% CI) | 1.00 | 0.22 (0.12–0.41) |
| Adjusted OR (95% CI)3 | 1.00 | 0.11 (0.97–1.05) |
| Adjusted OR (95% CI)4 | 1.00 | 0.34 (0.10–1.13) |
| Adjusted OR (95% CI)5 | 1.00 | 0.06 (0.02–0.18) |
| Men | ||
| High Waist Circumference2 (n, %) | 34 (70.8) | 37 (27.8) |
| Crude OR (95% CI) | 1.00 | 0.16 (0.08–0.33) |
| Adjusted OR (95% CI)3 | 1.00 | 0.17 (0.08–0.37) |
| Adjusted OR (95% CI)4 | 1.00 | 0.33 (0.09–1.30) |
| Triglycerides ≥150 mg/dL (n, %) | 31 (64.6) | 44 (33.1) |
| Crude OR (95% CI) | 1.00 | 0.27 (0.14–0.54) |
| Adjusted OR (95% CI)3 | 1.00 | 0.28(0.14–0.58) |
| Adjusted OR (95% CI)4 | 1.00 | 0.36 (0.16–0.80) |
| Adjusted OR (95% CI)5 | 1.00 | 0.34 (0.16–0.74) |
| Low HDL6 n(%) | 17 (35.4) | 31 (23.3) |
| Crude OR (95% CI) | 1.00 | 0.55 (0.27–1.13) |
| Adjusted OR (95% CI)3 | 1.00 | 0.61 (0.29–1.30) |
| Adjusted OR (95% CI)4 | 1.00 | 0.78 (0.33–1.83) |
| Adjusted OR (95% CI)5 | 1.00 | 0.67 (0.30–1.51) |
| Blood Pressure ≥130/≥85 mmHg (n, %) | 37 (77.1) | 68 (51.1) |
| Crude OR (95% CI) | 1.00 | 0.31 (0.15–0.66) |
| Adjusted OR (95% CI)3 | 1.00 | 0.28 (0.13–0.62) |
| Adjusted OR (95% CI)4 | 1.00 | 0.36 (0.15–0.86) |
| Adjusted OR (95% CI)5 | 1.00 | 0.28 (0.12–0.65) |
| Fasting glucose ≥100mg/dL (n, %) | 35 (72.9) | 78 (58.7) |
| Crude OR (95% CI) | 1.00 | 0.53 (0.26–1.09) |
| Adjusted OR (95% CI)3 | 1.00 | 0.53 (0.25–1.12) |
| Adjusted OR (95% CI)4 | 1.00 | 0.75 (0.32–1.75) |
| Adjusted OR (95% CI)5 | 1.00 | 0.57 (0.26–1.28) |
| Metabolic Syndrome (n, %)7 | 35 (72.9) | 40 (30.1) |
| Crude OR (95% CI) | 1.00 | 0.16 (0.08–0.33) |
| Adjusted OR (95% CI)3 | 1.00 | 0.15 (0.07–0.34) |
| Adjusted OR (95% CI)4 | 1.00 | 0.29 (0.12–0.71) |
| Adjusted OR (95% CI)5 | 1.00 | 0.20 (0.09–0.45) |
| Metabolic Syndrome (n, %)8 | 30 (88.2) | 23 (31.1) |
| Crude OR (95% CI) | 1.00 | 0.06 (0.02–0.19) |
| Adjusted OR (95% CI)3 | 1.00 | 0.06 (0.02–0.21) |
| Adjusted OR (95% CI)4 | 1.00 | 0.13 (0.01–2.08) |
| Adjusted OR (95% CI)5 | 1.00 | 0.08 (0.02–0.35) |
| Women | ||
| High Waist Circumference2 (n, %) | 35 (50.0) | 8 (25.0) |
| Crude OR (95% CI) | 1.00 | 0.33 (0.13–0.84) |
| Adjusted OR (95% CI)3 | 1.00 | 0.36 (0.14–0.96) |
| Adjusted OR (95% CI)4 | 1.00 | 0.70 (0.17–2.94) |
| Triglycerides ≥150 mg/dL (n, %) | 34 (48.6) | 7 (21.9) |
| Crude OR (95% CI) | 1.00 | 0.30 (0.11–0.77) |
| Adjusted OR (95% CI)3 | 1.00 | 0.32 (0.12–0.85) |
| Adjusted OR (95% CI)4 | 1.00 | 0.42 (0.15–1.21) |
| Adjusted OR (95% CI)5 | 1.00 | 0.48 (0.17–1.39) |
| Low HDL6 (n, %) | 26 (37.1) | 3 (9.4) |
| Crude OR (95% CI) | 1.00 | 0.18 (0.05–0.63) |
| Adjusted OR (95% CI)3 | 1.00 | 0.14 (0.04–0.55) |
| Adjusted OR (95% CI)4 | 1.00 | 0.19 (0.04–0.74) |
| Adjusted OR (95% CI)5 | 1.00 | 0.23 (0.06–0.91) |
| Blood Pressure ≥130/≥85 mmHg (n, %) | 34 (48.6) | 13 (40.6) |
| Crude OR (95% CI) | 1.00 | 0.72 (0.31–1.69) |
| Adjusted OR (95% CI)3 | 1.00 | 0.99 (0.40–2.49) |
| Adjusted OR (95% CI)4 | 1.00 | 1.69 (0.61–4.71) |
| Adjusted OR (95% CI)5 | 1.00 | 1.32 (0.49–3.53) |
| Fasting glucose ≥100mg/dL (n, %) | 28 (40.0) | 6 (18.8) |
| Crude OR (95% CI) | 1.00 | 0.35 (0.13–0.95) |
| Adjusted OR (95% CI)3 | 1.00 | 0.40 (0.14–1.13) |
| Adjusted OR (95% CI)4 | 1.00 | 0.49 (0.16–1.49) |
| Adjusted OR (95% CI)5 | 1.00 | 0.55 (0.18–1.66) |
| Metabolic Syndrome (n, %)7 | 28 (40.0) | 6 (18.8) |
| Crude OR (95% CI) | 1.00 | 0.35 (0.13–0.95) |
| Adjusted OR (95% CI)3 | 1.00 | 0.40 (0.14–1.12) |
| Adjusted OR (95% CI)4 | 1.00 | 0.55 (0.17–1.76) |
| Adjusted OR (95% CI)5 | 1.00 | 0.70 (0.21–2.37) |
| Metabolic Syndrome (n, %)8,9 | 28 (48.3) | 4 (16.7) |
| Crude OR (95% CI) | 1.00 | 0.21 (0.07–0.70) |
| Adjusted OR (95% CI)3 | 1.00 | 0.25 (0.07–0.86) |
| Adjusted OR (95% CI)4 | 1.00 | 0.29 (0.03–2.76) |
| Adjusted OR (95% CI)5 | 1.00 | 0.51 (0.11–2.47) |
Classification of high vitamin D profile is 25(OH)D concentrations ≥ 30 ng/ml and 1,25(OH)2D concentrations ≥ 38.4 pg/ml. Low vitamin D profile is 25(OH)D <20 ng/ml and 1,25(OH)2D < 28.5 pg/ml.
High waist circumference classification for Men >40 inches; for Women >35 inches.
Model adjusted for age, race/ethnicity, supplemental calcium; also adjusted for sex in analyses that included the total population.
Model adjusted for age, race/ethnicity, supplemental calcium and BMI; also adjusted for sex in analyses that included the total population.
Model adjusted for age, race/ethnicity, supplemental calcium, and WHR; also adjusted for sex in analyses that included the total population.
Low HDL cholesterol classification for Men <40mg/dL; for Women <50mg/dL.
Metabolic syndrome defined by modified ATP-III criteria.
Metabolic syndrome defined by IDF criteria.
4. Discussion
In our study, higher concentrations of 1,25(OH)2D and 25(OH)D were both associated with lower risk of higher concentrations of triglycerides and metabolic syndrome. Also, higher 1,25(OH)2D concentrations were associated with low HDL cholesterol, and higher 25(OH)D concentrations were associated with lower risk of having large waist circumference. Sex-stratified analyses revealed similar findings for men, though for women, only the association with low HDL was significant after adjusting for confounders. Further, the vitamin D metabolite profile analyses demonstrated lower odds for MetS, high triglycerides, and low HDL among those with high 25(OH)D and 1,25(OH)2D compared to those with low levels of both.
The active vitamin D metabolite 1,25(OH)2D has been studied far less frequently in epidemiological studies than 25(OH)D due to several of its properties, including its comparatively narrow range in circulation. However, it has been suggested that study of 1,25(OH)2D as a biomarker relative to 25(OH)D may yield useful information regarding supply of the active hormone available to tissue targets[40]. Furthermore, the evidence for potential mechanisms of action for vitamin D on MetS and its components have largely been demonstrated in experimental models to result from the activity of 1,25(OH)2D binding to the vitamin D receptor (VDR). Although 1,25(OH)2D may be produced at the cellular level in many tissue types, circulating concentrations may also affect availability of this potent hormone to target tissues. Therefore, we sought to investigate whether circulating concentrations of 1,25(OH)2D might be related to MetS and its components.
The results for 1,25(OH)2D demonstrated significant associations for triglyceride levels and MetS. In contrast to the findings for 25(OH)D, 1,25(OH)2D was also statistically significantly associated with low HDL levels in the overall population, as well as among men and women in stratified analyses. These findings are in agreement with those of the only other published study of 1,25(OH)2D and MetS[27], the results of which demonstrated statistically significant associations between 1,25(OH)2D and waist circumference, triglycerides, HDL, and glucose in a Taiwanese population. Given the hypothesized mechanisms of action for vitamin D in reducing risk of MetS, these findings support a role for the active vitamin D metabolite via VDR binding and transcriptional regulation of key genes related to metabolic dysregulation.[41,42]
To date, a statistically significant association between measured concentrations of 25(OH)D and MetS has been reported in several studies[8–19], though others have not found a relationship[23–26]. The present study is concordant with the majority of previously published work, demonstrating a significant association between 25(OH)D and MetS, which remained after adjustment for BMI. Comparatively few published studies have included analyses stratified for sex[15,26]. The results of one investigation reported no association between 25(OH)D and MetS among men and women combined, nor for sex-stratified analyses[26], while results of another report showed that while the relationship was stronger for women, an interaction term for sex was not statistically significant[15]. In contrast, our results suggest a stronger relationship between 25(OH)D and MetS among men compared to women, although as with the prior study by Ford et al.[15], the interaction term was not significant (pint=0.16). Nonetheless, in the present study there was a general pattern for stronger associations between 25(OH)D, MetS and its components for men as compared to women. These findings could be the result of the comparatively small sample size among women, or may reflect real metabolic differences between men and women in relation to body size, vitamin D metabolite concentrations, and MetS.
Regarding the components of MetS, significant associations were observed for 25(OH)D and high waist circumference and triglycerides. The findings for waist circumference are in agreement with the majority of published work [8–14,19,24,26]. These results are not surprising given the well-established inverse association between BMI and concentrations of 25(OH)D in circulation. Thought be multifactorial in nature, the comparatively low concentrations of 25(OH)D among those who are overweight and obese may result from a combination of factors. These include potential sequestration of this fat-soluble vitamin in adipose tissue or reduced sun exposure in relation to lower physical activity levels. Because of the documented relationship between BMI and concentrations of 25(OH)D, statistical models were conducted with and without BMI, and results were generally attenuated when BMI was added. As with waist circumference, the findings for triglycerides were similar to previously published work [8–10,12–14,17,19,23–25]. While the precise mechanism by which vitamin D may affect triglyceride levels is currently unknown, activity may be mediated via calcium-mediated suppression of fecal cholesterol excretion[43].
It is possible that the similar findings for 25(OH)D and 1,25(OH)2D indicated that each biomarker may capture information regarding the same pathway of vitamin D endocrine system activity in MetS. To explore this hypothesis, a variable for participants’ vitamin D metabolite profile was created, and the association between having high concentrations of both metabolites vs. low levels of both compounds and odds of MetS and its components was examined. The findings presented in Table 4 demonstrate a statistically significant inverse relationship between having a high vitamin D metabolite profile and triglyceride level, low HDL, and MetS. In general, the findings for vitamin D profile were modestly stronger than those for 25(OH)D or 1,25(OH)2D alone; however, these differences in effect did not reach statistical significance.
The strengths of present work include the measurement of both 25(OH)D and 1,25(OH)2D, the latter of which has been studied in relation to MetS in only one prior study, as well as the large sample size. Weaknesses include the single measure of each vitamin D metabolite, as well as the inability to ascertain a causal relationship from the cross-sectional design of the study. We were also unable to consider medications that may affect concentrations of vitamin D metabolites in this analysis and did not have data for levels of related biomarkers such as parathyroid hormone. In addition, for body size considerations we did not have measures of adiposity from highly accurate technologies such as dual-energy X-ray absorptiometry; rather, waist circumference measurements were performed by study participants, which may have resulted in misclassification of some participants. Further, there was little variation with regard to race or ethnicity; future work in this area should include more diverse study populations, as generalizability of these findings is thus limited. Further, as the study sample was comprised of individuals with a previous adenoma, there were double the number of men versus women, which may have reduced the statistical power to detect associations in women as well as potentially limiting the generalizability of the study.
5. Conclusions
The findings of this study demonstrate associations between the vitamin D metabolite 1,25(OH)2D and MetS, as well as its components. In addition, the present work confirms the findings of several other studies that 25(OH)D is also related to MetS. As discussed in the comprehensive review by Challoumas[44], clinical trial findings for the efficacy of vitamin D on blood lipid levels, important components of MetS, have been largely discouraging. However, some work has indicated that interventions aimed at improving vitamin D status can increase HDL concentrations and reduce hypertension[45]. The finding of a significant association for the active vitamin D metabolite 1,25(OH)2D indicates that more research in this area is warranted.
Acknowledgments
We are grateful to the members of the Phoenix Colon Cancer Prevention Physicians’ Network for their dedication to the WBF trial and thank the UDCA study group for their outstanding contribution to this project.
Funding: The project was supported by Public Health Service grants R01CA140285, CA-110814, CA-41108, CA-23074, and CA-77145. The funding source had no role in the design, interpretation, data collection, or decision to publish this study.
Abbreviations
- MetS
Metabolic Syndrome
- 25(OH)D
25-hydroxycholecalciferol
- 1,25(OH)2D
1,25-dihydroxycholecalciferol
- HDL
high density lipoprotein
- WBF
Wheat Bran Fiber trial
- UDCA
Ursodeoxycholic Acid trial
Footnotes
Disclosure Statement: The authors have declared no conflicts of interest.
Author Contributions: JWB and ETJ were primarily responsible for project conception and development of overall project plan. PL and PAT were involved in the hands-on conduct of the data collection for the WBF and UDCA trial. ETJ and JWB created the original and pooled databases necessary for the research; ETJ performed the statistical analysis in consultation with JWB and expert statistical review by DR; ETJ and JWB wrote and formatted the paper with input from all authors; all authors, particularly PWJ, EAH, and CLM contributed to interpretation of the data during the review process; JWB and ETJ had primary responsibility for final content; all authors approved the final manuscript before submission and none had a conflict of interest with regard to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM. Metabolic syndrome pandemic. Arteriosclerosis, Thrombosis & Vascular Biology. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher EJ, LeRoith D. Epidemiology and molecular mechanisms tying obesity, diabetes, and the metabolic syndrome with cancer. Diabetes Care. 2013;36(Suppl 2):S233–239. doi: 10.2337/dcS13-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltran-Sanchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. Journal of the American College of Cardiology. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 92:77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 6.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 7.Hibler EA, Sardo Molmenti CL, Lance P, Jurutka PW, Jacobs ET. Associations between circulating 1,25(OH)2D concentration and odds of metachronous colorectal adenoma. Cancer Causes Control. 2014;25:809–817. doi: 10.1007/s10552-014-0382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha C-KH, T-K, Lee S-H, Cho J-K, Kang H-S. Association between serum vitamin D status and metabolic syndrome in Korean young men. Medicine and Science in Sports and Exercise. 2014;46:513–519. doi: 10.1249/MSS.0b013e3182a6834a. [DOI] [PubMed] [Google Scholar]
- 9.Yin X, Sun Q, Zhang X, Lu Y, Sun C, et al. Serum 25(OH)D is inversely associated with metabolic syndrome risk profile among urban middle-aged Chinese population. Nutrition Journal. 2012;11:68. doi: 10.1186/1475-2891-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chacko SA, Song Y, Manson JE, Van Horn L, Eaton C, et al. Serum 25-hydroxyvitamin D concentrations in relation to cardiometabolic risk factors and metabolic syndrome in postmenopausal women. American Journal of Clinical Nutrition. 2011;94:209–217. doi: 10.3945/ajcn.110.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MK, Il Kang M, Won Oh K, Kwon HS, Lee JH, et al. The association of serum vitamin D level with presence of metabolic syndrome and hypertension in middle-aged Korean subjects. Clinical Endocrinology. 2010;73:330–338. doi: 10.1111/j.1365-2265.2010.03798.x. [DOI] [PubMed] [Google Scholar]
- 12.Maki KC, Fulgoni VL, 3rd, Keast DR, Rains TM, Park KM, et al. Vitamin D intake and status are associated with lower prevalence of metabolic syndrome in U.S. adults: National Health and Nutrition Examination Surveys 2003–2006. Metabolic Syndrome & Related Disorders. 2012;10:363–372. doi: 10.1089/met.2012.0020. [DOI] [PubMed] [Google Scholar]
- 13.Lee DM, Rutter MK, O’Neill TW, Boonen S, Vanderschueren D, et al. Vitamin D, parathyroid hormone and the metabolic syndrome in middle-aged and older European men. European Journal of Endocrinology. 2009;161:947–954. doi: 10.1530/EJE-09-0496. [DOI] [PubMed] [Google Scholar]
- 14.Lu L, Yu Z, Pan A, Hu FB, Franco OH, et al. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. 2009;32:1278–1283. doi: 10.2337/dc09-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford ES, Zhao G, Li C, Pearson WS. Serum concentrations of vitamin D and parathyroid hormone and prevalent metabolic syndrome among adults in the United States. Journal Of Diabetes. 2009;1:296–303. doi: 10.1111/j.1753-0407.2009.00046.x. [DOI] [PubMed] [Google Scholar]
- 16.Hypponen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes. 2008;57:298–305. doi: 10.2337/db07-1122. [DOI] [PubMed] [Google Scholar]
- 17.Reis JP, von Muhlen D, Miller ER., 3rd Relation of 25-hydroxyvitamin D and parathyroid hormone levels with metabolic syndrome among US adults. European Journal of Endocrinology. 2008;159:41–48. doi: 10.1530/EJE-08-0072. [DOI] [PubMed] [Google Scholar]
- 18.Rueda S, Fernandez-Fernandez C, Romero F, Martinez de Osaba J, Vidal J. Vitamin D, PTH, and the metabolic syndrome in severely obese subjects. Obesity Surgery. 2008;18:151–154. doi: 10.1007/s11695-007-9352-3. [DOI] [PubMed] [Google Scholar]
- 19.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28:1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 20.Brenner DR, Arora P, Garcia-Bailo B, Wolever TM, Morrison H, et al. Plasma vitamin D levels and risk of metabolic syndrome in Canadians. Clin Invest Med. 2011;34:E377. doi: 10.25011/cim.v34i6.15899. [DOI] [PubMed] [Google Scholar]
- 21.Hong HC, Lee JS, Choi HY, Yang SJ, Yoo HJ, et al. Liver enzymes and vitamin D levels in metabolically healthy but obese individuals: Korean National Health and Nutrition Examination Survey. Metabolism: Clinical & Experimental. 2013;62:1305–1312. doi: 10.1016/j.metabol.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Li HW, Brereton RE, Anderson RA, Wallace AM, Ho CK. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism: Clinical & Experimental. 2011;60:1475–1481. doi: 10.1016/j.metabol.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Chon SJYBH, Jung YS, Cho SH, Choi YS, Kim SY, Lee BS, Seo SK. Association between vitamin D status and risk of metabolic syndrome among Korean postmenopausal women. PLoS One. 2014:9. doi: 10.1371/journal.pone.0089721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S, Lim J, Kye S, Joung H. Association between vitamin D status and metabolic syndrome risk among Korean population: based on the Korean National Health and Nutrition Examination Survey IV-2, 2008. Diabetes Research & Clinical Practice. 2012;96:230–236. doi: 10.1016/j.diabres.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Hjelmesaeth J, Roislien J, Hofso D, Bollerslev J. Plasma 25-hydroxyvitamin d concentration and metabolic syndrome among middle-aged and elderly chinese individuals: response to Lu et al. Diabetes Care. 2010;33:e13. doi: 10.2337/dc09-1568. author reply e14. [DOI] [PubMed] [Google Scholar]
- 26.Reis JP, von Muhlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30:1549–1555. doi: 10.2337/dc06-2438. [DOI] [PubMed] [Google Scholar]
- 27.Cheng KH, Huang SP, Huang CN, Lee YC, Chu CS, et al. The impact of estradiol and 1,25(OH)2D3 on metabolic syndrome in middle-aged Taiwanese males. PLoS One. 2013;8:e60295. doi: 10.1371/journal.pone.0060295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberts DS, Martinez ME, Roe DJ, Guillen-Rodriguez JM, Marshall JR, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. N Engl J Med. 2000;342:1156–1162. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 29.Alberts DS, Martínez ME, Hess LH, Einspahr JG, Green SB, et al. Phase III Trial of Ursodeoxycholic Acid to Prevent Colorectal Adenoma Recurrence. J Natl Cancer Inst. 2005 doi: 10.1093/jnci/dji144. In Press. [DOI] [PubMed] [Google Scholar]
- 30.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: Development and validation. Epidemiology. 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Ritenbaugh C, Aickin M, Taren D, Teufel N, Graver E, et al. Use of a food frequency questionnaire to screen for dietary eligibility in a randomized cancer prevention phase III trial. Cancer Epidemiol, Biomarkers & Prev. 1997;6:347–354. [PubMed] [Google Scholar]
- 32.American Heart A, National Heart L, Blood I, Grundy SM, Cleeman JI, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiology in Review. 2005;13:322–327. [PubMed] [Google Scholar]
- 33.Alberti KG, Zimmet P, Shaw J Group IDFETFC. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 34.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–186. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 35.Hollis BW. Assessment of circulating 25(OH)D and 1,25(OH)2D: emergence as clinically important diagnostic tools. Nutr Rev. 2007;65:S87–90. doi: 10.1111/j.1753-4887.2007.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs ET, Alberts DS, Foote JA, Green SB, Hollis BW, et al. Vitamin D insufficiency in southern Arizona. Am J Clin Nutr. 2008;87:608–613. doi: 10.1093/ajcn/87.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 38.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 39.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 40.Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: physiology and biomarkers. Am J Clin Nutr. 2008;88:500S–506S. doi: 10.1093/ajcn/88.2.500S. [DOI] [PubMed] [Google Scholar]
- 41.Maestro B, Molero S, Bajo S, Davila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3) Cell biochemistry and function. 2002;20:227–232. doi: 10.1002/cbf.951. [DOI] [PubMed] [Google Scholar]
- 42.Maestro B, Campion J, Davila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocrine journal. 2000;47:383–391. doi: 10.1507/endocrj.47.383. [DOI] [PubMed] [Google Scholar]
- 43.Welberg JW, Monkelbaan JF, de Vries EG, Muskiet FA, Cats A, et al. Effects of supplemental dietary calcium on quantitative and qualitative fecal fat excretion in man. Ann Nutr Metab. 1994;38:185–191. doi: 10.1159/000177810. [DOI] [PubMed] [Google Scholar]
- 44.Challoumas D. Vitamin D supplementation and lipid profile: What does the best available evidence show? Atherosclerosis. 2014;235:130–139. doi: 10.1016/j.atherosclerosis.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 45.Al-Daghri NM, Alkharfy KM, Al-Saleh Y, Al-Attas OS, Alokail MS, et al. Modest reversal of metabolic syndrome manifestations with vitamin D status correction: a 12-month prospective study. Metabolism: Clinical & Experimental. 2012;61:661–666. doi: 10.1016/j.metabol.2011.09.017. [DOI] [PubMed] [Google Scholar]