Abstract
Objective(s)
The maternal-fetal inflammatory response contributes to both preterm premature rupture of membranes (PPROM) and adverse neurological outcomes. Additionally, cytokines associated with fetal placental inflammation can be detrimental to brain development regardless of inciting infection. We investigated whether differential patterns of cytokine markers in maternal and fetal plasma samples reflect subtypes of placental inflammation and neurological outcomes at 6 months in infants born to mothers with PPROM.
Study Design
Within a prospective cohort study of 25 women with PPROM, plasma cytokines (IL-1β, IL-6, IL-8, and TNF-α) were measured by ELISA from maternal blood samples at rupture and delivery, and from fetal umbilical cord blood samples. Patterns of cytokine expression were correlated with specific placenta pathologies. Infants underwent cranial ultrasound after birth and standardized neurological examinations at 6 months corrected gestational age. Predictors of inflammation and adverse neurological outcome were assessed by logistic regression, adjusting for gestational age at birth.
Results
Inflammation of the fetal side of the placenta was associated with elevated maternal IL-6 and IL-8 at delivery and fetal IL-1β, IL-6, IL-8, and TNF-α. Worse neurological outcome at 6 months was associated with inflammation of the fetal side of the placenta and shorter duration from rupture of membrane to delivery, independent of gestational age at birth or cranial ultrasound results.
Conclusion(s)
Our findings support the connection between fetal inflammation with adverse neurological outcome with PPROM, regardless of cranial ultrasound results. Further longitudinal studies are needed to adequately examine these patterns, and will aid in risk assessment and intervention strategies.
Keywords: cerebral palsy, chorioamnionitis, cytokines, funisitis, placenta
Introduction
Preterm birth is a major risk factor for long-term neurological disabilities, including cerebral palsy (CP).1 Despite accounting for 30-40% of preterm deliveries,2 preterm premature rupture of membranes (PPROM) is understudied in relation to neurologic disability. Infection/inflammation of the placenta and its membranes (“chorioamnionitis”) is considered a risk factor for CP, and the main contributor to PPROM.3-7 Although widely used, antibiotics in PPROM have failed to avert the fetal inflammatory response,8 been unsuccessful in improving long-term neurological outcomes,9-11 or have been associated with worse neurologic outcome.12 These findings highlight the complexity of the maternal-fetal inflammatory response and its alteration in the pathogenesis of abnormal neurological outcome within pregnancies complicated by PPROM.
Reliable clinical signs of placental infection/inflammation are not evident,13 necessitating alternative methods of early identification before preventive strategies can be developed. Because of their involvement in inflammatory processes and their distinct signatures depending on cause, duration, and organ(s) site, cytokines have been proposed as surrogate blood markers of placental inflammation. For instance, when compared to preterm newborns without PPROM, maternal serum and umbilical cord blood IL-8 levels were elevated in PPROM, yet IL-6 levels were only elevated in the cord blood.14 Additionally, umbilical cord IL-6 levels have been shown to be elevated in PPROM pregnancies when both microbial invasion of the amniotic cavity and histological chorioamnionitis (HCA) were present, rather than with HCA alone.15 Interestingly, maternal serum IL-6 levels within PPROM were shown in one study to predict funisitis after antibiotic completion, and to predict preclinical asymptomatic infection in another.8, 16
Cytokines have likewise been profiled in studies of perinatal infection, inflammation, and CP with varying results. 4, 6, 17-19 Inconsistencies might result from mixing heterogeneous conditions without sufficient pathological and clinical stratification between the distinct subsets of placental/membrane inflammatory insults, and their various impacts on offsprings' neurooutcome. Animal studies, performed in various species, establish a causal link between placental inflammation, maternal inflammatory mediators (notably, IL-1 and its close and interconnected “partners” such as IL-6, IL-8, and TNF-α, or their equivalent in animals), and perinatal brain injury.20-26 In fact, IL-1 and TNF-α activation is linked with fetal placental inflammation and perinatal brain injury, even without infection.23, 27 However, there is currently no preclinical experimental animal design adequately modeling the specific combination of gestational age and placental abnormalities seen in human PPROM. Thus, the precise set of inflammatory mediators involved in placental pathologies and perinatal brain injuries in PPROM pregnancies—with presumed baseline inflammation—remain unclear.
Recently, we identified two distinct pathological patterns in PPROM placentas; almost 40% did not show evidence of histologic chorioamnionitis (HCA), suggesting a non-inflammatory mechanism of preterm rupture.28 Additionally, we and others have demonstrated isolated funisitis without evidence of chorioamnionitis.28, 29 We therefore hypothesize that patterns of cytokine elevation and clinical characteristics will associate with patterns of maternal placental inflammation (chorioamnionitis) and fetal side placental inflammation (funisitis and chorionic plate vasculitis). We further hypothesize that patterns of cytokine elevation combined with placental pathologic findings and maternal and fetal clinical characteristics will emerge, which will inform our overall goal to create a high-risk multivariate index predictor model for perinatal brain injury in pregnancies complicated by PPROM. In this study, we investigate whether plasma titers of key placenta- and neuro-inflammatory markers (IL-1β, IL-6, IL-8, and TNF-α, targeted from preclinical and human epidemiological studies) in maternal samples at time of rupture of membranes (ROM) as well as maternal and fetal samples at delivery are associated with placental inflammatory patterns and adverse neurological outcomes at 6 months corrected gestational age in preterm infants born to mothers with PPROM. It is of the upmost importance to improve knowledge and diagnostic markers in this group, to ultimately aid in the development of new therapeutic approaches to beneficially modulate the maternal-fetal inflammatory response and prevent adverse neurological outcome.
Materials and Methods
Women with PPROM were enrolled in a prospective cohort study at the University of Colorado Hospital (UCH) from July 1, 2010 to June 30, 2012. PPROM was confirmed by standard clinical characteristics of alkaline pH, ferning, and pooling of amniotic fluid in the vagina on speculum examination in women > 24 or < 34 weeks gestation prior to labor onset. Clinical chorioamnionitis (CCA) was defined as 2 or more of the following: maternal fever > 38.0°C, maternal tachycardia (>100 beats per minute), fetal tachycardia (> 160 beats per minute), maternal fundal tenderness, maternal leukocytosis (WBC > 15,000), and purulent vaginal discharge OR amniocentesis with findings consistent with chorioamnionitis (glucose < 15 mg/dl, WBC count > 30 per cubic mm, leukocyte esterase positive, Gram stain with bacteria or > 6 WBC per high power field).30 Multiple gestation or non-viable pregnancy were excluded. Research was conducted in accordance with the 2004 Declaration of Helsinki, with signed informed consent (Colorado Multiple Institutional Review Board study 09-1107).
Demographic and clinical data were abstracted from the UCH Perinatal Database by a trained research assistant using a standardized protocol; ambiguity of maternal data was clarified by a maternal-fetal medicine specialist (V.D.W.) and for neonatal data by a neonatal neurologist (J.A.W.). Data included: 1.) demographics (maternal age, parity, and ethnicity, newborn gender and birth weight, prenatal care onset); 2.) maternal predictors (underlying condition requiring medical supervision, gestational diabetes, pregnancy-induced hypertension, preeclampsia, chorioamnionitis) 3.) intrapartum predictors (Category II or III fetal heart tracing leading to cesarean section, emergency cesarean section); and 4.) neonatal complications (respiratory distress, hemodynamic failure, sepsis, hypoglycemia, seizures). Although smoking, auto-immune disease, pregestational diabetes, and infertility/assisted reproduction were also predictors of interest, there were no cases within our cohort.
Cytokine analysis
Maternal venous blood samples were collected at time of enrollment and within 2 hours after delivery. Fetal cord blood venous samples were obtained immediately after delivery by trained perinatal research nurses with experience in venous cord blood collection. Samples were collected in EDTA tubes and centrifuged for 20 minutes at 1,600 × g at 4°, then transferred and centrifuged again. Platelet poor plasma was aliquotted, frozen, and sent for cytokine analysis (IL-1β, IL-6, IL-8, and TNF-α) via the Luminex Multicode Assay platform (Luminex Corp. Austin, TX)..
Placenta analysis
Assessments were conducted by a placental pathologist (M.D.P.). Placentas were immediately collected after delivery, placed in 10% neutral buffered formalin, and examined within 72 hours. A minimum of four formalin-fixed paraffin-embedded blocks were generated, including cross sections of umbilical cord, free fetal membranes, and full-thickness placental parenchyma; 5 mm sections from each block were stained with hematoxylin and eosin. HCA was defined as inflammation composed of maternal acute inflammatory cells (polymorphonuclear cells) within the amnion or chorion. The fetal inflammatory response (FIR), being composed of inflammatory cells of fetal origin, is histologically manifested by umbilical cord vasculitis and/or chorionic plate vasculitis; fetal side inflammation was defined as the presence of polymorphonuclear cells in the walls of fetal vessels of the chorionic plate (chorionic plate vasculitis), of the umbilical cord (vasculitis or phlebitis), or fetal inflammatory cells extending into Wharton's jelly (funisitis).
Post-natal evaluation
Newborns underwent cranial ultrasounds at least 4 days after birth.31 Infants underwent standardized neurological examinations (Pediatric Stroke Outcome Measure – “PSOM”) at 6 months corrected gestational age, with the examiner (J.A.W.) blinded to cytokine and placental pathology results. The PSOM is a validated tool for quantifying disability related to acquired brain injury that can be adapted for children < 2 years old. Subscale scoring (graded on level of severity) is 0 (no deficit), 0.5 (mild deficit, normal function), 1 (moderate deficit, decreased function, or 2 (severe deficit, no function); the total PSOM score ranges from 0 (no deficit) to 10 (maximum deficit).32
Statistical analysis
Dichotomous variables were compared using χ2 analysis (or Fisher's exact test) and continuous variables using Student's t-test or Mann Whitney U when appropriate. Linear regression was utilized to assess correlation of continuous variables. To assess predictors of 1.) placental pathology; 2.) elevated cytokine levels (dichotomized and defined a priori as individual level greater than the pooled sample median); and 3.) adverse neurological outcome at 6 months corrected gestational age (PSOM score dichotomized into normal/mild versus moderate/severe outcome for analysis), we calculated odds ratios (ORs) and 95% confidence intervals (CIs) using logistic regression. Univariate predictors included demographic, clinical, and laboratory data as described above. Gestational age at delivery was analyzed as both continuous and dichotomous variables (≤ 32 weeks and > 32 weeks); rupture to delivery interval was likewise analyzed as continuous and dichotomous variables (≤ 7 days, > 7 days). To determine independent predictors of outcomes, we created multivariate models; the model corrected for gestational age at delivery and included all covariates with a p-value of ≤ 0.10 in the initial analysis. Stata 12.0 (Stata Corp, College Station, TX) was used for all statistical analysis. All tests were two-sided and p < 0.05 was considered significant.
Results
Clinical characteristics and associated cytokine profiles
Twenty-five maternal-newborn dyads were examined (Table 1). There was no difference in the rate of CCA according to gestational age at PPROM, gestational age at delivery, or ROM interval. There was no difference in mean ROM interval between placentas with and without HCA (8 versus 9 days; p = 0.78). All cranial ultrasounds were read as normal, with the exception of a single newborn with a known grade III intraventricular hemorrhage on prenatal ultrasound.
Table 1. Selected clinical characteristics of PPROM pregnancies (N = 25).
| Characteristic | N |
|---|---|
| Maternal age (mean, range) | 29 years (19-40) |
| Nulliparity | 7 (28%) |
| Maternal race/ethnicity | |
| Caucasian (not of Hispanic heritage) | 12 (48%) |
| Caucasian (of Hispanic heritage) | 12 (48%) |
| Non-caucasian | 1 (4%) |
| Gestational age (mean, range) | |
| Rupture | 29.9 weeks (24.6 – 33.6) |
| Delivery | 31.2 weeks (25.6 – 34.2) |
| Rupture of membrane interval (mean, range) | 8.7 days (1 – 36) |
| Magnesium sulfate | 10 (40%) |
| Antibiotics | 25 (100%) |
| Betamethasone | 25 (100%) |
| Clinical chorioamnionitis | 11 (44% |
| Cesarean section | 7 (28%) |
| Male newborn | 16 (64%) |
PPROM, preterm premature rupture of membranes
Median maternal IL-6 and IL-8 at delivery, as well as fetal IL-1β, IL-6, and IL-8 were higher in gestational age of ≤ 32 weeks at delivery (Table 2), regardless of HCA presence. Maternal IL-8 plasma levels at rupture and delivery were significantly correlated (R2 = 0.33; p = 0.02). There were significant positive correlations between maternal and fetal levels of IL-1β (R2 = 0.81; p = 0.001) and IL-6 (R2 = 0.34; p = 0.04) at delivery. There was no significant correlation between maternal cytokines at ROM with fetal cytokines at delivery.
Table 2. Median values (with interquartile ranges) for cytokines in relation to clinical characteristics.
| Cytokine (pg/ml) | GAD ≤ 32 weeks | GAD > 32 weeks | ROM ≤ 7 days | ROM > 7 days | MAG + | MAG - |
|---|---|---|---|---|---|---|
| N = 13 | N = 12 | N = 12 | N = 13 | N = 10 | N= 15 | |
| IL-1β | ||||||
|
|
||||||
| Maternal rupture | 0 (0-2.7) | 0 (0-1.0) | 0.8 (0-2.2) | 0 (0-1.0) | 0 (0-2.7) | 0 (0-1.0) |
| Maternal delivery | 1.0 (0-2.9) | 0 (0-1.0) | 0.5 (0-1.7) | 0 (0-2.0) | 0 (0-0) | 1.0 (0-4.5) |
| Fetal cord | 8.1 (0.5-42.0) | 0 (0-0) | 0.5 (0-42.0) | 0 (0-8.1) | 0.5 (0-0.5) | 0 (0-42.0) |
| IL-6 | ||||||
|
|
||||||
| Maternal rupture | 2.3 (0-5.0) | 2.0 (2.0-2.0) | 3.0 (1.0-4.5) | 2.0 (1.5-2.2) | 2.0 (2.0-16.5) | 2.0 (1.5-2.0) |
| Maternal delivery | 90.2 (53.5-117.2) | 39.1 (20.3-49.5) | 30.2 (13.8-115.6) | 65.1 (38.7-155.1) | 39.1 (18.8-90.2) | 84.6 (34.3-272.0) |
| Fetal cord | 2137 (72.7-9569.0) | 12.2 (3.7-74.2) | 72.7 (3.7-9569.0) | 74.2 (10.5-2137) | 44.4 (12.2-545.0) | 88.1 (3.7-9569.0) |
| IL-8 | ||||||
|
|
||||||
| Maternal rupture | 4.4 (2.0-6.2) | 2.0 (2.0-2.0) | 5.2 (2.0-6.2) | 2.0 (2.0-3.5) | 2.0 (2.0-6.3) | 2.0 (2.0-5.9) |
| Maternal delivery | 32.9 (12.7-45.6) | 7.6 (6.2-15.2) | 8.7 (7.6-32.9) | 16.1 (6.6-34.3) | 8.4 (6.2-35.1) | 23.9 (7.6-33.5) |
| Fetal cord | 687.6 (114.8-1293.5) | 14.3 (9.7-29.8) | 114.8 (9.7-1200) | 29.8 (14.3 – 687.6) | 65.9 (14.3-263.6) | 57.9 (12.7-1293.5) |
| TNF-α | ||||||
|
|
||||||
| Maternal rupture | 3.4 (2.5-5.6) | 4.4 (3.0-7.6) | 5.7 (4.2-7.8) | 3.2 (2.5-4.8) | 3.3 (2.5-5.6) | 4.8 (3.0-5.8) |
| Maternal delivery | 7.6 (4.0-11.8) | 7.5 (4.8-8.7) | 7.2 (4.7-11.3) | 7.2 (4.7-11.3) | 7.5 (4.6-11.8) | 9.2 (4.8-10.8) |
| Fetal cord | 32.6 (15.9-55.8) | 20.5 (18.8-24.0) | 21.0 (13.4-32.6) | 24.0 (19.1-38.4) | 21.6 (18.8-29.2) | 26.1 (10.5-38.4) |
GAD, Gestational age at delivery; ROM, rupture of membrane interval; MAG, magnesium sulfate
Placental pathology
Gestational age at delivery ≤ 32 weeks was the only independent predictor of HCA (OR = 6.7, C.I. = 1.1 - 38.8, p = 0.04). There were no independent predictors of CCA. HCA and CCA were concordant for only 6/14 (43%) of the subjects. Additionally, fetal side placental inflammation without CCA (n = 5/13; 39%) or HCA (n = 3/13; 23%) was seen.
Median maternal IL-8 at ROM, maternal IL-6 and IL-8 at delivery, and fetal IL-1β, IL-6, IL-8, and TNF-α were elevated when fetal side placental inflammation was present (Table 4).
Table 4. Median values (with interquartile ranges) for cytokines in relation to placental pathology.
| Cytokine (pg/ml) | CCA + | CCA - | HCA + | HCA - | FETAL + | FETAL - |
|---|---|---|---|---|---|---|
| n = 11 | n = 14 | n = 14 | n = 11 | n = 13 | n = 12 | |
| IL-1β | ||||||
|
|
||||||
| Maternal rupture | 0 (0-0.5) | 0.5 (0-2.2) | 0 (0-0.5) | 1.3 (0-2.2) | 0 (0-1.0) | 1.0 (0-1.6) |
| Maternal delivery | 0.5 (0-6.6) | 0 (0-1.0) | 0 (0-1.0) | 1.0 (0-8.7) | 0 (0-4.5) | 0 (0-1.0) |
| Fetal cord | 6.0 (0-42.0) | 0.3 (0-0.5) | 0.5 (0-12.0) | 0 (0-0.5) | 8.1 (0-42.0) | 0 (0-0.5) |
| IL-6 | ||||||
|
|
||||||
| Maternal rupture | 2.2 (0.8-4.0) | 2.0 (1.8-3.5) | 2.2 (0.8-10.3) | 2.0 (1.8-2.0) | 2.0 (1.5-4.0) | 2.0 (1.5-2.0) |
| Maternal delivery | 96.1 (46.3-456.9 | ) 38.7 (18.8-90.2) | 76.6 (39.1-117.2) | 21.3 (18.8-272.0) | 115.6 (53.5-272.0) | 20.3 (13.8-34.3) |
| Fetal cord | 3459.8 (12.2-9569.0) | 73.45 (4.6-316.6) | 545.0 (16.1-6903.4) | 12.2 (3.7-88.1) | 2137.0 (74.2-9569.0) | 12.2 (3.7-72.7) |
| IL-8 | ||||||
|
|
||||||
| Maternal rupture | 3.5 (2.0-6.1) | 2.0 (2.0 – 5.25) | 3.5 (2.0-6.3) | 2.0 (2.0-3.2) | 2.1 (2.0-6.2) | 2.0 (2.0-4.4) |
| Maternal delivery | 31.8 (10.6-39.6) | 8.3 (5.6-17.0) | 17.0 (8.4-35.1) | 7.6 (5.6-33.5) | 32.9 (15.2-45.6) | 6.9 (4.7-8.4) |
| Fetal cord | 608.5 (14.2-1293.5) | 43.9 (13.5-189.2) | 114.8 (14.2-1200.0) | 17.0 (12.7-263.6) | 687.6 (29.8-1293.5) | 14.3 (9.7-114.8) |
| TNF-α | ||||||
|
|
||||||
| Maternal rupture | 5.3 (3.0-5.7) | 3.2 (2.5-6.6) | 3.2 (2.5-5.7) | 4.9 (3.1-6.6) | 3.4 (2.5-5.5) | 5.3 (2.9-7.6) |
| Maternal delivery | 8.2 (5.8-13.1) | 6.2 (4.0-9.4) | 6.9 (4.0-10.8) | 8.7 (4.6-15.4) | 8.7 (4.8-15.4) | 4.6 (2.6-8.7) |
| Fetal cord | 28.3 (18.8-38.4) | 20.8 (16.3-27.7) | 29.2 (18.8-55.8) | 21.0 (13.4-26.1) | 32.6 (20.5-55.8) | 19.1 (13.4-24.0) |
CCA, clinical chorioamnionitis; HCA, histological chorioamnionitis; FETAL, funisitis + chorionic plate vasculitis
Despite a tendency for higher odds of funisitis with male gender (OR = 6, p = 0.05), Hispanic ethnicity (OR = 4.7, p = 0.08), or HCA (OR = 6, p = 0.06) on univariate analysis, the only independent risk factor for funisitis was length of membrane rupture ≤ 7 days, although the confidence interval was wide (OR = 21, C.I. = 1.3 – 332, p = 0.03).
Neurological outcome
Forty-percent (n = 10) of infants had adverse neurological outcome at 6 months (PSOM score of 1.5 or higher); disabilities commonly included moderate-to-severe hemiparesis and/or global appendicular hypertonia sufficient to impair motor development. Fetal side placental inflammation was the only independent predictor of adverse neurological outcome at 6 months corrected gestational age, regardless of gestational age at birth or magnesium sulfate administration (OR = 7.8, C.I. = 1.1 – 55.6 ; p = 0.04). Worse neurological outcome was more common when both HCA and fetal side placental inflammation were present (p = 0.04). Worse neurological outcome at 6 months was highly correlated with the combination of HCA plus fetal placental inflammation (correlation coefficient = 0.73, p = 0.01) as well as with shorter ROM interval (Figure 1, R2 = 0.75, correlation coefficient = -0.87, p = 0.01).
Figure 1.
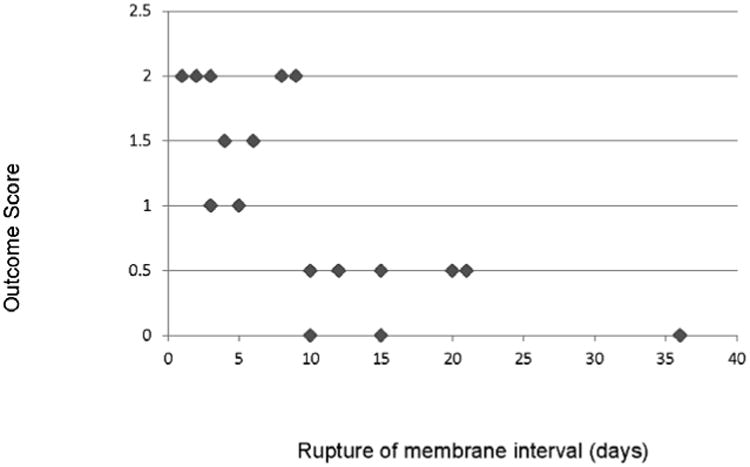
Correlation of neurological outcome at 6 months with rupture of membrane interval. Figure 1. Higher score denotes worse outcome (0 = no disability, 0.5 = mild disability, 1.0 = moderate disability, ≥ 1.5 = severe disability. Diamonds represent aggregate rupture interval data points for outcome scores.
Comment
In this prospective cohort pilot study of maternal-infant dyads from pregnancies complicated by PPROM, fetal side placental inflammation and shorter ROM duration were associated with worse neurological outcome at 6 months corrected gestational age, regardless of gestational age at birth or cranial ultrasound results. Furthermore, maternal and fetal IL-6 and IL-8 were elevated at delivery when placental inflammation on the fetal side was present, supporting the connection between maternal and fetal inflammation with adverse neurologic outcome in a PPROM population. Similar to animal models,23, 27 IL-1β and TNF-α levels were increased in fetal cord blood when fetal side placental inflammation was present.
While HCA is considered an indicator of maternal placental inflammation – with the clinical manifestations of maternal fever, maternal or fetal tachycardia, and fundal tenderness – funisitis and chorionic plate vasculitis are indicators of fetal side placental inflammation. Elevated cord blood cytokines combined with funisitis have been associated with cerebral palsy development in preterm and extremely low birth weight children, and detrimental to fetal brain development in animal models.6, 23, 27, 33 We found that fetal side placental inflammation – not HCA – was associated with adverse neurological outcome at 6 months, similar to findings described by Redline et al in an extremely low birthweight population.33 Consistent with other studies, IL-1β, IL-6, IL-8, and TNF-α were elevated in fetal cord blood when fetal side placental inflammation was present. 14, 34-37 Similar to animal models, the combination of elevated cord blood cytokines with fetal side placental inflammation appears to have detrimental effects on brain development, even without an infection.23, 27 Our data also suggest that funisitis – as a marker for the fetal inflammatory response – may not manifest with clinically evident symptoms in the pregnant woman. These data may argue against the concept that all funisitis is a progression of infection from HCA, but support the concept that funisitis (in some instances) is a response to another unidentified insult, a de novo fetal inflammatory response, or a partially treated maternal infection.8, 38, 39
While HCA is considered an indicator of maternal placental inflammation – with the clinical manifestations of maternal fever, maternal or fetal tachycardia, and fundal tenderness – funisitis and chorionic plate vasculitis are indicators of fetal side placental inflammation. Elevated cord blood cytokines combined with funisitis have been associated with cerebral palsy development in preterm and extremely low birth weight children, and detrimental to fetal brain development in animal models.6, 23, 27, 33 We found that fetal side placental inflammation – not HCA – was associated with adverse neurological outcome at 6 months, similar to findings described by Redline et al in an extremely low birthweight population.33 Consistent with other studies, IL-1β, IL-6, IL-8, and TNF-α were elevated in fetal cord blood when fetal side placental inflammation was present. 14, 34-37 Similar to animal models, the combination of elevated cord blood cytokines with fetal side placental inflammation appears to have detrimental effects on brain development, even without an infection.23, 27 Our data also suggest that funisitis – as a marker for the fetal inflammatory response – may not manifest with clinically evident symptoms in the pregnant woman. These data may argue against the concept that all funisitis is a progression of infection from HCA, but support the concept that funisitis (in some instances) is a response to another unidentified insult, a de novo fetal inflammatory response, or a partially treated maternal infection.8, 38, 39
Although unknown at this time, one can speculate how differing mechanisms of PPROM may give insight into downstream effects on offspring neurological outcomes and overall fetal programming pathways. ROM of ≤ 7 days was an independent predictor of fetal side placental inflammation, and fetal side placental inflammation was an independent predictor of worse neurological outcome at 6 months; subsequently, ROM of ≤ 7 days was highly correlated with worse neurological outcome at 6 months, regardless of gestational age at delivery. These data support the idea that a robust inflammatory response at membrane rupture (without clinical signs of infection) may make it impossible to maintain the pregnancy in some instances,40 while a mild inflammatory event may not lead to immediate delivery and resolve over time. Timing of corticosteroid administration may contribute to the resultant inflammatory response.41 We speculate that ROM duration may signify differing pathways to labor progression after PPROM, falling into hyperacute (< 72 hours; maternal-derived placental inflammation), acute (≤ 7 days; fetal-derived placental inflammation) or chronic (> 7 days; “non-inflammatory”).28 These intervals may relate to divergent long-term outcomes in the offspring.
In a study similar in design, IL-6 and IL-8 concentrations were significantly higher in fetal cord blood when fetal vasculitis was present, but no correlation was found with cytokine level and newborn MRI findings; however, post-natal neurological evaluations were not performed.42 We likewise found that despite higher levels of fetal cord IL-6 and IL-8 wtih funisitis, there was no evidence of brain injury on neonatal cranial ultrasounds. Yet, 40% of infants clearly had abnormal neurological exams at 6 months, regardless of gestational age. Although routinely performed in the neonatal intensive care nursery, single cranial ultrasound has a low yield for diagnosing periventricular leukomalacia and stroke.31, 43 Our findings utilizing standardized neurological exams suggest that this current standard of care may give a false sense of security regarding long-term neurological outcomes. We postulate that newborn cranial ultrasounds may underrepresent clinically significant deficits seen on later neurological exams, and should not be relied upon solely for prognostic indication in either the clinical or research setting. In addition, umbilical cord cytokine analysis may provide a superior indicator of prognosis and warrants further investigation for routine clinical use.
Our study has several limitations. The most evident is our small sample size; these data serve as a launching point for further inquiries into mechanisms of adverse neurological outcome after PPROM. For instance, our finding of very high levels of fetal cord IL-6 in gestational age < 32 weeks suggests that most early PPROM is associated with the fetal response syndrome; our sample size was not adequate to corroborate a link between gestational age and adverse neurological outcome. Data are further limited by limiting outcome analysis at 6 months, as outcomes such as language and behavior cannot be assessed in infancy. Additionally, we did not incorporate a matched control group. Instead, we designed the study to have PPROM serve as controls for themselves (poor neurological outcomes versus normal neurological outcomes), as births secondary to other causes of presumed non-infectious mechanisms of prematurity may improperly skew the pathological and inflammatory effects. We attempted to identify patterns that would give clues to the women at highest risk for delivering a child with brain injury apparent in infancy, within the defined high-risk population of PPROM.
Mechanisms of PPROM involving leukocyte subtypes other than polymorphonuclear cells,44 may also contribute to outcome. Therefore, other methods of placental examination, including immunohistochemistry, may be insightful in discerning the pathophysiology of PPROM-related outcomes. Finally, the study of elevated cytokines as causative agents can be problematic. Are cytokines a cause, association, or mediator? Although our study builds a more comprehensive view by examining both maternal and fetal cytokines, along with placental findings, to gain understanding into mechanisms of poor neurological outcomes, the answer is more complex than mere cytokine levels, pathological associations, and radiologic correlates.
In this prospective cohort study, patterns of heightened maternal-fetal inflammatory response, placental pathology, and clinical characteristics emerge that are associated with adverse neurological outcomes after PPROM. Our findings support the connection between adverse neurologic outcome in infancy with maternal and fetal inflammation in a PPROM population. Additionally, we found that cranial ultrasounds were not helpful for predicting adverse neurological outcomes in this at-risk population. Large longitudinal studies of PPROM are needed to adequately examine the inflammatory patterns seen in this study, assess comprehensive long-term neurologic outcome, and to aid in the ultimate design of a multimodal risk assessment and intervention tool.
Table 3. Median values (with interquartile ranges) for cytokines in relation to the neonate.
| Cytokine (pg/ml) | Male | Female | Hispanic | Non-Hispanic | None/mild disability | Moderate/severe disability |
|---|---|---|---|---|---|---|
| N = 16 | N = 9 | N = 12 | N = 13 | N = 15 | N= 10 | |
| IL-1β | ||||||
|
|
||||||
| Maternal rupture | 0 (0-1.2) | 0 (0-2.1) | 0 (0-0) | 1.0 (0-2.7) | 0 (0-0.5) | 0 (0-0) |
| Maternal delivery | 0 (0-1.7) | 0 (0-2.9) | 0 (0-1.0) | 0.5 (0-2.3) | 0.5 (0-2.8) | 0 (0-0) |
| Fetal cord | 0 (0-0.3) | 0.5 (0-12.0) | 0 (0-12.0) | 0.5 (0-0.5) | 0.5 (0-12.0) | 0.3 (0-0.5) |
| IL-6 | ||||||
|
|
||||||
| Maternal rupture | 2.0 (0.8-2.2) | 2.0 (1.8-10.8) | 2.0 (1.5-2.3) | 2.0 (1.5-5.0) | 1.8 (0.8-14.5) | 1.0 (0-2.0) |
| Maternal delivery | 49.5 (21.3-76.6) | 90.2 (20.3-192.9) | 96.1 (49.5-192.9) | 36.7 (20.1-66.7) | 36.7 (26.6-340.4) | 65.1 (32.2-116.4) |
| Fetal cord | 49.3 (7.1-317.6) | 73.5 (12.2-6903.4) | 74.2 (10.5-6903.4) | 72.7 (5.5-545.0) | 16.1 (15.5-6903.4) | 309.6 (74.2-545.0) |
| IL-8 | ||||||
|
|
||||||
| Maternal rupture | 2.0 (2.0-3.5) | 3.2 (2.0-6.3) | 2.0 (2.0-4.8) | 2.0 (2.0-6.1) | 2.0 (1.8-4.2) | 2.0 (2.0-2.0) |
| Maternal delivery | 12.7 (7.6-30.7) | 17.0 (5.6-45.6) | 23.9 (6.9-33.5) | 8.7 (6.6-85.6) | 7.0 (5.2-27.0) | 13.0 (9.0-17.0) |
| Fetal cord | 36.1 (13.5-86.4) | 146.7 (14.3-1200.0) | 29.8 (14.2-1200.0) | 57.9 (12.7-263.6) | 14.3 (6.9-1399.8) | 72.3 (29.8-114.8) |
| TNF-α | ||||||
|
|
||||||
| Maternal rupture | 3.5 (2.5-5.4) | 4.4 (3.0-6.9) | 3.4 (2.5-5.3) | 4.4 (2.5-7.6) | 3.4 (2.9-5.7) | 2.8 (2.5-3.0) |
| Maternal delivery | 6.9 (4.0-9.4) | 7.6 (4.6-11.8) | 4.9 (4.6-10.8) | 8.7 (5.8-13.1) | 8.1 (5.0-9.8) | 4.4 (4.0-4.8) |
| Fetal cord | 23.6 (18.5-27.7) | 22.3 (18.8-38.4) | 24.0 (15.9-55.8) | 21.0 (18.8-29.2) | 19.1 (18.8-38.4) | 24.9 (20.5-29.2) |
Clinical Implications.
Maternal and fetal inflammation is connected to adverse infant neurological outcome in pregnancies complicated by PPROM
IL-6 and IL-8 – with funisitis - appear to be particulary important in the fetal inflammatory response and neurological outcome
Adverse neurological outcome is also associated with decreased rupture of membrane interval
Larger, prospective studies of PPROM are needed to adequately assess the patterns and implications found in this study
Footnotes
This study was conducted in Aurora, CO, U.S.A.
Disclosure Statement: The authors report no conflicts of interest.
Financial Support: NIH BIRCWH K12 KD HDO57022 (J.A.W.), American Heart Association SouthWest Affiliates Clinical Research Grant 10CRP3670014 (J.A.W.), Colorado CCTSA Grant 5UL1RR025780 (J.A.W.), UCD Academic Enrichment Fund (J.A.W.). CIHR MOP201257 (G.S.), Foundation of Stars (G.S.), Centre de Recherche Mère-Enfant de l'Université de Sherbrooke (G.S.), Canada.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Larroque B, Ancel PY, Marret S, et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet. 2008;371:813–20. doi: 10.1016/S0140-6736(08)60380-3. [DOI] [PubMed] [Google Scholar]
- 2.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med. 2006;19:773–82. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- 3.Grether JK, Nelson KB, Walsh E, Willoughby RE, Redline RW. Intrauterine exposure to infection and risk of cerebral palsy in very preterm infants. Arch Pediatr Adolesc Med. 2003;157:26–32. doi: 10.1001/archpedi.157.1.26. [DOI] [PubMed] [Google Scholar]
- 4.Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–75. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- 5.Wu YW, Colford JM., JR Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284:1417–24. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 6.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG. 2003;110(Suppl 20):124–7. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 7.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canzoneri BJ, Grotegut CA, Swamy GK, et al. Maternal serum interleukin-6 levels predict impending funisitis in preterm premature rupture of membranes after completion of antibiotics. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25:1329–32. doi: 10.3109/14767058.2011.632794. [DOI] [PubMed] [Google Scholar]
- 9.Hutzal CE, Boyle EM, Kenyon SL, et al. Use of antibiotics for the treatment of preterm parturition and prevention of neonatal morbidity: a metaanalysis. Am J Obstet Gynecol. 2008;199:620 e1–8. doi: 10.1016/j.ajog.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. The Cochrane database of systematic reviews. 2010:CD001058. doi: 10.1002/14651858.CD001058.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Kenyon S, Pike K, Jones DR, et al. Childhood outcomes after prescription of antibiotics to pregnant women with preterm rupture of the membranes: 7-year follow-up of the ORACLE I trial. Lancet. 2008;372:1310–8. doi: 10.1016/S0140-6736(08)61202-7. [DOI] [PubMed] [Google Scholar]
- 12.Marlow N, Pike K, Bower E, et al. Characteristics of children with cerebral palsy in the ORACLE children study. Dev Med Child Neurol. 2012;54:640–6. doi: 10.1111/j.1469-8749.2012.04274.x. [DOI] [PubMed] [Google Scholar]
- 13.Bek KM, Nielsen FR, Qvist I, Rasmussen PE, Tobiassen M. C-reactive protein (CRP) and pregnancy. An early indicator of chorioamnionitis. A review. European journal of obstetrics, gynecology, and reproductive biology. 1990;35:29–33. doi: 10.1016/0028-2243(90)90139-r. [DOI] [PubMed] [Google Scholar]
- 14.Satar M, Turhan E, Yapicioglu H, Narli N, Ozgunen FT, Cetiner S. Cord blood cytokine levels in neonates born to mothers with prolonged premature rupture of membranes and its relationship with morbidity and mortality. European cytokine network. 2008;19:37–41. doi: 10.1684/ecn.2008.0118. [DOI] [PubMed] [Google Scholar]
- 15.Kacerovsky M, Cobo T, Andrys C, et al. The fetal inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2013;26:795–801. doi: 10.3109/14767058.2013.765404. [DOI] [PubMed] [Google Scholar]
- 16.Gulati S, Agrawal S, Raghunandan C, et al. Maternal serum interleukin-6 and its association with clinicopathological infectious morbidity in preterm premature rupture of membranes: a prospective cohort study. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25:1428–32. doi: 10.3109/14767058.2011.638952. [DOI] [PubMed] [Google Scholar]
- 17.Nelson KB, Grether JK, Dambrosia JM, et al. Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res. 2003;53:600–7. doi: 10.1203/01.PDR.0000056802.22454.AB. [DOI] [PubMed] [Google Scholar]
- 18.Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–81. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 19.Yoon BH, Romero R, Yang SH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–40. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe KB, Snyder CC, Gisslen T, et al. Modulation of lipopolysaccharide-induced chorioamnionitis in fetal sheep by maternal betamethasone. Reproductive sciences. 2013;20:1447–54. doi: 10.1177/1933719113488445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry CA, Nitsos I, HILLMAN NH, et al. Interleukin-1 in lipopolysaccharide induced chorioamnionitis in the fetal sheep. Reproductive sciences. 2011;18:1092–102. doi: 10.1177/1933719111404609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girard S, Tremblay L, Lepage M, Sebire G. Early detection of placental inflammation by MRI enabling protection by clinically relevant IL-1Ra administration. American journal of obstetrics and gynecology. 2012;206:358 e1–9. doi: 10.1016/j.ajog.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Girard S, Tremblay L, Lepage M, Sebire G. IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J Immunol. 2010;184:3997–4005. doi: 10.4049/jimmunol.0903349. [DOI] [PubMed] [Google Scholar]
- 24.Brochu ME, Girard S, Lavoie K, Sebire G. Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: An experimental study. Journal of neuroinflammation. 2011;8:55. doi: 10.1186/1742-2094-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larouche A, Roy M, Kadhim H, Tsanaclis AM, Fortin D, Sebire G. Neuronal injuries induced by perinatal hypoxic-ischemic insults are potentiated by prenatal exposure to lipopolysaccharide: animal model for perinatally acquired encephalopathy. Dev Neurosci. 2005;27:134–42. doi: 10.1159/000085985. [DOI] [PubMed] [Google Scholar]
- 26.Yoon BH, Kim CJ, Romero R, et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol. 1997;177:797–802. doi: 10.1016/s0002-9378(97)70271-0. [DOI] [PubMed] [Google Scholar]
- 27.Girard S, Sebire H, Brochu ME, Briota S, Sarret P, Sebire G. Postnatal administration of IL-1Ra exerts neuroprotective effects following perinatal inflammation and/or hypoxic-ischemic injuries. Brain, behavior, and immunity. 2012;26:1331–9. doi: 10.1016/j.bbi.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong-Wells J, Post MD, Donnelly M, Manco-Johnson MJ, Fisher BM, Winn VD. Patterns of placental pathology in preterm premature rupture of membranes. Journal of developmental origins of health and disease. 2013;4:249–55. doi: 10.1017/S2040174413000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht JL, Allred EN, Kliman HJ, et al. Histological characteristics of singleton placentas delivered before the 28th week of gestation. Pathology. 2008;40:372–6. doi: 10.1080/00313020802035865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37:339–54. doi: 10.1016/j.clp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Vries LS, Cowan FM. Should cranial MRI screening of preterm infants become routine? Nat Clin Pract Neurol. 2007;3:532–3. doi: 10.1038/ncpneuro0608. [DOI] [PubMed] [Google Scholar]
- 32.Kitchen L, Westmacott R, Friefeld S, et al. The pediatric stroke outcome measure: a validation and reliability study. Stroke. 2012;43:1602–8. doi: 10.1161/STROKEAHA.111.639583. [DOI] [PubMed] [Google Scholar]
- 33.Redline RW, Minich N, Taylor HG, Hack M. Placental lesions as predictors of cerebral palsy and abnormal neurocognitive function at school age in extremely low birth weight infants (<1 kg) Pediatr Dev Pathol. 2007;10:282–92. doi: 10.2350/06-12-0203.1. [DOI] [PubMed] [Google Scholar]
- 34.Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 35.Hecht JL, Fichorova RN, Tang VF, et al. Relationship Between Neonatal Blood Protein Concentrations and Placenta Histologic Characteristics in Extremely Low GA Newborns. Pediatr Res. 2011;69:68–73. doi: 10.1203/PDR.0b013e3181fed334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Alquen D, Kramer BW, Seidenspinner S, et al. Activation of umbilical cord endothelial cells and fetal inflammatory response in preterm infants with chorioamnionitis and funisitis. Pediatr Res. 2005;57:263–9. doi: 10.1203/01.PDR.0000148713.48218.86. [DOI] [PubMed] [Google Scholar]
- 37.Andrys C, Drahosova M, Hornychova H, et al. Umbilical cord blood concentrations of IL-6, IL-8, and MMP-8 in pregnancy complicated by preterm premature rupture of the membranes and histological chorioamnionitis. Neuro endocrinology letters. 2010;31:857–63. [PubMed] [Google Scholar]
- 38.Chaiworapongsa T, Romero R, Kim JC, et al. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol. 2002;186:1178–82. doi: 10.1067/mob.2002.124042. [DOI] [PubMed] [Google Scholar]
- 39.Redline RW. Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med. 2012;17:20–5. doi: 10.1016/j.siny.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Gargano JW, Holzman C, Senagore P, et al. Mid-pregnancy circulating cytokine levels, histologic chorioamnionitis and spontaneous preterm birth. Journal of reproductive immunology. 2008;79:100–10. doi: 10.1016/j.jri.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramaniam A, Cliver SP, Andrews WW, Faye-Petersen OM, Goldenberg RL, Biggio JR. Effect of corticosteroid interval on markers of inflammation in spontaneous preterm birth. American journal of obstetrics and gynecology. 2013;209:379 e1–6. doi: 10.1016/j.ajog.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 42.Sorensen LC, Skogstrand K, Hougaard DM, et al. Cord blood inflammatory markers, foetal vasculitis and cerebral MRI abnormalities in preterm infants. Acta Paediatr. 2007;96:1362–4. doi: 10.1111/j.1651-2227.2007.00437.x. [DOI] [PubMed] [Google Scholar]
- 43.De Vries LS, Benders MJ, Groenendaal F. Imaging the premature brain: ultrasound or MRI? Neuroradiology. 2013 doi: 10.1007/s00234-013-1233-y. [DOI] [PubMed] [Google Scholar]
- 44.Holzman C, Senagore PK, Wang J. Mononuclear leukocyte infiltrate in extraplacental membranes and preterm delivery. American journal of epidemiology. 2013;177:1053–64. doi: 10.1093/aje/kws351. [DOI] [PMC free article] [PubMed] [Google Scholar]


