Abstract
Recent advances on human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs) have brought us closer to the realization of their clinical potential. Nonetheless, tissue engineering and regenerative medicine applications will require the generation of hPSC products well beyond the laboratory scale. This also mandates the production of hPSC therapeutics in fully-defined, xeno-free systems and in a reproducible manner. Toward this goal, we summarize current developments in defined media free of animal-derived components for hPSC culture. Bioinspired and synthetic extracellular matrices for the attachment growth and differentiation of hPSCs are also reviewed. Given that most progress in xeno-free medium and substrate development has been demonstrated in two-dimensional rather than three dimensional culture systems, translation from the former to the latter poses unique difficulties. These challenges are discussed in the context of cultivation platforms of hPSCs as aggregates, on microcarriers or after encapsulation in biocompatible scaffolds.
Keywords: Human pluripotent stem cells, chemically defined media, xeno-free biomaterials, 3D culture, stem cell processing
1. Introduction
Since their isolation and derivation in 1998 [1] hESCs have been considered a promising inexhaustible cellular source for treating currently incurable diseases such as diabetes, Parkinson and heart failure. Stem cells exhibit two fundamental attributes: extensive self-renewal and the potential for differentiation into all types of somatic cells. Ethical concerns relating to the derivation of hESCs from fertilized eggs have largely abated with the reprogramming of terminally differentiated adult cells to stem cells termed iPSCs [2-4]. Human iPSCs and hESCs share many key properties including pluripotency and prolonged self-renewal under appropriate conditions making feasible their propagation in traditional static cultures and scalable stirred-suspension vessels [5-9]. These cells also provide a means toward patient-specific therapies and disease model development [10, 11].
Therapeutic use of hPSCs necessitates their expansion and efficient differentiation in large-scale under well-defined conditions. Scalable production is necessitated by most current cell therapy protocols and those under development requiring 108-1010 cells per patient [12]. For example, myocardial infarction results in the damage or ablation of at least 1-2×109 myocytes [13, 14] and approximately 1.3×109 β-cells are required for insulin independence in diabetes patients [15, 16]. Stirred suspension bioreactor systems affording densities of 106-107 cells/ml are appealing for generating stem cell therapeutics, especially given the limitations for scale-up of traditional dish cultures.
Large-scale production of hPSC derivatives goes hand in hand with the development of xeno-free environments excluding animal-derived products such as serum and cytokines commonly used in traditional mammalian cell culture [17-21]. The promise of stem cells for regenerative medicine and the rapid advances in recent years have intensified efforts toward the development of xeno-free scalable systems for stem cell products.
Yet such advances are contingent upon addressing hPSC survival, proliferation, and differentiation issues whose exact dependencies on intracellular signals and extrinsic factors require further elucidation. The microenvironment – commonly referred to as the niche – imposes its effects mainly through soluble factors, cell-cell and/or cell-matrix contacts and mechanotransduction (Figure 1). Here, we summarize the progress on the development of defined xeno-free media. Advances in extracellular matrices and synthetic substrates for the maintenance of uncommitted hPSCs on 2D surfaces are also reviewed. In the latter part of the article, the challenges are discussed for developing clinically relevant scalable systems for the culture of hPSCs as aggregates, after scaffold encapsulation and on microcarriers.
Figure 1.
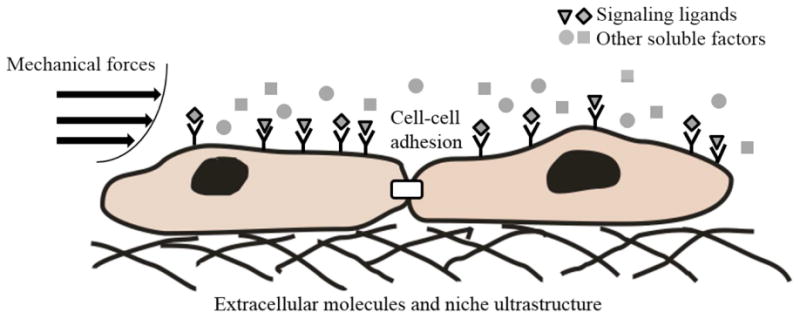
Schematic representation of microenvironmental cues encountered by human PSCs. Such cues include signaling ligands, other soluble factors, mechanical forces, interactions among cells and between cells and extracellular matrix molecules presented in a fashion dictated by the niche ultrastructure. Signaling ligands include bFGF (basic fibroblast growth factor), Wnt ligands, BMPs (bone morphogenetic proteins), activin A, and insulin. Other soluble factors include salts, vitamins and lipids.
2. Xeno-free media for hPSC culture
Limiting the aberrant differentiation of cultured hPSCs is a key consideration as the cells self-renew or their fate is directed along particular lineages. Our knowledge of appropriate conditions supporting hPSC self-renewal is based on heterologous systems of development, mainly that of the mouse embryo. A significant body of studies on signaling of hPSCs in culture has extended our understanding of the dependence of self-renewal on extracellular parameters. It is well accepted that the pluripotency of hESCs is controlled through common genetic networks of transcriptional factors [22-24]. Examples of such factors include Nanog, Pou5f1 (also known as Oct4) and Sox2 with cooperative interactions among them underlying the maintenance or loss of pluripotent state early in embryonic development and in vitro [23, 25, 26].
Multiple signaling pathways such as the transforming growth factor-beta (TGF-β) super family-activated cascades, receptor tyrosine kinase (RTK) signaling (downstream of the basic fibroblast growth factor (bFGF)), canonical Wnt signaling [22, 27], and pathways related to insulin or insulin-like growth factors (IGFs) [28, 29] regulate pluripotency gene levels [30, 31]. Based on signal transduction findings, a key approach to develop media for hPSCs is to identify and supply extrinsic growth factors which work through cascades with direct access to hPSC pluripotency programs. Bone morphogenetic proteins (e.g. BMP4) and the leukemia inhibitory factor (LIF; a JAK/STAT signaling activator) are sufficient to preserve the undifferentiated state of cultured mouse ESCs (mESCs) [32] even in serum-free conditions [33] but not of hESCs [1, 34]. Human PSC pluripotency depends on TGFβ signaling [35] with TGFβ1, Activin A and Nodal directly activating Nanog expression via a promoter site for SMAD2/3 binding [36, 37]. Because these molecules are produced by hPSCs to varying degrees, they are not part of all medium formulations.
Basic FGF though is a universal supplement which is critical for sustaining hESC self-renewal in vitro [38, 39]. For hPSC culture on mouse embryonic fibroblast (mEF) feeder cell layers [40] or in mEF-conditioned medium [41], the bFGF concentration (4 ng/ml) is lower than in feeder-free cultures (40-100 ng/ml) [38, 42, 43]. Interestingly, the BMP antagonist noggin supports the growth of undifferentiated hESCs in unconditioned medium with 40 ng/ml bFGF but does not appear to have an effect when bFGF is increased to 100 ng/ml [44].
Canonical Wnt/β-catenin signaling has also been implicated in hPSC self-renewal [45, 46]. Even so, others reported that recombinant Wnt3a is not sufficient to maintain hESCs undifferentiated without feeder cells and β-catenin-mediated transcriptional activity is upregulated during differentiation [47]. The effects of Wnt signaling in hESC pluripotency have been difficult to unravel because different hPSC lines exhibit disparate levels of endogenous Wnt activity. Further, Wnt has been implicated in the specification of stem and progenitor cells along multiple and often developmentally distant lineages suggesting that exposure of hPSCs to Wnt ligands should be finely customized.
These and other -often unidentified- factors are traditionally provided through supplementation of the medium with fetal bovine serum (FBS). Nonetheless, the use of non-human components (e.g. Neu5Gc; [48]) is incompatible with clinical applications driving efforts to design xeno-free culture systems for hPSCs and their products. Serum replacers (e.g. knockout serum replacer (KSR)) [49] have proprietary composition and may also contain animal-derived components such as bovine serum albumin (BSA).
Media composed of chemically defined, non-xenogeneic compounds for the propagation and differentiation of hPSCs are highly desirable [18, 30, 50, 51]. Approaches to develop defined media for hPSCs consist of identifying both a suitable basal medium and additional signaling factors promoting cell growth and preservation of pluripotency or induction of (directed) differentiation. Basal media such as DMEM and DMEM/F12 provide mainly glucose, vitamins and salts (at appropriate osmolarity) to cells whereas factors (e.g. bFGF) eventually activate or repress genetic programs for hPSC self-renewal or specification. For example, a defined medium based on DMEM/F12 with 100 ng/ml bFGF and components such as TGF-β, LiCl, insulin, GABA and BSA or human serum albumin (HSA) is extensively used in hPSC cultivation [52, 53]. Other formulations are show in Table 1. DMEM/F12 with 20 ng/ml bFGF and B27, N2 and BSA has been used to maintain hESCs for over 27 passages. And in the absence of BSA, DMEM/F12 combined with N2, B27 and high concentration of bFGF (40-100 ng/ml) is adequate for hESC maintenance. The X-Vivo 10 medium supplied with recombinant bFGF, stem cell factor (SCF), LIF and Flt3 ligands has also been successfully used for hESC maintenance. Nonetheless, the aforementioned media typically contain BSA (or the more expensive HSA). Recently, a fully defined medium (E8) containing 8 factors (including bFGF) without BSA was described for the long-term propagation of hPSCs [54].
Table 1.
Composition of defined and xeno-free media for hPSC culture.
| Name | Basal medium | Select supplements | Xeno -free? | Ref. |
|---|---|---|---|---|
| Commercially available media | ||||
|
| ||||
| mTeSR | DMEM/F12 | bFGF, TGFβ, Insulin, Transferrin, Cholesterol, Lipids, Pipecolic, BSA, GABA, LiCl, Amino acids, L-glutamine, β-mercaptoethanol | No | [53] |
| StemPro | DMEM/F12 | bFGF, Activin A, Transferrin, Lipids, NEAA, L-glutamine, β-mercaptoethanol, HRG1β, LR3-IGF1 | No | [28] |
| TeSR | DMEM/F12 | bFGF, TGFβ, Insulin, Transferrin, Cholesterol, Lipids, Pipecolic, HSA, GABA, LiCl, Amino acids, L-glutamine, β-mercaptoethanol | Yes | [52] |
| E8 | DMEM/F12 | bFGF, TGFβ1, Insulin, Transferrin, Seleniun, L-ascorbic acid | Yes | [54] |
|
| ||||
| Other media | ||||
|
| ||||
| DMEM/F12 | N2, B27, BSA, bFGF, L-glutamine | No | [55] | |
| IMDM/F12 | bFGF, Activin A, Insulin, Transferrin, BSA, Activin A, L-glutamine, β-mercaptoethanol, Monothioglycerol | No | [39] | |
| DMEM/F12 | bFGF, Insulin, Transferrin, Cholesterol, Lipid-rich BSA, L-glutamine, β-mercaptoethanol | No | [56] | |
| DMEM/F12 | bFGF, N2, B27, BSA, L-glutamine, β-mercaptoethanol | No | [57] | |
| ESF | bFGF, Insulin, Transferrin, β-mercaptoethanol, 2-ethanolamine, selenite, Ascorbic acid, albumin conjugated with oleic acid | No | [58] | |
| XVIVO-10 | bFGF, hFLT3*, L-glutamine, β-mercaptoethanol | Yes | [59] | |
hFLT3: human FMS-like tyrosine kinase-3, Animal-origin components are underlined.
Despite the significant advances in the development of defined and xeno-free media, there are still unresolved issues. For instance, side-by-side comparison by our laboratory and others of the performance of commercially available xeno-free media indicates differences in the fold-expansion of cells over the same period, particularly over multiple passages. Although the reason(s) for such discrepancies are unclear, the quality of supplements used in these medium formulations may be a suspect. The generation even of recombinant growth factors and other proteins (e.g. recombinant albumin) requires separation steps (e.g. isolation from bacterial cultures, purification etc.) which do not always result in impurity-free preparations. Traces of impurities may affect the propagation of cells and their long-term potential.
Moreover, almost all current protocols for hPSC culture require daily medium replacement increasing the cost and associated labor. Fluctuation of growth factor levels in the medium contributes to the variability of hPSC cultivation. Soluble human or zebrafish bFGF loses most of its activity in culture after 24 hours [60]. This may be circumvented with the controlled release of bFGF (or other factors) in culture. Basic FGF-loaded PLGA microspheres added to hPSC cultures reduce the frequency of medium from daily to every three days or biweekly [61].
Hence, creating supplements with extended shelf life while keeping the cost low are highly desirable. Small molecules promoting hPSC self-renewal have been suggested as candidates which may fit the bill. Using high-content screening methods, small molecules such as trimipramine and ethopropazine which can diffuse easily through multi-layer cellular configurations and have much longer degradation times, have been reported to maintain the self-renewal of hESCs replacing exogenous bFGF [62, 63].
3. Extracellular matrices for hPSC cultivation
Despite the availability of chemically defined media for hPSC cultivation, the quest for relevant xeno-free substrates, particularly for use in large-scale production of stem cell products of wide utility. Beyond the obvious requirement for promoting cell adhesion, the design of defined surfaces is subjected to a unique constraint of unimpeded hPSC self-renewal and differentiation. Efforts in this direction are hampered by the incomplete knowledge of the regulation of human stem and progenitor cell fate within complex niches in vivo.
Traditionally, hPSCs have been maintained on layers of inactivated mEFs, which secrete factors supporting hPSCs. Thus, early efforts focused on feeder cell surrogates of human origin including human fetal foreskin fibroblasts [64-67], adult epithelial cells [68], bone marrow cells [69, 70] and placenta-derived feeder cells [71, 72]. Apparent difficulties in the sourcing – including variability due to donor age and condition [73], derivation, preparation and preservation of human feeder cells limit their use in stem cell culture. Importantly, co-culturing hPSCs with feeder cells adds a requirement for separation and removal of the latter thereby imposing significant technical and economic burdens on envisioned bioprocesses.
The introduction of the extracellular matrix protein (ECM) mixture Matrigel produced by Engelbreth-Holm-Swarm mouse sarcoma cells led to successful expansion of stem cells without the need for feeder cells. Matrigel contains laminin, collagen type IV, heparan sulfate, proteoglycans, entactin, and nidogen [1, 74], and its use as an hPSC substrate is fairly straightforward and not time-consuming. However, its undefined composition precludes its use in applications calling for the xeno-free production of stem cell progeny. These facts have elicited efforts to develop defined substrates for the generation of therapeutic products from stem cell cultures.
3.1 Human ECM protein-based substrata
Natural ECM glycoproteins such as laminin, fibronectin, vitronectin, entactin, tenascin and collagen influence stem cell adhesion, survival, growth and differentiation [75, 76] through their interactions with cell surface moieties. Each ECM component exhibits distinct domains for binding to surface receptors (e.g. integrins) mediating adhesion and triggering signaling cascades linked to cell fate selection [77-79]. Among different motifs, the integrin-interacting arginine-glycine-aspartic acid (‘RGD’) motif is shared by various ECM proteins including laminin, vitronectin and fibronectin [80, 81]. In fact, mutations in the RGD sequence result in greatly reduced cell adhesion [82]. A mixture of recombinant human collagen IV, vitronectin, fibronectin and laminin supports the derivation and growth of undifferentiated hESCs over multiple passages [52]. Along this vein, the use of ECM proteins and peptides from tissue isolates or in recombinant form has been investigated extensively for the culture of hPSCs.
Among the 24 different known integrin heterodimers, α1β1, α2β1, α3β1, α6β1, α6β4, α7β1, α9β1, and αvβ3 in various cell types have been reported to bind laminin [83]. The α6β1 integrin is expressed by hESCs and plays a significant role in adhesion [74] suggesting that laminin is a critical ECM protein for supporting hESC proliferation. Indeed, natural or recombinant laminin in lieu of Matrigel was reported to maintain the growth and pluripotency of hESCs in mEF-conditioned medium [74, 84]. However, human placenta-derived laminin was shown to only support hESC self-renewal for 3 passages in chemically defined medium [85] while over longer periods (>10 passages), hESCs grew significantly more slowly with evident spontaneous differentiation and poor adhesion [86]. The presence of ECM molecules (besides laminin) secreted by feeder cells may be a potential explanation for the discrepancy in the findings of these studies. Moreover, although laminin-511 (but not laminin-332) supports the culture of multiple hESC lines under xeno-free conditions [87], laminin peptides failed to promote hESC attachment and growth in a concurrent study [88]. Thus, the utility of laminin alone as hPSC substrate remains unsettled.
Like laminin, the use of vitronectin has been explored for the culture of hPSCs. Vitronectin mediates hPSC adhesion through αVβ5 integrins as shown in integrin-blocking antibody experiments [86]. The proliferation of three hESC lines (HUES1, HES2, HESC-NL3) in mTESR1 medium was supported by vitronectin in a manner comparable to that of Matrigel and in contrast to fibronectin- (acting through the α5β1 integrin) coated surfaces requiring feeder cell-conditioned (but not defined) medium for hESCs to grow. Interestingly enough, Liu et al. reported that cultured hESCs could not be maintained on vitronectin for more than 7 days [55] in defined medium containing bFGF, N2 and B27 supplements. These results illustrate the complexity of pinpointing individual matrix components supporting hPSC culture and emphasize the need for considering multiple aspects of the culture system including the medium used for hPSC maintenance.
Nonetheless, vitronectin from human plasma promotes self-renewal for over 20 passages without compromising the potential of hPSCs for differentiation [89]. Notably, a threshold surface density of 250 ng of vitronectin/cm2 was estimated for successful hPSC culture. This value applies to whole-molecule vitronectin and should be adjusted when utilizing vitronectin derivatives or fragments. This may explain the differential support of cultured hPSCs by variants of vitronectin [54]. For hESCs grown in E8 medium, two truncated vitronectin molecules (amino acids 62-398 and 62-478) promote initial attachment and survival of hESCs as single cells (with ROCK inhibitor or blebbistatin) and as clumps. A chimeric glycoprotein of vitronectin and IGF1 also maintains hESCs in defined medium [78].
Fibronectin also features the RGD domain interacting with α3β1, α5β1, α8β1, αvβ1, αvβ3, αvβ5, and αvβ6 integrins [90, 91]. Based on available studies, the role of fibronectin in stem cell adhesion and culture is still unclear. Human-plasma fibronectin promotes hESC proliferation and pluripotency in defined medium for at least 10 [85] to 13 passages [55]. Yet, cultivation of hESCs on fibronectin-coated surfaces failed in mTeSR1 but not in mEF-conditioned medium as mentioned above [86]. As with vitronectin, a threshold density of 80 ng/cm2 of plasma-fibronectin was determined for hESC culture in a serum-free medium [92]. This density also applied to the 120 kDa fragment of fibronectin with the central cell-binding domain containing the RGD motif (1–10 type III repeats), while other fibronectin fragments did not support the maintenance of hESCs.
Capitalizing on the central role of the RGD domain of ECM molecules on hPSC adhesion, various groups implemented an approach of building ECM substrata with synthetic RGD-containing peptides [88, 93]. A cyclic RGD peptide covalently bound to tissue culture surface at 10-30 fmol/cm2 was used to culture hESCs in conditioned medium (10 passages) or mTeSR1 (5 days) [94]. It should be noted however that such substrata should promote not only hPSC adhesion but self-renewal and unhindered differentiation as well. For example, one peptide featuring the YIGSR domain promotes hESC adhesion but cultured cells display significantly reduced expression of OCT4 and SSEA4 [95] in contrast to other integrin-binding peptides (e.g. GKKQRFRHRNRKG, FHRRIKA and GWQPPARARI). Peptides derived from the bone sialoprotein and vitronectin (but not from fibronectin) and covalently attached onto acrylate-coated surfaces facilitate the adhesion of hESCs [96]. Thus, although the presence of binding (e.g., RGD) domains of natural ECM molecules may point to candidate peptides for hPSC culture, additional optimization of the whole peptide sequences is necessary for the development of appropriate substrata.
A summary of natural ECM proteins or their derivatives used as substrata for hPSC culture is presented in Table 2. The type of medium utilized in each study is also shown.
Table 2.
Summary of extracellular protein based defined xeno-free substrates for hPSC culture on 2D surfaces.
| Matrices | Medium | Cell line | Ref. |
|---|---|---|---|
| Human laminin (2 μg/cm2, absorption) | NC-SFM | H1 | [59] |
| Human fibronectin (25 μg/ml, absorption) | HESCO | H9, BG01 | [56] |
| Human plasma fibronectin | DMEM/F12 plus N2, B27, bFGF, Activin A, and neurotrophin 4 | MAN 1, HUES 1, HUES7 | [85] |
| Recombinant human E-cadherin and mouse IgG1 Fc domain fusion protein (absorption) | mTeSR | H1, H9, hiPSC2a*, hiPSC3a*, hiPSC6a* | [97] |
| Recombinant human laminin-511 (20 μg/ml, absorption) | O3 (a variant of mTeSR1) or H3 (a variant of TeSR1) | H1, H9, HS420, HS207, HS401, BJ#12* LDS1.4* | [87] |
| Recombinant human Vitronectin (5 ng/μl, absorption) | mTeSR1 | HUES1, HUES2, HESCNL3 | [86] |
| Combination of cyclic RGD and vitronectin derived heparin-binding peptides (Biotinylated peptides attached to streptavidin-coated surface) | mTeSR1 | H1, H7, H9, H14, IMR-90-1* | [95] |
| a combination of human collagen IV, vitronectin fibronectin and laminin (10 μg/cm2, 0.2 μg/cm2, 5 μg/cm2, 5 μg/cm2, respectively, absorption) | TeSR1 | H1, H7, H9, H14 | [52] |
| Bone sialoprotein and vitronectin derived RGD containing peptides (peptides conjugated to acrylate-coated surface) | X-VIVO plus bFGF and TGF-β1 | H1, H7 | [96] |
| Recombinant Truncated forms of human Vitronectin | E8 | H1, H9, IMR90*, iPSC-foreskin*, iPSC-DF19* | [54] |
hiPSC lines
3.2 Synthetic ECMs
The varied performance of natural or recombinant human ECM proteins and the associated high cost have motivated the development of synthetic ECMs. Recent studies have demonstrated success in culturing hPSCs on such synthetically prepared polymer surfaces [98]. Synthetic polymers have been widely investigated as extracellular matrices for the cultivation of PSCs because of their low cost and high availability [99]. Synthetic ECMs that are biocompatible and mimic natural ECMs have been researched extensively. Li et al.,[100] synthesized a 3D hydrogel scaffold made of poly(N-isopropylacrylamide-co-acrylic acid)[p(NIPAA-co-AAc)] with Gln-Pro-Gln-Gly-Leu-Ala-Lys, an acrylated peptide crosslinker that can be digested by collagenase. Polyacrylic acid-graft-Ac-CGGNGEPRGDTYRAY-NH2, a linear polymeric chain containing synthetic peptides also assisted in enhancing cell adhesion. A more extensive collection of 91 different polyacrylamide polymers was also investigated. Sixteen of those supported pluripotent hESCs (HUES9) for five days [99] with poly(methyl vinyl ether-alt-maleic anhydride) [PMVE-alt-MA] exhibiting the best performance for propagation of pluripotent stem cells (HUES7 and HUES9 hESCs and an unnamed iPSC line) for five passages. Stem cells on PMVE-alt-MA expressed integrins α5 (ITGA5) and αv (ITGAV) more than cells cultured on Matrigel, thereby strengthening attachment to the matrix and were capable of giving rise to endoderm, mesoderm and ectoderm cells. Other polymers successfully used as ECMs for PSC culture are listed in Table 3. In addition, polymers with ester ions and cyclic polymer ions have also been shown to promote hESC adhesion [101].
Table 3.
Synthetic polymers used for hPSC culture.
| Surface coating | Cell line | Duration and success of pluripotency maintenance | Tested differentiation | Ref. |
|---|---|---|---|---|
| poly[2- (methacryloyloxy)ethy l dimethyl-(3- sulfopropyl) ammonium hydroxide] (PMEDSAH) | *H9, BG01 [102, 103], iPSCs [104] | 25 passages with pluripotency maintained at levels similar to that of Matrigel [102]; 3 passages for BG01; 10 passages for H9[103]; 15 passages for iPSCs [104] | Yes; differentiated into mesenchy- mal stem cells that were later differentiated into adipogenic, chondrogenic and osteogenic lineages [104] | [102- 104] |
| aminopropylmethacryl amide (APMAAm) | H1s, H9- hOct4- pGZs, | 10 passages for H1; 22 passages for H9-hOct4-pGZs; H1 cells attached to the substrate maintained pluripotency at levels similar with Matrigel cultures. The H9-hOct4-pGZs line had higher pluripotency levels on the substrate than on Matrigel. H1 cells: 63.3% higher attachment efficiency on the substrate than on Matrigel. H9-hOct4-pGZ cells, though attached less, proliferated faster than cells on Matrigel. | Yes; differentiated into embryoid bodies | [105] |
| poly(methyl vinyl ether-alt-maleicanhydride) [PM VE-alt-MA] | HUES7, HUES9 and unnamed hiPSC line | 5 passages | Yes; differentiated to endoderm, mesoderm and ectoderm. | [99] |
Taken together, both the composition and chemical structure of synthetic matrices considered for hPSC culture are important determinants of the attachment and maintenance of pluripotency or capacity for specification.
3.3 Substrates for 3D hPSC culture
Findings reviewed thus far pertain mostly to flat surfaces coated with ECM or synthetic molecules for hPSC culture. Considerable efforts however have been geared toward the design of 3D scaffolds, some of which mimic natural stem/progenitor cell niches. Hydrogels are commonly used to create 3D environments for stem cells in culture. For example, scaffolds of 2.4% (w/v) alginate and 2.4% (w/v) chitosan prepared by lyophilization [106] support BG01V hESCs over 21 days of culture. Human H1 ESCs encapsulated in alginate beads and cultured in dishes maintain their undifferentiated state expressing OCT4, NANOG, SSEA-4, TRA-1-60 and TRA-1-81 after 260 days [107]. These cells were also coaxed toward type II pneumocytes after 160 days of encapsulation, and to neuronal and chondrogenic lineages after 200 days of culture. Scaffolds of alginate and chitin support HUES7, BG01V/hOG and hFib2-iPS4 cells for 10 passages [108]. Human ESCs in 3D alginate capsules show more rapid commitment into midbrain dopamine neurons than in 2D cultures [109]. In fact, when combined with poly(γ-glutamic acid) (γ-PGA), alginate promotes neural differentiation of iPSCs in matrices coated with nerve growth factor (NGF) [110]. Lastly, defined hydrogels consisting of hyaluronic acid, which is present during early embryo development, have also been used to maintain and differentiate hESCs [111].
In addition to the composition, the scaffold ultrastructure affects stem cell growth. Fibrous scaffolds support the proliferation of stem cells (H1, H9 hESCs) cultured for 14 days in poly(desaminotyrosyl tyrosine ethyl ester carbonate) (pDTEc) matrices coated with poly-D-lysine. The cells can be differentiated into neuronal, smooth muscle, and hepatic-like lineages [112]. Similarly, hESCs (HES3, Endeavour-1, Envy) adhere to 3D PLGA cylindrical (2 mm thick) matrix slices coated with laminin [113]. Two days after seeding, the cells within the scaffold can be coaxed to mesoderm. Poly(methacrylic acid)-coated carbon nanotubes, which are similar in scale to collagen and laminin moieties, promote neuronal differentiation of hESCs [114, 115].
From this discussion becomes obvious that there are many potential avenues for manufacturing materials for hPSC cultivation. Hybrid biomaterials are promising as they combine the adhesion properties and biological functionality of natural or bioinspired ECMs with the low cost and prolonged shelf life of synthetic molecules.
4. Scalable hPSC culture systems
The development of xeno-free culture media and substrates is driven largely by the therapeutic applications envisioned for hPSCs and their progeny. The generation of large quantities of cells under strictly defined conditions and in a reproducible fashion is a prerequisite for the use of hPSC products in the clinic. Moreover, the production of larger batches of cells is more economical motivating scale-up of stem cell cultivation.
Different designs of bioreactors offer alternatives for the large-scale culture of hPSC products [116, 117]. Among those, automated systems afford the handling of higher culture volumes per run while eliminating operator errors. Such systems include the CompacT SelectT [118] and Cellhost systems [119], which were utilized for large-throughput culture of hPSCs in tissue culture flasks. Other automated platforms designed for general scalable mammalian cell culture such as the CELLROLL roller bottle system (Integra Biosciences, Hudson, NH) and the CellCube (Corning Inc., Acton, MA) may also be suitable for hPSC cultivation. Other bioreactor modalities have also been demonstrated in conjunction with PSC culture including rotary cell culture systems [120] and slow-turning lateral vessels [121].
To that end, stirred suspension bioreactor is an appealing choice for large-scale cultures due to the homogenous environment and ease of operation and monitoring of culture. These bioreactors afford multiple culture modes including the cultivation of cells encapsulated, on microcarriers or as aggregates (Figure 2). These modes have been demonstrated for the culture of hPSCs and will be discussed below in the context of xeno-free generation of stem cells.
Figure 2. Different modes of hPSC cultivation in stirred-suspension vessels.
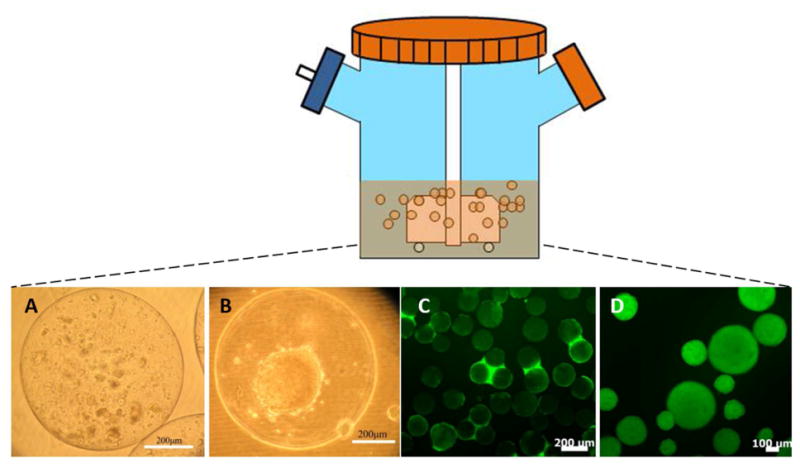
(A-B) Human ESCs cultured in alginate capsules (A) without or (B) with a liquefied core. (C) Human ESCs cultured on vitronectin-coated microcarriers. (D) Human ESCs cultured as aggregates.
It should be noted that selecting biomaterials for scalable application e.g. by mere translation of materials used statically might not be straightforward. In stirred suspension, both cells and scaffolds face a different hydrodynamic environment than that in static culture. Agitation is necessary to keep cells and scaffold suspended and ensures a homogeneous environment. However, stirring exposes cells and scaffolds to shear stresses. Apparently, biomaterials should bear certain mechanical properties for preservation of structural integrity under flow in the bioreactor. Shear stress induces cell removal from surfaces and reduces cell viability [17]. In a recent study, we reported that a peptide-conjugated polystyrene matrix, which supported attachment and proliferation of hiPSCs under static condition, was not sufficient to achieve the same goal under agitation [9]. Human ESCs cultured on vitronectin-coated microcarriers also show reduced growth rate compared to cells cultured on dishes coated with the same protein [122]. In addition to desired mechanical properties, affordable, biocompatible and biodegradable materials are highly preferred for large-scale bioprocessing aiming at serving future clinical applications.
4.1 Cultivation of hPSCs after encapsulation
Cells cultivated in stirred suspension after their encapsulation in matrices (typically hydrogels) are protected from hydrodynamic shear and excessive agglomeration of clusters. The materials employed for encapsulation allow control of their permeability and therefore of the molecules exchanged between cells and the culture environment. For example, tight control of the permeability of encapsulation materials aims to allow the transport of O2 and nutrients while blocking the penetration of immune cells and antibodies. These cell-laden scaffolds may be transplanted directly with minimal immunological rejection [123] serving as a basis for scalable systems intended for expanding and differentiating hPSCs to therapy-grade cells.
The general procedure of encapsulation entails the formation of cell-gel droplets and gel cross-linking. In this respect, biocompatible materials requiring mild cross-linking conditions are advantageous. Alginate is the most common material used for encapsulation [124] with appealing attributes such as biocompatibility, inertness toward cells [125] and a relatively straightforward protocol for generating micron-size capsules laden with cells under physiologically relevant conditions. A cell suspension (a few million cells per milliliter) of sodium alginate solution (normally 1-2% (w/v)) is dispersed in droplets, which solidify upon contact with a CaCl2 solution [126]. Cells can be maintained in solid or liquefied-core capsules with external coating. We previously demonstrated that both mESCs and hESCs can be entrapped in alginate beads coated with poly-L-lysine (pLL) and cultured in spinner flasks [127]. The pLL coating allows the liquefaction of the bead core using Ca+2-chelating agents thereby facilitating the controlled aggregation of the cells. Besides enhancing the mechanical strength of the beads, the pLL layer is also permeable to soluble differentiation factors (e.g. Wnt3a, Activin A, BMP4) as shown with the coaxing of encapsulated hESCs to cardiomyocytes-like cells. Combining alginate microencapsulation with microcarriers allowed the hESC expansion in spinner flasks for two weeks noting a 20-fold increase in concentration and a 3-fold improvement of post-thawing viability after cryopreservation [128]. Alginate can also be mixed with other materials for stem cell entrapment. For example, mESCs encapsulated in a mixture of 1.1% (w/v) alginate and 0.1% (v/v) gelatin have been cultured in a rotary high-aspect-ratio vessel (HARV) [129]. The cells were successfully induced into alveolar epithelial cells with shorter times of differentiation compared to dish cultures. Mouse ESCs cultured in rotary bioreactors have also been induced to cardiomyocytes [130] and osteogenic lineages [131].
Besides alginate, agarose is another choice for hydrogel encapsulation of ESCs. Mouse ESCs encapsulated in size-controlled agarose capsules can be cultured in stirred suspension at high density and become hematopoietic progenitors [132]. Agarose-encapsulated mESCs propagated in 250-ml spinner flasks have also been differentiated into cardiomyocytes [133].
Despite all the advantages that cell encapsulation offers, it may pose considerable hindrance to the transfer of O2, nutrients, waste and factors as shown in Figure 3. Such limitations may affect the control of cell proliferation and/or the differentiation along particular lineages. If cell purification is required, the separation and harvesting of cells from the scaffolding material(s) not only increases the cost of the process but potentially contributes to the reduction in cell number and viability. Moreover, the use of UV for cross-linking certain gels after cell loading is another concern.
Figure 3.
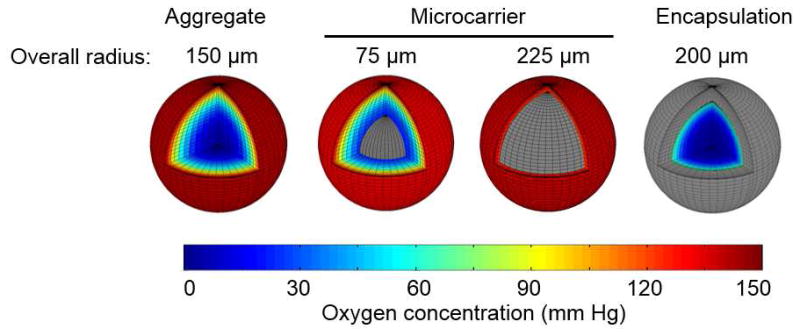
Comparison of the profile of O2 in different 3D culture modes, i.e. hPSCs cultured as aggregates, on microcarrier or after encapsulation in alginate beads. Profiles were generated using a reaction-diffusion model assuming Michaelis-Menten kinetics for O2 consumption by cells and pertinent parameters for diffusion in 1% alginate matrices [161, 162]. Color regions represent the O2 profile of hPSCs and gray regions indicate biomaterials (microcarrier or alginate). For aggregate culture, hPSC clusters with 150 μm radius were modeled. Two profiles are shown of hPSCs grown on the surface of microcarriers with radii of 75 and 200 μm. Alginate beads were taken having a radius of 200 μm with 1% composition encapsulating a 150 μm hPSC aggregate.
4.2 Microcarrier culture of hPSCs
Microcarrier bioreactors have been utilized since the early 1970's for the large-scale culture of different (particularly anchorage-dependent) cell types intended to generate a wide gamut of products including, viruses, vaccines and proteins [134, 135]. Microcarriers afford distinct advantages such as high surface-to-volume ratio and flexibility in accommodating the adhesion needs of various cells via surface modification [136] in conjunction with the benefits of stirred suspension bioreactors such as real-time monitoring and controlling of the culture environment. Microcarrier culture usually holds a higher volume fraction (ratio between cell and medium). Assuming an average volume of approximately 2000 μm3 per human cell (∼15 μm3 diameter), the cellular volume fraction in microcarrier suspension culture is about 0.4% (for 2×106 cells/ml) compared to 0.2%-0.3% for a confluent dish culture. Compared to bioreactor aggregate cultures, hPSCs attached on microcarriers are also exposed more readily and uniformly to the medium bulk concentrations of oxygen, nutrients and factors (Figure 1).
Current embodiments of the microcarrier culture systems however, require the separation of cells from the beads unless secreted metabolites or other non-cell products are desired. This requirement increases the downstream processing time and overall cost and may reduce the recovery of cellular products. Furthermore, high levels of agitation-induced shear are detrimental to cells while the effects of stress from lower stirring speeds especially on stem cells are still unclear. Compared to other cell types (e.g. CHO or Vero cells) traditionally cultured on microcarriers, hPSCs exhibit a more pronounced tendency for cell-cell aggregation. Thus, multi-bead cell clusters may be formed at low agitation speeds. The presence of shear in microcarrier bioreactors hampers the direct application of xeno-free substrates from 2D to 3D environments [9]. Indeed, much of the discussion in the previous sections centered on xeno-free matrices developed for hPSC cultured on flat surfaces (e.g. dishes) but there are significant differences between 2D and 3D substrata (e.g., with respect to curvature and elasticity affecting stem cell shape, spreading, and ultimately specification). For example, the growth rate of mesenchymal stem cells cultured on peptide-modified alginate beads is inversely related to the diameter of the beads due to differences in shear stresses acting on cells [137]. Proliferation of hESCs on vitronectin (full molecule)-coated microcarriers was reportedly hampered compared to tissue culture dishes layered with the same protein [122]. Attachment and proliferation of hESCs on microcarriers coated with laminin-111, which supports hESC growth on dishes, were sensitive to shear [122]. Human PSCs attach and spread on vitronectin-derived peptide conjugated-microcarriers in static culture. However, cells readily peel off of microcarriers and form aggregates in agitated suspension [9]. Taken together these findings demonstrate that surface modifications for 2D hPSC cultures do not translate directly to dynamic 3D cultures.
The composition of microcarriers affects the overall surface charge and functional group availability for cell adhesion thereby dictating largely their suitability for cultivation of particular cell types. Various commercially available microcarrier types have been tested in multiple reports for hPSC culture [8, 122]. Microcarriers layered with Matrigel exhibit consistent performance in stirred suspension bioreactors (Table 4) but the matrix's undefined composition and animal origin prevent its use in clinical-grade hPSC products.
Table 4.
Summary of microcarrier culture of hPSCs in stirred suspension bioreactors.
| Microcarrier base material and/or brand | Coating | Cell line | Cell number fold increase/culture length (days)/passages | Ref. |
|---|---|---|---|---|
| cellulose | Matrigel | HES-2, HES-3 | 4 / 6 / 25 | [138] |
| trimethyl ammonium coated polystyrene (Hillex II) | None | ESI017, HUES9 | 2.2/ 5 / 6 | [139] |
| Collagen coated polystyrene (HyClone) | Matrigel | H1, H9 | 34 / 8 / 1 | [140] |
| Cytodex 3 | Matrigel | H1, H9 | 3.25 / 5 | [8] |
| DE53(Watman) | Mouse laminin-111 | HES2, HES3 | 10 passages | [122] |
| Cytodex 3 | None | H9 | 6.8 / 14 / 1 | [141] |
| Collagen(HyClone) | Matrigel | B12-3 (iPSC) | 7 / 7 / 1 | [17] |
| Cellulose (Whatman) | Matrigel | HES-2, HES-3 | 4 / 6 / 25 | [142] |
| Cytodex 3 | Matrigel | SCED461 | 15 / 14 / 1 | [143] |
| Cultispher S | Gelatin | SHEF3 | 10 / 7 / 1 | [144] |
| Peptide-conjugated polystyrene | Poly-L-lysine | IMR90 | 23.3 / 6 / 5 | [9] |
Microcarriers with positive surface charges appear to perform better than those with negative or neutral charge [122, 145]. Indeed, microcarriers with surface-conjugated peptides support hPSC attachment and growth under agitation, only after coating with pLL, which is a positively charged synthetic polymer. Despite the lower seeding efficiency than on Matrigel-coated microcarriers (38% vs. 77%), a similar fold-increase (23.3 vs. 20.7) is achieved for hPSCs on pLL-coated, peptide microcarriers over multiple 6-day passages. The cells maintain a normal karyotype and consistent expression of pluripotency genes and proteins (Nanog, OCT4 and SSEA4) during 5 consecutive passages while subsequently they form embryoid bodies and their specification can be directed to all three germ layers [9].
In the future, functional modifications of microcarriers will aim to not only support the expansion of uncommitted hPSCs but also their lineage-specific differentiation so that the two culture segments can be integrated in a single process. To better meet the needs of clinical applications, materials should be utilized for microcarrier construction which are biocompatible and biodegradable allowing direct transplantation to patients thereby eliminating expensive downstream processing steps. Certain clinical applications, for example, may call for particular degradation rates, which can be adjusted by controlling the biomaterial composition, for better integration of the implanted cells with the host tissue. Obviously, such considerations should be viewed in conjunction with the overall bioprocess cost.
4.3 Cultivation of hPSCs as aggregates
Undifferentiated hPSCs aggregate forming embryoid bodies (EBs) on non-adhesive surfaces or in suspension. Methods for EB formation include suspension in low-affinity culture dishes and hanging drops [146] although control of the aggregate size can be challenging. Conversely, cells can be cultured in microwells of specific size [147] or microchannel devices [148] resulting in aggregates with a narrow size distribution. The scalability of most of these methods for producing large quantities of EBs for bioreactor culture is debatable. This issue may be addressed with the use of rotary orbital suspension culture systems yielding EBs which are homogeneous in size and shape [149]. Yet, single dispersed hPSCs can be seeded directly into suspension bioreactors [17, 150] in the presence of ROCK inhibitor (Y-27632) [151]. Since then, several reports emerged of hPSCs successfully expanded as aggregates in stirred suspension systems [152-154].
A major advantage of culturing hPSCs as aggregates in stirred suspension is the absence of extraneous scaffolds. This reduces the downstream separation steps for obtaining pure cell populations and make the whole process easy to set up and economic. However, aggregates formed by hPSCs are usually uneven in size, which can be caused by initial heterogeneous aggregate formation and agglomeration during culture. As EBs increase in size, cells near the aggregates' core are subjected to limited transport of nutrients and O2. Spatial gradients may further modulate the propensity of stem cells for proliferation, differentiation and apoptosis [133, 155]. Shear stress encountered by aggregates when cultured under agitation affects cell proliferation and differentiation. It was reported that moderate shear (1.5 to 15 dyne/cm2) promotes hematopoietic and endothelial differentiation of hESCs [156].
There have been a few reports combining the bioreactor culture of hPSC aggregates with materials for structural support of the clusters and for promoting cell adhesion, self-renewal and differentiation. During normal embryogenesis, stem cells form complex 3D structures and differentiate along disparate lineages. To that end, the EB system may serve as an in vitro platform of stem cells differentiation mimicking aspects of in vivo development [157]. Microparticles (10-15 μm diameter) can be incorporated into cell aggregates to affect cell fate decisions via controlled release of soluble factors. Such localized delivery of cues promotes differentiation by altering their local concentration. Incorporation of gelatin microparticles loaded with bone morphogenetic protein-4 (BMP4) and thrombopoietin (TPO) promotes mesoderm differentiation of hESCs compared to the traditional medium supplementation with soluble stimuli [158]. Microparticles made of different materials including agarose, PLGA and gelatin have been embedded within mESC aggregates. When mESC clusters are cultured with retinoic acid (RA)-releasing PLGA beads, the fate and organization of the cells changes compared to aggregates without the particles [159]. Vascular differentiation is also enhanced by PLGA microparticles (diameter of 0.24-25 μm) releasing vascular endothelial growth factor (VEGF), placenta growth factor (PLGF) and bFGF [160].
Conclusions
Our review of the current state of the art in defined xeno-free media and substrates for hPSC culture underlines the great advances noted in recent years but also the issues remaining to be resolved. Tackling these challenges will require expanding our knowledge of mechanisms governing stem cell self-renewal and commitment and most importantly, how these mechanisms can be exploited in synthetic culture environments. It is also becoming apparent that bridging stem cell research with the commercial scale production of hPSC therapeutics in a good manufacturing practice (GMP) fashion can be done effectively and efficiently through multidisciplinary approaches. Such efforts will be instrumental in the design and development of bioprocesses for the standardized and cost-effective production of stem cell products.
Acknowledgments
Funding support has been provided by the National Institutes of Health (NHLBI, R01HL103709) and the New York Stem Cell Science Trust (NYSTEM, contracts C024355 and C026714) to EST.
Footnotes
The authors declare no potential conflicts of interest.
Contributor Information
Yongjia Fan, Email: Yongjia.Fan@tufts.edu.
Jincheng Wu, Email: Jincheng.Wu@tufts.edu.
Preeti Ashok, Email: Preeti.Ashok@tufts.edu.
Michael Hsiung, Email: mhsiung@buffalo.edu.
Emmanuel S. Tzanakakis, Email: Emmanuel.Tzanakakis@tufts.edu.
References
- 1.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Huangfu DW, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nature Biotechnology. 2008;26(11):1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Lebkowski JS, et al. Human embryonic stem cells: culture, differentiation, and genetic modification for regenerative medicine applications. Cancer J. 2001;7(Suppl 2):S83–93. [PubMed] [Google Scholar]
- 6.Kehoe DE, et al. Scalable Stirred-suspension Bioreactor Culture of Human Pluripotent Stem Cells. Tissue Eng Part A. 2010;16(2):405–21. doi: 10.1089/ten.tea.2009.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dang SM, et al. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells. 2004;22(3):275–282. doi: 10.1634/stemcells.22-3-275. [DOI] [PubMed] [Google Scholar]
- 8.Nie Y, et al. Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnol Prog. 2009;25(1):20–31. doi: 10.1002/btpr.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan Y, et al. Facile Engineering of Xeno-Free Microcarriers for the Scalable Cultivation of Human Pluripotent Stem Cells in Stirred Suspension. Tissue Eng Part A. 2013 doi: 10.1089/ten.tea.2013.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park IH, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soldner F, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136(5):964–77. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palsson BO, Bhatia SN. Tissue Engineering. Prentice Hall; Upper Saddle River: 2003. [Google Scholar]
- 13.Jing D, et al. Stem Cells for Heart Cell Therapies. Tissue Eng Part B Rev. 2008;14(4):393–406. doi: 10.1089/ten.teb.2008.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Want AJ, et al. Large-scale expansion and exploitation of pluripotent stem cells for regenerative medicine purposes: beyond the T flask. Regenerative Medicine. 2012;7(1):71–84. doi: 10.2217/rme.11.101. [DOI] [PubMed] [Google Scholar]
- 15.Lock LT, Tzanakakis ES. Stem/Progenitor Cell Sources of Insulin-Producing Cells for the Treatment of Diabetes. Tissue Eng. 2007;13(7):1399–1412. doi: 10.1089/ten.2007.0047. [DOI] [PubMed] [Google Scholar]
- 16.Ryan EA, et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51(7):2148–57. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 17.Kehoe DE, et al. Scalable Stirred-Suspension Bioreactor Culture of Human Pluripotent Stem Cells. Tissue Engineering Part A. 2010;16(2):405–421. doi: 10.1089/ten.tea.2009.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallon BS, et al. Toward xeno-free culture of human embryonic stem cells. Int J Biochem Cell Biol. 2006;38(7):1063–75. doi: 10.1016/j.biocel.2005.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei T, et al. Xeno-free derivation and culture of human embryonic stem cells: current status, problems and challenges. Cell Res. 2007;17(8):682–8. doi: 10.1038/cr.2007.61. [DOI] [PubMed] [Google Scholar]
- 20.Vemuri MC, et al. Derivation of human embryonic stem cells in xeno-free conditions. Methods Mol Biol. 2007;407:1–10. doi: 10.1007/978-1-59745-536-7_1. [DOI] [PubMed] [Google Scholar]
- 21.Rajala K, et al. Testing of nine different xeno-free culture media for human embryonic stem cell cultures. Hum Reprod. 2007;22(5):1231–8. doi: 10.1093/humrep/del523. [DOI] [PubMed] [Google Scholar]
- 22.Pera MF, Tam PP. Extrinsic regulation of pluripotent stem cells. Nature. 2010;465(7299):713–20. doi: 10.1038/nature09228. [DOI] [PubMed] [Google Scholar]
- 23.Chambers I, Tomlinson SR. The transcriptional foundation of pluripotency. Development. 2009;136(14):2311–22. doi: 10.1242/dev.024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bibikova M, et al. Unraveling epigenetic regulation in embryonic stem cells. Cell Stem Cell. 2008;2(2):123–34. doi: 10.1016/j.stem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Boyer LA, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122(6):947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodda DJ, et al. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280(26):24731–7. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- 27.Boiani M, Scholer HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005;6(11):872–84. doi: 10.1038/nrm1744. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110(12):4111–9. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bendall SC, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448(7157):1015–21. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- 30.Chase LG, Firpo MT. Development of serum-free culture systems for human embryonic stem cells. Current Opinion in Chemical Biology. 2007;11(4):367–72. doi: 10.1016/j.cbpa.2007.06.421. [DOI] [PubMed] [Google Scholar]
- 31.Unger C, et al. Good manufacturing practice and clinical-grade human embryonic stem cell lines. Hum Mol Genet. 2008;17(R1):R48–53. doi: 10.1093/hmg/ddn079. [DOI] [PubMed] [Google Scholar]
- 32.Welling M, Geijsen N. Uncovering the true identity of naive pluripotent stem cells. Trends Cell Biol. 2013;23(9):442–8. doi: 10.1016/j.tcb.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Ying QL, et al. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115(3):281–92. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 34.Xu RH, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20(12):1261–4. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 35.Ginis I, et al. Differences between human and mouse embryonic stem cells. Developmental Biology. 2004;269(2):360–380. doi: 10.1016/j.ydbio.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 36.Xu RH, et al. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3(2):196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vallier L, et al. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development. 2009;136(8):1339–49. doi: 10.1242/dev.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu RH, et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2(3):185–90. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 39.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118(Pt 19):4495–509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 40.Amit M, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227(2):271–8. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 41.Xu C, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19(10):971–4. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, et al. Human embryonic stem cells maintained in the absence of mouse embryonic fibroblasts or conditioned media are capable of hematopoietic development. Blood. 2005;105(12):4598–603. doi: 10.1182/blood-2004-10-4065. [DOI] [PubMed] [Google Scholar]
- 43.Xu C, et al. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells. 2005;23(3):315–23. doi: 10.1634/stemcells.2004-0211. [DOI] [PubMed] [Google Scholar]
- 44.Levenstein ME, et al. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24(3):568–74. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato N, et al. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Medicine. 2004;10(1):55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez A, et al. The WNT receptor FZD7 is required for maintenance of the pluripotent state in human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(4):1409–1414. doi: 10.1073/pnas.1323697111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dravid G, et al. Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells. 2005;23(10):1489–501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- 48.Martin MJ, et al. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11(2):228–32. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Gonzalo FR, Izpisua Belmonte JC. Albumin-associated lipids regulate human embryonic stem cell self-renewal. PLoS One. 2008;3(1):e1384. doi: 10.1371/journal.pone.0001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akopian V, et al. Comparison of defined culture systems for feeder cell free propagation of human embryonic stem cells. In Vitro Cell Dev Biol Anim. 2010;46(3-4):247–58. doi: 10.1007/s11626-010-9297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valamehr B, et al. Developing defined culture systems for human pluripotent stem cells. Regenerative Medicine. 2011;6(5):623–34. doi: 10.2217/rme.11.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ludwig TE, et al. Derivation of human embryonic stem cells in defined conditions. Nature Biotechnology. 2006;24(2):185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 53.Ludwig TE, et al. Feeder-independent culture of human embryonic stem cells. Nature Methods. 2006;3(8):637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 54.Chen G, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8(5):424–9. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y, et al. A novel chemical-defined medium with bFGF and N2B27 supplements supports undifferentiated growth in human embryonic stem cells. Biochem Biophys Res Commun. 2006;346(1):131–9. doi: 10.1016/j.bbrc.2006.05.086. [DOI] [PubMed] [Google Scholar]
- 56.Lu J, et al. Defined culture conditions of human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103(15):5688–93. doi: 10.1073/pnas.0601383103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao S, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103(18):6907–12. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furue MK, et al. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc Natl Acad Sci U S A. 2008;105(36):13409–14. doi: 10.1073/pnas.0806136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, et al. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnology and Bioengineering. 2005;91(6):688–98. doi: 10.1002/bit.20536. [DOI] [PubMed] [Google Scholar]
- 60.Chen G, et al. Thermal stability of fibroblast growth factor protein is a determinant factor in regulating self-renewal, differentiation, and reprogramming in human pluripotent stem cells. Stem Cells. 2012;30(4):623–30. doi: 10.1002/stem.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lotz S, et al. Sustained levels of FGF2 maintain undifferentiated stem cell cultures with biweekly feeding. PLoS One. 2013;8(2):e56289. doi: 10.1371/journal.pone.0056289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumagai H, et al. Identification of small molecules that promote human embryonic stem cell self-renewal. Biochemical and Biophysical Research Communications. 2013;434(4):710–716. doi: 10.1016/j.bbrc.2013.03.061. [DOI] [PubMed] [Google Scholar]
- 63.Desbordes SC, et al. High-throughput screening assay for the identification of compounds regulating self-renewal and differentiation in human embryonic stem cells. Cell Stem Cell. 2008;2(6):602–612. doi: 10.1016/j.stem.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meng G, et al. Extracellular matrix isolated from foreskin fibroblasts supports long-term xeno-free human embryonic stem cell culture. Stem Cells Dev. 2010;19(4):547–56. doi: 10.1089/scd.2009.0303. [DOI] [PubMed] [Google Scholar]
- 65.Choo AB, et al. Expansion of pluripotent human embryonic stem cells on human feeders. Biotechnology and Bioengineering. 2004;88(3):321–31. doi: 10.1002/bit.20247. [DOI] [PubMed] [Google Scholar]
- 66.Hovatta O, et al. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Human Reproduction. 2003;18(7):1404–1409. doi: 10.1093/humrep/deg290. [DOI] [PubMed] [Google Scholar]
- 67.Amit M, et al. Human feeder layers for human embryonic stem cells. Biology of Reproduction. 2003;68(6):2150–6. doi: 10.1095/biolreprod.102.012583. [DOI] [PubMed] [Google Scholar]
- 68.Richards M, et al. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20(9):933–6. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 69.Cheng LZ, et al. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells. 2003;21(2):131–142. doi: 10.1634/stemcells.21-2-131. [DOI] [PubMed] [Google Scholar]
- 70.Zhang K, et al. Utilization of human amniotic mesenchymal cells as feeder layers to sustain propagation of human embryonic stem cells in the undifferentiated state. Cell Reprogram. 2011;13(4):281–8. doi: 10.1089/cell.2010.0103. [DOI] [PubMed] [Google Scholar]
- 71.Kim SJ, et al. Human placenta-derived feeders support prolonged undifferentiated propagation of a human embryonic stem cell line, SNUhES3: comparison with human bone marrow-derived feeders. Stem Cells and Development. 2007;16(3):421–8. doi: 10.1089/scd.2006.0098. [DOI] [PubMed] [Google Scholar]
- 72.Miyamoto K, et al. Human placenta feeder layers support undifferentiated growth of primate embryonic stem cells. Stem Cells. 2004;22(4):433–40. doi: 10.1634/stemcells.22-4-433. [DOI] [PubMed] [Google Scholar]
- 73.Escobedo-Lucea C, et al. Development of a Human Extracellular Matrix for Applications Related with Stem Cells and Tissue Engineering. Stem Cell Reviews and Reports. 2012;8(1):170–183. doi: 10.1007/s12015-011-9270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu CH, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnology. 2001;19(10):971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 75.Prowse ABJ, et al. Stem cell integrins: Implications for ex-vivo culture and cellular therapies. Stem Cell Research. 2011;6(1):1–12. doi: 10.1016/j.scr.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287(5457):1427–30. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 77.Braam SR, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26(9):2257–65. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 78.Manton KJ, et al. A chimeric vitronectin: IGF-I protein supports feeder-cell-free and serum-free culture of human embryonic stem cells. Stem Cells Dev. 2010;19(9):1297–305. doi: 10.1089/scd.2009.0504. [DOI] [PubMed] [Google Scholar]
- 79.Guilak F, et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5(1):17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruoslahti E. The RGD story: a personal account. Matrix Biol. 2003;22(6):459–65. doi: 10.1016/s0945-053x(03)00083-0. [DOI] [PubMed] [Google Scholar]
- 81.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238(4826):491–7. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 82.Obara M, Kang MS, Yamada KM. Site-directed mutagenesis of the cell-binding domain of human fibronectin: separable, synergistic sites mediate adhesive function. Cell. 1988;53(4):649–57. doi: 10.1016/0092-8674(88)90580-6. [DOI] [PubMed] [Google Scholar]
- 83.Nishiuchi R, et al. Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol. 2006;25(3):189–97. doi: 10.1016/j.matbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 84.Humphrey RK, et al. Maintenance of pluripotency in human embryonic stem cells is STAT3 independent. Stem Cells. 2004;22(4):522–530. doi: 10.1634/stemcells.22-4-522. [DOI] [PubMed] [Google Scholar]
- 85.Baxter MA, et al. Analysis of the distinct functions of growth factors and tissue culture substrates necessary for the long-term self-renewal of human embryonic stem cell lines. Stem Cell Res. 2009;3(1):28–38. doi: 10.1016/j.scr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 86.Braam SR, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alpha V beta 5 integrin. Stem Cells. 2008;26(9):2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 87.Rodin S, et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28(6):611–5. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 88.Melkoumian Z, et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol. 2010;28(6):606–10. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- 89.Yap LY, et al. Defining a threshold surface density of vitronectin for the stable expansion of human embryonic stem cells. Tissue Eng Part C Methods. 2011;17(2):193–207. doi: 10.1089/ten.TEC.2010.0328. [DOI] [PubMed] [Google Scholar]
- 90.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. Journal of Cell Science. 2006;119(19):3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leiss M, et al. The role of integrin binding sites in fibronectin matrix assembly in vivo. Current Opinion in Cell Biology. 2008;20(5):502–507. doi: 10.1016/j.ceb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 92.Kalaskar DM, et al. Characterization of the interface between adsorbed fibronectin and human embryonic stem cells. Journal of the Royal Society Interface. 2013;10(83) doi: 10.1098/rsif.2013.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doran MR, et al. Defined high protein content surfaces for stem cell culture. Biomaterials. 2010;31(19):5137–42. doi: 10.1016/j.biomaterials.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 94.Kolhar P, et al. Synthetic surfaces for human embryonic stem cell culture. J Biotechnol. 2010;146(3):143–6. doi: 10.1016/j.jbiotec.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 95.Klim JR, et al. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat Methods. 2010;7(12):989–94. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Melkoumian Z, et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nature Biotechnology. 2010;28(6):606–U95. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- 97.Nagaoka M, et al. Culture of human pluripotent stem cells using completely defined conditions on a recombinant E-cadherin substratum. Bmc Developmental Biology. 2010;10 doi: 10.1186/1471-213X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Villa-Diaz LG, et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat Biotechnol. 2010;28(6):581–3. doi: 10.1038/nbt.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brafman DA, et al. Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials. 2010;31:9135–9144. doi: 10.1016/j.biomaterials.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li YJ, et al. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. Journal of Biomedical Materials Research PArt A. 2006;79A:1–5. doi: 10.1002/jbm.a.30732. [DOI] [PubMed] [Google Scholar]
- 101.Mei Y, et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nature Materials. 2010;9:768–778. doi: 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Villa-Diaz LG, et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nature Biotechnology. 2010;28:581–583. doi: 10.1038/nbt.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nandivada H, et al. Fabrication of synthetic polymer coatings and their use in feeder-free culture of human embryonic stem cells. Nature Protocols. 2011;6:1037–1043. doi: 10.1038/nprot.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Villa-Diaz LG, et al. Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells. 2012;30:1174–1181. doi: 10.1002/stem.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Irwin EF, et al. Engineered polymer-media interfaces for the long-term self-renewal of human embryonic stem cells. Biomaterials. 2011;32:6912–6919. doi: 10.1016/j.biomaterials.2011.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Z, et al. Feeder-free self-renewal of human embryonic stem cells in 3D porous natural polymer scaffolds. Biomaterials. 2010;31:404–412. doi: 10.1016/j.biomaterials.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 107.Siti-Ismail N, et al. The benefit of human embryonic stem cell encapsulation for prolonged feeder-free maintenance. Biomaterials. 2008;29:3946–3952. doi: 10.1016/j.biomaterials.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 108.Lu HF, et al. A 3D microfibrous scaffold for long-term human pluripotent stem cell self-renewal under chemically defined conditions. Biomaterials. 2012;33:2419–2430. doi: 10.1016/j.biomaterials.2011.11.077. [DOI] [PubMed] [Google Scholar]
- 109.Kim J, Sachdev P, Sidhu K. Alginate microcapsule as a 3D platform for the efficient differentiation of human embryonic stem cells to dopamine neurons. Stem Cell Research. 2013;11(3):978–989. doi: 10.1016/j.scr.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 110.Kuo YC, Chang YH. Differentiation of induced pluripotent stem cells toward neurons in hydrogel biomaterials. Colloids Surf B Biointerfaces. 2013;102:405–11. doi: 10.1016/j.colsurfb.2012.08.061. [DOI] [PubMed] [Google Scholar]
- 111.Gerecht S, et al. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104(27):11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carlson AL, et al. Microfibrous substrate geometry as a critical trigger for organization, self-renewal, and differentiation of human embryonic stem cells within synthetic 3-dimensional microenvironments. FASEB. 2012;26:3240–3251. doi: 10.1096/fj.11-192732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gao SY, et al. Modeling the adhesion of human embryonic stem cells to poly(lactic-co-glycolic acid) surfaces in a 3D environment. Journal of Biomedical Materials Research. 2010;92A:683–692. doi: 10.1002/jbm.a.32401. [DOI] [PubMed] [Google Scholar]
- 114.Chao TI, et al. Poly(methacrylic acid)-grafted carbon nanotube scaffolds enhance differentiation of hESCs into neuronal cells. Adv Mater. 2010;22(32):3542–7. doi: 10.1002/adma.201000262. [DOI] [PubMed] [Google Scholar]
- 115.Chao TI, et al. Carbon nanotubes promote neuron differentiation from human embryonic stem cells. Biochem Biophys Res Commun. 2009;384(4):426–30. doi: 10.1016/j.bbrc.2009.04.157. [DOI] [PubMed] [Google Scholar]
- 116.Placzek MR, et al. Stem cell bioprocessing: fundamentals and principles. J R Soc Interface. 2009;6(32):209–32. doi: 10.1098/rsif.2008.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kirouac DC, Zandstra PW. The systematic production of cells for cell therapies. Cell Stem Cell. 2008;3(4):369–81. doi: 10.1016/j.stem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 118.Thomas RJ, et al. Automated, scalable culture of human embryonic stem cells in feeder-free conditions. Biotechnol Bioeng. 2009;102(6):1636–44. doi: 10.1002/bit.22187. [DOI] [PubMed] [Google Scholar]
- 119.Terstegge S, et al. Automated maintenance of embryonic stem cell cultures. Biotechnol Bioeng. 2007;96(1):195–201. doi: 10.1002/bit.21061. [DOI] [PubMed] [Google Scholar]
- 120.Wang X, et al. Scalable producing embryoid bodies by rotary cell culture system and constructing engineered cardiac tissue with ES-derived cardiomyocytes in vitro. Biotechnol Prog. 2006;22(3):811–8. doi: 10.1021/bp060018z. [DOI] [PubMed] [Google Scholar]
- 121.Gerecht-Nir S, Cohen S, Itskovitz-Eldor J. Bioreactor cultivation enhances the efficiency of human embryoid body (hEB) formation and differentiation. Biotechnol Bioeng. 2004;86(5):493–502. doi: 10.1002/bit.20045. [DOI] [PubMed] [Google Scholar]
- 122.Chen AK, et al. Critical microcarrier properties affecting the expansion of undifferentiated human embryonic stem cells. Stem Cell Research. 2011;7(2):97–111. doi: 10.1016/j.scr.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 123.Uludag H, De Vos P, Tresco PA. Technology of mammalian cell encapsulation. Adv Drug Deliv Rev. 2000;42(1-2):29–64. doi: 10.1016/s0169-409x(00)00053-3. [DOI] [PubMed] [Google Scholar]
- 124.Orive G, et al. Biocompatibility of microcapsules for cell immobilization elaborated with different type of alginates. Biomaterials. 2002;23:3825–3831. doi: 10.1016/s0142-9612(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 125.Pasparakis G, Bouropoulos N. Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate–chitosan beads. International Journal of Pharmaceutics. 2006;323:34–42. doi: 10.1016/j.ijpharm.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 126.Erickson GR, et al. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochemical and Biophysical Research Communications. 2002;290:763–769. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 127.Jing D, Parikh A, Tzanakakis ES. Cardiac cell generation from encapsulated embryonic stem cells in static and scalable culture systems. Cell. Transplantation. 2010;19:1397–1412. doi: 10.3727/096368910X513955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Serra M, et al. Microencapsulation Technology: A Powerful Tool for Integrating Expansion and Cryopreservation of Human Embryonic Stem Cells. Plos One. 2011;6(8) doi: 10.1371/journal.pone.0023212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Siti-Ismail N, et al. Development of a Novel Three-Dimensional, Automatable and Integrated Bioprocess for the Differentiation of Embryonic Stem Cells into Pulmonary Alveolar Cells in a Rotating Vessel Bioreactor System. Tissue Engineering Part C-Methods. 2012;18(4):263–272. doi: 10.1089/ten.TEC.2011.0299. [DOI] [PubMed] [Google Scholar]
- 130.Consolo F, et al. Computational modeling for the optimization of a cardiogenic 3D bioprocess of encapsulated embryonic stem cells. Biomechanics and Modeling in Mechanobiology. 2012;11(1-2):261–277. doi: 10.1007/s10237-011-0308-0. [DOI] [PubMed] [Google Scholar]
- 131.Hwang YS, et al. The use of murine embryonic stem cells, alginate encapsulation, and rotary microgravity bioreactor in bone tissue engineering. Biomaterials. 2009;30(4):499–507. doi: 10.1016/j.biomaterials.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 132.Dang SM, et al. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells. 2004;22(3):275–82. doi: 10.1634/stemcells.22-3-275. [DOI] [PubMed] [Google Scholar]
- 133.Bauwens C, et al. Development of a perfusion fed bioreactor for embryonic stem cell-derived cardiomyocyte generation: Oxygen-mediated enhancement of cardiomyocyte output. Biotechnology and Bioengineering. 2005;90(4):452–461. doi: 10.1002/bit.20445. [DOI] [PubMed] [Google Scholar]
- 134.Clark JM, Hirtenstein MD. Optimizing culture conditions for the production of animal cells in microcarrier culture. Ann N Y Acad Sci. 1981;369:33–46. doi: 10.1111/j.1749-6632.1981.tb14175.x. [DOI] [PubMed] [Google Scholar]
- 135.van Wezel AL. Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nature. 1967;216(110):64–5. doi: 10.1038/216064a0. [DOI] [PubMed] [Google Scholar]
- 136.Varani J, et al. Use of recombinant and synthetic peptides as attachment factors for cells on microcarriers. Cytotechnology. 1993;13(2):89–98. doi: 10.1007/BF00749935. [DOI] [PubMed] [Google Scholar]
- 137.Schmidt JJ, Jeong J, Kong H. The Interplay Between Cell Adhesion Cues and Curvature of Cell Adherent Alginate Microgels in Multipotent Stem Cell Culture. Tissue Engineering Part A. 2011;17(21-22):2687–2694. doi: 10.1089/ten.tea.2010.0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oh SKW, et al. Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Research. 2009;2(3):219–230. doi: 10.1016/j.scr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 139.Phillips BW, et al. Attachment and growth of human embryonic stem cells on microcarriers. J Biotechnol. 2008;138(1-2):24–32. doi: 10.1016/j.jbiotec.2008.07.1997. [DOI] [PubMed] [Google Scholar]
- 140.Lock LT, Tzanakakis ES. Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng Part A. 2009;15(8):2051–63. doi: 10.1089/ten.tea.2008.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fernandes AM, et al. Successful scale-up of human embryonic stem cell production in a stirred microcarrier culture system. Braz J Med Biol Res. 2009;42(6):515–22. doi: 10.1590/s0100-879x2009000600007. [DOI] [PubMed] [Google Scholar]
- 142.Oh SKW, et al. Agitation can Induce Differentiation of Human Pluripotent Stem Cells in Microcarrier Cultures. Tissue Engineering Part C-Methods. 2011;17(2):165–172. doi: 10.1089/ten.TEC.2010.0320. [DOI] [PubMed] [Google Scholar]
- 143.Serra M, et al. Improving expansion of pluripotent human embryonic stem cells in perfused bioreactors through oxygen control. Journal of Biotechnology. 2010;148(4):208–215. doi: 10.1016/j.jbiotec.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 144.Storm MP, et al. Three-dimensional culture systems for the expansion of pluripotent embryonic stem cells. Biotechnology and Bioengineering. 2010;107(4):683–95. doi: 10.1002/bit.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marinho PA, et al. Xeno-free production of human embryonic stem cells in stirred microcarrier systems using a novel animal/human-component-free medium. Tissue Eng Part C Methods. 2012 doi: 10.1089/ten.TEC.2012.0141. [DOI] [PubMed] [Google Scholar]
- 146.Kurosawa S, et al. Olanzapine potentiates neuronal survival and neural stem cell differentiation: regulation of endoplasmic reticulum stress response proteins. J Neural Transm. 2007;114(9):1121–8. doi: 10.1007/s00702-007-0747-z. [DOI] [PubMed] [Google Scholar]
- 147.Ungrin MD, et al. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS One. 2008;3(2):e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Torisawa YS, et al. Efficient formation of uniform-sized embryoid bodies using a compartmentalized microchannel device. Lab on a Chip. 2007;7(6):770–776. doi: 10.1039/b618439a. [DOI] [PubMed] [Google Scholar]
- 149.Carpenedo RL, Sargent CY, McDevitt TC. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells. 2007;25(9):2224–34. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 150.Krawetz R, et al. Large-scale expansion of pluripotent human embryonic stem cells in stirred-suspension bioreactors. Tissue Eng Part C Methods. 2010;16(4):573–82. doi: 10.1089/ten.TEC.2009.0228. [DOI] [PubMed] [Google Scholar]
- 151.Watanabe K, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25(6):681–6. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 152.Abbasalizadeh S, et al. Bioprocess development for mass production of size-controlled human pluripotent stem cell aggregates in stirred suspension bioreactor. Tissue Eng Part C Methods. 2012;18(11):831–51. doi: 10.1089/ten.TEC.2012.0161. [DOI] [PubMed] [Google Scholar]
- 153.Schulz TC, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One. 2012;7(5):e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Amit M, et al. Dynamic suspension culture for scalable expansion of undifferentiated human pluripotent stem cells. Nature Protocols. 2011;6(5):572–9. doi: 10.1038/nprot.2011.325. [DOI] [PubMed] [Google Scholar]
- 155.Powers DE, et al. Effects of oxygen on mouse embryonic stem cell growth, phenotype retention, and cellular energetics. Biotechnology and Bioengineering. 2008;101(2):241–254. doi: 10.1002/bit.21986. [DOI] [PubMed] [Google Scholar]
- 156.Wolfe RP, Ahsan T. Shear stress during early embryonic stem cell differentiation promotes hematopoietic and endothelial phenotypes. Biotechnol Bioeng. 2013;110(4):1231–42. doi: 10.1002/bit.24782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.O'Shea KS. Embryonic stem cell models of development. Anat Rec. 1999;257(1):32–41. doi: 10.1002/(SICI)1097-0185(19990215)257:1<32::AID-AR6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 158.Purpura KA, et al. Systematic engineering of 3D pluripotent stem cell niches to guide blood development. Biomaterials. 2012;33(5):1271–80. doi: 10.1016/j.biomaterials.2011.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carpenedo RL, et al. Homogeneous and organized differentiation within embryoid bodies induced by microsphere-mediated delivery of small molecules. Biomaterials. 2009;30(13):2507–2515. doi: 10.1016/j.biomaterials.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ferreira L, et al. Human embryoid bodies containing nano- and microparticulate delivery vehicles. Advanced Materials. 2008;20(12):2285. + [Google Scholar]
- 161.Hulst AC, H HJH, Buitelaar RM, Tramper J. Determination of the effective diffusion coefficient of oxygen in gel materials in relation to gel concentration. Biotechnology Techniques. 1989;3(3):199–204. [Google Scholar]
- 162.Wu J, et al. Oxygen Transport and Stem Cell Aggregation in Stirred-Suspension Bioreactor Cultures. PLOS ONE. 2014 doi: 10.1371/journal.pone.0102486. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]


