Abstract
INTRODUCTION
Recent data suggests and increased risk of cardiovascular events and mortality in men on testosterone therapy (TT). To date there are no long term, prospective studies to determine safety. In such cases, retrospective observational studies can be helpful. We examined our patient database to determine if TT altered a man’s risk of all cause mortality.
METHODS
We queried our hormone database for all men with a serum testosterone level and then examined charts to determine testosterone status. In all, 509 men had charts available for review. We linked our patient records to the National Death Index to determine morality.
RESULTS
Of the 509 men who met inclusion criteria, 284 were on testosterone therapy and 225 did not use testosterone. Age (mean 54 years) and follow up time (mean 10 years) were similar for both groups. In all, 19 men died—10 (4.4%) of the men not on TT and 9 (3.2%) of the men on TT. After adjusting for age and year of evaluation, there was no significant difference in the risk of death based on TT (HR 1.0, 95% CI 0.39 – 2.57, p=1.0).
CONCLUSIONS
There appears to be no change in mortality risk overall for men utilizing long-term testosterone therapy.
Keywords: testosterone, hormones, hypogonadism, death
Introduction
Low testosterone can negatively impact quality and quantity of life in men.1–3 Treatment of hypogonadism with testosterone therapy (TT) has been shown to improve muscle mass and strength, sexual function and desire, mood, bone mineral density, and mortality.3–7 However, there remains concern about possible negative health effects of TT.
A recent randomized controlled trial of older men with hypogonadism and limited mobility demonstrated an increased risk of cardiovascular events when on testosterone compared to placebo.8 For this reason, the trial was stopped after approximately 9 months. However a separate trial conducted using a similar patient population failed to demonstrate an impact on mortality after 6 month.9 Furthermore, a retrospective analysis of VA data identified a survival advantage for men on TT with up to 4 years of follow up.10 In contrast, another retrospective study of veterans demonstrated a higher risk of mortality, myocardial infarctions, and ischemic strokes while on TT.11 Recently, a retrospective study utilizing US claims data identified a higher risk of nonfatal myocardial infarction for men after initiating TT.12
Given the heterogeneity in the literature and the uncertain applicability of previous findings to middle aged men on TT, we sought to determine the impact on mortality of TT in men 40 years and older. By linking men treated with testosterone therapy over the past 20 years with the National Death index, we examined the association between mortality and TT.
Patients and Methods
After Institutional Review Board approval, an initial study cohort was identified with available data from 1989 to 2009 contained in the andrology database at the Baylor College of Medicine Special Procedures Laboratory in the Scott Department of Urology. The laboratory performs a high volume of hormone analyses for the treatment and management of sexual dysfunction, hypogonadism, and male infertility. Within this group, we reviewed available charts of men to identify a cohort of men with known testosterone therapy status. Dates and type (transdermal versus injection) of testosterone therapy were recorded. Men who had used both injection and transdermal therapy were counted in the injection group.
National Death Index Linkage
All eligible men in the andrology database were linked to the National Death Index (NDI). The NDI is a central computerized index of death record information compiled from data submitted to the National Center for Health Statistics (NCHS) from each state’s vital statistics office. The NDI contains nearly all deaths in the United States beginning in 1979 until December 31, 2010 at the time of record linkage. Automated, probabilistic matching was performed using social security number, first name, last name, middle initial, and date of birth. All matches were reviewed by ME and SL.
Statistical analysis
Men accrued at risk time from their initial hormone analysis until death or December 31, 2010 (the final year that complete death data was available). The rate of death in our cohort was compared to the general Texas population given that >90% of the cohort lived in Texas. We calculated the expected number of deaths by multiplying the number of years at risk by the 10-year age strata death rates from the Texas Department of State Health Services for the study period. Standardized Mortality Rates (SMRs) were calculated by dividing the observed number of deaths by the expected number of deaths. Analyses were performed on the entire cohort as well as subgroups (e.g. testosterone therapy status, type of testosterone therapy, baseline testosterone level).
We also analyzed the risk of death in our cohort after stratifying based on testosterone status using a Cox proportional hazards regression model while adjusting for age and year of evaluation. Comparison between Kaplan Meier curves was performed using log rank function. All p values were two sided with p<0.05 considered statistically significant. Analyses were performed using SAS (version 9.3, SAS Institute, Inc, Cary, NC).
Results
In all, 509 men met inclusion criteria with 80 on injection and 204 men on transdermal therapy. There was no significant difference between men on and not on testosterone therapy with regard to age, date of evaluation, or age at last follow up. The mean follow up for the group was 10.3 years with some men followed up to 20 years. Baseline testosterone was significantly lower for the men who were started on TT (325 vs 356, p<0.01; Table 1).
Table 1.
Characteristics of Cohort
| Characteristic | TT No | TT Yes | p value | |
|---|---|---|---|---|
| n | 225 | 284 | ||
|
| ||||
| Age at Evaluation | mean (SD) | 54.89 (11.14) | 54.1 (8.7) | 0.99 |
|
|
||||
| 40–49 | 91(40.44) | 105(36.97) | ||
| 50–59 | 66(29.33) | 108(38.03) | ||
| 60+ | 68(30.22) | 71(25.0) | ||
|
| ||||
| Age at last follow up or death, mean (SD) | 65.2 (11.43) | 64.4 (8.99) | 0.80 | |
|
| ||||
| Follow-up time, mean (SD) | 10.31 (3.25) | 10.31(3.43) | 0.69 | |
|
| ||||
| Year of Evaluation | 1991–1995 | 12(5.33) | 28(9.86) | 0.17 |
| 1996–2000 | 90(40.00) | 101(35.56) | ||
| 2001–2005 | 115(51.11) | 149(52.46) | ||
| 2006-present | 8(3.56) | 6(2.11) | ||
|
| ||||
| Type of TT | injectable | 80 | ||
| Transdermal | 204 | |||
|
| ||||
| Baseline testosterone (ng/dL) | 356.1 (139.01) | 325.21 (204.78) | <0.01 | |
A total of 19 men died in the cohort, 3.2% of men on TT and 4.4% of men not on TT. Compared to the general Texas population, the men in our cohort had a lower risk of overall death with 19 cases observed with 59 expected (SMR 0.33, 95% CI 0.20–0.51). The rates did not differ based on TT status (Table 2)
Table 2.
All cause mortality stratified by testosterone therapy status. SMR (Standardized mortality rate). Hazard ratio (HR) represents Cox regression multivariable model adjusted for patient age and year of evaluation.
| n | Observed | Expected | SIR (95% CI) | HR (95% CI) | p | |
|---|---|---|---|---|---|---|
| All men | 781 | 19 | 62.8 | 0.30 (0.18, 0.47) | ||
| TT Yes | 368 | 9 | 30.7 | 0.29 (0.13, 0.56) | Ref | 0.86 |
| TT No | 413 | 10 | 32.1 | 0.31 (0.15, 0.57) | 0.92 (0.36 – 2.35) |
No meaningful changes in the conclusions occurred when stratifying men by baseline testosterone levels. (i.e. <300 ng/dL versus ≥300 ng/dL (Table 3). Table 4. In addition, similar standardized incidence rates were seen for men after stratification based on type of TT (i.e. injection versus transdermal) with similar death rates identified between the groups (p=0.16)).
Table 3.
All cause mortality rate stratified by baseline testosterone level (< versus ≥ 300 ng/dL). SMR (standardized mortality rates).
| n | Observed | Expected | SMR (95% CI) | ||
|---|---|---|---|---|---|
| Testosterone<300 | All men | 246 | 10 | 30.0 | 0.33 (0.16, 0.61) |
| TRT Yes | 168 | 7 | 19.0 | 0.37 (0.15, 0.76) | |
| TRT No | 78 | 3 | 11.0 | 0.27 (0.05, 0.79) | |
| Testosterone≥300 | All men | 262 | 9 | 27.9 | 0.32 (0.15, 0.61) |
| TRT Yes | 116 | 2 | 10.1 | 0.20 (0.02, 0.72) | |
| TRT No | 146 | 7 | 17.8 | 0.39 (0.16, 0.81) |
Discussion
The current report found no increased risk of death for men on testosterone therapy for up to 15 years. Moreover, on subgroup analysis, there was no difference in risk based on the type of testosterone therapy or baseline testosterone levels.
Testosterone therapy has been shown to benefit men in quality of life measures such as sexual function and desire and mood.3,13,14 In addition, health benefits have also been demonstrated including increased muscle mass and strength, bone mineral density, and even overall mortality.4–6,10 Despite benefits, there are concerns about the risk of TT for cardiovascular health.
While two meta-analyses did not report an increased risk of cardiovascular events with testosterone, a recent trial in older men found a higher rate of cardiovascular events in men on TT.8,15,16 In addition, a study on the Colorado VA system reported a higher risk of mortality, heart attack, and stroke in men on TT.11 In contrast, another trial of older men found no increased risk of death.9 Moreover, a retrospective study demonstrated a mortality benefit to TT.10 Thus, there is heterogeneity in the literature. Moreover, given that dyslipidemia and polycythemia can result from testosterone, biologic plausibility does exist for a higher incidence of cardiovascular disease. In addition, a limitation of most studies is the relatively short duration of follow up which complicates issues of long term safety.
The current report suggests that TT does not alter a man’s mortality risk. Indeed, all subanalyses which compared testosterone methods and baseline patient characteristics demonstrated no alteration in mortality risk. On subanalyses, cardiovascular mortality did not show any trends toward increased incidence with testosterone.
It is important to note that the mortality rate is lower in our cohort than the general Texas population. However, no difference was noted based on TT status suggesting that testosterone does not meaningfully affect this risk. The lower SMR is perhaps not surprising given that men who seek medical care represent higher socioeconomic and educated groups. Indeed, socioeconomic status indicators are associated with mortality.17 In addition, similar to the “healthy worker effect,” observed in occupational cohorts, men seeking regular medical care for men’s health related issues likely represent a healthier group than the overall population.18
Several limitations warrant mention. The retrospective, observational study design limits the interpretation of the findings because subjects were not randomized to treatment and were, instead, treated based on clinical condition. As such, bias may be introduced as we may not be able to adequately control for confounding factors that may have affected both treatment and outcome. Our number of deaths was relatively small, which limited our power to detect differences based on TT status
Nevertheless, the current report demonstrates no increased risk of death for men on TT in men on long term TT. Further studies are warranted to confirm the generalizability of the current findings.
Figure 1.
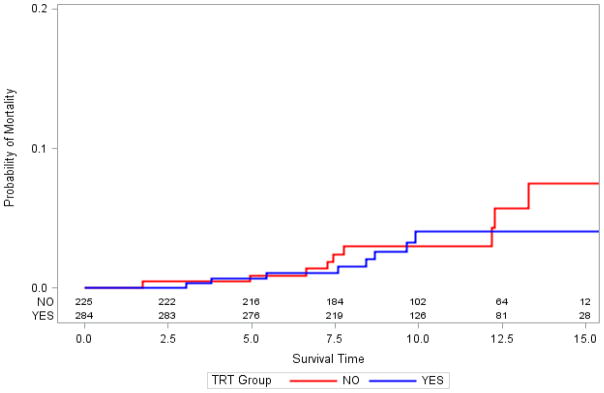
Kaplan Meier curves examining mortality after stratifying based on testosterone replacement therapy (TRT) status.
Acknowledgments
This study is supported in part by P01HD36289 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development, National Institutes of Health (to DJL and LIL). The project was also partially supported by an investigator initiated grant from Endo Pharmaceuticals (to MLE and LIL).
References
- 1.Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Archives of internal medicine. 2006;166(15):1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- 2.Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the Testim Registry in the US (TRiUS) Aging Male. 2011 doi: 10.3109/13685538.2011.606513. [DOI] [PubMed] [Google Scholar]
- 3.Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. Improved sexual function with testosterone replacement therapy in hypogonadal men: real-world data from the Testim Registry in the United States (TRiUS) J Sex Med. 2011;8(11):3204–3213. doi: 10.1111/j.1743-6109.2011.02436.x. [DOI] [PubMed] [Google Scholar]
- 4.Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, et al. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997;82(2):407–413. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya RK, Khera M, Blick G, Kushner H, Nguyen D, Miner MM. Effect of 12 months of testosterone replacement therapy on metabolic syndrome components in hypogonadal men: data from the Testim Registry in the US (TRiUS) BMC Endocr Disord. 2011;11(1):18. doi: 10.1186/1472-6823-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, Judge JO, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010;58(6):1134–1143. doi: 10.1111/j.1532-5415.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. Changes in prostate specific antigen in hypogonadal men after 12 months of testosterone replacement therapy: support for the prostate saturation theory. The Journal of urology. 2011;186(3):1005–1011. doi: 10.1016/j.juro.2011.04.065. [DOI] [PubMed] [Google Scholar]
- 8.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, Oldham JA, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95(2):639–650. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 10.Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97(6):2050–2058. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- 11.Vigen R, O’Donnell CI, Baron AE, Grunwald GK, Maddox TM, Bradley SM, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA: the journal of the American Medical Association. 2013;310(17):1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 12.Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the Testim Registry in the US (TRiUS) Aging Male. 2012;15(1):14–21. doi: 10.3109/13685538.2011.606513. [DOI] [PubMed] [Google Scholar]
- 14.Shores MM, Kivlahan DR, Sadak TI, Li EJ, Matsumoto AM. A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression) J Clin Psychiatry. 2009;70(7):1009–1016. doi: 10.4088/jcp.08m04478. [DOI] [PubMed] [Google Scholar]
- 15.Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60(11):1451–1457. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95(6):2560–2575. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 17.Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA: the journal of the American Medical Association. 1998;279(21):1703–1708. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- 18.Park RM. The healthy worker survivor effect and mortality at two automotive engine manufacturing plants. Am J Ind Med. 1996;30(6):655–663. doi: 10.1002/(SICI)1097-0274(199612)30:6<655::AID-AJIM2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]


