Abstract
Objective
Arteriogenesis represents the maturation of pre-formed vascular connections in response to flow changes and shear stress. These collateral vessels can restore up to 60% of the native blood flow. Shear stress and vascular injury can induce the release of nucleotides from vascular smooth muscle cells and platelets that can serve as signaling ligands, suggesting they may be involved in mediating arteriogenesis. The nucleotide receptor P2Y2R has also been shown to mediate smooth muscle migration and arterial remodeling. Thus, we hypothesize that the P2Y2 nucleotide receptor (P2Y2R) mediates arteriogenesis in response to ischemia.
Methods
Hindlimb ischemia was induced by femoral artery ligation (FAL) in C57Bl/6NJ or P2Y2R negative mice (P2Y2-/-). Hindlimb perfusion was measured with laser Doppler imaging (LDPI) in comparison to the sham-operated contralateral limb immediately and at 3, 7, 14, 21, and 28 days post-ligation. Collateral vessel size was measured by Microfil casting. Muscle specimens were harvested and analyzed with immunohistochemistry for Ki67, VCAM-1, macrophages, and muscle viability by H&E.
Results
Hindlimb ischemia as induced by FAL in C57Bl/6NJ mice resulted in significant ischemia as measured by LDPI. There was rapid recovery to near normal levels of perfusion by 2 weeks. In P2Y2R negative mice (P2Y2-/-), arterial ligation resulted in severe ischemia with greater tissue loss. Recovery of perfusion was impaired, achieving only 40% of wild type mice by 28 days. Collateral vessels in the P2Y2-/- mice were underdeveloped with reduced vascular cell proliferation and smaller vessel size. The collaterals were ~65% the size of WT collateral vessels (P=0.011). Angiogenesis at 28 days in the ischemic muscle, however, was greater in the P2Y2-/- mice (P<0.001), possibly related to persistent ischemia leading and angiogenic drive. Early macrophage recruitment was reduced by nearly 70% in P2Y2-/- despite significantly more myocyte necrosis. However, inflammation was greater at the 28 day time point in the P2Y2-/-mice.
Conclusions
P2Y2R deficiency does not alter baseline collateral vessel formation. However, it does significantly impair collateral maturation with resultant persistent limb ischemia despite enhanced angiogenesis. These findings reinforce the importance of arteriogenesis in the recovery of perfusion in ischemic tissues as compared to angiogenesis. They also support the role of P2Y2R in mediating this process. The mechanism by which P2Y2R mediates arteriogenesis may involve the recruitment of inflammatory cells to the ischemic tissues which is essential to arteriogenesis. Approaches to target P2Y2R may yield new therapeutic strategies for the treatment of arterial occlusive disease.
Keywords: P2Y2 receptor, arteriogenesis, collateral vessels, ischemia, vascular biology
Introduction
Cardiovascular disease is the leading cause of morbidity and mortality in the developed world,1 accounting for myocardial infarction, stroke and peripheral vascular disease. However, the arterial system has the innate ability to adapt to occlusive disease through arteriogenesis.2 Arteriogenesis is an adaptive response to flow changes involving the development of functional collateral arterioles that circumvent arterial occlusion and restores continuous flow to the affected downstream tissues and organs to a degree that often approximates normal blood flow. These collateral arteries arise from pre-existing inter-arteriolar connections that form during development and are critical to the preservation of tissue perfusion. The absence of these preexisting networks or an impairment in arteriogenesis results in severe ischemic changes following vascular occlusion.3,4
Arteriogenesis is a complex process that is still poorly understood. It involves vascular cell activation, proliferation, migration, and inflammatory cell recruitment that ultimately result in outward vessel growth.5 It is driven by the mechanical forces that accompany the hemodynamic shifts following arterial occlusion rather than tissue ischemia. When occlusion of a conductance vessel occurs, large pressure gradients rapidly develop across small arteriolar connections, increasing flow and fluid shear stress that provide the mechanical impetus for remodeling of these vessels into functional collaterals. These collaterals have a much greater capacity for blood delivery than angiogenesis.5 Therefore, approaches that enhance arteriogenesis may be more effective in the treatment of vasoocclusive disease than those that focus on angiogenesis. Physiologic flow-induced shear stress stimulates the release of nucleotides into the extracellular space, thereby altering their tightly regulated pericellular concentrations.6-8 Mechanisms for nonlytic release of nucleotides from certain cells, such as endothelial cells (EC), include vesicular transport or passage through electrochemical pores.9,10 Extracellular nucleotides such as adenosine-5’-triphosphate (ATP) and uridine-5’-triphosphate (UTP) then activate cell surface nucleotide receptors which can mediate cellular processes such as proliferation and migration. Vascular cells as well as bone marrow-derived inflammatory cells express the P2 class of nucleotide receptors.11 In particular, the P2Y2 receptor (P2Y2R) is upregulated in vascular cells exposed to increased shear stress and following vascular injury in vitro and in vivo.12,13 These receptors mediate intimal hyperplasia in rabbits12 and smooth muscle cell (SMC) proliferation and migration.14 Thus, we hypothesize that P2Y2R and nucleotides may mediate arteriogenesis in the setting of vascular occlusion. In this study, the role of P2Y2R was investigated in a murine model of hindlimb ischemia, focusing on collateral maturation and limb perfusion. Our findings suggest that this receptor may play an important role in effective collateral growth and the recovery of perfusion.
Methods
Animal Models
All animal procedures were performed in accordance with the Institutional Animal Care and Use Committee of the University of Pittsburgh (Protocol 0911093B-5). Adult C57Bl/6 NJ (WT) or P2Y2R knockout on C57Bl/6 NJ background (P2Y2 -/-; strain B6.129P2-P2ry2tm1Bhk/J) mice (20-25 gm; Jackson Labs; Bar Harbor, ME) were used. The absence of P2Y2R expression in the P2Y2-/- mice was confirmed by PCR. Mice were anesthetized with intraperitoneal sodium pentobarbital (75 mg/kg) and inhalational isoflurane. Femoral artery ligation (FAL) was performed on the right hindlimb via a transverse skin incision. The femoral artery was ligated with 6-0 silk immediately distal to the deep femoral artery and at the proximal popliteal artery. A sham exposure was performed on the contralateral leg without ligation. Buprenorphine (0.1 mg/kg) was given for post-operative analgesia.
Laser Doppler Perfusion Imaging (LDPI)
LDPI (Perimed III, Perimed; North Royalton, OH) was used to assess perfusion of the mouse hindlimb as described.15 Mice were anesthetized using an isoflurane regulator and positioned on a warming pad. Measurements were obtained at 1 hour and 3, 7, 14, 21, and 28 days post-FAL in each animal with a total of 8 mice per strain Regions of interest for perfusion measurements encompassed the plantar surface of the foot. Mean perfusion was represented as a ratio of the ligated limb to the contralateral, nonischemic limb.
Tissue processing
Mice were sacrificed after 3, 7, or 28 days and limbs were perfused in situ with phosphate buffered saline (pH 7.4) and 4% paraformaldehyde via the abdominal aorta. Tissue specimens were then collected en bloc and fixed in 4% paraformaldehyde at 4°C overnight. Thigh adductor and tibialis anterior (TA) muscles were cryoprotected in 30% sucrose at 4°C for 24 hours. Tissues were frozen with 2-methylbutane and liquid nitrogen and then sectioned (7μm thickness). The superficial thigh muscles were used for collateral vessel immunohistochemistry while the TA was used for general histology and immunohistochemistry. H&E staining was performed on TA sections, spaced ~200μm apart, for a total of 3 sections per animal, and 4 animals per group. Images were obtained with an Olympus Provis microscope (Tokyo, Japan).
Immunohistochemistry
Immunohistochemistry was performed with antibodies against CD45 (cat# ab10558), VCAM-1, and α-actin (1:100, Abcam; Cambridge, MA); CD31(cat# 550274) and Ki67 (1:200, BD Pharmingen; San Diego, CA); and F4/80 (1:1000, Abcam). Cryosections were blocked with 2% BSA, incubated with primary antibody for 1 hour, washed, and then incubated with fluorophoreconjugated secondary antibody for 1 hour (goat anti-rabbit or goat anti-rat, Cy3 or Alexa fluor 488, 1:1000 dilution; Amersham). Nuclei were counterstained with 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma; St. Louis, MO) for 30 seconds. Images were acquired using an Olympus Fluoview 1000 confocal microscope (Tokyo, Japan). Quantification of positive staining was expressed as a ratio to either DAPI positive structures or myocytes. Muscle necrosis was determined as cells with cytoplasmic vacuoles. Regenerating cells had centrally located nuclei.
Microfil injection and morphometric analysis of collaterals:
To assess collateral vessel formation, half of the mice (N=4/strain) were sacrificed on day 28 and perfused through the left ventricle with PBS plus 10U/mmol/L heparin. The descending thoracic aorta was cannulated and Microfil (MV120-blue, Flow Tech Inc.; Carver, MA) was injected with a flow pump until it flowed out through the vented IVC. Aorta and IVC were ligated and the Microfil was allowed to polymerize overnight at 4°C as described.16 Specimens were dissected free of surrounding tissues, leaving vascular structures and muscles intact, and cleared using graded ethanol immersion for 24 hours each followed by methyl salicylate (12 hours). Collateral vessels were imaged at 6X magnification. Vessel diameters were measured using a calibrated optical micrometer using Image J (NIH) by a blinded observer.
Statistical Analysis
Results are expressed as mean ± SEM. Differences among multiple groups were analyzed with one-way analysis of variance and the Holm-Sidak method was employed for all pair-wise comparisons (SigmaStat;SPSS). Differences between two groups were analyzed using the Student's t-test. Statistical significance was indicated by a P value < 0.05.
Results
Impaired recovery of hindlimb perfusion following femoral ligation in P2Y2 -/- mice
FAL markedly reduced foot perfusion in all mice as measured by LDPI with similar levels of cutaneous perfusion in WT and P2Y2-/- mice at day 0 (Figure 1A,B). Perfusion gradually recovered in both strains of mice. By day 7, we observed a sharp divergence in the recovery curves where the P2Y2-/- mice experienced little improvement in perfusion beyond that time point while the WT mice continued to improve (Figure 1B). By day 28, WT mice exhibited full perfusion recovery while the P2Y2-/- mice reached <40% of the perfusion levels of the contralateral limb (Figure 1B).
Figure 1.
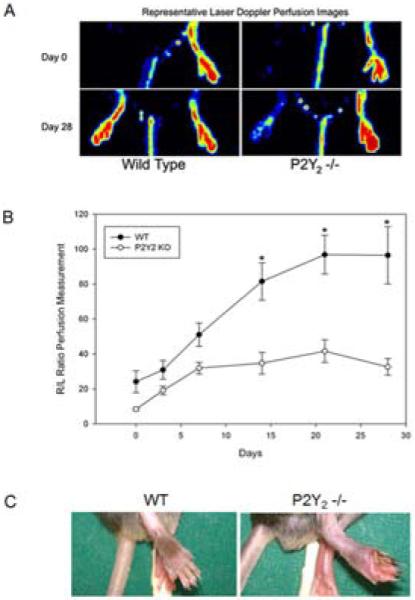
P2Y2-/- mice have blunted perfusion recovery and develop ischemic wounds following hindlimb ischemia. A) Representative Laser Doppler Perfusion Imaging at days 0 and 28 following right femoral artery ligation in WT and P2Y2-/- mice. B) Perfusion was calculated from the LDPI of the plantar foot immediately post-ligation and then at 3, 7, 14, 21 and 28 days. Perfusion was expressed as a ratio of the ischemic/nonischemic (R/L) limbs (N=8 animals/group, mean ± SEM). Significant differences were observed between groups from day 14 through day 28 (one-way ANOVA; *P<.001 vs. all other groups). C) Representative photographs of the ischemic foot at day 14 demonstrated gangrene and tissue loss in the P2Y2-/- mouse. 50% (N=4/8) of the P2Y2 -/- animals developed wounds or gangrene of the ischemic foot.
All mice had impaired motor function of the ischemic hindlimb with paresis and clawing of the foot immediately after FAL but it was more severe in the KO mice. WT mice had a more rapid recovery of limb function than the P2Y2-/- mice. By day 14, 50% of P2Y2 -/- mice developed toe gangrene and tissue loss while none of the WT mice did (Figure 1C). The mice with toe gangrene went on to autoamputate while those with ulceration showed some evidence of healing at 28 days. No new lesions developed after the 14 day time point. All mice recovered near normal function of the ischemic hindlimb by day 28 despite persistently reduced perfusion in the P2Y2-/- mice.
Increased inflammation and skeletal muscle regeneration in P2Y2-/- mice at day 28
TA muscle collected 28 days post-FAL was analyzed by immunohistochemistry for CD45 as a nonspecific marker of inflammation (Figure 2A,B). The number of CD45+ cells in muscle sections was similar in the nonischemic hindlimbs from WT and P2Y2-/- mice. In muscle from the ischemic hindlimb of WT mice, there was minimal inflammatory infiltration detected at this late time point. In contrast, there was a three-fold increase in inflammatory cells in the P2Y2-/- mice (P<.001), suggesting an increased inflammatory response to FAL in the absence of P2Y2R function (Figure 2A,B) at 28 days.
Figure 2.
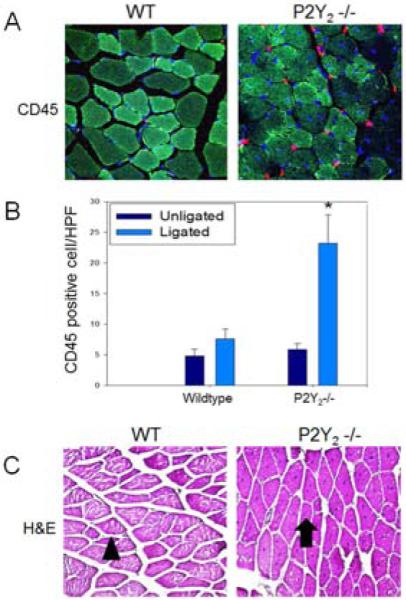
P2Y2-/- mice exhibited increased inflammation and muscle regeneration in the ischemic hindlimb compared with control mice. A) Representative photomicrographs of TA muscle stained with anti-CD45 (red) and DAPI (blue) at 60x magnification. B) Representative photomicrograph of TA muscle stained with H&E. WT mice exhibited normal muscle architecture and normal-appearing mature myocytes with peripheral nuclei (arrowhead). Muscle from P2Y2 -/- mice had a high predominance of regenerating myocytes which are characterized by centralized nuclei (arrow) (N=4/group, 3 sections analyzed per animal). C) CD45 positive cells were counted and expressed as number per high power field. There were significantly more CD45 positive cells identified in the P2Y2-/- ischemic muscles than in WT muscle (N=4/group, 3 sections analyzed per animal; *P<.001 vs. all other groups).
By H&E, the TA muscles exhibited preserved muscle architecture in WT and P2Y2-/- mice with no evidence of muscle necrosis by 28 days. However, the muscle from P2Y2 -/- mice showed a predominance of regenerating myocytes, noted by centrally located nuclei in 3 of 4 animals (Figure 2C, arrow). Only 1 of 4 WT mice had evidence of active muscle regeneration with the majority of the cells appearing to be mature myocytes (Figure 2C, arrowhead) as indicated by peripherally located nuclei. These findings suggest near full recovery from the muscle ischemia in the WT mice while the KO mice experienced persistent inflammation and ongoing myocyte regeneration.
P2Y2-/- mice exhibited greater angiogenesis in TA muscle following FAL
CD31 staining of the TA muscle collected at 28 days following FAL was performed to detect ECs and capillary formation (Figure 3A). These structures were quantified by calculating a CD31+ structure/ myocyte ratio (Figure 3B). In WT mice, there was a modest increase in CD31 positive structures following hindlimb ischemia. In P2Y2-/- mice, the capillary density in the ischemic hindlimb was significantly increased compared with the WT group (N=4 mice/group; 1.97 ± 0.25 vs. 1.31 ± 0.06 fold increase, P<.05). These findings support increased angiogenesis in the P2Y2-/- mice. CD31 is also expressed on leukocytes but at a much lower level. The intensity of the staining suggests that the CD31 positive structures are EC structures but may include some inflammatory cells.
Figure 3.
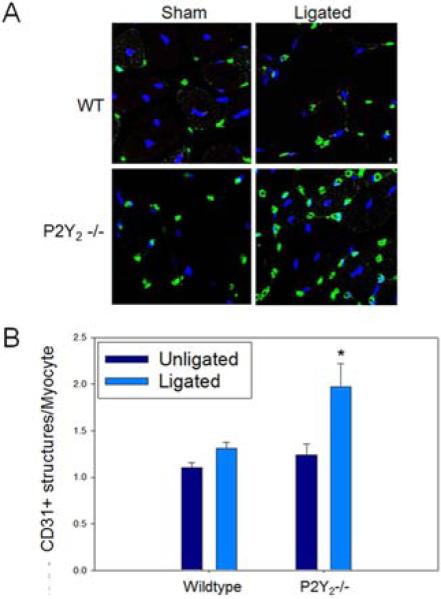
Angiogenesis was increased in P2Y2-/- mice following FAL. TA muscles from WT and P2Y2-/- mice were sectioned and stained at 28 days following hindlimb ischemia. Endothelial cell and capillary structures were stained with anti-CD31. A) Representative confocal photomicrographs of TA muscle sections stained with anti-CD31 (green) and DAPI (blue) and imaged at 60x. B) CD31 positive structures were counted and expressed as a ratio to number of total myocytes per section (N=4/group; 4 sections per animal; p<0.05).
Collateral maturation in WT mice was more pronounced than in P2Y2-/- mice
Three days after FAL, the superficial adductor muscles were evaluated for collateral artery development in the ischemic and control hindlimbs. Sections of muscle were stained for Ki67 to quantify proliferation of the resident vascular cells as a marker of collateral vessel maturation. Three collateral vessels per animal were analyzed for Ki67 staining (Figure 4A). There was significantly more Ki67 staining cells in the collateral vessels of the ischemic adductor muscle from WT mice versus P2Y2 -/- mice (Figure 4B, P<.001). VCAM-1 has been shown to be upregulated in the setting of arteriogenesis and recruits the inflammatory cells that are required for collateral maturation.18,24 Thus, adductor sections were stained for VCAM-1. Among collateral vessels in WT mice, diffuse VCAM-1 staining was often found in the endothelium. However, VCAM-1 staining was reduced in the collateral ECs in P2Y2 -/- mice (Figure 5).
Figure 4.
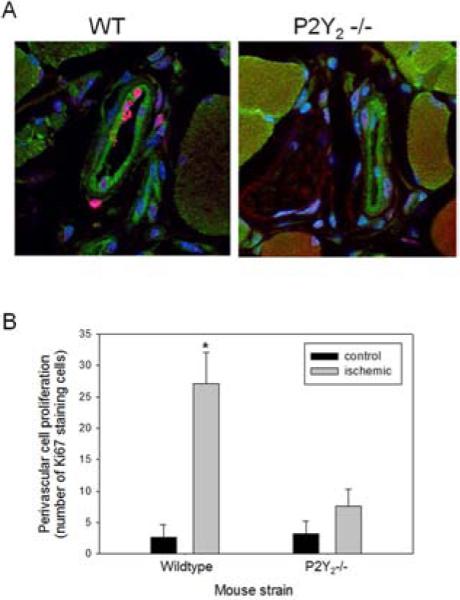
Proliferative activity in the collateral vessels was increased in WT mice compare to P2Y2-/- mice. Collateral vessels were examined 3 days following FAL. Tissue sections were stained for Ki67 (red), CD31 (green), and DAPI (blue). A) Representative confocal photomicrographs are shown at 100x magnification. B) Quantification of Ki67 positive cells in the collateral vessels, expressed as % total number of perivascular cells (N=5 mice/group, three sections analyzed per animal; *P<.001 vs. all other groups).
Figure 5.
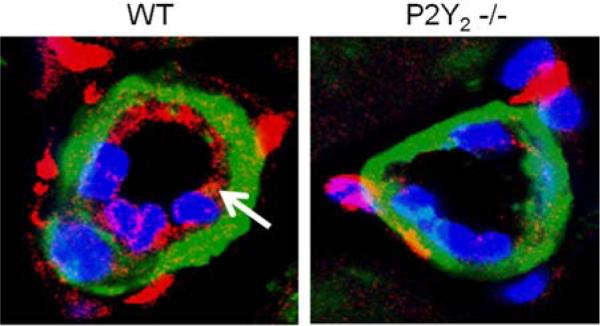
VCAM-1 expression is collateral vessels following hindlimb ischemia was increased in WT versus P2Y2-/- mice. Sections made muscle isolated 3 days following FAL were stained for VCAM-1 (red) and α-smooth muscle actin (green), and DAPI (blue). Collateral vessels were imaged via confocal microscopy at 100x magnification. Representative images are shown for WT and P2Y2 -/- animals. Endothelial staining for VCAM-1 was seen in the collateral vessels from WT mice (arrow) but not the P2Y2 -/- mice. (N=5 mice/ group, three sections analyzed per animal)
Collateral vessel anatomy and morphometric analysis
At 28 days post-FAL, vascular casts of the collateral vessels were created with Microfil and used for morphometric analysis. In the nonischemic limbs, preexisting inter-arteriolar connections were similar between the WT and KO mice (data not shown), suggesting that the P2Y2R deletion did not alter the embryologic development of these collaterals. Following ischemia, there was no apparent difference in collateral vessel numbers or location between the two strains (Figure 6). The superficial adductor collaterals were significantly larger in diameter in WT mice than in the P2Y2-/- mice (Table I). The diameters of collaterals in the ischemic hindlimb were also compared to the undeveloped collaterals of the contralateral sham limbs. The P2Y2 -/- mice exhibited a significantly blunted maturation of these vessels compared to the WT animals.
Figure 6.
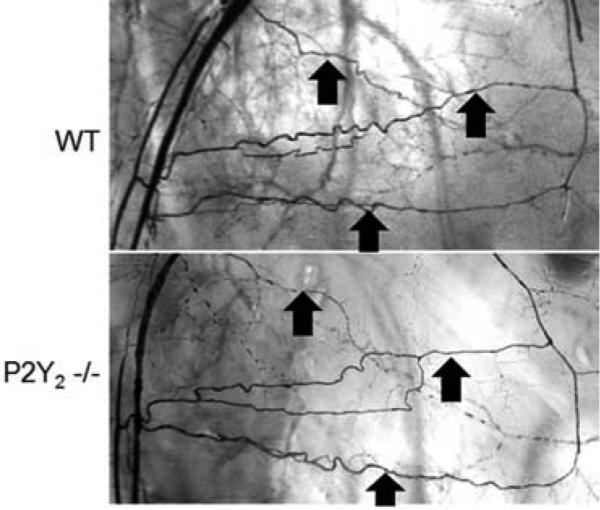
Representative photomicrographs of Microfil casting of the hindlimb arterial system are shown. Animals were casted at 28 days following FAL. Collateral vessels are noted by arrows showing similar distributions between WT and P2Y2-/- mice but less robust development in the P2Y2-/- mice (see Table I for quantification)(N=4 mice/group).
Table I Quantification of collateral vessel growth following hindlimb ischemia in WT and P2Y2 −/− mice.
| WT | P2Y2 −/− | P value | |
|---|---|---|---|
| Diameter (μm) | 53.2 ± 6.7 | 34.5 ± 2.8 | .011 |
| Percent Growth (%) | 77.7 ± 28.1 | 11.6 ± 4.8 | .041 |
Quantification of collateral artery size at 28 days by microfilm casting. Values are mean ± SEM. Four animals per treatment group and 3 collaterals were assessed per mouse. Comparisons were made to the sham operated contralateral vessel. Statistical analysis was performed using Student's t-test.
Reduced early macrophage recruitment following ischemia in P2Y2-/- mice despite increased muscle necrosis
Muscle from the ischemic hindlimbs of WT and P2Y2-/- mice at day 7 were stained for F4/80 to determine macrophage recruitment (Figure 7). There were significantly more macrophages in the ischemic muscle from WT than the P2Y2-/- mice (Figure 7A,B). Interestingly, there was marked muscle necrosis observed in the KO muscle as denoted by cells with vacuolization versus the WT muscle (Figure 7B). Despite this difference in necrosis, early macrophage recruitment was increased in the WT. These findings suggest that P2Y2R may play a role in macrophage recruitment in the setting of ischemia. While muscle regeneration appeared to be increased in the WT mice versus P2Y2-/- mice, this was not statistically significant (Figure 7C).
Figure 7.
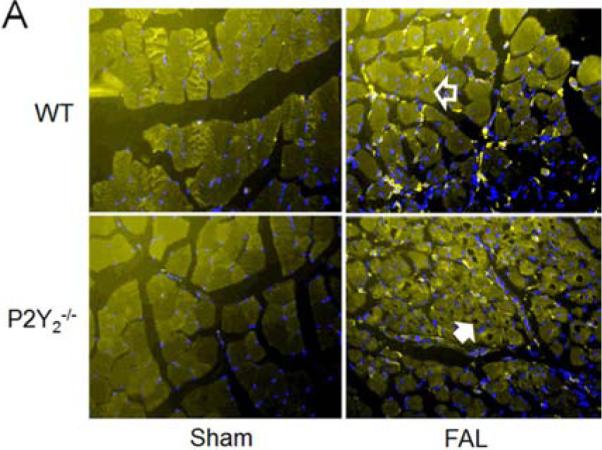
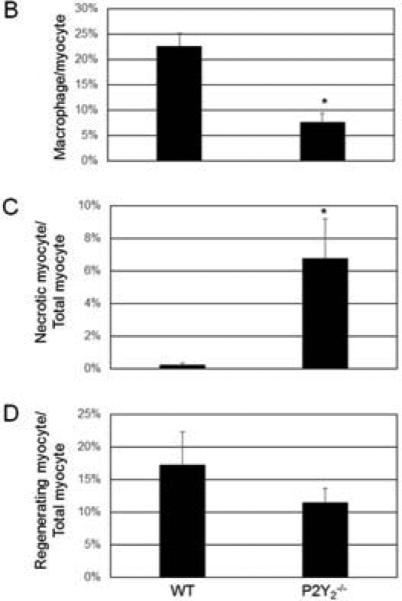
Early inflammation was reduced in the ischemic hindlimb from P2Y2-/- mice compared to WT mice at 7 days post-FAL. However, myocyte necrosis was increased in the P2Y2-/- mice. A) Representative photomicrographs demonstrating F4/80 (macrophages, yellow) and DAPI (blue) staining of WT and P2Y2-/- hindlimb muscle at day 7. Myocyte necrosis was denoted by vacuolated cells (solid white arrow) while regenerating myocytes had centrally located nuclei (open arrow) (N=4-5 mice/group, 3 sections per animal). B) Quantification of macrophage infiltration is reported as number of macrophages/total myocyte per HPF and expressed as percent (mean ± SEM). C) Quantification of myocyte necrosis is reported as number of necrotic myocyte/total myocyte per HPF. D) Quantification of regenerating myocyte is reported as number of regenerating myocyte/total myocyte per HPF. (N=4-5 mice/group, 3 sections per animal; *P>0.05)
Discussion
The development of novel therapies for tissue ischemia arising from vaso-occlusive disease has mainly been directed toward the stimulation of angiogenesis with growth factors or stem cell administration. However, the human body has a built-in mechanism to restore tissue perfusion after major arterial occlusion through pre-existing collateral connections that can mature into significant arterial channels.2 Driven by changes in fluid shear stress, these collaterals mature into large diameter vessels that can restore up to 40% of the maximal conductance of a normal artery.17,18 Thus, there is potential benefit to deciphering the mechanical and biochemical stimuli that drive arteriogenesis. This understanding may allow us to harness these pre-existing vascular connections to overcome limb ischemia.
In this study, we demonstrated a role for the P2Y2R in arteriogenesis. Mice deficient in P2Y2R exhibited profound early ischemia with tissue loss and delayed functional recovery following FAL versus WT mice. The absence of P2Y2R did not alter baseline collateral vasculature but arteriogenesis was significantly impaired with reduced collateral vessel growth in response to ischemia. This deficient collateral development resulted in delayed muscle regeneration and increased inflammation. When we examined macrophage recruitment at 7 days post-FAL, we found reduced macrophage accumulation in the P2Y2-/- mice. Together with the 28 day findings, this suggests that there is a delay in the inflammatory response in these knockout mice. By the end of 28 days, there was less than 40% recovery in cutaneous perfusion in the ischemic hindlimb of P2Y2-/- mice as compared to WT mice, supporting the significant contribution of collateral maturation to the recovery of tissue perfusion.
P2Y2R is a G-protein coupled receptor which is activated equally by ATP and UTP. Its canonical signaling pathway involves phospholipase C activation and the release of intracellular calcium stores. P2Y2R is upregulated in vascular cells in response to mechanical stimulation or injury in vitro13 as well as in injured arteries in vivo.12 P2Y2R has been reported to mediate vessel remodeling and the growth of vascular EC and SMC.19-21 Nucleotides such as ATP and UTP are released from these cells in response to shear stress and vascular injury and stimulates both EC and SMC proliferation and migration6-8,11-14. Yu et al reported that UTP can stimulate SMC migration through intracellular P2Y2 R interactions with filamin A,14 supporting the relevance of this receptor in vascular responses and remodeling. Similarly, P2Y2R stimulation with UTP increased intimal hyperplasia in rodents through the promotion of proliferative activity.12 In parallel with this role in mechanotransduction, P2Y2R associates with v 3/5 integrins by virtue of an integrin binding motif, Arg-Gly-Asp (RGD), located on the first extracellular loop22 and can transactivate integrin signaling pathways.20 v 3 integrins have an established role in vascular remodeling and SMC migration and growth,23,24 and have recently been linked to arteriogenesis.25 In keeping with these reports, we demonstrated that P2Y2-/- mice exhibited reduced proliferation in the collateral vessel wall with smaller resulting arterioles, supporting reduced growth/maturation of these vessels as compared with WT mice. Taken together, our data suggest a local release of nucleotides in response to ischemia or shear stress following arterial ligation that may mediate arteriogenesis through P2Y2R. We identified large numbers of capillaries in the distal calf muscle of the P2Y2-/- mice as compared with WT, suggesting enhanced angiogenesis in the KO mice. A plausible explanation is that reduced collateral vessel maturation results in prolonged ischemia of the distal leg that then drives angiogenesis. It has been reported that when arteriogenesis is efficient in compensating for an initial arterial occlusion, the angiogenic response in the distal limb is inhibited.2 An important limitation is that the EC marker CD31 is also expressed on inflammatory cells such as monocytes and neutrophils, raising the possibility that the angiogenesis we detected may represent inflammatory cells. However, the CD31 expression is greatest in the ECs26 and suggests that most of the structures we detected represent endothelial structures. Despite greater capillary development, the tissue perfusion remained reduced in the P2Y2-/- mice, providing further demonstration of the inferiority of angiogenesis compared to arteriogenesis in reestablishing tissue perfusion. Our findings support a greater emphasis should be placed on promoting arteriogenesis.
The responses of P2Y2-/- mice to FAL are very similar to reported observations in Balb/C mice.13 Different strains of mice have variable ischemic responses to FAL. C57Bl6 mice, the parent strain for the P2Y2-/-, develop modest ischemic injury with a great capacity to recover, achieving baseline levels of perfusion and negligible tissue loss.15 In contrast, Balb/C mice develop severe ischemia with frequent gangrene and necrosis.2 It has been suggested that the increased susceptibility to ischemia of this strain is due to an inherent lack of collateral density.3,27 We found that the baseline collateral density of P2Y2 -/- mice was similar to the C57Bl6 strain, whereas we observed decreased collaterals in Balb/C mice (data not shown). The lack of pre-formed collateral pathways forms the basis for the severe ischemic injury seen in Balb/C mice.27 Similar to our findings in the P2Y2-/- mice, FAL in Balb/C mice results in very high angiogenic activity in the distal calf muscles. These findings again support the very important role that proximal collaterals play in regulating angiogenesis. While the P2Y2-/- mice do possess a normal network of immature collateral vessels, the inability of these networks to mature properly in the setting of ischemia likely contributed to the exaggerated distal angiogenesis. Genetic studies identified a locus associated with the ischemia susceptibility traits of the Balb/C mice on chromosome 7, near the mapped location of the P2Y2R gene.3,28 Our preliminary studies revealed that Balb/C mice express full-length P2Y2R mRNA transcripts similar to WT mice (data not shown). However, the defect in Balb/C mice might be located in regulatory elements that control P2Y2R expression or responses to ischemia and needs to be further investigated.
Inflammatory cell recruitment is necessary for the outward remodeling process.29,30 van Weel et al demonstrated that T lymphocytes and NK cells are required for arteriogenesis with impaired arteriole maturation in mice depleted of these cells.30 They also showed that Balb/C mice exhibit very different T lymphocyte and NK cell responses to infection, suggesting this may be the mechanism behind the poor collateral vessel formation in these mice. More recently, Bastiaansen et al31 reported on the essential role of inflammation and monocytes in arteriogenesis. Mice lacking the transcriptional co-activator p300-CBP-associated factor (PCAF), a histone acetyltransferase that facilitates the regulation of inflammatory genes, experience reduced monocyte recruitment to perivascular tissues and impaired arteriogenesis following ischemia. Following FAL, mononuclear cells infiltrate the remodeling collateral wall early to promote maturation.32 P2Y2R may facilitate this infiltration during arteriogenesis by mediating adhesion molecule and cytokine expression. The stimulation of P2Y2R on vascular cells has been shown to upregulate VCAM-1 and ICAM-1 as well as lymphotoxin-alpha expression, all of which promote leukocyte recruitment.33,34 We demonstrated early luminal VCAM-1 expression in maturing collateral vessels of WT mice but to a much lower degree in the P2Y2-/- mice, suggesting that P2Y2R participates in the inflammatory response required for vascular remodeling. Similarly, we identified a significant reduction in early macrophage recruitment to the ischemic hindlimb in these mice. This finding was contrary to our expectations given the marked muscle necrosis detected in the P2Y2-/- mice which should have promoted inflammation. Thus, P2Y2R may mediate the early inflammation that is critical to collateral maturation.
Our study has important limitations. Although P2Y2R was observed to mediate arteriogenesis in the present study, the use of global knockout mice does not allow us to determine which cells have the greatest influence in this response. For example, leukocytes are important in arteriogenesis. Purinergic signaling is an integral part of autocrine and paracrine signaling of inflammatory cells, and P2Y2 -/- mice are known to have impaired neutrophil chemotaxis with susceptibility to pulmonary infections. The use of bone marrow chimeras or cell type-specific knockout mice would aid in differentiating the influence of P2Y2R signaling in resident vascular cells versus inflammatory cells. We were also unable to quantify the release of nucleotide ligands in the hindlimb ischemia model because of the complexities of isolating extracellular fluids from the hindlimb muscle. Future studies will elucidate the impact of these specific molecular pathways upstream and downstream of P2Y2R activation on regulating collateral remodeling.
In conclusion, these studies demonstrate the importance of the P2Y2R for arteriogenesis in a murine model of FAL, potentially through the early regulation of inflammatory responses to ischemia and muscle necrosis. The impaired arteriogenesis in P2Y2-/- mice delayed muscle regeneration and reduced perfusion recovery which provided ongoing stimulus for angiogenesis and inflammation. The greater capacity for restoring adequate distal perfusion arteriogenesis versus angiogenesis is the impetus for continued investigation into this process and the molecular signals involved. This understanding may yield pharmacological therapies that target collateral maturation as a treatment for severe arterial occlusive disease.
Clinical Relevance.
Many patients with advanced cardiovascular disease have limited or no options for revascularization. Unfortunately, no effective medical therapies exist to improve perfusion in these patients. Arteriogenesis is the process by which collateral arteries develop from preexisting vessels and is more effective at restoring perfusion than angiogenesis, the process of growing new vessels. The mechanisms of arteriogenesis, though, are not well understood. We aim to identify a key molecular signal in this adaptive phenomenon. With a better understanding of the mechanisms governing arteriogenesis, pharmacological targets that could enhance collateral formation and improve limb salvage may be identified.
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the VA Pittsburgh Healthcare System (ET, RMM, MCM, AS). The work was supported by funding through funding from NIH T32 HL098036 (ET). The contents of this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin E, Berry JD, Bravata DM, et al. Heart disease and stroke statistics - 2012 Update. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, et al. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 3.Chalothorn D, Clayton J, Zhang H, Pomp D, Faber J. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30:179–91. doi: 10.1152/physiolgenomics.00047.2007. [DOI] [PubMed] [Google Scholar]
- 4.Chalothorn D, Faber JE. Strain-dependent variation in collateral circulatory function in mouse hindlimb. Physiol. Genomics. 2010;42:469–479. doi: 10.1152/physiolgenomics.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol. 2003;23:1143–1151. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 6.Milner P, Bodin P, Loesch A, Burnstock G. Rapid release of endothelin and ATP from isolated aortic endothelial cells exposed to increased flow. Biochem Biophys Res Commun. 1990;170(2):649–56. doi: 10.1016/0006-291x(90)92141-l. [DOI] [PubMed] [Google Scholar]
- 7.Korenaga R, Ando J, Tsuboi H, Yang W, Sakuma I, Toyo-oka T, et al. Laminar flow stimulates ATP- and shear stress-dependent nitric oxide production in cultured bovine endothelial cells. Biochem Biophys Res Commun. 1994;198(1):213–9. doi: 10.1006/bbrc.1994.1030. [DOI] [PubMed] [Google Scholar]
- 8.Bodin P, Bailey DJ, Burnstock G. Increased flow-induced ATP release from isolated vascular endothelial but not smooth muscle cells. Br J Pharmacol. 1991;103:1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sipos A, Vargas SL, Toma I, Hanner F, Willecke K. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol. 2009:1724–1732. doi: 10.1681/ASN.2008101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International union of pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seye CI, Kong Q, Erb Laurie, Garrad RC, Krugh B, Wang M, et al. Functional P2Y2 nucleotide receptors mediate uridine 5′-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation. 2002;106:2720–26. doi: 10.1161/01.cir.0000038111.00518.35. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Andersson M, Karlsson L, Watson MA, Cousens DJ, Jern S, et al. Incereased mitogenic and decreased contractile P2 receptors in smooth muscle cells by shear stress in human vessels with intact endothelium. Arterioscler Thromb Vasc Biol. 2003;23:1370–1376. doi: 10.1161/01.ATV.0000080350.37408.5A. [DOI] [PubMed] [Google Scholar]
- 14.Yu N, Erb L, Shivaji R, Weisman GA, Seye CI. Binding of the P2Y2 nucleotide receptor to filamin A regulates migration of vascular smooth muscle cells. Circ Res. 2008;102:581–8. doi: 10.1161/CIRCRESAHA.107.162271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachdev U, Cui X, McEnaney R, Wang T, Benabou K, Tzeng E. TLR2 and TLR4 mediate differential responses to limb ischemia through MyD88-dependent and independent pathways. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y, Luo Y, Tang S, Rajantie I, Salven P, Heil M, et al. Critical function of BMX/Etk in ischemia-mediated arteriogenesis and angiogenesis. J Clin Invest. 2006;116:2344–55. doi: 10.1172/JCI28123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito WD, Arras M, Scholz D, Winkler B, Htun P, Schaper W. Angiogenesis but not collateral growth is associated with ischemia after femoral artery occlusion. Am J Physiol. 1997:H1255–65. doi: 10.1152/ajpheart.1997.273.3.H1255. [DOI] [PubMed] [Google Scholar]
- 18.Lazarous DF, Shou M, Scheinowitz M, Hodge E, Thirumurti V, Kitsiou AN, et al. Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation. 1996;94:1074–82. doi: 10.1161/01.cir.94.5.1074. [DOI] [PubMed] [Google Scholar]
- 19.Kaczmarek E, Erb L, Koziak K, Jarzyna R, Wink MR, Guckelberger O, et al. Modulation of endothelial cell migration by extracellular nucleotides. Involvement of focal adhesion kinase and phosphatidylinositol 3-kinase - mediated pathways. Thromb Haemost. 2005;93(4):735–742. doi: 10.1267/THRO05040735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagchi S, Lio Z, Gonzalez FA, Chorna NE, Seye CI, Weisman GA, et al. The P2Y2 nucleotide receptor requires interaction with alpha-v integrins to communicate with G0 and stimulate chemotaxis. J Biol Chem. 2005;280:39058–39066. [Google Scholar]
- 21.Wilden PA, Agazie YM, Kaufman R, Halenda SP. ATP-stimulated smooth muscle proliferation requires independent ERK and PI3K signaling pathways. Am J Physiol. 1998;275:H1209–H1215. doi: 10.1152/ajpheart.1998.275.4.H1209. [DOI] [PubMed] [Google Scholar]
- 22.Erb L, Liu J, Ockerhausen J, Kong Q, Garrad RC, Griffin K, et al. An RGD sequence in the P2Y2 receptor interacts with avb3 integrins and is required for Go-mediated signal transduction. J Cell Biol. 2001;153(3):491–501. doi: 10.1083/jcb.153.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li JM, Fan LM, Shah A, Brooks G. Targeting avb3 and a5b1 for gene delivery to proliferating VSMCs: synergistic effect of TGF-b1. Am J Physiol. 2003:H1123–H1131285. doi: 10.1152/ajpheart.00103.2003. [DOI] [PubMed] [Google Scholar]
- 24.Hutchings H, Ortega N, Plouet J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesioin, migration, and survival through integrin ligation. FASEB J. 2003;17:1520–1522. doi: 10.1096/fj.02-0691fje. [DOI] [PubMed] [Google Scholar]
- 25.Cai WJ, Li MB, Wu X, Wu S, Zhu W, Chen D, et al. Activation of the integrins a5b1 and avb3 and focal adhesion kinase (FAK) during arteriogenesis. Mol Cell Biochem. 2009;322:161–169. doi: 10.1007/s11010-008-9953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton J, Americh L, Gayon R, Julien D, Mansat M, Mansat P, et al. A comparative study of endothelial cell markers expressed in chronically inflamed human tissues: MECA-79, Duffy antigen receptor for chemokines, von Willebrand fractor, CD31, CD34, CD105, and CD146. J Pathol. 2005;206:260–8. doi: 10.1002/path.1788. [DOI] [PubMed] [Google Scholar]
- 27.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, et al. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–26. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 28.Dokun A, Keum S, Hazarika S, Li Y, Lamonte GM, Wheeler F, et al. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation. 2008;117:1207–15. doi: 10.1161/CIRCULATIONAHA.107.736447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergmann CE, Hoefer IE, Meder B, Roth H, van Royen N, Breit SM. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukoc Biol. 2006;80:59–65. doi: 10.1189/jlb.0206087. [DOI] [PubMed] [Google Scholar]
- 30.van Weel V, Toes RE, Seghers L, Deckers MM, de Vries MR, Eilers PH. Natural killer cells and CD4+ T-cells modulate collateral artery development. Arterioscler Thromb Vasc Biol. 2007;27:2310–8. doi: 10.1161/ATVBAHA.107.151407. [DOI] [PubMed] [Google Scholar]
- 31.Bastiaansen AJNM, Ewing MM, de Boer HC, van der Pouw Kraan TC, de Vries MR, Peters EAB, et al. Lysine acetyltransferase PCAF is a key regulator of arteriogenesis. Arterioscler Thromb Vasc Biol. 2013;33:1902–10. doi: 10.1161/ATVBAHA.113.301579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis). Circ Res. 2004;95:449–458. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 33.Seye CI, Yu N, Jain R, Kong Q, Minor T, Newton J, et al. The P2Y2 nucleotide receptor mediates UTP-induced vascular cell adhesion molecule-1 expression in coronary artery endothelial cells. J Biol Chem. 2003;278:24960–24965. doi: 10.1074/jbc.M301439200. [DOI] [PubMed] [Google Scholar]
- 34.Seye CI, Agca Y, Agca C, Derbigny W. P2Y2 receptor-mediated lymphotoxin-alpha (LTA) secretioin regulates intercellular cell adhesion molecule (ICAM)-1 expression in vascular smooth muscle cells. J Biol Chem. 2012;287:10535–43. doi: 10.1074/jbc.M111.313189. [DOI] [PMC free article] [PubMed] [Google Scholar]


