Abstract
Background
Histologically identified intraprostatic incision (IPI) into malignant glands is associated with an increase in biochemical recurrence following radical prostatectomy (RP). However, the predictor of IPI is poorly evaluated.
Objective
To evaluate the risk factors for IPI into cancer during RP for clinically localized prostate cancer (PCa).
Design, setting, and participants
Between January 1993 and July 2013, 19 986 men with clinically localized PCa underwent RP at our institution. This study includes 14 434 cases that had complete clinicopathologic data. IPI was defined as an iatrogenic incision into the prostate resulting in the presence of malignant glands at the inked surgical margin, regardless of accompanying pathologic features.
Intervention
Open, retropubic, robot-assisted laparoscopic and pure laparoscopic RP.
Outcome measurements and statistical analysis
Univariate and multivariable logistic regression analyses were conducted for risk factors of IPI in RP specimens.
Results and limitations
The overall incidence of IPI into malignant tissue was noted in 410 (2.8%) cases. In multivariable analysis, obesity, lower prostate weight, surgeon experience, and pure laparoscopic RP were associated with a higher risk of IPI. The odds ratios (OR) for body mass index and prostate weight were 1.05 (95% confidence interval [CI], 1.03–1.08; p < 0.001) and 0.99 (95% CI, 0.98–0.99, p < 0.001), respectively. The ORs for surgeon experience (>250 cases) and pure laparoscopic RP compared to open RP were 0.71 (95% CI, 0.55–0.90, p = 0.005) and 2.05 (95% CI, 1.35–3.11; p = 0.001), respectively.
Conclusions
The risk of IPI during RP is higher in men with obesity and lower prostate weight. In addition, a pure laparoscopic RP and the early series of each surgeon were associated with a higher risk of IPI. However, tumor characteristics were not associated with the IPI occurrence.
Patient summary
Intraprostatic incision occurrence is associated with obesity, small prostate, and surgeon experience and laparoscopic technique but not Gleason score and tumor stage.
Keywords: Pathology, Prostate cancer, Prostatectomy
1. Introduction
Radical prostatectomy (RP) is a commonly performed procedure for treating clinically localized prostate cancer (PCa). Attempts have been made to evaluate the quality of RP through assessment of surgical margin status [1]. Positive surgical margin (PSM) is associated with decreased biochemical recurrence-free survival, as well as PCa specific survival [2]. However, PSM is significantly influenced by tumor characteristics such as Gleason score and pathologic stage. Therefore, it may not be the best quality tool for assessing the surgical technique.
PSM may occur as a consequence of intraprostatic incision (IPI), also known as capsular incision, when a surgeon inadvertently transects into an intraprostatic tumor [3–6]. Because histologic boundaries of the prostate are vague and benign prostate glands are seen admixed with skeletal muscle in the apex [5], a recent update recommended using IPI, not capsular incision, to describe this condition [4]. IPI has a significant negative impact on patient outcome following RP [6–9]. A high probability of IPI in obese patients could predict difficulty in achieving the optimal surgical approach and outcome, and it could also negatively impact disease-free survival of these men [10,11]. If the IPI rate is similar across the pathologic stage, IPI may be potentially used as a marker of violation of the surgical plane independent of tumor characteristics, and a tool to assess surgical quality. In addition, it is unclear what perioperative factors influence IPI.
In this study, we examined the prevalence of IPI according to pathologic stage using a large cohort of patients who underwent RP in a single center with standardized pathologic examination of surgical specimens. Then we investigated the independent preoperative predictors of an IPI.
2. Patients and methods
Between January 1993 and July 2013, 19 986 men with clinically localized PCa underwent RP at our institution. This study included 14 434 men who had complete clinicopathologic data and those who received no neoadjuvant hormonal therapy. Cases with IPI into tumor were identified from RP final pathology reports. Our previous study on the impact of IPI on survival included only men with organ-confined disease, excluding those with extraprostatic extension (EPE), seminal vesicle invasion, and/or lymph node metastasis [8]. However, in this current study, all men were included regardless of accompanying pathologic features.
RP specimens were sectioned as previously described [5]. IPI was defined as an iatrogenic incision into the prostate resulting in the presence of malignant glands at the inked surgical margin. Cases with tumor extending to the inked margins in the same plane where benign prostatic glands also extended to those margins were considered to have a PSM due to IPI. At the apex, if the tumor was unassociated with benign prostatic glands at the inked edge, the tumor was classified as having a positive margin in an area where it was unclear if there was a PSM associated with EPE or IPI due to ambiguities of where the edge of the prostate was in this region; these cases were not considered in the current study as having IPI. Equivocal cases of whether or not IPI was present were reviewed and reclassified [5]. Prostate weight was determined by measuring gross RP specimen weight, including the seminal vesicles and vasal tips before October 2010, and excluding those after this date.
Differences in age, preoperative prostate-specific antigen (PSA), body mass index (BMI), prostate weight, surgery year, race, clinical stage, biopsy Gleason sum, operation type (open radical retropubic prostatectomy, pure laparoscopic RP, or robot-assisted laparoscopic RP [RARP]), and surgeon experience according to presence of IPI were compared using the student t test for continuous variables and the chi-square test for categorical variables. Age at RP, preoperative PSA level, BMI, prostate weight, and surgery year were examined as continuous variables. Race (Caucasian, African American, and others), clinical stage (T1, T2, and T3), biopsy Gleason sum (≤6, 7, ≥8), operation type, and surgeon experience were examined as categorical variables. To consider surgeon experience, an expert was defined as a surgeon who performed >250 cases in each operation type [12–14]. Univariate and multivariable logistic regression analyses were conducted to assess the prognostic significance of preoperative variables. All tests were two-sided, with p < 0.05 considered statistically significant. STATA 11.0 (Stata Corp., College Station, TX, USA) was used for the statistical analyses.
3. Results
Overall, IPI into malignant glands was diagnosed in 410 of the 14 434 RP specimens (2.8%). IPI was found in 289 (2.9%) for pT2, 97 (2.8%) for pT3a, 15 (3.0%) for pT3b, and 9 (3.1%) for pN+ ( p = 0.975). Figure 1 shows that the probability of IPI was not associated with pathologic stages. However, the probability of PSM increased with advancing pathologic stage ( p < 0.001). The probabilities of PSM in pT2, pT3a, pT3b, and pN+ were 4.2%, 30.6%, 30.1%, and 36.9%, respectively. Unlike pathologic stages, surgeon experience was also associated with PSM and IPI.
Fig. 1.
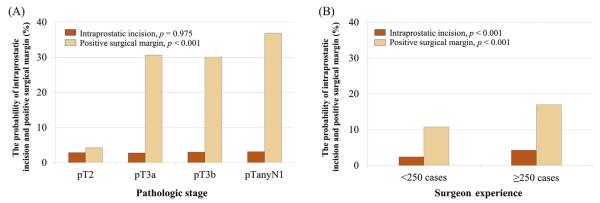
The probabilities of intraprostatic incision and positive surgical margin according to (A) pathologic stage and (B) surgeon experience. P values were calculated by chi-square test.
In univariate analysis, men with IPI had a lower prostate weight ( p < 0.001) and a higher BMI ( p < 0.001) in more recent series (surgery year, p = 0.036). The incidence of IPI was higher in RARP and pure laparoscopic RP than open RP ( p < 0.001). More IPIs were produced in each surgeon’s earlier series (<250 cases; p < 0.001) (Table 1).
Table 1.
Preoperative clinical and pathologic characteristics (N = 14 434)
| Intraprostatic incision |
p value | ||
|---|---|---|---|
| No | Yes | ||
| Patients, no. (%) | 14 024 (97.2) | 410 (2.8) | |
| Age, yr, median (IQR) | 58 (53–63) | 58 (53–62) | 0.133 |
| Race, no. (%) | 0.713 | ||
| Caucasian | 12 239 (87.3) | 360 (87.8) | |
| African American | 1192 (8.5) | 36 (8.8) | |
| Others | 593 (4.2) | 14 (3.4) | |
| PSA level, ng/ml, median (IQR) | 5.5 (4.1–7.7) | 5.5 (4.4–7.9) | 0.647 |
| Clinical stage, no. (%) | 0.618 | ||
| T1 | 10 081 (71.9) | 303 (73.9) | |
| T2 | 3888 (27.7) | 105 (25.6) | |
| T3 | 55 (0.4) | 2 (0.5) | |
| Biopsy Gleason sum, no. (%) | 0.608 | ||
| 4–6 | 10 084 (71.9) | 301 (73.4) | |
| 7 | 3368 (24.0) | 96 (23.4) | |
| 8–10 | 572 (4.1) | 13 (3.2) | |
| BMI, kg/m2, median (IQR) | 26.6 (24.8–29.0) | 27.3 (25.1–30.1) | <0.001 |
| Prostate weight, g, median (IQR) | 50 (41–62) | 47 (39–56) | <0.001 |
| Surgery year, median (IQR) | 2002 (1999–2008) | 2003 (2000–2007) | 0.036 |
| Cases by experts, no. (%) | 10 799 (77.0) | 266 (64.9) | <0.001 |
| Operation type, no. (%) | <0.001 | ||
| Open | 11 910 (84.9) | 303 (73.9) | |
| Robot-assisted laparoscopic | 1628 (11.6) | 75 (18.3) | |
| Laparoscopic | 486 (3.5) | 32 (7.8) | |
BMI = body mass index; IQR = interquartile range; PSA = prostate-specific antigen.
In multivariable analysis, obesity, lower prostate weight, pure laparoscopic RP, and surgeon experience were independently associated with a higher risk of IPI into tumor. The odds ratios (OR) of IPI for BMI and prostate weight was 1.05 (95% confidence interval [CI], 1.03–1.08; p < 0.001) and 0.99 (95% CI, 0.98–0.99; p < 0.001), respectively. The ORs of IPI for RARP and pure laparoscopic RP compared to open RP was 1.40 (95% CI, 1.00–1.97; p = 0.052) and 2.05 (95% CI, 1.35–3.11; p = 0.001), respectively. The risk of IPI for a surgeon with 250 prior cases decreased 29% (OR: 0.71; 95% CI, 0.55–0.90; p = 0.005) (Table 2).
Table 2.
Univariate and multivariable analyses for risk of intraprostatic incision according to preoperative variables in men undergoing radical prostatectomy between 1993 and 2013 (N = 14 434)
| Univariate |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | p value | OR | (95% CI) | p value | |
| Race (Caucasian) | 1.00 | |||||
| African American | 1.03 | (0.73–1.45) | 0.882 | – | – | – |
| Others | 0.80 | (0.47–1.38) | 0.425 | – | – | – |
| Age, yr/10 | 0.89 | (0.77–1.05) | 0.133 | – | – | – |
| PSA level, ng/ml | 1.01 | (0.99–1.02) | 0.647 | – | – | – |
| BMI, kg/m2 | 1.05 | (1.03–1.08) | <0.001 | 1.05 | (1.03–1.08) | <0.001 |
| Prostate weight, g/10 | 0.87 | (0.81–0.92) | <0.001 | 0.88 | (0.82–0.93) | <0.001 |
| Surgery year | 1.02 | (1.00–1.04) | 0.036 | 0.99 | (0.97–1.02) | 0.575 |
| Clinical stage (T1) | 1.00 | |||||
| T2 | 0.90 | (0.72–1.13) | 0.351 | – | – | – |
| T3 | 1.21 | (0.29–4.98) | 0.792 | – | – | – |
| Biopsy Gleason sum (≤6) | 1.00 | |||||
| 7 | 0.96 | (0.76–1.21) | 0.698 | – | – | – |
| 8–10 | 0.76 | (0.43–1.34) | 0.341 | – | – | – |
| Experts (No: cases ≤250, no.) | 1.00 | 1.00 | ||||
| (Yes: Cases >250, no.) | 0.55 | (0.45–0.68) | <0.001 | 0.71 | (0.55–0.90) | 0.005 |
| Operation type (Open) | 1.00 | 1.00 | ||||
| Robot-assisted laparoscopic | 1.81 | (1.40–2.34) | <0.001 | 1.40 | (1.00–1.97) | 0.052 |
| Laparoscopic | 2.59 | (1.78–3.77) | <0.001 | 2.05 | (1.35–3.11) | 0.001 |
BMI = body mass index; CI = confidence interval; OR = odds ratio; PSA = prostate-specific antigen.
4. Discussion
IPI in the RP specimen is associated with an increased risk of progression following surgery [5–7,9]. Most studies that evaluated the prognostic significance of IPI reported the IPI rate in men with organ-confined (OC) disease only [6–9,15]. The current study was performed to evaluate the IPI rate across different pathologic stages and to identify predictors of IPI. Of 14 434 RP cases, 410 (2.8%) had an IPI. More importantly, the IPI rate across different pathologic stages was similar, suggesting that IPI may be a better indicator of surgical skills than PSM. The independent predictors of IPI included obesity, smaller prostate size, surgeon experience, and pure laparoscopic technique.
There is a significant variation in the reported IPI rate in men with OC disease. In the current study population of 10 105 men with OC disease, 289 (2.9%) were diagnosed with IPI. In our previous reports, isolated IPI rates in OC disease ranged from 1.8% to 2.3% [8,16]. However, others reported the IPI rates in OC disease as high as 20% [9,15]. This variation in IPI rate can be partly attributed to pathologic interpretation. For example, it is challenging to distinguish IPI from a PSM associated with EPE or equivocal PSM in an area where it is difficult to distinguish OC disease with tumor close to resection margins [5].
IPI occurs most commonly on the posterolateral section of the RP specimen [7–9]. One of the potential causes for IPI at this site may be the neurovascular bundle–sparing technique. There is also the potential risk of overcalling IPI if pathologists are less experienced in evaluating RPs. PCa extending out of the prostate may induce a desmoplastic reaction such that extraprostatic tumor is not seen in periprostatic adipose tissue. If pathologists do not recognize these foci as EPE, because they incorrectly require seeing tumor in adipose tissue to diagnose EPE, then a PSM associated with IPI will be diagnosed, as opposed to the correct diagnosis of a PSM with EPE (Fig. 2) [3,17,18]. Increased education of pathologists to recognize EPE in the absence of adipose tissue involvement may improve the accurate diagnosis of IPI.
Fig. 2.
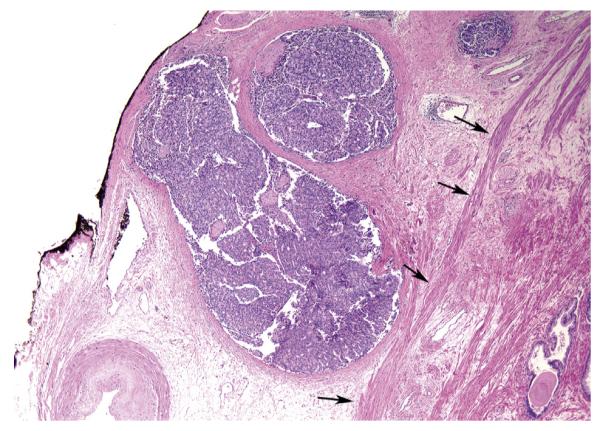
High-grade adenocarcinoma extending out of the prostate associated with a desmoplastic reaction, such that tumor is not seen with periprostatic adipose tissue (Hematoxylin & Eosin, reduced from X4). The edge of the prostate where the condensed smooth muscle of the prostate ends is noted by the arrows. If this tumor was at the margin, it would be extraprostatic extension with a positive surgical margin. If not recognized as extraprostatic extension because of the lack of fat invasion, it would incorrectly be designated as organ-confined disease with intraprostatic incision.
There are few studies on the predictors of IPI in the RP specimen. In an open retropubic RP series, Freedland et al concluded that obesity is a risk factor for IPI [10]. They also showed that men with an IPI were younger, had lower Gleason sum, and a smaller prostate [10]. In the current study, we found obesity and smaller prostate weight were independent predictors of IPI in multivariable analysis. In addition, we found that laparoscopic technique was associated with an increased IPI rate. There is lack of tactile or visual feedback in pure laparoscopic surgery. RARP was also associated with a higher risk of IPI in earlier cases. In supplementary analysis, IPI rate of RARP has decreased to that of open RP. Five years of data show no significant differences of IPI rates between RARP and open RP (2.4% vs 1.7%, respectively; p = 0.130).
Surgeon experience is an important predictor of PSM in various types of surgery [12,14,19]. To define the acquisition of experience in each RP type, many studies tried to show cutoff numbers of surgery already performed. Vickers et al reported that the probability of PSM in open RP decreased to 25% for a surgeon with 250 prior cases [12]. Thompson et al showed that the risk of PSM for advanced stage in RARP gradually decreased, and reached a plateau at 200 to 300 cases [14]. In the current study, the probability of IPI (OR: 0.71; p = 0.005) decreased for experienced surgeons with 250 prior cases of each operation type.
Most studies on risk factors for PSM demonstrated that preoperative tumor characteristics, such as preoperative PSA level, biopsy Gleason score, clinical stage, and multiple positive biopsies, are strongly associated with the PSM [1,20,21]. Similar to the current study, obesity and smaller prostate weight were also associated with PSM [22–24]. Furthermore, surgery-related factors, such as operation type and surgeon experience, led to a change in PSM [25–29]. The risk factors for PSM are similar to those of IPI with the exception of tumor pathologic characteristics. Therefore, we suggest using IPI, rather than PSM, to assess the quality of surgery.
There are several limitations to our study. In our study, significantly more open RPs were performed compared to minimally invasive surgery. The majority of these open RP cases were performed by more experienced surgeons, although this relative experience gap has decreased in recent years. We did not consider the number of positive cores and their location in our model because complete data on these variables were not available. Tumor volume might be correlated with IPI occurrence, although IPI was not associated with tumor grade and stage in the current study. In addition, the location and extent of IPI were not considered in this study. We also did not consider neurovascular-bundle preservation technique, although higher PSM rate was observed in cases with that technique [14,30]. Additional study including neurovascular-bundle preservation information may add more insight. Finally, to adjust for surgeon experience, we used a binary variable: whether or not a surgeon had already performed >250 cases in each surgery type. However, surgeons might need different numbers of surgery to overcome the learning curve in each surgery type.
5. Conclusions
The incidence of IPI is low (2.8%). Unlike PSMs, the rate of IPI into malignant glands is similar across different pathologic stages. Independent predictors of IPI in the RP specimen were obesity, lower prostate weight, pure laparoscopic RP technique, and surgeon experience. We suggest using IPI, rather than PSM, to assess the quality of surgery.
Acknowledgments
Funding/Support and role of the sponsor: This study was supported by a SPORE grant P50CA58236 from the National Institutes of Health, which was involved in data collection, management, analysis, and interpretation.
Footnotes
Author contributions: Sung-Woo Park had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Park, Han.
Acquisition of data: Humphreys.
Analysis and interpretation of data: Park, Han.
Drafting of the manuscript: Park, Readal, Han.
Critical revision of the manuscript for important intellectual content: Epstein, Partin, Han.
Statistical analysis: Park, Han.
Obtaining funding: Partin, Han.
Administrative, technical, or material support: Jeong, Humphreys, Partin.
Supervision: Partin, Han.
Other (specify): None.
Financial disclosures: Sung-Woo Park certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- [1].Wieder JA, Soloway MS. Incidence, etiology, location, prevention and treatment of positive surgical margins after radical prostatectomy for prostate cancer. J Urol. 1998;160:299–315. [PubMed] [Google Scholar]
- [2].Chalfin HJ, Dinizo M, Trock BJ, et al. Impact of surgical margin status on prostate-cancer-specific mortality. BJU Int. 2012;110:1684–9. doi: 10.1111/j.1464-410X.2012.11371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Epstein JI. Incidence and significance of positive margins in radical prostatectomy specimens. Urol Clin North Am. 1996;23:651–63. doi: 10.1016/s0094-0143(05)70343-8. [DOI] [PubMed] [Google Scholar]
- [4].Yossepowitch O, Briganti A, Eastham JA, et al. Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol. 2014;65:303–13. doi: 10.1016/j.eururo.2013.07.039. [DOI] [PubMed] [Google Scholar]
- [5].Chuang AY, Epstein JI. Positive surgical margins in areas of capsular incision in otherwise organ-confined disease at radical prostatectomy: histologic features and pitfalls. Am J Surg Pathol. 2008;32:1201–6. doi: 10.1097/PAS.0b013e318162a8bf. [DOI] [PubMed] [Google Scholar]
- [6].Preston MA, Carrière M, Raju G, et al. The prognostic significance of capsular incision into tumor during radical prostatectomy. Eur Urol. 2011;59:613–8. doi: 10.1016/j.eururo.2010.12.005. [DOI] [PubMed] [Google Scholar]
- [7].Shuford MD, Cookson MS, Chang SS, et al. Adverse prognostic significance of capsular incision with radical retropubic prostatectomy. J Urol. 2004;172:119–23. doi: 10.1097/01.ju.0000132137.02846.ec. [DOI] [PubMed] [Google Scholar]
- [8].Chuang AY, Nielsen ME, Hernandez DJ, Walsh PC, Epstein JI. The significance of positive surgical margin in areas of capsular incision in otherwise organ confined disease at radical prostatectomy. J Urol. 2007;178:1306–10. doi: 10.1016/j.juro.2007.05.159. [DOI] [PubMed] [Google Scholar]
- [9].Kwak KW, Lee HM, Choi HY. Impact of capsular incision on biochemical recurrence after radical perineal prostatectomy. Prostate Cancer Prostatic Dis. 2010;13:28–33. doi: 10.1038/pcan.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Freedland SJ, Grubb KA, Yiu SK, et al. Obesity and capsular incision at the time of open retropubic radical prostatectomy. J Urol. 2005;174:1798–801. doi: 10.1097/01.ju.0000177077.53037.72. discussion 1801. [DOI] [PubMed] [Google Scholar]
- [11].Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–53. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- [12].Vickers A, Bianco F, Cronin A, et al. The learning curve for surgical margins after open radical prostatectomy: implications for margin status as an oncological end point. J Urol. 2010;183:1360–5. doi: 10.1016/j.juro.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Doumerc N, Yuen C, Savdie R, et al. Should experienced open prostatic surgeons convert to robotic surgery? The real learning curve for one surgeon over 3 years. BJU Int. 2010;106:378–84. doi: 10.1111/j.1464-410X.2009.09158.x. [DOI] [PubMed] [Google Scholar]
- [14].Thompson JE, Egger S, Böhm M, et al. Superior quality of life and improved surgical margins are achievable with robotic radical prostatectomy after a long learning curve: a prospective single-surgeon study of 1552 consecutive cases. Eur Urol. 2014;65:521–31. doi: 10.1016/j.eururo.2013.10.030. [DOI] [PubMed] [Google Scholar]
- [15].Kumano M, Miyake H, Muramaki M, Kurahashi T, Takenaka A, Fujisawa M. Adverse prognostic impact of capsular incision at radical prostatectomy for Japanese men with clinically localized prostate cancer. Int Urol Nephrol. 2009;41:581–6. doi: 10.1007/s11255-008-9467-z. [DOI] [PubMed] [Google Scholar]
- [16].Barocas DA, Han M, Epstein JI, et al. Does capsular incision at radical retropubic prostatectomy affect disease-free survival in otherwise organ-confined prostate cancer? Urology. 2001;58:746–51. doi: 10.1016/s0090-4295(01)01336-x. [DOI] [PubMed] [Google Scholar]
- [17].Epstein JI. Radical prostatectomy: processing, staging, and prognosis. Parts I and II. Int J Surg Pathol. 2010;18:118S–23S. doi: 10.1177/1066896910370473. [DOI] [PubMed] [Google Scholar]
- [18].Epstein JI, Amin M, Boccon-Gibod L, et al. Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scand J Urol Nephrol Suppl. 2005:34–63. doi: 10.1080/03008880510030932. [DOI] [PubMed] [Google Scholar]
- [19].Magheli A, Gonzalgo ML, Su LM, et al. Impact of surgical technique (open vs laparoscopic vs robotic-assisted) on pathological and biochemical outcomes following radical prostatectomy: an analysis using propensity score matching. BJU Int. 2011;107:1956–62. doi: 10.1111/j.1464-410X.2010.09795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cheng L, Slezak J, Bergstralh EJ, Myers RP, Zincke H, Bostwick DG. Preoperative prediction of surgical margin status in patients with prostate cancer treated by radical prostatectomy. J Clin Oncol. 2000;18:2862–8. doi: 10.1200/JCO.2000.18.15.2862. [DOI] [PubMed] [Google Scholar]
- [21].Coelho RF, Chauhan S, Orvieto MA, Palmer KJ, Rocco B, Patel VR. Predictive factors for positive surgical margins and their locations after robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2010;57:1022–9. doi: 10.1016/j.eururo.2010.01.040. [DOI] [PubMed] [Google Scholar]
- [22].Patel VR, Coelho RF, Rocco B, et al. Positive surgical margins after robotic assisted radical prostatectomy: a multi-institutional study. J Urol. 2011;186:511–6. doi: 10.1016/j.juro.2011.03.112. [DOI] [PubMed] [Google Scholar]
- [23].Marchetti PE, Shikanov S, Razmaria AA, Zagaja GP, Shalhav AL. Impact of prostate weight on probability of positive surgical margins in patients with low-risk prostate cancer after robotic-assisted laparoscopic radical prostatectomy. Urology. 2011;77:677–81. doi: 10.1016/j.urology.2010.07.512. [DOI] [PubMed] [Google Scholar]
- [24].Lee SE, Lee WK, Jeong MS, et al. Is body mass index associated with pathological outcomes after radical prostatectomy in Korean men? BJU Int. 2011;107:1250–5. doi: 10.1111/j.1464-410X.2010.09592.x. [DOI] [PubMed] [Google Scholar]
- [25].Atug F, Castle EP, Srivastav SK, Burgess SV, Thomas R, Davis R. Positive surgical margins in robotic-assisted radical prostatectomy: impact of learning curve on oncologic outcomes. Eur Urol. 2006;49:866–71. doi: 10.1016/j.eururo.2006.02.054. discussion 871–2. [DOI] [PubMed] [Google Scholar]
- [26].Eastham JA, Kattan MW, Riedel E, et al. Variations among individual surgeons in the rate of positive surgical margins in radical prostatectomy specimens. J Urol. 2003;170:2292–5. doi: 10.1097/01.ju.0000091100.83725.51. [DOI] [PubMed] [Google Scholar]
- [27].Smith JA, Jr, Chan RC, Chang SS, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical pros-tatectomy. J Urol. 2007;178:2385–9. doi: 10.1016/j.juro.2007.08.008. discussion 2389–90. [DOI] [PubMed] [Google Scholar]
- [28].Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–63. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- [29].Sooriakumaran P, Srivastava A, Shariat SF, et al. A multinational, multi-institutional study comparing positive surgical margin rates among 22 393 open, laparoscopic, and robot-assisted radical pros-tatectomy patients. Eur Urol. 2014;66:450–6. doi: 10.1016/j.eururo.2013.11.018. [DOI] [PubMed] [Google Scholar]
- [30].Williams SB, Chen MH, D’Amico AV, et al. Radical retropubic prostatectomy and robotic-assisted laparoscopic prostatectomy: likelihood of positive surgical margin(s) Urology. 2010;76:1097–101. doi: 10.1016/j.urology.2009.11.079. [DOI] [PubMed] [Google Scholar]


