Abstract
Through incentive learning the emotional experience of a reward in a relevant need state (e.g., hunger for food) sets the incentive value that guides the performance actions that earn that reward when the need state is encountered again. Opiate withdrawal has been proposed as a need state in which, through experience, opiate value can be increased resulting in escalated opiate self-administration. Endogenous opioid transmission plays anatomically dissociable roles in the positive emotional experience of reward consumption and incentive learning. We, therefore, sought to determine if chronic opiate exposure and withdrawal produces a disruption in the fundamental incentive learning process such that reward seeking, even for non-opiate rewards, can become maladaptive, inconsistent with the emotional experience of reward consumption and irrespective of need. Rats trained to earn sucrose or water on a reward-seeking chain were treated with morphine (10-30 mg/k.g., s.c.) daily for 11 d prior to testing in withdrawal. Opiate withdrawn rats showed elevated reward-seeking actions, but only after they experienced the reward in withdrawal, an effect that was strongest in early (1-3 d), as opposed to late (14-16 d) withdrawal. This was sufficient to overcome a negative reward value change induced by sucrose experience in satiety and, in certain circumstances, was inconsistent with the emotional experience of reward consumption. Lastly, we found that early opiate withdrawal-induced inflation of reward value was blocked by inactivation of basolateral amygdala mu opioid receptors. These data suggest that in early opiate withdrawal the incentive learning process is disrupted resulting in maladaptive reward seeking.
Keywords: Opiate withdrawal, incentive learning, instrumental conditioning, reward, chronic morphine
INTRODUCTION
Prescription opiates are highly efficacious for pain management, but carry a high abuse liability (Fields, 2011; Savage, 2003) and their use may lead to a higher propensity for non-narcotic substance abuse (Michna et al., 2004). Chronic opiate exposure is also associated with a preference for sugary foods and consequent weight gain (Mysels and Sullivan, 2010; Nolan and Scagnelli, 2007). Opiate abuse itself is characterized by compulsive drug seeking despite a decline in the experienced ‘high’ and often in the face of severe negative consequences. Here we evaluate a potential common psychological process that may be disrupted by chronic opiate exposure to result in these aberrant behaviors.
Opiate withdrawal is characterized by severe physical aversive symptoms and anhedonia (Hand et al., 1988; Koob et al., 1992; Stinus et al., 2000) and has been proposed to contribute to the compulsive nature of opiate seeking via a negative reinforcement process (Koob, 1996; Koob and Le Moal, 2005; Koob et al., 1989). Disruption of positive incentive processes may, however, also play a role. Experiencing a reward (either earned or non-contingently) in a relevant motivational/need state sets - through the process referred to as instrumental incentive learning - the incentive value that is recalled when making a decision to engage in an instrumental action to earn that specific reward when in that same state in the future (Balleine, 1992; Dickinson and Balleine, 1994). It is well-established that opiate self-administration in rodents can be increased during opiate withdrawal (Goldberg et al., 1969; Kenny et al., 2006; Wikler and Pescor, 1967). Interestingly, experience with the opiate in this withdrawal state can enhance opiate-seeking behavior, an effect that occurs independent of an opportunity to learn that the drug-seeking action will lead to alleviation of withdrawal (i.e., negative reinforcement) (Hutcheson et al., 2001). This suggests that the opiate is more valued in the withdrawn state, thereby acting as a greater incentive for drug seeking. Because a reward’s incentive value is normally tightly-linked to the recent emotional experience derived from its consumption (Balleine, 2001; Cabanac, 1992; Damasio, 1996), it is possible that the elevated opiate seeking observed during opiate withdrawal is a secondary consequence of a boost in the emotional experience produced by opiate consumption. However, the observation that opiate addicts desire the drug more than would be expected from self-report of the pleasure derived from its consumption (Lamb et al., 1991) suggests another potential interpretation. That is, that chronic opiate administration and withdrawal disrupts the fundamental incentive learning process whereby the reward experience is translated into the incentive value used to guide future behavior, such that the latter is inflated out of proportion to the former. This account predicts that incentive learning for natural rewards, such as food and water, would also be impacted by opiate treatment and withdrawal.
In support of this account, recent work in rodents has shown that the emotional experience of sucrose consumption, and the updating of the incentive value of that reward (information encoded in the reward representation used to guide reward-seeking actions) were found to rely on anatomically dissociable opioid-dependent neural processes (Wassum et al., 2009). While the former involved endogenous opioid transmission in the nucleus accumbens shell and ventral pallidum, the latter required mu opioid receptor activation in the basolateral amygdala (BLA) (Wassum et al., 2011a; Wassum et al., 2009). Chronic opiate administration dysregulates endogenous opioid signaling throughout the brain, including in the BLA (Brady et al., 1989). It is possible, therefore, that plasticity in endogenous opioid transmission induced by chronic opiate administration and withdrawal increases the gain on the BLA mu opioid receptor-dependent incentive learning process through which the emotional experience of reward is translated into incentive value, such that rewards generally, not just opiates, experienced in the opiate-withdrawn state become over-valued, out of line with the emotional experience induced by their consumption. In support of this idea, food-motivated behavior is reportedly enhanced following chronic opiate exposure under some circumstances (Babbini et al., 1976; Cooper et al., 2010; Ford and Balster, 1976; Ranaldi et al., 2009).
We, therefore, assessed the impact of chronic opiate exposure and withdrawal on food- and water-seeking actions and palatability in a rodent incentive learning procedure. Using such an approach, we sought to determine: 1) Can the opiate-withdrawn state support a shift in the incentive value of non-opiate rewards, such as sucrose or water? 2) If there is such a generalized effect of opiate withdrawal on reward seeking, is it consistent or discordant with the emotional experience observed during reward consumption? 3) If opiate withdrawal inflates reward value, does this effect have the potential to overshadow a negative reward experience, thereby producing ‘compulsive’ behavior? 4) Does this withdrawal-induced reward inflation depend on BLA opioid transmission?
MATERIALS AND METHODS
General Approach
The goal of these experiments was to evaluate if and how withdrawal from chronic opiate exposure alters the incentive learning process to disrupt subsequent value-driven reward-seeking actions. We assessed the effects of opiate withdrawal on value-driven reward seeking, palatability and goal approach for a sucrose (Experiment 1, 2 and 4) or water (Experiment 3) reward.
Each experiment followed the same general structure. Rats were trained on a 2-lever sequence of actions to earn a sucrose solution (Experiment 1, 2 and 4) or water (Experiment 3) reward wherein lever pressing on a “seeking” lever to the left of the magazine introduces a second “delivery” lever to the right of the magazine, pressing of which results in reward delivery to the magazine. The initial lever-press action in the sequence has been demonstrated to be selectively sensitive to changes in the learned incentive value of the earned reward and relatively immune to the general activational effects of motivational state (e.g., hunger or thirst) and of reward-paired cues (Balleine et al., 2005; Balleine et al., 1995; Corbit and Balleine, 2003; Wassum et al., 2011a; Wassum et al., 2009). Following training to stable response rates (see Supplemental Methods), rats were split into two groups: one given once-daily administration of morphine for 11 d (10-30 mg/kg s.c.) on an administration protocol known to induce opiate dependence (Harvey-Lewis et al., 2012) and one given saline vehicle control injections. Following drug treatment rats were tested in early (1-3 d) or late (14-16 d; Experiment 1) or only in early (Experiments 2-4) withdrawal for their reward-seeking performance on the chain of actions, first under non-rewarded conditions, to assess any general effect of chronic opiate exposure and the withdrawal state on reward seeking. Rats were then given an incentive learning opportunity wherein they were non-contingently re-exposed to the food or water reward in the opiate-withdrawn state. The effects of this incentive learning opportunity were then tested in a second, post-re-exposure test of reward seeking conducted in the absence of reward. Importantly, to prevent negative reinforcement from contributing to the results, rats were never given an opportunity to learn that their reward-seeking lever-press actions would lead to any potential alleviation of the negative withdrawal symptoms that might be conferred by sucrose or water experience in this state.
During the non-contingent sucrose/water re-exposure we evaluated the palatability responses elicited during consumption using a contact lickometer (see below for full description), a measure previously reported to provide a similar assessment of palatability to the commonly used taste reactivity measures that are often termed reward ‘liking’ (Berridge, 1991; Davis and Perez, 1993; Davis and Smith, 1988, 1992). This allowed us to assess whether chronic opiate exposure and withdrawal induced reward seeking that was discordant with the emotional impact of the reward. During this session we also evaluated the effects of opiate withdrawal on goal approach behavior signaled by the contextual or sucrose pump cues, measured as magazine entries with a photobeam detector. This procedure, therefore, allowed for within-subjects measures of the effects of opiate withdrawal on reward palatability, goal approach behavior and value-driven reward-seeking actions.
Subjects
Male, Long Evans rats (280-300g at the outset of the study; Experiment 1: Early Withdrawal Group n=15- 8 vehicle-treated, 7 morphine-treated, Late Withdrawal Group n=15- 8 vehicle-treated, 7 morphine-treated; Experiment 2: n=24- 12 vehicle-treated, 12 morphine-treated; Experiment 3: n=15- 8 vehicle-treated, 7 morphine-treated; Experiment 4: n=36- 11 in group vehicle-treated, intra-BLA vehicle, 8 vehicle-treated, intra-BLA CTOP, 8 morphine-treated, intra-BLA vehicle, 9 morphine-treated, intra-BLA CTOP; Charles River Laboratories, Wilmington, MA) were group housed and handled daily prior to training for 5-7 d. Rats were maintained on a food- or water-restriction schedule whereby they were either deprived of food for 4 h (Experiment 1 and 4) or 22 h (Experiment 2), or water-deprived for 18 h (Experiment 3) prior to each day’s training or testing session (see each experiment description below). Food or water was returned 2-4 h after each daily training session. Unless it was restricted as stated rats were provided free access to food or filtered tap water in the home cage. All procedures were conducted in accordance with the National Research Council’s Guide for the Care and use of Laboratory Animals and were approved by the UCLA Institutional Animal Care and Use Committee. Training and testing took place during the dark phase of a 12:12 h reverse dark:light cycle in 16 Med Associates (St. Albans, VT) operant chambers described in the Supplemental Methods.
Chronic morphine treatment
Following training as described previously (Wassum et al., 2011a; Wassum et al., 2011b; Wassum et al., 2009) and in the Supplemental Methods rats underwent chronic drug treatment. The food/water deprivation schedule of training was maintained during drug treatment. Drug injections were conducted in a room different from that of all behavioral training and testing to avoid any effects of a morphine-paired context on test performance. Morphine (generously provided by NIDA) was dissolved in sterile saline vehicle and injected at a volume of 1 ml/kg. Half of the rats in each group were administered morphine once daily on an escalating dose regime: 2 d of 10 mg/kg, 2 d of 20 mg/kg, 1 d of 25 mg/kg, 6 d of 30 mg/kg, s.c.. The remaining rats were administered sterile saline vehicle (1 ml/kg s.c.). This dosing regime has been previously shown to result in physical dependence marked by tolerance to morphine analgesia and enhancement of naloxone precipitated withdrawal, as well as altered reward seeking in a delay-discounting task (Harvey-Lewis et al., 2012). Moreover, we show evidence of withdrawal in these rats marked by a significant loss in body weight (Martin et al., 1963) compared to the last day of drug exposure and to vehicle-treated controls (see Supplemental Figure 1). A passive drug administration procedure was chosen so that solely the pharmacological effects of the drug treatment and withdrawal from it could be assessed without the confound of previous drug-seeking experience. Drug groups were counterbalanced based on seeking lever-press rate on the last day of training. All behavioral training (pre-drug) and testing (opiate withdrawal) was conducted drug free.
Experiment 1
The training and testing procedures for Experiment 1 are outlined in Figure 1. Rats were maintained 4 h food deprived throughout training and testing to avoid ceiling effects on lever pressing and sucrose incentive value. Following training and chronic morphine treatment rats were tested either 1-3 d (Group Early Withdrawal) or 14-16 d (Group Late Withdrawal) after the last drug injection. For both groups testing was identical and began with a test (Test 1a) of responding on the instrumental chain under non-rewarded conditions for 5 min. This non-rewarded test was conducted just as in training, with rats responding on the seeking lever on random ratio (RR) 4 schedule to receive the second delivery lever, which was retracted once pressed, but no reward was delivered allowing evaluation of the immediate (i.e., pre-exposure) impact of opiate withdrawal on reward seeking. The next day rats were given non-contingent re-exposure to the sucrose training outcome (30 exposures/35 min) in the operant box with the levers retracted. Lickometer measures were collected during this phase of the experiment to assess sucrose palatability. These non-contingent sucrose deliveries provide an incentive learning opportunity wherein the value of the sucrose reward may be updated in the new opiate-withdrawn state. The day following re-exposure rats were tested again (Test 1b) for their responding on the chain under non-rewarded conditions for 5 min in order to assess the effects of the previous day’s incentive learning opportunity on reward-seeking actions.
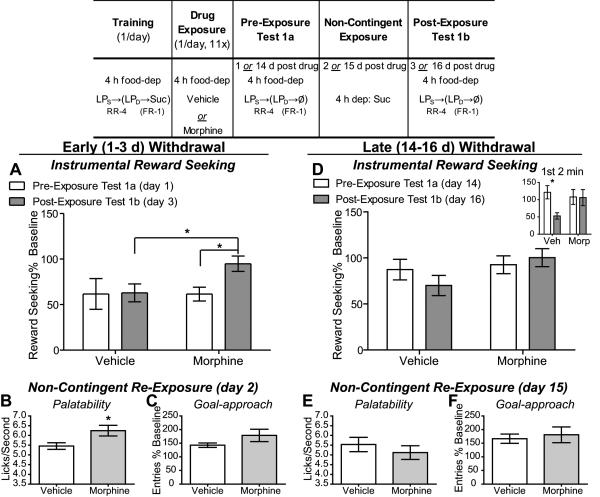
Figure 1. Experiment 1: Value-driven reward-seeking actions are elevated after reward experience/incentive learning opportunity in early opiate withdrawal.
Table: Experiment 1 Design- Rats were trained 4 h food deprived on the seeking-delivery chain of actions to earn sucrose. Following training rats were treated with either vehicle (early withdrawal: n=8; late withdrawal n=8) or morphine (early withdrawal: n=7; late withdrawal n=7) 1/d for 11 d. Testing commenced 24 h after the last drug injection and for Group Early Withdrawal and 14 d after last injection for Group Late Withdrawal. Rats were tested for the effects of opiate withdrawal on reward seeking (in 5 min non-rewarded extinction tests) both prior to and after an incentive learning opportunity (non-contingent exposure to sucrose in opiate withdrawal). A. Effects of early opiate withdrawal on reward-seeking action performance (normalized to pre-drug baseline reward-seeking response rate on the last pre-drug training session) prior to (Test 1a- open bars) and after (Test 1b- shaded bars) an opportunity to experience the sucrose training outcome in the opiate withdrawn state. B. Effects of early opiate withdrawal on sucrose palatability, assessed as lick frequency, during the non-contingent re-exposure to the sucrose in opiate withdrawal. C. Effects of early opiate withdrawal on goal approach (normalized to pre-drug baseline entry rate on the last pre-drug training session) during the non-contingent re-exposure to the sucrose in opiate withdrawal. D. Effects of protracted (i.e., late) opiate withdrawal on reward seeking (normalized to pre-drug baseline response rate) prior to (Test 1a- open bars) and after (Test 1b- shaded bars) an opportunity to experience the sucrose training outcome in the late opiate withdrawn state. The inset shows the effects of late opiate withdrawal (Morph) compared to vehicle-treated control (Veh) on reward seeking prior to and after sucrose re-exposure during the first two min of each test. E. Effects of late opiate withdrawal on sucrose palatability, assessed as lick frequency, during the non-contingent re-exposure to the sucrose in opiate withdrawal. F. Effects of late opiate withdrawal on goal approach (normalized to pre-drug baseline entry rate) during the non-contingent re-exposure to the sucrose in opiate withdrawal. *, p<0.05. RR-4, random-ratio 4; FR-1, fixed-ratio 1; LPS, seeking lever press, LPD, delivery lever press; Suc, 20% sucrose solution; Ø, no reward delivery; dep, deprived; *, p<0.05.
Experiment 2
The training and testing procedures for Experiment 2 are outlined in Figure 2 and were similar to Experiment 1 with the exception that rats were maintained 22 h food deprived throughout training and were tested in early withdrawal 1 h food deprived to assess the effects of a downshift in need state on reward seeking. In this experiment the sucrose re-exposure provided rats the opportunity to experience, for the first time, the sucrose while sated; a negative incentive learning opportunity wherein the rats learn that, when sated, the sucrose is less valuable (Balleine, 1992; Dickinson and Balleine, 1994; Wassum et al., 2011a). The effect of this negative incentive value change on sucrose seeking was evaluated by comparing reward-seeking response rate prior to (Test 1a) and after (Test 1b) the re-exposure. Rats were given both a reward-seeking (Test 2a) and re-exposure test 22 h food deprived to serve as a control. The order of testing was not counterbalanced to allow for the critical comparison between the sated test in this experiment and the effects seen in early withdrawal in Experiment 1.
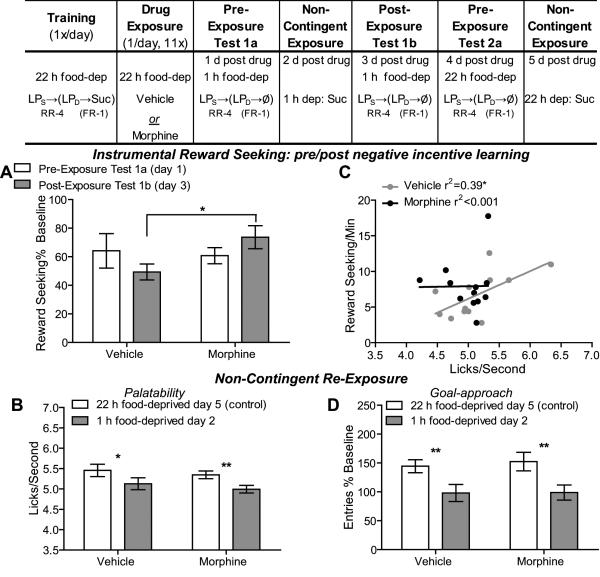
Figure 2. Experiment 2: In early opiate withdrawal a negative change in reward value is blocked resulting in reward seeking inconsistent with reward palatability.
Table: Experiment 2 Design- Rats were trained 22 h food deprived on the chain of actions to earn sucrose. Following chronic morphine (n=12) or vehicle (n=12) treatment all rats were tested in early withdrawal (1-5 d off drug). For the first testing phase all rats were deprived of food for 1 h prior to each day’s test. On the first test day rats were allowed to lever press on the sequence of actions non-rewarded in order to assess the general effects of satiety on reward seeking. On the second test day rats were given non-contingent re-exposure to the sucrose outcome sated (1 h food-deprived) for the first time, creating an opportunity for negative incentive learning in which the rat should learn that, when sated, the sucrose is less valuable (Balleine, 1992; Dickinson and Balleine, 1994; Wassum et al., 2011a). The next day rats were then tested for their reward-seeking performance (in a 5 min non-rewarded extinction test) following this opportunity for negative incentive learning. As a control rats were given both a reward seeking and palatability test in the 22 h food-deprived state. A. Effects of early opiate withdrawal on reward seeking (normalized to pre-drug baseline response rate) prior to (Test 1a- open bars) and after (Test 1b- shaded bars) a negative incentive learning opportunity- experience with sucrose training outcome sated (1 h food-deprived) for the first time. B. Effects of early opiate withdrawal on sucrose palatability, assessed as lick frequency, when in a hungry 22 h food-deprived control state relative to a novel 1 h food-deprived state. C. Effects of early opiate withdrawal on goal approach (normalized to pre-drug baseline entry rate) during the non-contingent re-exposure to the sucrose in opiate withdrawal when in a hungry 22 h food deprived control state relative to a 1 h food-deprived state. D. Correlation between sucrose palatability (lick frequency) during the 1 h food-deprived non-contingent re-exposure and subsequent reward-seeking rate 1 h food deprived the next day- separated for the vehicle-treated and opiate withdrawn (morphine) rats. RR-4, random-ratio 4; FR-1, fixed-ratio 1; LPS, seeking lever press, LPD, delivery lever press; Suc, 20% sucrose solution; Ø, no reward delivery; dep, deprived; *, p<0.05; **, p<0.01.
Experiment 3
The training and testing procedures for Experiment 3, in which we tested the effects of opiate withdrawal on the palatability, goal approach and reward seeking for a water reward, are outlined in Figure 3. Rats were maintained 18 h water-deprived with full access to food throughout training and testing and testing was conducted entirely in early withdrawal. Training and testing were otherwise identical to Experiment 1. In addition to weight, food and water consumption were monitored in this experiment (see Supplemental Figure 2).
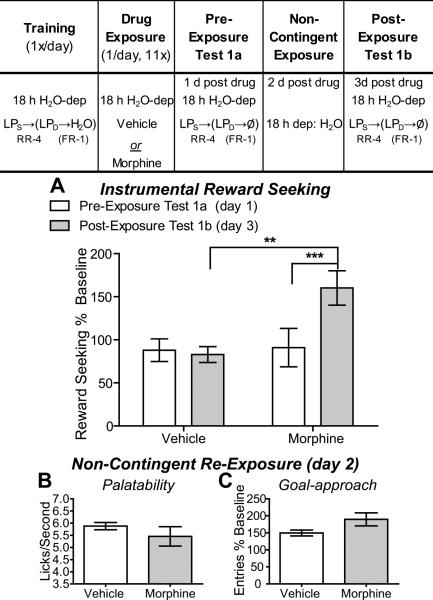
Figure 3. Experiment 3: In early opiate withdrawal the value and subsequent seeking actions for a water reward are inflated.
Table: Experiment 3 Design- Rats were trained 18 h water deprived on a heterogeneous seeking-delivery chain of actions to earn water. Following training rats were treated with either vehicle (n=8) or morphine (n=7) 1/d for 11d. Testing commenced 24h after the last drug injection and, in all cases, was conducted drug free (i.e., in early withdrawal). Rats were tested for the effects of early opiate withdrawal on reward seeking (in 5 min non-rewarded extinction tests) both prior to and after an incentive learning opportunity (non-contingent exposure to the water training outcome in the opiate withdrawn state). A. Effects of early opiate withdrawal on water seeking (normalized to pre-drug baseline reward-seeking response rate) prior to (Test 1a- open bars) and after (Test 1b- shaded bars) an opportunity to experience the water training outcome in the opiate withdrawn state. B. Effects of early opiate withdrawal on water palatability, assessed as lick frequency, during the non-contingent re-exposure to the water in opiate withdrawal. C. Effects of early opiate withdrawal on goal approach (normalized to pre-drug baseline entry rate) during the non-contingent re-exposure to the water in opiate withdrawal. RR-4, random-ratio 4; FR-1, fixed-ratio 1; LPS, seeking lever press, LPD, delivery lever press; H2O, water; Ø, no reward delivery; dep, deprived; **, p<0.01; ***, p<0.001.
Experiment 4
The training and testing procedures for Experiment 4 were largely similar to Experiment 1 and are shown in Figure 4. Following training rats underwent surgery for implantation of guide cannula targeted above the BLA (see below). Rats were single-housed following surgery. After a 5 d recovery period rats were retrained for 2 d prior to the start of morphine treatment as described above. Testing was conducted in early withdrawal and was identical to Experiment 1 with the exception that rats received an infusion of either the selective mu opioid receptor antagonist, CTOP (D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2; 1μg/0.5μl/side- see Supplemental Methods), or sterile water vehicle, into the BLA immediately prior to non-contingent re-exposure to sucrose. There were 4 groups, counterbalanced based on seeking lever-press rate on the last day of instrumental training, as follows: chronic morphine-treated intra-BLA vehicle; chronic morphine-treated intra-BLA CTOP; chronic vehicle-treated intra-BLA vehicle; chronic vehicle-treated intra-BLA CTOP. Sucrose was used as the training outcome, as in Experiments 1 and 3, to allow for absolute control over reward exposure, which was critical to assess the effects of intra-BLA CTOP on incentive learning.
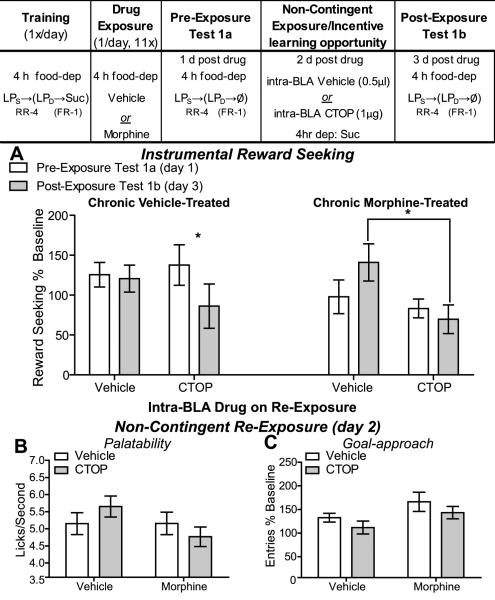
Figure 4. Experiment 4: Early Opiate withdrawal-induced inflated incentive value is dependent upon BLA mu opioid receptor activation.
Table: Experiment 4 Design- Rats were trained 4 h food deprived on a heterogeneous seeking-delivery chain of actions to earn sucrose. Following training rats were treated with either vehicle or morphine 1/d for 11 d. Testing commenced 24 h after the last drug injection and was conducted in early withdrawal. Rats were tested for the effects of opiate withdrawal on reward seeking (in 5 min non-rewarded extinction tests) both prior to and after non-contingent exposure to the sucrose training outcome in the opiate withdrawn state. During this incentive learning opportunity rats were given an infusion of either intra-BLA sterile water vehicle (vehicle-treated n=11, morphine-treated n=8) or CTOP (1µg/side; vehicle-treated n=8, morphine-treated n=9) to block mu opioid receptors. A. Effects of opiate withdrawal on reward seeking (normalized to pre-drug baseline reward-seeking response rate) prior to (Test 1a- open bars) and after (Test 1b- shaded bars) an opportunity to experience the sucrose training outcome in the opiate withdrawn state. B. Effects of early opiate withdrawal and intra-BLA CTOP on sucrose palatability, assessed as lick frequency, during the non-contingent re-exposure to the sucrose in opiate withdrawal. C. Effects of early opiate withdrawal and intra-BLA CTOP on goal approach (normalized to pre-drug baseline entry rate) during the non-contingent re-exposure to the sucrose in opiate withdrawal. RR-4, random-ratio 4; FR-1, fixed-ratio 1; LPS, seeking lever press, LPD, delivery lever press; Suc, 20% sucrose solution; Ø, no reward delivery; dep, deprived; *, p<0.05.
Surgery
Standard stereotaxic procedures were used for implantation of bilateral guide cannulae (Wassum et al., 2011a; Wassum et al., 2009). Rats were anesthetized with isoflurane (4–5% induction, 1–2% maintenance) and implanted bilaterally with 22 gauge stainless steel guide cannulae (Plastics One) 1 mm above the intended BLA injection site (coordinates from bregma and the skull surface: anterior-posterior, −3.0; medial-lateral, ±5.1; ventral, 8.0 mm).
Histological Analysis
Histology was conducted as described previously (Wassum et al., 2009). See Supplemental Figure 5.
Palatability analysis
The sucrose solution or water was delivered into a custom-built electrically-isolated magazine through a stainless steel tube. A lickometer circuit (Med Associates), connecting the grid floor of the box and the stainless steel tubes, with the circuit closed by the rats’ tongue, allowed recording of individual lick events. Lickometer measures were amplified and fed through an interface to a PC programmed to record the time of each lick to the nearest 1 msec. Based on previous reports (Baird et al., 2006; Davis and Smith, 1992; Kaplan et al., 1995; Thornton-Jones et al., 2007; Wassum et al., 2011b; Wassum et al., 2009) we used licking frequency as a measure of sucrose or water palatability. This measure of licking microstructure during consumption provides a similar analysis of palatability changes as those assessing taste reactivity following oral infusions (Davis and Perez, 1993).
Data Analysis
In order to control for pre-test response variability and allow comparison across tests conducted in different deprivation states (see (Wassum et al., 2011a; Wassum et al., 2011b; Wassum et al., 2009)) lever pressing and magazine entry data are presented as a percentage of pre-drug baseline response rate, with the baseline as the rate of performance (seeking lever press or head entry rate) during the last training session before drug treatment and test. For all hypothesis tests, the α level for significance was set to p < 0.05. Data were analyzed with t-tests and ANOVAs as described below using SPSS, GraphPad Prism and Excel. Bonferroni post-hoc analyses correcting for multiple comparisons were used to clarify main effects and interactions.
RESULTS
Experiment 1: Early opiate withdrawal disrupts incentive learning to elevate value-driven reward seeking
Experiment 1 was designed to test the hypothesis that chronic opiate administration and withdrawal disrupts the fundamental incentive learning process whereby the reward experience is translated into the incentive value used to guide future behavior. As illustrated in Figure 1, rats were trained 4 h food deprived on a seeking-delivery chain of lever-pressing actions to earn sucrose. All rats acquired the action chain and at the end of training, prior to drug-treatment and testing, performed the instrumental reward-seeking response at similar rates. For the group tested in early withdrawal the seeking response rate was 9.94 seeking presses/min for the future vehicle-treated group (SEM=0.93) and 9.64 seeking presses/min (SEM=1.19; t13=0.21, p=0.84) for the future morphine-treated group. For those tested in late withdrawal the seeking response rate was 9.53 seeking presses/min for the future vehicle-treated group (SEM=0.49) and 9.17 seeking presses/min (SEM=0.99; t13=0.34, p=0.74) for the future morphine-treated group. Testing was conducted drug-free (i.e., in withdrawal; Figure 1) and commenced 24 h after the last drug injection for Group Early Withdrawal and 14 d after the last injection for Group Late Withdrawal. Rats were tested for the effects of opiate withdrawal on reward-seeking both prior to and after an incentive learning opportunity (non-contingent re-exposure to sucrose in the opiate withdrawn state).
As seen in Figure 1A, there was no significant difference in reward-seeking response rate between the vehicle- and morphine-treated rats during the pre-exposure reward-seeking test in early withdrawal, but there is a readily apparent difference in reward seeking during the second post-exposure test, due to an increase in reward seeking in the morphine-treated rats after sucrose exposure in early withdrawal, an effect that was absent in vehicle-treated rats. These observations were confirmed by ANOVA, which indicated a significant main effect of Exposure (F1,13=6.32, p=0.03), no significant main effect of Drug Treatment (F1,13=1.11, p=0.31), but a significant Drug × Exposure interaction (F1,13=5.47, p=0.04). Post-hoc analyses, controlling for multiple comparisons, clarify this interaction to reveal that there was no significant difference in seeking rate between the vehicle- and morphine-treated rats during the first pre-exposure test of reward seeking (Test 1a, p>0.05), but that there was a significant difference in reward-seeking rate during the second post-exposure reward- seeking test (Test 1b, p<0.05). Indeed, rats in early opiate withdrawal showed a significant increase in their seeking response rate after exposure to the sucrose training outcome in the withdrawn state (p<0.05), while control, vehicle-treated rats showed no significant change (p>0.05). These data suggest that early opiate withdrawal has no general effect on reward seeking prior to an opportunity for incentive learning, but after such an opportunity reward-seeking actions are increased, relative to controls. Analysis of these data taking body weight into consideration is provided in the Supplemental Results and Supplemental Figure 2.
Incentive value is normally related to, although can be dissociated from, the emotional experience of reward consumption (Wassum et al., 2009), which we assayed here with a lick frequency measure (Berridge and Kringelbach, 2008; Wassum et al., 2009) during sucrose re-exposure. Rats in opiate withdrawal displayed significantly higher sucrose lick rates than those treated with vehicle (t13=2.53, p=0.03; Figure 1B), suggesting that the elevation in reward seeking was concomitant with an increase in sucrose palatability. No significant effect of Drug Treatment on goal approach was detected (t13=1.57, p=0.14; Figure 1C), suggesting that, as expected, the effects of early withdrawal following chronic opiate exposure were limited to instrumental actions.
These data suggest that in acute opiate withdrawal the value of a sucrose reward can be inflated upon experience in the withdrawn state driving elevated reward-seeking actions. In an additional experiment (see Supplemental Methods, Results and Supplemental Figure 3) we evaluated the persistence of this effect into late withdrawal. Here we find that the value encoded during sucrose experience in early withdrawal continues to influence reward-seeking 14 d into withdrawal - a time point at which the palatability of the sucrose is no-longer elevated above controls (Supplemental Figure 3). To fully evaluate this ‘late withdrawal’ stage a second group of rats in Experiment 1 was tested beginning 14 d after the last drug injection (Figure 1D-F). As seen in Figure 1D, there was no apparent difference in reward-seeking rate between vehicle- and morphine-treated groups in either the pre- or post-exposure test conducted in late withdrawal. This was confirmed by ANOVA, which showed no main effect of Exposure (F1,13=0.28, p=0.61) or Drug (F1,13=2.27, p=0.16), and no significant interaction between these factors (F1,13=1.86, p=0.20). These data seem to suggest that the late opiate withdrawn state is not effective in altering instrumental incentive learning. Interestingly however, time course analysis shows a significant a 3-way interaction between Exposure, Time in Test and Drug Exposure (F4,52=2.47, p=0.05) (see Supplemental Results for full analysis), providing a hint that the late withdrawal state may be effective in altering instrumental incentive learning. Based on this analysis, we next re-examined the data including only the first two minutes of the reward-seeking tests (Figure 1D- inset), a period in which we have previously shown incentive learning effects to be largest (Wassum et al., 2011b). Analysis of these data still shows no overall main effect of Drug (F1,13=0.88, p=0.37), but does reveal a significant effect of Exposure (F1,13=4.90, p=0.05) and, importantly, an Exposure × Drug interaction (F1,13=4.38, p=0.05); the vehicle-treated group showed a significant decrease in reward seeking after sucrose re-exposure (i.e., in Test 1b; p<0.05), while the morphine group showed no such change (p>0.05). This is interesting when one considers that 14 d in withdrawal both morphine- and vehicle-treated rats show greater sucrose reward seeking during the pre-exposure test than rats tested 1 d off drug (main effect of Drug-To-Test Time: F1,26=5.37, p=0.03). This may represent an incubation of craving effect (Grimm et al., 2005) such that when, during the sucrose re-exposure session, this ‘incubated’ sucrose value is not realized vehicle-treated rats adjust their reward seeking down accordingly. Indeed, experiencing sucrose after a period of forced abstinence following training can attenuate the abstinence-induced elevation of un-cued, but not conditioned reinforced, sucrose-seeking behavior (Grimm et al., 2005). In this interpretation opiate withdrawal may create a state that prevents this value down-shift.
When tested in late withdrawal there was no effect of drug treatment on sucrose lick frequency (t13=0.81, p=0.43; Figure 1E), suggesting that the effects of opiate withdrawal to alter sucrose palatability are short lasting. These results suggest that opiate withdrawal has the ability to alter the emotional experience of a sucrose reward, but only in the short-term, and also has the ability to alter the incentive learning process so as to inflate the instrumental incentive value of sucrose - an effect that is clearly strongest in early withdrawal, but remains present after 2 weeks of withdrawal. The temporal dissociability of these effects suggests that the ability of opiate withdrawal to alter incentive learning may not depend on its reward palatability effect.
Experiment 2: Opiate withdrawn rats fail to reduce sucrose reward seeking following satiety-induced reduction in experienced sucrose palatability
We next evaluated if opiate withdrawal would inflate the value of a reward in the face of a negative shift in the reward experience. Experiment 2 was designed to test the hypothesis that opiate withdrawal-induced inflation of incentive value would be sufficient to counteract the normal reduction in such value induced by experience of the sucrose reward in a sated state (Figure 2), perhaps reflecting ‘compulsive’ behavior. Rats were trained 22 h food-deprived to earn sucrose reward. At the conclusion of training, prospective vehicle and morphine groups displayed similar reward-seeking rates (Vehicle-treated: 14.30 presses/min SEM=2.02, Morphine-treated: 11.29 presses/min SEM=1.22; t22=1.27, p=0.22). Following chronic morphine or vehicle treatment all rats were tested in early withdrawal. The first series of tests was conducted in a novel sated state (1 h food-deprived) to assess the effects of opiate withdrawal on negative incentive learning i.e., reduction in reward seeking after experiencing the sucrose reward under sated conditions for the first time (Balleine, 1992; Wassum et al., 2011a). Following initial testing rats were tested again for their reward seeking and sucrose palatability in the control 22 h food-deprived state.
Observation of the data in Figure 2A suggests equivalent response rates in the vehicle- and morphine-treated groups during the pre-exposure test, but higher responding in morphine relative to vehicle groups in the post-exposure test due to the opposite direction of the exposure effect on reward seeking across drug groups. This was confirmed by statistical analysis: there was neither a significant main effect of Exposure (F1,22=0.02, p=0.89), nor of Drug Treatment (F1,22=1.15, p=0.30) on reward seeking in this negative incentive learning procedure, but importantly there was a significant Drug × Exposure interaction (F1,22=4.69, p=0.04). Post-hoc analysis confirmed the significance of the difference between the drug treatment groups in the second test conducted after negative incentive learning (p<0.05). A negative incentive learning effect is marked by a decrease in reward seeking for a food reward when sated relative to hungry, but only after the reward has been experienced sated (Balleine et al., 1995; Dickinson and Balleine, 1994). We, therefore, used planned comparisons to show that the vehicle control group’s reward-seeking response rate was not reduced relative to the 22 h control training state (see Supplemental Figure 4) when tested 1 h food deprived (Test 1a, t12=0.45, p=0.66) until the rats had the opportunity to experience the sucrose reward in the 1 h deprived state (i.e., on the post-exposure reward-seeking Test 1b; t12=3.62, p=0.004). This was not the case for the opiate withdrawn rats; after the negative incentive learning opportunity opiate-withdrawn rats reward-seeking rate was not different from their 22 h food-deprived control state (t12=0.73, p=0.45).
As seen in Figure 2B, there was a significant main effect of Deprivation (1 h v 22 h food-deprived; F1,22=19.76, p=0.0002) on sucrose lick frequency, with no significant main effect of Drug (F1,22=0.58, p=0.45) and no interaction between these factors (F1,22=0.02, p=0.88); sucrose lick frequency was significantly lower when sated relative to hungry for both vehicle- (p<0.05) and opiate-withdrawn rats (p<0.01). These data suggest that while opiate withdrawal may increase sucrose palatability (Figure 1), this effect is not sufficient to overcome a satiety-induced reduction in palatability (Figure 2B). Opiate withdrawal is, however, capable of overcoming the reduction in reward value induced by satiety (Figure 2A), highlighting the dissociability of the effects of opiate withdrawal on the emotional experience of reward consumption and value-driven reward seeking. Indeed, there was a significant positive correlation between sucrose palatability (lick frequency) and subsequent sucrose reward seeking (r2=0.39, p=0.03) in vehicle-treated rats, while no such correlation was apparent in opiate-withdrawn rats (r2=0.0002, p=0.97; Figure 2C), suggesting that, in opiate withdrawal, reward seeking can be inconsistent with the most recent emotional experience derived from reward consumption.
Lastly, we found only a main effect of Deprivation on goal approach (Figure 2D; F1,22=26.36, p<0.0001), with no effect of Drug Treatment (F1,22=0.06, p=0.80), and no Drug × Exposure interaction (F1,22=0.13, p=0.72); both vehicle- and chronic morphine-treated rats approached the magazine less when sated than when hungry (p<0.01).
Experiment 3: In opiate withdrawal the value and subsequent seeking actions for a water reward are inflated
Evidence suggests that opiate exposure alters weight gain and metabolism (Ferenczi et al., 2010; Gosnell et al., 1983; Levine and Atkinson, 1987; Levine et al., 1985), which could potentially account for the withdrawal-induced changes in sucrose value and palatability detected in Experiment 1 and 2. Opiate-withdrawn rats may value sucrose more because of a greater metabolic need for food rather than a primary effect of the withdrawal itself. Indeed, our morphine-treated rats weighed significantly less than their vehicle-treated counterparts (See Supplemental Results and Supplemental Figure 1, 2) and this was taken as evidence of withdrawal symptoms. Although we can statistically rule out that these effects on weight or metabolism explain the effects of opiate withdrawal on value-guided reward seeking actions for sucrose (see Supplemental Results), Experiment 3 was designed to experimentally rule this out using a water reward, which provides no calories. This also provided an opportunity to test the generality of the effect.
All rats were trained on the action chain to earn water while 18 h water-deprived and at the end of training pressed the seeking lever at an average rate of 9.89 (SEM=1.13) presses/min (prospective vehicle group) and 9.23 (SEM=0.99) presses/min (prospective morphine-treated group: t13=0.43, p=0.67). Morphine-treated rats weighed less at the end of drug treatment than vehicle-treated controls and during drug treatment did, on some days, consume less food, but, importantly, not less water (Supplemental Figure 2 and Supplemental Results). Testing was conducted in early withdrawal and was identical to Experiment 1.
As can be seen in Figure 3A, there was no significant difference in water-seeking response rate between the vehicle- and morphine-treated rats during the pre-exposure reward-seeking test, but after water exposure in withdrawal morphine-, but not vehicle-treated rats escalated their water seeking. Analysis reveals a marginally insignificant main effect of Drug (F1,13=3.56, p=0.08) on water seeking, a significant main effect of Exposure (F1,13=12.23, p=0.004), and, importantly, a significant Exposure × Drug treatment interaction (F1,13=16.47, p=0.001). There was no significant difference in water seeking between the vehicle- and morphine-treated rats during the first pre-exposure test of reward seeking (p>0.05), but there was a significant difference in water seeking during the second post-exposure test (p<0.01); opiate-withdrawn rats showed a significant increase in water seeking after water re-exposure in withdrawal (p<0.001), while vehicle-treated rats showed no change (p>0.05), suggesting that, as with a sucrose reward, the opiate-withdrawal state inflates the value of a water reward resulting in elevated water seeking.
Unlike Experiments 1, where sucrose was the reward, in this experiment there was no significant difference between opiate-withdrawn and vehicle-treated rats in lick frequency (t13=1.05, p=0.31; Figure 3B), suggesting that opiate withdrawal-inflated water value is not commensurate with emotional experience of water consumption. As in previous studies there was no significant effect of opiate withdrawal on goal approach during the re-exposure test (t13=1.99, p=0.07; Figure 3C).
Experiment 4: Opiate withdrawal-induced inflated incentive value is blocked by BLA mu opioid receptor inactivation
BLA mu opioid receptor activation is necessary for a hunger-induced increase in sucrose reward value, but not for increases in sucrose palatability (Wassum et al., 2011a; Wassum et al., 2009). Moreover, BLA mu opioid receptor binding sites are upregulated following chronic opiate exposure (Brady et al., 1989). Therefore, we next tested the hypothesis that BLA mu opioid receptor activation is necessary for opiate withdrawal-induced increases in sucrose instrumental incentive value by replicating Experiment 1, with the exception that prior to the non-contingent re-exposure/opportunity for incentive learning in early withdrawal rats were given an infusion of either vehicle, or the selective mu opioid receptor antagonist, CTOP, into the BLA (Figure 4). Following training, there was no difference in reward seeking between the prospective groups (chronic vehicle/intra-BLA vehicle: 6.96 (SEM=0.97) seeking presses/min; chronic morphine/intra-BLA vehicle 6.10 (SEM=0.81); chronic vehicle/intra-BLA CTOP: 7.54 (SEM=1.08); chronic morphine/intra-BLA CTOP 7.34 (SEM=0.64): F3,32=0.48, p=0.70).
Observation of the data in Figure 4A suggests that intra-BLA CTOP during re-exposure blocks the post-exposure increase in reward seeking evident in intra-BLA vehicle-treated animals (right side of panel). This supported by an initial ANOVA, which demonstrates no overall main effect of Chronic Drug (vehicle or morphine treated; F1,32=1.50, p=0.23), but a marginally insignificant effect of Intra-BLA Drug (vehicle or CTOP; F1,32=2.88, p=0.10), a significant Exposure × Intra-BLA Drug interaction (F1,32=4.66, p=0.04) and a marginally insignificant Exposure × Chronic Drug interaction (F1,32=3.23, p=0.08). All other main effects and interactions were non-significant (p>0.05). Given the interactions this analysis was clarified with separate analyses on the data divided by chronic drug treatment. In morphine-treated rats there was no effect of Exposure (F1,15=0.50, p=0.49), but there was a significant effect of Intra-BLA Drug (F1,16=6.97, p=0.02). While there was no significant interaction (F1,16=1.85, p=0.19), reward-seeking rate was significantly higher during the post-exposure test in the rats that experienced the sucrose in opiate withdrawal under intra-BLA vehicle relative to those rats who had this exposure under BLA mu opioid receptor blockade (p<0.05). Within the chronic vehicle-treated group there was no main effect of Intra-BLA Drug (F1,17=0.18, p=0.69), but rather only an effect of Exposure (F1,17=4.82, p=0.04) with a moderately insignificant interaction between these factors (F1,17=3.30, p=0.09). The negative effect of exposure is significant (p<0.05) in the rats that received intra-BLA CTOP on the re-exposure test. In both the vehicle- and morphine-treated rats, response rates on the first pre-exposure test (Figure 4A- open bars) were higher than those seen in Experiment 1 (see Figure 1A- open bars), despite the fact that the deprivation, training and testing procedures were identical with the exception of intra-BLA drug infusion. This difference may be due to stress from surgery/implant or other differences between these experiments, including differences in the time of day that the training and testing was conducted. Overall, these data suggest that early opiate withdrawal creates a state in which the value of a reward is increased and that this effect is blocked by inactivation of BLA mu opioid receptors.
There was neither a main effect of Chronic Drug (F1,32=1.93, p=0.17) nor Intra-BLA Drug (F1,32=0.03, p=0.86) on sucrose lick frequency and no interaction between these factors (F1,32=1.98, p=0.17; Figure 4B). Contrary to our findings in Experiments 1, but similar to Experiment 2 and 3, these data suggest that opiate withdrawal did not induce a palatability increase, perhaps because of the infusion stress. In this experiment a main effect of Chronic Drug on goal approach behavior (F1,32=6.21, p=0.02) was detected, with neither an effect of Intra-BLA Drug (F1,32=1.87, p=0.18), nor an interaction between these factors (F1,32=0.01, p=0.91; Figure 4C).
DISCUSSION
This study evaluated the effect of opiate withdrawal on value-driven reward seeking and reward palatability. The data show that in early opiate withdrawal the experience-dependent incentive value of both a sucrose and water reward was increased resulting in enhanced value-driven reward seeking. Importantly, in certain circumstances, such reward seeking was inconsistent with the emotional experience of reward consumption and occurred in the face of circumstances that would otherwise negatively impact reward value. These data, therefore, suggest that early opiate withdrawal is a distinct motivational state in which the general incentive learning process is altered resulting in maladaptive reward seeking. The incentive value inflated in early withdrawal continued to influence reward seeking in late withdrawal, but the late withdrawal stage itself was only modestly effective in elevating sucrose incentive value, suggesting this mechanism might contribute to escalation and increased frequency of opiate use, short-term relapse and other effects of specifically early withdrawal on natural reward consumption and non-opiate drug use. This effect of early opiate withdrawal on incentive learning was dependent upon BLA mu opioid receptor activation.
In opiate withdrawal opiate self-administration is elevated (Goldberg et al., 1969; Kenny et al., 2006; Wikler and Pescor, 1967). Previous experience with an opiate in withdrawal (i.e., an incentive learning opportunity) can enhance opiate seeking, relative to rats in withdrawal who have not had this experience and this occurs in the absence of an opportunity for negative reinforcement, i.e., the opportunity to learn that a specific drug-seeking action will lead to alleviation of negative withdrawal symptoms (Hutcheson et al., 2001). The latter study supports an incentive motivational account of opiate addiction, wherein opiate withdrawal serves as a motivational state in which the incentive value of opiates used to inform drug-seeking decisions is elevated (Hutcheson et al., 2001). While this could result from an enhanced emotional impact of the drug in the withdrawn state, it is also possible that repeated opiate administration and withdrawal disrupts the fundamental endogenous opioid-dependent incentive learning process such that the incentive value assigned to the reward when experienced in the withdrawn state may be inflated beyond the level appropriate for its emotional impact. A prediction of such an account is that opiate withdrawal should function as a state in which the incentive value of non-opiate rewards is also increased, producing general maladaptive reward-based decision making. Our data provide evidence in support of this possibility. Opiate withdrawal-induced elevation of reward value was not always consistent with an elevated emotional experience. Indeed, although we found no evidence of elevated palatability responses to water in withdrawal, water seeking was nonetheless increased after water was experienced in withdrawal. Moreover, the inflation of incentive value in the opiate-withdrawal state was also sufficient to counter an opposing negative reward value change and decline in the emotional experience of the reward brought about by experiencing a food reward when sated, a finding that is particularly pertinent to the drug-addicted condition, wherein the abused substance continues to be desired and compulsively sought out irrespective of need and despite adverse consequences.
Importantly, in these experiments rats were never given the opportunity for the reward-seeking action to be negatively reinforced by any potential alleviation of the withdrawal symptoms that may have been conferred by food or water consumption, effectively ruling out a negative reinforcement account. Opiate withdrawal-inflated reward seeking was found to critically depend on reward evaluation and was found not to be the result of an effect on locomotor activity, generally elevated appetitive behavior or on instrumental learning per se. Moreover, this was not the result of a secondary effect of morphine exposure on weight or hunger, because identical effects were detected for a food and water reward even though water intake was not significantly impacted by morphine exposure/withdrawal.
A commonly held view is that drug addiction is associated with strong urges to obtain the drug and reduced responding for natural rewards (Kalivas et al., 2005). The current data provide evidence of a potential mechanism for the first part of this assumption, but could been seen as contradictory to the second, given the finding of elevated responding for natural rewards in early opiate withdrawal. Natural rewards were not directly compared to drug rewards in this study, nonetheless our data are consistent with findings of elevated food- (Babbini et al., 1976; Cooper et al., 2010; Ford and Balster, 1976; Ranaldi et al., 2009) and cocaine-seeking behavior (He and Grasing, 2004) in opiate withdrawal. Moreover, our data may be considered as contrasting to other data showing that opiate withdrawn rats display reduced preference for food-associated places (Harris and Aston-Jones, 2003, 2007) and attenuated acquisition of a food-reinforced lever press response (Harris and Aston-Jones, 2003). The duration (8 days in Harris and Aston-Jones, 2003 v. 2-3 days in the current study) and type of withdrawal (continuous morphine treatment in Harris and Aston-Jones, 2003 v. intermittent in the current study), in addition to the different instrumental tasks used, likely accounts for the disparate effects on reward seeking and perhaps suggests that temporally distinct neural adaptations in withdrawal differentially alter reward-seeking behaviors. Additionally, it has long been established that cues associated with naloxone-precipitated withdrawal are effective conditioned suppressors of operant behavior (Baldwin and Koob, 1993; Koob et al., 1992) suggesting that the negative affective state of withdrawal has a detrimental effect on natural reward-related behavior. Indeed, systemic naloxone treatment, which has also been shown to produce a negative emotional state (Mucha and Iversen, 1984; Skoubis et al., 2005), can reduce sucrose seeking in an experience-dependent manner (Wassum et al., 2009). This effect is in the opposite direction to those detected here in which reward seeking was enhanced in opiate withdrawal following exposure to the reward in the withdrawn state, suggesting the negative affective state induced by early withdrawal cannot fully account for the current results. Rather a chronic opiate-induced disruption in the fundamental incentive learning process more readily explains the escalation of food- and water-seeking in early opiate withdrawal. Indeed, early opiate withdrawal-induced enhanced incentive value was found to be dependent upon BLA mu opioid receptor activation- the very same mechanism required for encoding normal hunger-induced increases in incentive value used to drive sucrose reward-seeking (Wassum et al., 2009). That the late opiate withdrawal state was not as effective as the early state in elevating sucrose incentive value perhaps suggests that any adaptations in the BLA mu opioid receptor system subside after 14 d off drug.
Taken together these data suggest that early opiate withdrawal disrupts the fundamental incentive learning process such that reward value becomes inflated, out of proportion with reward experience, resulting in maladaptive reward seeking. In addition to providing a potential mechanism for the escalation of opiate seeking in addicts during early opiate withdrawal, these data may also help to explain why recovering opiate addicts and those treated with chronic prescription opiates are both more likely to abuse other drugs and to over-eat (Michna et al., 2004; Mysels and Sullivan, 2010; Nolan and Scagnelli, 2007).
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Nathaniel Tom and Fiona Lao for their technical assistance as well as Sam Brotherton for his assistance with data analysis software. This work was supported by startup funds to Kate Wassum from the UCLA Life Sciences Division, DA035443 from the NIH to Kate Wassum, DA029035 from the NIH to Sean Ostlund, DA05010 and DA09359 from the NIH to Nigel Maidment and by the Shirley and Stefan Hatos Center for Neuropharmacology.
REFERENCES
- Babbini M, Gaiardi M, Bartoletti M. Changes in fixed-interval behavior during chronic morphine treatment and morphine abstinence in rats. Psychopharmacologia. 1976;45:255–259. doi: 10.1007/BF00421136. [DOI] [PubMed] [Google Scholar]
- Baird JP, Rios C, Gray NE, Walsh CE, Fischer SG, Pecora AL. Effects of melanin-concentrating hormone on licking microstructure and brief-access taste responses. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1265–1274. doi: 10.1152/ajpregu.00143.2006. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Koob GF. Rapid induction of conditioned opiate withdrawal in the rat. Neuropsychopharmacology. 1993;8:15–21. doi: 10.1038/npp.1993.3. [DOI] [PubMed] [Google Scholar]
- Balleine B. Instrumental performance following a shift in primary motivation depends on incentive learning. J Exp Psychol Anim Behav Process. 1992;18:236–250. [PubMed] [Google Scholar]
- Balleine B. Incentive processes in instrumental conditioning. In: RaKS Mowrer., editor. Handbook of Contemporary Learning Theories. Erlbaum; Hillsdale, New Jersey: 2001. pp. 307–366. [Google Scholar]
- Balleine B, Paredes-Olay C, Dickinson A. Effects of Outcome Devaluation on the Performance of a Heterogeneous Instrumental Chain. International Journal of Comparative Psychology. 2005;18:257–272. [Google Scholar]
- Balleine BW, Garner C, Gonzalez F, Dickinson A. Motivational control of heterogeneous instrumental chains. J Exp Psychol Anim Behav Process. 1995;21:203–217. [Google Scholar]
- Berridge KC. Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite. 1991;16:103–120. doi: 10.1016/0195-6663(91)90036-r. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady LS, Herkenham M, Long JB, Rothman RB. Chronic morphine increases mu-opiate receptor binding in rat brain: a quantitative autoradiographic study. Brain Res. 1989;477:382–386. doi: 10.1016/0006-8993(89)91432-7. [DOI] [PubMed] [Google Scholar]
- Cabanac M. Pleasure: the common currency. J Theor Biol. 1992;155:173–200. doi: 10.1016/s0022-5193(05)80594-6. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Shi YG, Woods JH. Reinforcer-dependent enhancement of operant responding in opioid-withdrawn rats. Psychopharmacology (Berl) 2010;212:369–378. doi: 10.1007/s00213-010-1966-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Instrumental and Pavlovian incentive processes have dissociable effects on components of a heterogeneous instrumental chain. J Exp Psychol Anim Behav Process. 2003;29:99–106. doi: 10.1037/0097-7403.29.2.99. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol. 1993;264:R97–103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of lick rate measures the positive and negative feedback effects of carbohydrates on eating. Appetite. 1988;11:229–238. doi: 10.1016/s0195-6663(88)80005-9. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci. 1992;106:217–228. [PubMed] [Google Scholar]
- Dickinson A, Balleine BW. Motivational control over goal-directed action. Animal Learning and Behavior. 1994;22:1–18. [Google Scholar]
- Ferenczi S, Núñez C, Pintér-Kübler B, Földes A, Martín F, Márkus VL, Milanés MV, Kovács KJ. Changes in metabolic-related variables during chronic morphine treatment. Neurochem Int. 2010;57:323–330. doi: 10.1016/j.neuint.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Fields HL. The doctor's dilemma: opiate analgesics and chronic pain. Neuron. 2011;69:591–594. doi: 10.1016/j.neuron.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford RD, Balster RL. Schedule-controlled behavior in the morphine-dependent rat. Pharmacol Biochem Behav. 1976;4:569–573. doi: 10.1016/0091-3057(76)90199-4. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Woods JH, Schuster CR. Morphine: conditioned increases in self-administration in rhesus monkeys. Science. 1969;166:1306–1307. doi: 10.1126/science.166.3910.1306. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Levine AS, Morley JE. The effects of aging on opioid modulation of feeding in rats. Life Sci. 1983;32:2793–2799. doi: 10.1016/0024-3205(83)90401-0. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand TH, Koob GF, Stinus L, Le Moal M. Aversive properties of opiate receptor blockade: evidence for exclusively central mediation in naive and morphine-dependent rats. Brain Res. 1988;474:364–368. doi: 10.1016/0006-8993(88)90452-0. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Altered motivation and learning following opiate withdrawal: evidence for prolonged dysregulation of reward processing. Neuropsychopharmacology. 2003;28:865–871. doi: 10.1038/sj.npp.1300122. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behav Brain Res. 2007;176:251–258. doi: 10.1016/j.bbr.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey-Lewis C, Perdrizet J, Franklin KB. The effect of morphine dependence on impulsive choice in rats. Psychopharmacology (Berl) 2012;223:477–487. doi: 10.1007/s00213-012-2738-5. [DOI] [PubMed] [Google Scholar]
- He S, Grasing K. Chronic opiate treatment enhances both cocaine-reinforced and cocaine-seeking behaviors following opiate withdrawal. Drug Alcohol Depend. 2004;75:215–221. doi: 10.1016/j.drugalcdep.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci. 2001;4:943–947. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Roitman MF, Grill HJ. Ingestive taste reactivity as licking behavior. Neurosci Biobehav Rev. 1995;19:89–98. doi: 10.1016/0149-7634(94)00023-t. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Drug addiction: the yin and yang of hedonic homeostasis. Neuron. 1996;16:893–896. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M, Bloom FE. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev. 1989;13:135–140. doi: 10.1016/s0149-7634(89)80022-3. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Preston KL, Schindler CW, Meisch RA, Davis F, Katz JL, Henningfield JE, Goldberg SR. The reinforcing and subjective effects of morphine in post-addicts: a dose-response study. J Pharmacol Exp Ther. 1991;259:1165–1173. [PubMed] [Google Scholar]
- Levine AS, Atkinson RL. Opioids in the regulation of food intake and energy expenditure. Fed Proc. 1987;46:159–162. [PubMed] [Google Scholar]
- Levine AS, Morley JE, Gosnell BA, Billington CJ, Bartness TJ. Opioids and consummatory behavior. Brain Res Bull. 1985;14:663–672. doi: 10.1016/0361-9230(85)90116-9. [DOI] [PubMed] [Google Scholar]
- Martin WR, Wikler A, Eades CG, Pescor FT. Tolerance to and Physical Dependence on Morphine in Rats. Psychopharmacologia. 1963;4:247–260. doi: 10.1007/BF00408180. [DOI] [PubMed] [Google Scholar]
- Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, Palombi D, Jamison RN. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J Pain Symptom Manage. 2004;28:250–258. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Iversen SD. Reinforcing properties of morphine and naloxone revealed by conditioned place preferences: a procedural examination. Psychopharmacology (Berl) 1984;82:241–247. doi: 10.1007/BF00427782. [DOI] [PubMed] [Google Scholar]
- Mysels DJ, Sullivan MA. The relationship between opioid and sugar intake: review of evidence and clinical applications. J Opioid Manag. 2010;6:445–452. doi: 10.5055/jom.2010.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan LJ, Scagnelli LM. Preference for sweet foods and higher body mass index in patients being treated in long-term methadone maintenance. Subst Use Misuse. 2007;42:1555–1566. doi: 10.1080/10826080701517727. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Egan J, Kest K, Fein M, Delamater AR. Repeated heroin in rats produces locomotor sensitization and enhances appetitive Pavlovian and instrumental learning involving food reward. Pharmacol Biochem Behav. 2009;91:351–357. doi: 10.1016/j.pbb.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Savage SR. Opioid medications in the management of pain. In: Graham AW, Schultz TK, Mayo-Smith MF, Ries RK, Wilford BB, editors. Principles of Addiction Medicine. American Society of Addiction Medicine; 2003. pp. 1451–1463. [Google Scholar]
- Skoubis PD, Lam HA, Shoblock J, Narayanan S, Maidment NT. Endogenous enkephalins, not endorphins, modulate basal hedonic state in mice. Eur J Neurosci. 2005;21:1379–1384. doi: 10.1111/j.1460-9568.2005.03956.x. [DOI] [PubMed] [Google Scholar]
- Stinus L, Caille S, Koob GF. Opiate withdrawal-induced place aversion lasts for up to 16 weeks. Psychopharmacology (Berl) 2000;149:115–120. doi: 10.1007/s002139900358. [DOI] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Kennett GA, Vickers SP, Clifton PG. A comparison of the effects of the CB(1) receptor antagonist SR141716A, pre-feeding and changed palatability on the microstructure of ingestive behaviour. Psychopharmacology (Berl) 2007;193:1–9. doi: 10.1007/s00213-007-0745-8. [DOI] [PubMed] [Google Scholar]
- Wassum KM, Cely IC, Balleine BW, Maidment NT. Mu opioid receptor activation in the basolateral amygdala mediates the learning of increases but not decreases in the incentive value of a food reward. Journal of Neuroscience. 2011a;31:1583–1599. doi: 10.1523/JNEUROSCI.3102-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Balleine BW, Maidment NT. Differential dependence of Pavlovian incentive motivation and instrumental incentive learning processes on dopamine signaling. Learn Mem. 2011b;18:475–483. doi: 10.1101/lm.2229311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT, Balleine BW. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci U S A. 2009;106:12512–12517. doi: 10.1073/pnas.0905874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A, Pescor FT. Classical conditioning of a morphine abstinence phenomenon, reinforcement of opioid-drinking behavior and "relapse" in morphine-addicted rats. Psychopharmacologia. 1967;10:255–284. doi: 10.1007/BF00401386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.