Abstract
Despite recent therapeutic advances, malignant melanoma is an aggressive tumor in dogs and is associated with a poor outcome. Novel, targeted agents are necessary to improve survival. In this study, 6-bromoindirubin-3′-oxime (BIO), a serine/threonine kinase inhibitor with reported specificity for glycogen synthase kinase-3 beta (GSK-3β) inhibition, was evaluated in vitro in three canine melanoma cell lines (CML-10C2, UCDK9M2, and UCDK9M3) for β-catenin-mediated transcriptional activity, Axin2 gene and protein expression levels, cell proliferation, chemotoxicity, migration and invasion assays.
BIO treatment of canine malignant melanoma cell lines at 5 µM for 72 h enhanced β-catenin-mediated transcriptional activity, suggesting GSK-3β inhibition, and reduced cell proliferation and migration. There were no significant effects on invasion, chemotoxicity, or apoptosis. The results suggest that serine/ threonine kinases may be viable therapeutic targets for the treatment of canine malignant melanoma.
Keywords: Canine melanoma, Glycogen synthase kinase, Serine/threonine kinase, 6-Bromoindirubin-3′-oxime, Wnt signaling
Introduction
Malignant melanoma is a common neoplasm in the dog, accounting for approximately 4% of all malignant tumors and most frequently affecting the oral cavity, skin, digit, and eye (MacEwen et al., 1986; Ramos-Vara et al., 2000; Smedley et al., 2011). Melanoma is the most common oral tumor in dogs and is both locally aggressive and highly metastatic (Todoroff and Brodey, 1979; Wallace et al., 1992; Smith et al., 2002). Despite aggressive therapy including any combination of surgery, radiation therapy, chemotherapy, and immunotherapy, few affected dogs survive beyond 1 year and most affected dogs succumb to local tumor recurrence, metastasis, or both (Harvey et al., 1981; MacEwen et al., 1986; Kosovsky et al., 1991; Bateman et al., 1994; Freeman et al., 2003; Bergman et al., 2006; Brockley et al., 2013; Dank et al., 2014). While more targeted immunotherapeutic approaches have demonstrated safety and shown promise in the treatment of malignant melanoma in dogs, true efficacy is questionable and evidence for durable remissions is still lacking (Bergman et al., 2003; Alexander et al., 2006; Grosenbaugh et al., 2011; Ottnod et al., 2013).
Improved targeted therapies are needed to improve disease outcome. However, for therapeutic targets to be effective, they should be selected based on a working knowledge of the signaling pathways that contribute to the behavioral characteristics of the disease. Our previous work, as well as the work of others, suggests that the canonical Wnt signaling pathway is suppressed in malignant melanoma as evidenced by minimal β-catenin-mediated transcriptional activity (Chon et al., 2013). Interestingly, in humans with melanoma the activation of the canonical Wnt signaling pathway has been associated with reduced cell proliferation and improved survival (Chien et al., 2009).
The canonical Wnt signaling pathway is an evolutionarily conserved mechanism that is crucial for development and has been implicated in the regulation of several neoplastic processes (Huelsken and Birchmeier, 2001; Klaus and Birchmeier, 2008; Anastas and Moon, 2013). The cornerstone protein of the canonical Wnt signaling pathway is β-catenin, with activation of the pathway resulting in the cytoplasmic stabilization of β-catenin and its subsequent translocation into the nucleus to upregulate target genes. In the absence of active canonical Wnt signaling there is minimal cytoplasmic or nuclear β-catenin present due to its ubiquitin-mediated degradation. β-Catenin is targeted for ubiquitin-mediated degradation by a protein complex consisting of Axin, adenomatous polyposis coli protein (APC), casein kinase I alpha (CKIα), and glycogen synthase kinase-3 beta (GSK-3β). Casein kinase I alpha and GSK-3β are serine/ threonine kinases responsible for phosphorylating β-catenin on serine 45 and threonine 41, serines 37/33, respectively (MacDonald et al., 2009).
Physiologically, Axin2, a homolog of Axin, is a known negative regulator of the Wnt signaling pathway, promoting the phosphorylation and degradation of β-catenin (Leung et al., 2002; Chia and Costantini, 2005). The transcription of Axin2 is rapidly induced by active canonical Wnt signaling (Jho et al., 2002; Leung et al., 2002; Chia and Costantini, 2005), thereby serving as a negative feedback loop in regulation of the canonical Wnt signaling pathway. In the neoplastic setting, increased amounts of Axin2 protein have been detected in certain human cancers, including colon carcinoma, hepatoblastoma and lung carcinoma; in contrast, reduced amounts of Axin2 protein have been detected in most breast, bladder, pancreas, prostate, and melanoma cells (Lustig et al., 2002). There is only one report of an activating Axin2 germline mutation and concomitant inactivation of mismatch repair genes described in one human patient (Castiglia et al., 2008), indicating the rarity of constitutive activation of Axin2 in melanoma patients.
GSK-3β is a serine/threonine kinase responsible for regulating the function of multiple proteins through the phosphorylation of serine or threonine residues within the target proteins. The role of GSK-3β in cancer appears dependent on the cancer type. The presence of active GSK-3β has been associated with an improved prognosis in breast cancer, while reduced GSK-3β expression in hepatic cancers was associated with a poor prognosis (Quintayo et al., 2012; Huang et al., 2013). However, recent work suggests its presence or hyperactivation plays an oncogenic role in other human cancers, such as colon cancer, osteosarcoma, gliomas, and malignant melanoma (Kotliarova et al., 2008; Tang et al., 2012; Madhunapantula et al., 2013; Salim et al., 2013; Tsai et al., 2013). The role of GSK-3β in canine malignant melanoma, however, has not yet been defined.
Given our previous finding of minimal canonical Wnt signaling activity in canine malignant melanoma and the recent work of others suggesting that active GSK-3β may have an oncogenic role, we aimed in the present study to determine the impact of GSK-3β inhibition on β-catenin-mediated transcriptional activity in canine malignant melanoma cell lines and their behavior. 6-Bromoindirubin-3′-oxime (BIO) is a selective and potent GSK-3β inhibitor that is expected to activate the canonical Wnt signaling pathway by virtue of its mode of action. We therefore hypothesized that β-catenin-mediated transcriptional activity would be enhanced with BIO treatment and that BIO treatment would lead to a less biologically aggressive phenotype.
Materials and methods
Cell culture and reagents
Canine melanoma cell lines UCDK9M2, UCDK9M3 (gifts from Dr. M. Kent of the University of California, Davis, USA), and CML-10C2 (gift from Dr. L. Wolfe of Auburn University, USA) were maintained in modified Eagle’s medium-alpha (MEM-α) and supplemented with 10% fetal bovine serum (FBS), sodium pyruvate, L-glutamine, MEM vitamins, non-essential amino acids (all products from Fisher Scientific) at 37 °C in a humidified incubator with 5% CO2. UCDK9M2 was generated from lymph node metastasis of a canine oral malignant melanoma; UCDK9M3 was generated from a canine primary oral malignant melanoma, and CML-10C2 from a canine cutaneous malignant melanoma. (2′Z,3′E)-6-Bromoindirubin-3′-oxime (BIO, Sigma-Aldrich) was dissolved in dimethyl sulfoxide (DMSO, Fisher Scientific) to a concentration of 10 mM and stored at −20 °C.
Western blot analysis for GSK-3β protein in canine melanoma cell lines
Melanoma cells were lysed using a mammalian protein extraction reagent (MPER; Pierce) combined with a phosphatase and protease inhibitor (Pierce) and protein lysates were collected. Protein lysates were separated by electrophoresis on a 7.5% sodium dodecyl sulfate (SDS) polyacrylamide gel (BioRad) at 150 V for approximately 45 min. Proteins were then transferred onto a nitrocellulose membrane (Whatman) at 100 V for 1 h, then blocked with Tris-buffered saline/0.05% Tween 20 (TBST) containing 5% non-fat dry milk and 1% bovine serum albumin for 1 h (all reagents from Fisher Scientific). The membranes were probed overnight at 4 °C with either a rabbit anti-GSK-3β monoclonal antibody (9315, Cell Signaling) diluted 1:1000 in blocking solution or a rabbit anti-laminin polyclonal antibody (ab11575, Abcam) diluted 1:1000 in blocking solution. The antibodies are directed against the human protein and are expected to cross-react with canine protein. Excess primary antibody was removed by washing three times for 5 min with TBST. Membranes were incubated with 50 ng/mL horseradish peroxidase-conjugated anti-rabbit or anti-goat IgG secondary antibody (Thermo Scientific) diluted in blocking solution for 1 h at room temperature, then washed three times for 5 min with TBST, and treated with Clarity Western ECL Substrate (Bio-Rad). Blots were exposed to film, developed, and then imaged using a Gel Logic 100 Imaging System (Kodak).
Assessment of β-catenin-mediated transcriptional activity in canine melanoma cell lines
Cell lines were grown in 24-well plates at a density that would achieve 10– 20% confluence within 24 h. Cells were then treated with 5 nM, 1 µM, 5 µM BIO or the equivalent volume of DMSO. Following a 24 h treatment with BIO or DMSO, cells were transiently transfected using Lipofectamine LTX and PLUS reagents (Invitrogen) to introduce either 500 ng TOPflash or 500 ng FOPflash reporter plasmid, together with TK-Renilla luminescent reporter plasmid (TCF Reporter Plasmid Kit; Millipore) at a 5:1 (Flash:Renilla) ratio. For each well, canine melanoma cells were transfected with 600 ng total DNA using 1 µL PLUS reagent and 3 µL LTX. The TOPflash luciferase reporter plasmid contains TCF4 binding sites upstream of the luciferase gene, resulting in luciferase activity in the presence of active Wnt/β-catenin signaling, whereas the FOPflash reporter plasmid contains mutated TCF4 binding sites. The TK-Renilla plasmid served as a control for transfection efficiency.
Forty-eight hours after transfection of luciferase plasmids (for a total 72 h of BIO or DMSO treatment), cells were harvested and luciferase and Renilla luminescence were measured using the Dual-Luciferase Reporter Assay System (Promega) on a BioTek Synergy HT Multi-mode Microplate Reader, using Gen5 software (BioTek Instruments). The relative luciferase units for each transfection were adjusted by Renilla activity in the same sample, and each corrected TOPflash luciferase value was normalized to the corresponding corrected FOPflash value. The TOP/FOPflash ratios of BIO-treated cell lines were then normalized to the ratios of DMSO-treated cell lines. Three independent transfections were performed, with each sample assayed in triplicate.
Quantitative PCR (qPCR) for relative expression of β-catenin target gene (Axin2) in canine melanoma cell lines
β-Catenin-mediated transcriptional activity in the three canine malignant melanoma cell lines was determined by assessing downstream target gene expression by quantitative polymerase chain reaction (qPCR). Total RNA was isolated from cells using Trizol (Invitrogen), and purified by PureLink RNA Mini Kit (Ambion, Life Technologies) according to the manufacturer’s instructions. cDNA was synthesized from 250 ng of total RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Life Technologies) according to the manufacturer’s protocol. qPCR was performed using TaqMan Gene Expression Master Mix with TaqMan Gene Expression Assays (Applied Biosystems) according to the manufacturer’s protocol on a Bio-Rad iQ5 Multicolor Real-Time PCR Detection System with Bio-Rad iCycler machine and iQ5 software. The gene assessed was canine Axin2 (Cf02631333_m1, Applied Biosystems), and Ct values were normalized to 18S expression (4352930E, Applied Biosystems). Relative differences in mRNA expression of BIO-treated cells were compared and normalized to DMSO-treated (vehicle control) cells using the ΔΔCt method (Yuan et al., 2008). Gene expression of samples was measured in triplicate.
Western blot analysis for Axin2 protein in canine melanoma cell lines
After 72 h of treatment with either BIO 5 µM or equivalent volume of DMSO, cells were lysed using a mammalian protein extraction reagent (MPER; Pierce) combined with a phosphatase and protease inhibitor (Pierce) and protein lysates were collected. Protein lysates (approximately 50 µg) were separated by electrophoresis on a 7.5% SDS polyacrylamide gel (BioRad) at 150 V for approximately 45 min. Proteins were then transferred onto a nitrocellulose membrane (Whatman) at 100 V for 1 h, then blocked with TBST containing 5% non-fat dry milk and 1% bovine serum albumin for 1 h (all reagents from Fisher Scientific). The membranes were probed overnight at 4 °C with either a rabbit polyclonal anti-Axin2 (Conductin) antibody (sc-20784, Santa Cruz Biotechnology) diluted 1:200 in blocking solution or a goat polyclonal anti-actin (sc-1615, Santa Cruz Biotechnology) diluted 1:200 in blocking solution. The antibodies are directed against the human proteins and are expected to cross-react with the canine proteins.
Excess primary antibody was removed by washing three times for 5 min with TBST. Membranes were incubated with 50 ng/mL horseradish peroxidase-conjugated anti-rabbit or anti-goat IgG secondary antibody (Thermo Scientific) diluted in blocking solution for 1 h at room temperature, then washed three times for 5 min with TBST, and treated with Clarity Western ECL Substrate (Bio-Rad). Blots were exposed to film, developed, and then imaged using a Gel Logic 100 Imaging System (Kodak). The Gel Logic 100 Imaging System was used for quantification of band density and comparison of relative protein amounts between BIO- and DMSO-treated cells within each cell line.
Proliferation and chemotoxicity assays
Melanoma cells were plated in 96-well plates at a density such that untreated cells would reach an absorbance of approximately 1.0 at the conclusion of the experiment. To this end, we previously assessed multiple concentrations of each non-treated cell line with the MTS assay (data not shown) and determined the appropriate density of cells to plate to ensure that cells were not dead from overgrowth at the time of assay reading. Melanoma cells were plated in MEM media containing either BIO 5 µM or an equivalent volume of DMSO.
Following a 24 h incubation, media was removed and replaced with fresh MEM media (with 5 µM BIO or equivalent volume DMSO), either without (for proliferation assay) or with carboplatin (Hospira) at concentrations of 0, 0.1, 1.0, 10, 100, and 1000 µM. Forty-eight hours after media replacement, cell viability was assessed by adding 20 µL CellTiter96 AQueous One Solution Cell Proliferation Assay solution (Promega) per 100 µL media to each well.
Following a sufficient incubation period, absorbance was measured at 490 nm on a VersaMax tunable microplate reader using SoftMax Pro 4.7 software (Molecular Devices). Proliferation was assessed using the non-carboplatin treated cells; absorbance in cells incubated for 72 h with 5 µM BIO was compared to those treated with an equivalent volume of DMSO alone. Survival curves for chemotherapy treated cells were generated by dividing the absorbance reading for chemotherapy-treated cells at each concentration by the absorbance for the non-chemotherapy treated cells, with the resultant modified cell curve compared to the control cell curve. Assays were plated in quadruplicate and repeated in triplicate.
Migration and invasion assays
Migration and invasion assays were performed using BD control inserts and BD BioCoat Matrigel Invasion Chambers (BD Biosciences), respectively. Cells were treated with BIO 5 µM or equivalent volume of DMSO for 48 h. The cells were then counted to determine how many microliters of cells were needed to be plated to achieve a density of 5 × 104 cells/insert in EMEM media containing all supplements except serum in triplicate control or Matrigel-coated chambers. CMEM media was used as a chemoattractant. Both plated and attractant media contained 5 µM BIO for treated cells or DMSO for control cells. Plates were incubated for an additional 24 h at 37 °C in 5% CO2, then migrating/invading cells were fixed and stained with Diff-Quik Stain Set (Jorgensen Laboratories) and mounted onto microscope slides.
Cells were imaged on an inverted microscope at 10 × magnification and counted in one center field per insert. Migration was assessed by comparing the average number of migrating cells (counted cells) observed on the three uncoated control inserts per 10 × field for BIO-treated and DMSO-treated control cells. The migration index was calculated by dividing the average number of migrating cells in BIO-treated cells by the average number of migrating cells in the DMSO-treated cells, thereby normalizing the BIO-treated migrating numbers to DMSO-treated control cells.
Invasion was assessed by comparing the ratios of invading cells for BIO-treated and DMSO-treated control cells; this ratio (% invasion) was calculated by dividing the average number of cells present on three Matrigel-coated inserts by the average number of cells present on three uncoated control inserts for both the BIO-treated and DMSO-treated cells. The invasion index was then calculated by dividing the % invasion for BIO-treated cells by the % invasion for DMSO-treated cells, thereby normalizing the invading percentage of BIO-treated cells to DMSO-treated control cells. Assays (each with three control and three Matrigel-coated inserts) were repeated in triplicate.
To ensure that cells were not falling through the membrane pores (8 µm pores), we measured the smallest diameter of the smallest cells visualized on cytospun slides for each cell line. Measurements of the smallest diameter of the four smallest cells visualized in one 40 × field were averaged for each cell line.
Apoptosis assay
Melanoma cells were plated in 15-cm-round culture dishes in CMEM containing either BIO 5 µM or an equivalent volume of DMSO at a density such that approximately 75% confluency would be achieved within 72 h. After a 72 h incubation period, cells were collected and washed with Hank’s Balanced Salt Solution (HBSS) (Mediatech). Samples were divided and four conditions were prepared for analysis via flow cytometry: cells suspended in HBSS only (control), cells incubated with Annexin V-FITC (eBioscience) only (control), cells treated with propidium iodide (eBioscience) only (PI, control), and cells treated with both Annexin V-FITC and PI. Samples were prepared using Annexin V-FITC Apoptosis Detection Kit (eBioscience) according to the manufacturer’s instructions.
Cells were analyzed using FACSCalibur (BD BioSciences) and the cells were excited with the 488 nm laser. Annexin V-FITC was used to determine the percentage of cells undergoing apoptosis, PI was used to identify dead cells, and non-fluorescence detected live cells. The data were analyzed using FlowJo software and the results were reported as a percentage of the cell population. The entire apoptosis assay (from cell growth and treatment to apoptosis assay and flow cytometric evaluation) was repeated in triplicate per cell line per condition (DMSO- and BIO-treated cells).
Statistical analyses
All graphs were generated and statistical analyses were performed using Prism 6 for Mac OS X (GraphPad Software). All data are presented as means ± standard error of the mean (SEM) of three independently performed experiments. A two-way ANOVA test with Sidak post-test was performed on all chemotoxicity and apoptosis experiments. A paired t test was performed for the migration and invasion assays, qPCR, TOP/FOPflash, and proliferation experiments. P < 0.05 was considered statistically significant.
Results
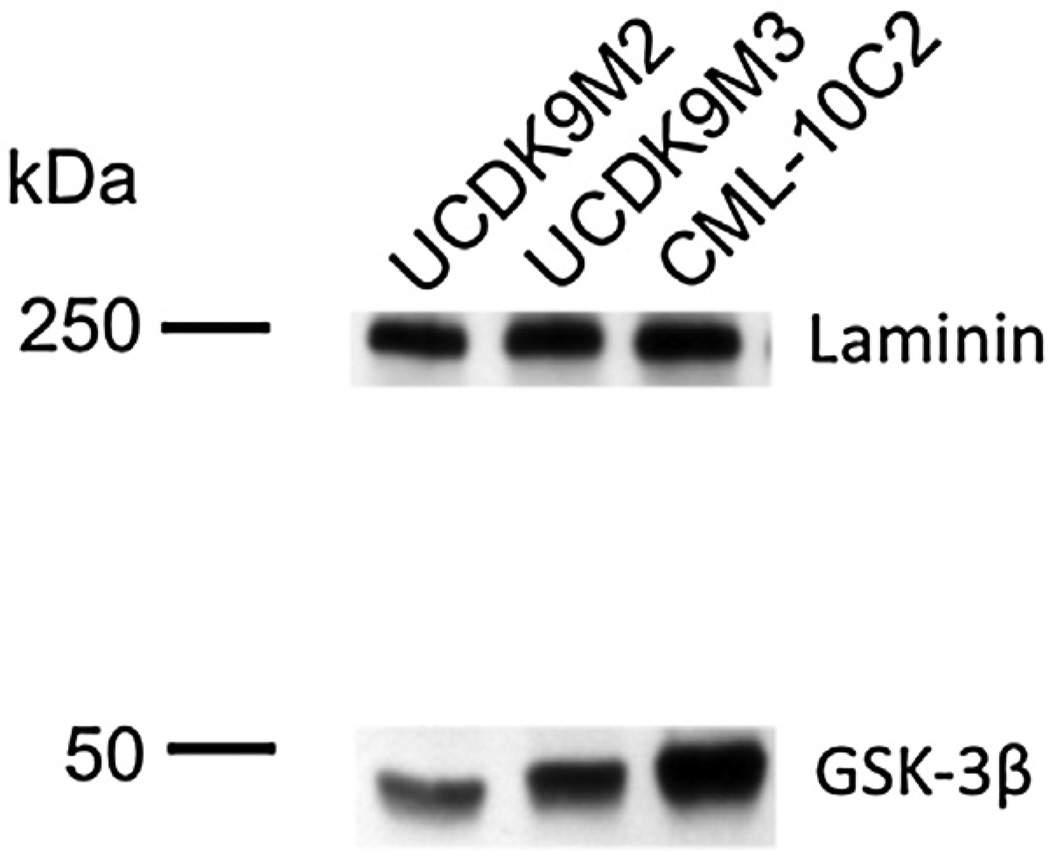
GSK-3β protein expression in canine melanoma cell lines
To validate the presence of GSK-3β protein in the canine melanoma cell lines, we performed Western blot analysis for total GSK-3β (Fig. 1). We confirmed the presence of GSK-3β, a negative regulator of canonical Wnt-signaling and a target of BIO inhibition, in all three canine melanoma cell lines. Therefore, these three cell lines were used in all subsequent experiments.
Fig. 1.
Western blot analysis for total GSK-3β in the canine melanoma cell lines CML-10C2, UCDK9M2, and UCDK9M3.
BIO effects on β-catenin transcriptional activity in canine malignant melanoma cell lines
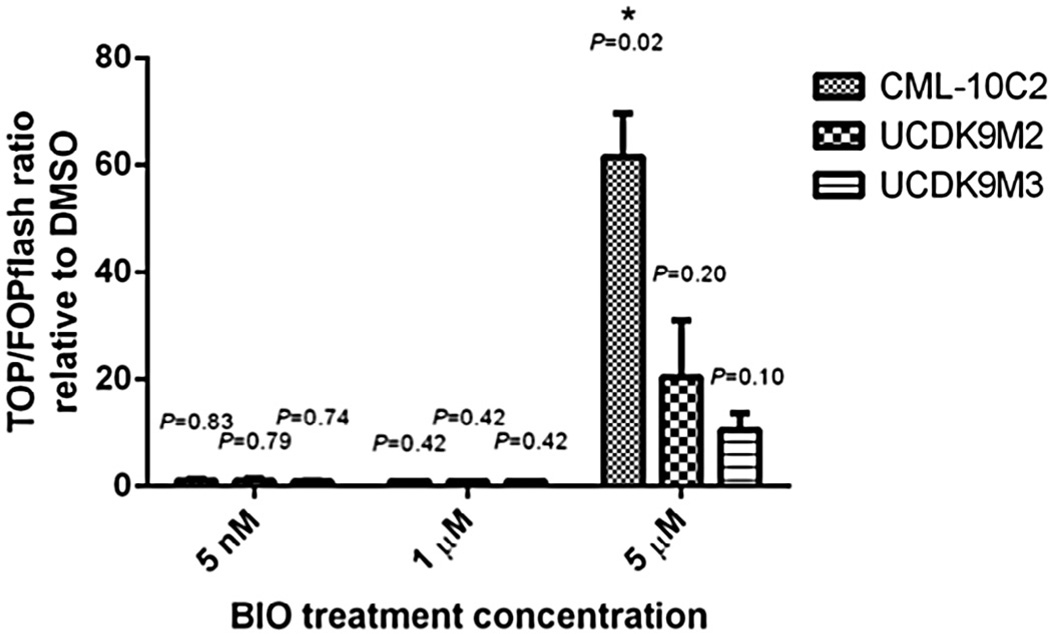
To evaluate the effects of BIO treatment, and thus presumed GSK-3β inhibition, on β-catenin-mediated transcriptional activity, the combination of a TOP/FOPflash luciferase reporter assay with qPCR and Western blot analysis of Axin2 was performed on the three canine melanoma cell lines. For the TOP/FOPflash assay, a ratio > 1 indicates that there is canonical Wnt signaling activity above background level. The TOP/FOPflash ratios of the BIO-treated cells were further normalized to those of the DMSO-treated control cells. Based on these normalized TOP/FOPflash ratios, there was no change in β-catenin-mediated transcriptional activity when cell lines were treated with 5 nM or 1 µM BIO. However, 5 µM BIO treatment increased (approximately 60-fold) β-catenin-mediated transcriptional activity in the CML-10C2 cell line compared to DMSO (vehicle control) treatment (Fig. 2).
Fig. 2.
Relative luciferase reporter assay to assess Wnt/β-catenin pathway activity in canine melanoma cell lines (CML-10C2, UCDK9M2, and UCDK9M3) dosed with 5 nM, 1 µM, and 5 µM BIO for 72 h. TOPflash and FOPflash values were corrected to respective Renilla luminescence as a transfection control. BIO-treated TOP/FOPflash ratios were then normalized to DMSO-treated TOP/FOPflash ratios. Data represent mean ± standard error of the mean from three independent experiments. *P < 0.05.
In the UCDK9M2 and UCDK9M3 cell lines, there was a trend toward increased β-catenin-mediated transcriptional activity in cells treated with 5 µM BIO compared to DMSO, although this difference failed to reach statistical significance (P = 0.2, P = 0.1, respectively). Given the lack of changes in β-catenin-mediated transcriptional activity at 5 nM and 1 µM BIO, the remainder of the experiments were completed using 5 µM BIO.
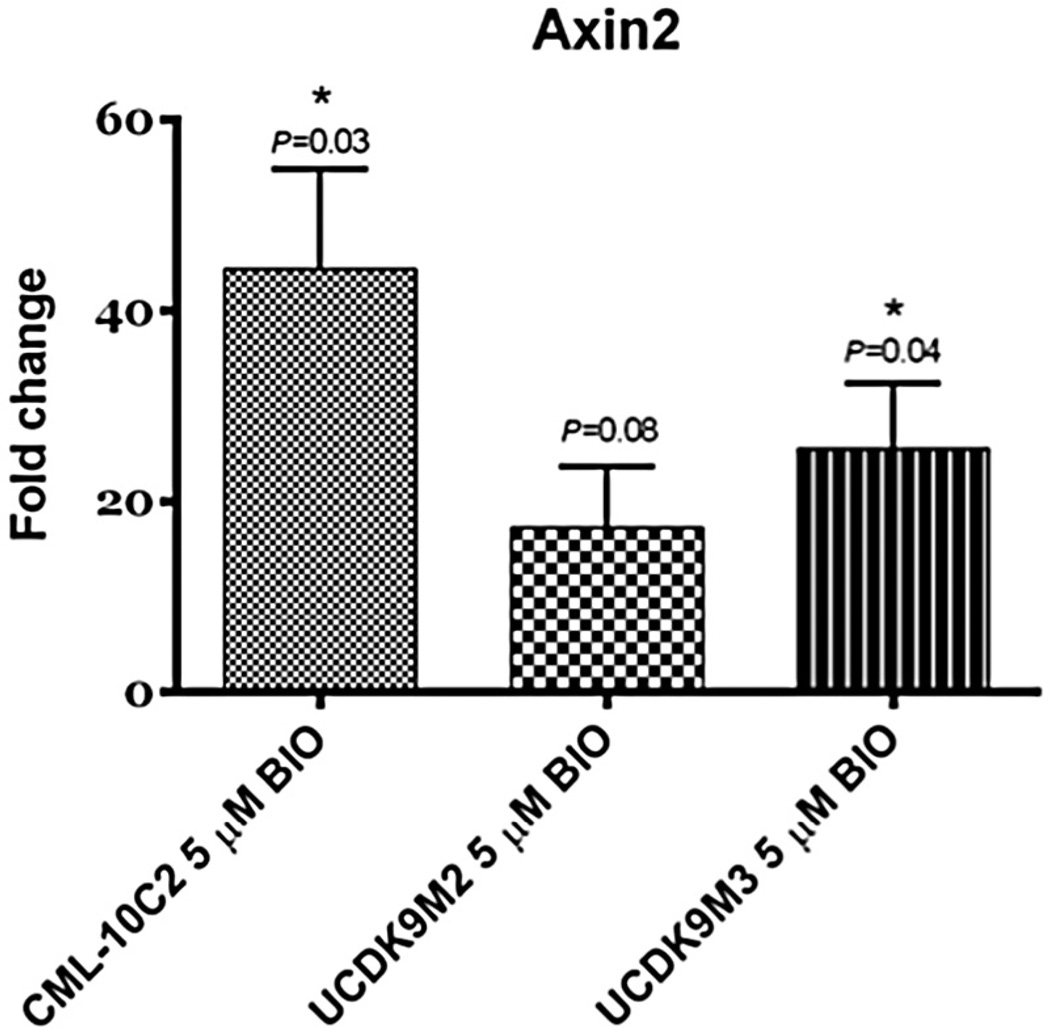
To validate the results of the TOP/FOPflash assays, the expression of a target gene downstream of β-catenin, Axin2, was assessed using qPCR. The relative mRNA expression of Axin2 was significantly increased with BIO treatment in both CML-10C2 and UCDK9M3 cell lines when compared to DMSO-treated cells (Fig. 3). In the UCDK9M2 cell line, Axin2 mRNA expression was increased with BIO treatment; however, the difference failed to reach statistical significance (P = 0.08). In further support of these findings, Axin2 protein was increased in all three BIO-treated cell lines compared to the DMSO-treated cell lines, as shown by increased relative expressions (RE) quantified by densitometry (Fig. 4).
Fig. 3.
Relative expression of the downstream β-catenin target gene, Axin2, in canine melanoma cell lines (CML-10C2, UCDK9M2, and UCDK9M3) treated with 5 µM BIO or equivalent volume of DMSO vehicle control for 72 h. Relative differences in Axin2 mRNA expression of BIO-treated cells were normalized to DMSO-treated cells. Data represent the mean ± standard error of the mean from three independent experiments. *P < 0.05.
Fig. 4.
Western blot analysis for Axin2 in canine melanoma cell lines (CML-10C2, UCDK9M2, and UCDK9M3) treated with 5 µM BIO or equivalent volume of DMSO vehicle control for 72 h. Quantification of blots was performed using the Gel Logic 100 Imaging System (Kodak). Numerical values, i.e. relative expression (RE), represent the density of Axin2 in BIO-treated cells relative to its corresponding DMSO-treated cells of the same cell line, adjusted by the densities of the corresponding loading controls (actin).
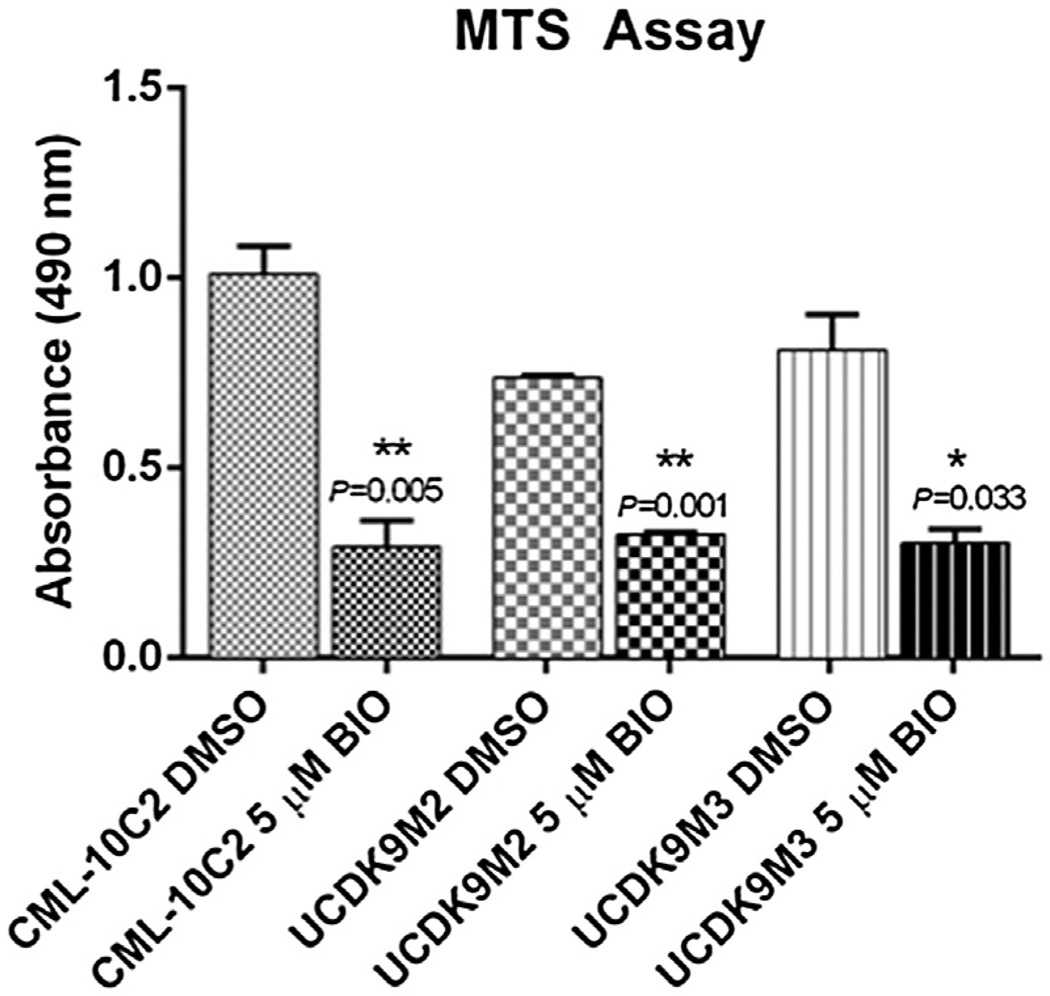
BIO effects on canine melanoma cell behavior
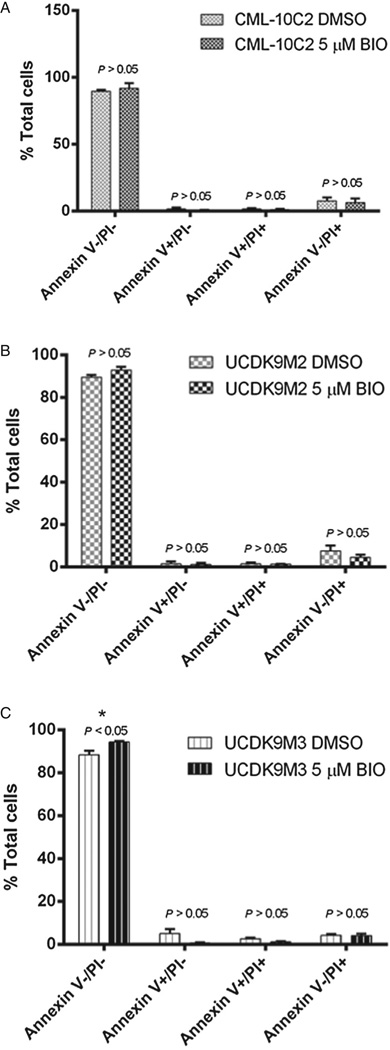
To determine if cell behavior was altered subsequent to BIO treatment, in vitro behavioral assays were performed. These assays included cellular proliferation and apoptosis (assessed at 72 h), chemotoxicity to carboplatin, migration, and invasion. BIO treatment of all three cell lines resulted in decreased cellular proliferation relative to control-treated cells (P < 0.05; Fig. 5). To determine whether decreased cell proliferation resulted from cells undergoing apoptosis, cells were treated with Annexin-V and PI. There was no difference in apoptosis of any of the three cell lines between the BIO- and control-treated cells (Figs. 6A – C). In the UCDK9M3 cell line, there was an increased percentage of viable cells in the BIO-treated cells compared to the DMSO-treated control group; this difference was not noted in the other two cell lines, nor was there a difference in early or late apoptotic cells in any of the three lines.
Fig. 5.
MTS assay assessing proliferation in canine melanoma cells treated with either 5 µM BIO or equivalent volume of DMSO for 72 h. Results show decreased proliferation in cells treated with 5 µM BIO for all three melanoma cell lines. Data represent the mean ± standard error of the mean from three independent experiments. *P < 0.05, **P < 0.01.
Fig. 6.
Apoptosis assessed with flow cytometry in three melanoma cell lines (A: CML-10C2; B: UCDK9M2; C: UCDK9M3) treated with 5 µM BIO or equivalent volume of DMSO for 72 h. Analysis with FACSCalibur after excitation with 488 nm laser showed no significant difference in percentage of late apoptotic cells between BIO- vs. DMSO-treated cells. Data represent the mean ± standard error of the mean from three independent experiments. *P < 0.05.
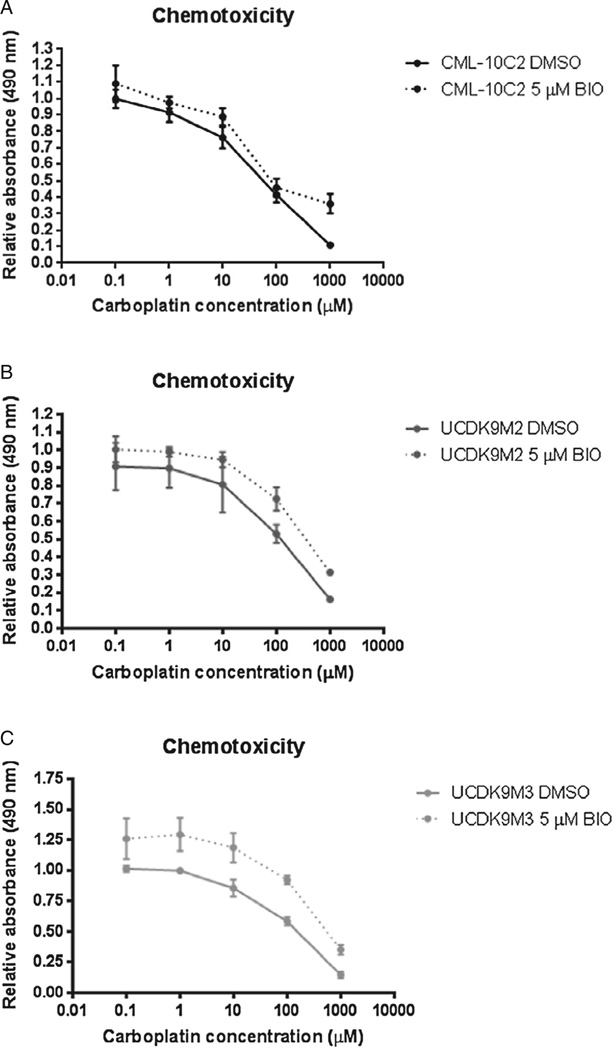
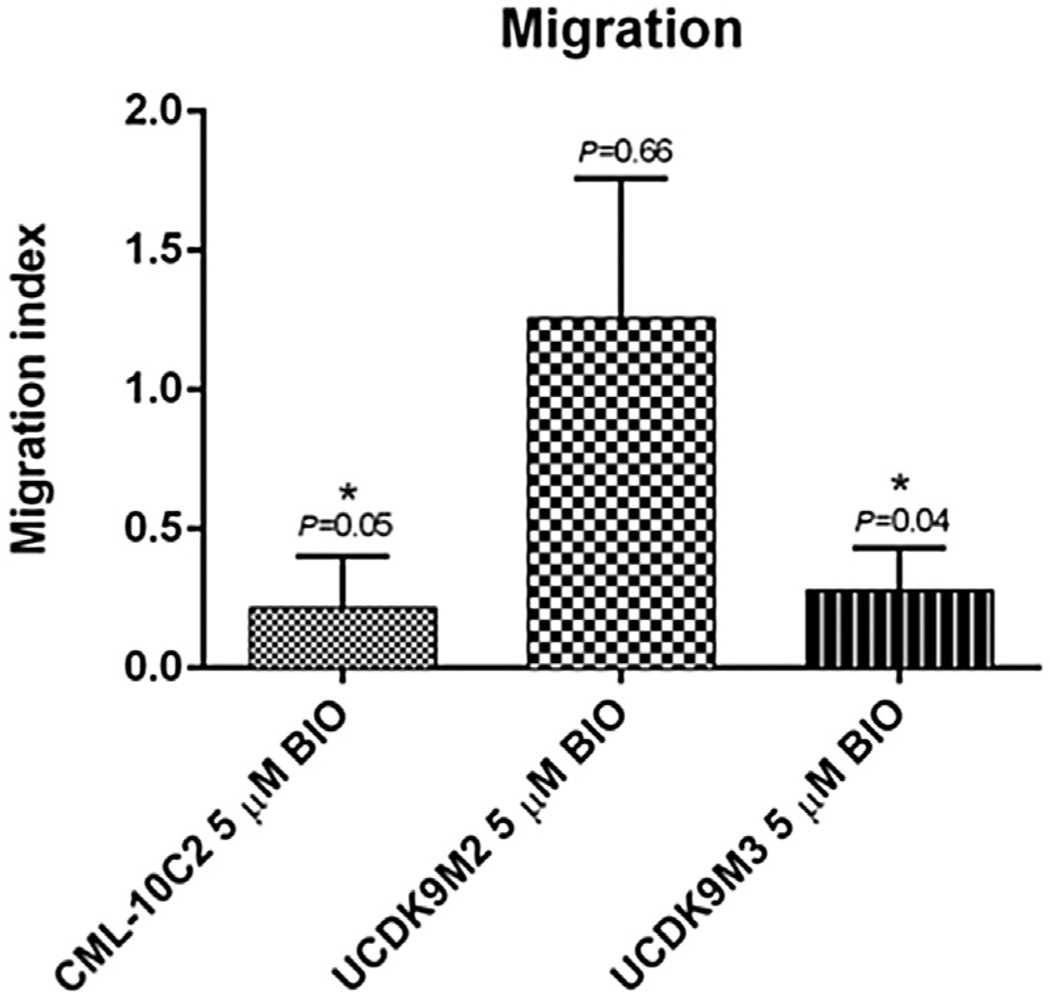
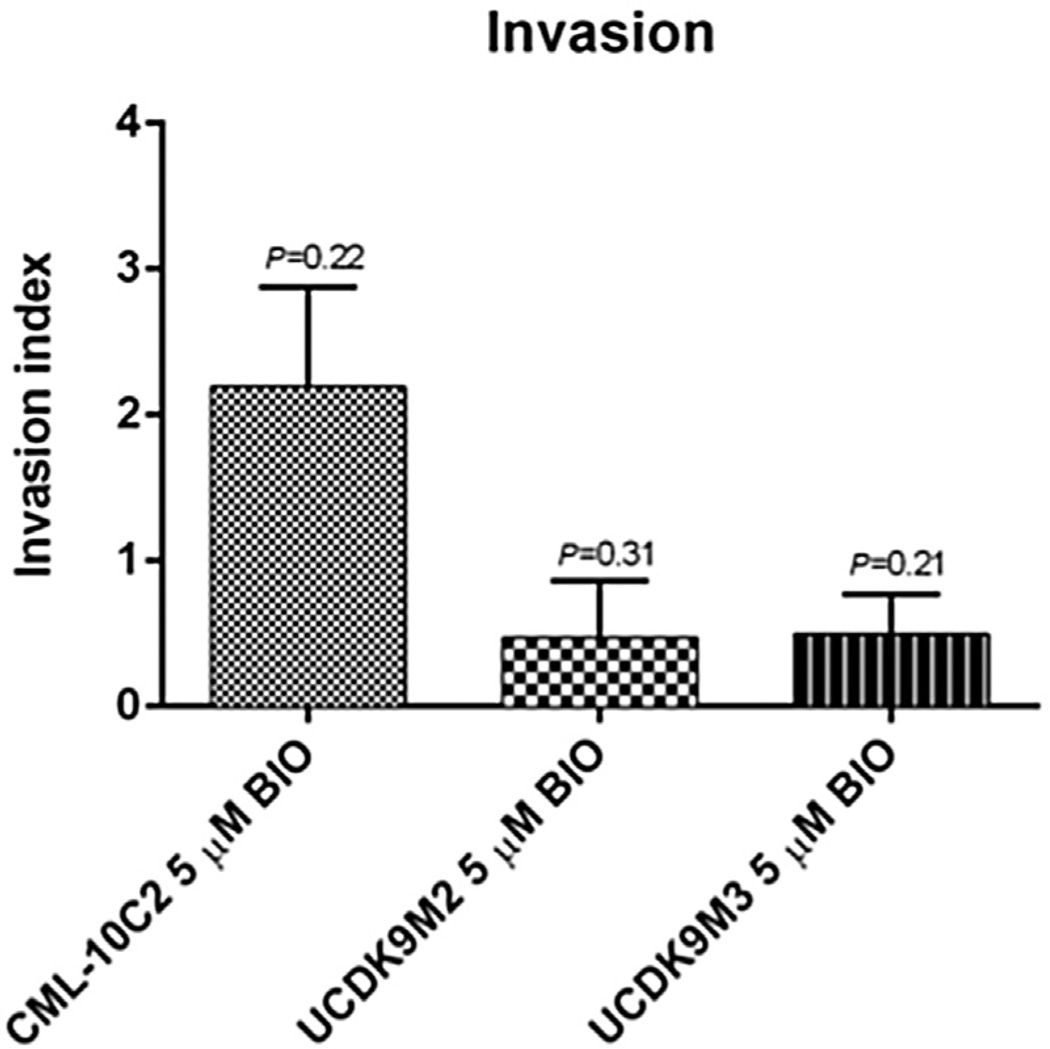
Control and BIO-treated cells displayed a dose-dependent response to carboplatin chemotherapy (Figs. 7A – C). However, BIO treatment did not change carboplatin chemotoxicity of any of the three cell lines relative to control-treated cells (Figs. 7A – C). BIO treatment of CML-10C2 and UCDK9M3 cell lines reduced their ability to migrate compared to control-treated cells (Fig. 8). There was no difference in the migration ability of BIO-treated UCDK9M2 cells (Fig. 8). BIO treatment did not impact the invasion of any of the three cells lines (Fig. 9). Mean cell counts ± SEM for three independent trials performed in triplicate for each cell line per condition (DMSO or BIO) for both the migration and invasion assays are shown in Table 1.
Fig. 7.
Assessment of chemotoxicity to varying concentrations of carboplatin in three melanoma cell lines (A: CML-10C2; B: UCDK9M2; C: UCDK9M3) showed no significant difference in chemotoxicity between melanoma cells treated with 5 µM BIO or equivalent volume of DMSO for 72 h. Data represent the mean ± standard error of the mean from three independent experiments.
Fig. 8.
Migration assay using uncoated BD control inserts revealed significantly decreased migration in melanoma cells treated with 5 µM BIO or equivalent volume of DMSO for 72 h in two melanoma cell lines CML-10C2 and UCDK9M3. Data represent the mean ± standard error of the mean from three independent experiments. *P < 0.05.
Fig. 9.
Invasion assay using BD BioCoat Matrigel-coated inserts revealed no significant difference in the invasiveness of melanoma cells treated with 5 µM BIO or equivalent volume of DMSO for 72 h. Data represent the mean ± standard error of the mean from three independent experiments.
Table 1.
Number of cells counted for the three canine melanoma cell lines under dimethylsulfoxide (DMSO) and 6-bromoindirubin-3′oxime (BIO)-treated conditions for migration and invasion assays.
| CML-10C2 DMSO | CML-10C2 5 µM BIO | UCDK9M2 DMSO | UCDK9M2 5 µM BIO | UCDK9M3 DMSO | UCDK9M3 5 µM BIO | |
|---|---|---|---|---|---|---|
| Migration | 361.6 ± 76.4 | 28.9 ± 7.2 | 194.6 ± 38.1 | 187.3 ± 68.6 | 458.6 ± 119.6 | 194.9 ± 81.7 |
| Invasion | 218 ± 58.7 | 38.1 ± 10.8 | 144.4 ± 40.7 | 28.4 ± 7.7 | 218.8 ± 65.6 | 23.0 ± 6.4 |
Data are presented as the mean ± standard error of mean for three independent trials performed in triplicate.
To ensure that cells were not falling through the 8 µm pores of the migration and invasion assay insert membranes, the smallest diameter of the smallest cells visualized on cytospun slides was measured. The average smallest cell diameters for cell lines UCDK9M2, UCDK9M3, and CML-10C2 were larger than 8 µm (14, 15.3 and 14.7 µm, respectively), confirming that the cells were not too small to fall through the membrane pores.
Discussion
Canine oral malignant melanoma is a highly aggressive disease that is currently associated with a poor prognosis due to an inability to adequately control local and distant disease. While immunomodulatory therapy for canine malignant melanoma may hold promise, the true impact of this approach is not yet fully understood. Targeted therapies in the form of tyrosine kinase inhibitors (e.g. vemurafenib for BRAF-mutations) or tumor-specific responses with immunomodulating agents (e.g. ipilimumab) have had greater success for human melanoma patients, particularly for late-stage patients (Ascierto et al., 2013). However, such therapies are not entirely applicable to dogs due to the differences in molecular mechanisms that contribute to canine melanoma formation (Richter et al., 2005; Shelly et al., 2005). New therapeutic advances that target specific pathways involved in disease development and progression are thus necessary for the treatment of malignant melanoma in dogs as well as people.
GSK-3β plays an important role in suppressing the canonical Wnt signaling pathway by its ability to phosphorylate β-catenin, thereby targeting β-catenin for degradation. As we have previously reported, β-catenin-transcriptional activity is minimal in canine malignant melanoma (Chon et al., 2013). Importantly, a number of in vitro and in vivo studies have demonstrated that BIO, a selective and potent inhibitor of GSK-3β, has anti-oncogenic effects against breast and pancreatic carcinoma stem cells, osteosarcoma, and melanoma (Liu et al., 2011; Cao et al., 2012; Dastjerdi et al., 2012; Nicolaou et al., 2012). However, its effect has not been assessed on canine malignant melanoma cell lines. Therefore BIO may represent a logical compound for evaluation as it has known anti-cancer effects both in vitro and in vivo and it targets a pathway known to be suppressed in canine malignant melanoma (Liu et al., 2011; Chon et al., 2013).
Our findings indicate that the canonical Wnt signaling pathway is activated in canine melanoma cell lines, as evidenced by increased β-catenin-mediated transcriptional activity. This finding is significant, as the activation of the canonical Wnt signaling pathway has been associated with reduced cell proliferation and improved survival in humans with melanoma (Chien et al., 2009). The reduced proliferation and improved survival associated with activated canonical Wnt signaling in melanoma may be due to a more differentiated phenotype and therefore, less aggressive tumor (Chien et al., 2009; O’Connell and Weeraratna, 2009). Therefore, the upregulation of canonical Wnt signaling may be an attractive therapeutic target.
In addition to the finding that BIO treatment can activate the canonical Wnt signaling pathway in canine melanoma cells, the behavior of canine melanoma cell lines was affected by BIO treatment. BIO treatment decreased cellular proliferation in all three of the cell lines tested. The decreased cell proliferation was not associated with changes in apoptosis, suggesting that BIO acts in a cytostatic manner against the canine malignant melanoma cell lines. In the flow cytometric assay, one of the cell lines (UCDK9M3) had a higher percentage of viable cells compared to the DMSO-treated control cells, in contrast to the other two cell lines. The mechanism is unclear, although it is possible that the non-treated cells were starting to die due to over-growth, whereas the BIO treatment by slowing cell growth was preventing cell over-growth and artificially enhancing viability relative to non-treated controls.
With the exception of the UCDK9M3 cell line, our findings correlate with those of Liu et al. (2011), who noted reduced cell viability in human melanoma cell lines treated with BIO at concentrations ranging from 5 to 20 µM. However, in that study a concurrent increase in apoptosis of BIO-treated cell lines was noted in contrast to our findings. In our study, BIO-treatment reduced cell migration in two of the three cell lines (CML-10C2 and UCDK9M3), but had no detectable effect on invasion or carboplatin chemosensitiv-ity. The small, but not statistically significant, increase in resistance to carboplatin chemotherapy in BIO-treated cells may be secondary to decreased cell proliferation and the resulting lower sensitivity to chemotherapy. Importantly, BIO treatment has been shown to maintain its anti-melanoma activity in vivo as melanoma tumor growth was suppressed in a mouse xenograft model (Liu et al., 2011).
Two limitations must be highlighted when interpreting the results of this study. Firstly, GSK-3β inhibition may result in the modulation of non-β-catenin-associated pathways. Therefore, the resulting phenotype cannot be solely attributed to the enhancement of canonical Wnt pathway activity. The NF-κB pathway is another prominent signaling pathway that utilizes GSK-3β as a key regulator. In osteosarcoma, GSK-3β has been found to enhance the activity of NF-κB, which is an important regulator of genes involved with cell proliferation and survival (Tang et al., 2012). Tang et al. (2012) demonstrated that inhibition of GSK-3β activity reduced the aggressiveness of human osteosarcoma cell lines, perhaps through its impact on NF-κB activation. Recently, Ito et al. (2013) have demonstrated that bortezomib treatment reduces the viability of canine melanoma cell lines, potentially through inhibition of NF-κB (Ito et al., 2013). It is possible that inhibition of NF-κB pathway activity has contributed to the resulting phenotype in our study, either alone or in combination with inhibition of the Wnt signaling pathway. Further experiments are required to address this limitation.
Secondly, the specificity of BIO for GSK-3β at the concentration used for the behavioral assays is unclear. The reported half maximal inhibitory concentration (IC50) for GSK-3β inhibition by BIO is 5 nM (Meijer et al., 2003). In our study, increased β-catenin-associated transcriptional activity was observed at 5 µM, which is a 1000-times greater concentration than the reported IC50 concentration for GSK-3β inhibition of this compound. It is conceivable that much higher concentrations of BIO are required for GSK-3β inhibition in canine malignant melanoma cells if there is aberrant activation of GSK-3β. Alternatively, these BIO-associated phenotypes may be the result of inhibiting non-GSK-3β targets, such as other tyrosine or serine-threonine kinases. Further studies are necessary to address this possibility and to potentially identify these kinases.
In human melanoma cell lines, BIO treatment is known to affect the JAK/STAT signaling pathway (Liu et al., 2011). According to Liu et al. (2011), activities of JAK family kinases were inhibited in a dose-dependent manner with IC50 values ranging from 0.3 µM to 8 µM for the different JAK kinases, suggesting that BIO is a pan-JAK small-molecule inhibitor. In that study, GSK-3β activity was not assessed and, similar to our study, they could not exclude the possibility that inhibition of other signaling pathways may have contributed to their results.
An additional concern regarding the clinical relevance of our findings is the ability to safely reach in vivo the BIO concentrations used in our studies. While additional pharmacodynamic and pharma-cokinetic studies are necessary, Liu et al. (2011) safely administered BIO in an approximately 20 mM solution via oral gavage to mice and demonstrated biological activity. Experiments to specifically inhibit GSK-3β (for example, GSK-3β knockdown through RNA interference) are warranted to determine the specific contribution of GSK-3β inhibition on these cellular behaviors, and we are conducting such experiments to further elucidate the role of GSK-3β in canine malignant melanoma.
Conclusions
BIO treatment can activate the Wnt/β-catenin signaling pathway in canine melanoma cell lines. Furthermore, BIO treatment of canine malignant melanoma cell lines is associated with decreased cell proliferation and migration. Although the phenotypic alterations of canine malignant melanoma cell lines treated with BIO cannot be attributed specifically to GSK-3β inhibition at this time, the findings suggest that BIO or similar kinase inhibitors represent promising candidate molecules to evaluate in canine malignant melanoma. However, further studies are necessary to determine the specific pathway or pathways that are responsible for the observed phenotype.
Acknowledgements
The authors wish to thank Drs. M. Kent and L. Wolfe for providing the cell lines utilized in this study. This project was supported by a grant from the Morris Animal Foundation (D13CA-030) (EC, TJS), a post-doctoral training fellowship grant from the Morris Animal Foundation (D14CA-405) (EC, TJS), the Merial-NIH Summer Scholars Program grant NIH T35 OD 011078 (BF, TJS), and in part by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427 (TJS). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the study sponsors. The study sponsors had no role in the study design, writing or the manuscript, interpretation of data, writing of the manuscript, or decision to submit. These data were presented in part at the Veterinary Cancer Society Annual Meeting, Minneapolis, MN, 2013.
Footnotes
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
References
- Alexander AN, Huelsmeyer MK, Mitzey A, Dubielzig RR, Kurzman ID, MacEwen EG, Vail DM. Development of an allogeneic whole-cell tumor vaccine expressing xenogeneic gp100 and its implementation in a phase II clinical trial in canine patients with malignant melanoma. Cancer Immunology and Immunotherapy. 2006;55:433–442. doi: 10.1007/s00262-005-0025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastas JN, Moon RT. Wnt signalling pathways as therapeutic targets in cancer. Nature Reviews Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- Ascierto PA, Bastholt L, Hersey P, Cinat G, Eggermont AM, Hauschild A, Espinosa E, Robert C. Side effects and toxicities of targeted therapies in stage IV melanoma. American Journal of Therapeutics. 2013 doi: 10.1097/MJT.0b013e3182a39858. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Bateman KE, Catton PA, Pennock PW, Kruth SA. 0-7-21 Radiation therapy for the treatment of canine oral melanoma. Journal of Veterinary Internal Medicine. 1994;8:267–272. doi: 10.1111/j.1939-1676.1994.tb03231.x. [DOI] [PubMed] [Google Scholar]
- Bergman PJ, McKnight J, Novosad A, Charney S, Farrelly J, Craft D, Wulderk M, Jeffers Y, Sadelain M, Hohenhaus AE, et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: A phase I trial. Clinical Cancer Research. 2003;9:1284–1290. [PubMed] [Google Scholar]
- Bergman PJ, Camps-Palau MA, McKnight JA, Leibman NF, Craft DM, Leung C, Liao J, Riviere I, Sadelain M, Hohenhaus AE, et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine. 2006;24:4582–4585. doi: 10.1016/j.vaccine.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Brockley LK, Cooper MA, Bennett PF. Malignant melanoma in 63 dogs (2001–2011): The effect of carboplatin chemotherapy on survival. New Zealand Veterinary Journal. 2013;61:25–31. doi: 10.1080/00480169.2012.699433. [DOI] [PubMed] [Google Scholar]
- Cao H, Chu Y, Lv X, Qiu P, Liu C, Zhang H, Li D, Peng S, Dou Z, Hua J. GSK3 inhibitor-BIO regulates proliferation of immortalized pancreatic mesenchymal stem cells (iPMSCs) Public Library of Science One. 2012;7:e31502. doi: 10.1371/journal.pone.0031502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglia D, Bernardini S, Alvino E, Pagani E, De Luca N, Falcinelli S, Pacchiarotti A, Bonmassar E, Zambruno G, D’Atri S. Concomitant activation of Wnt pathway and loss of mismatch repair function in human melanoma. Genes, Chromosomes and Cancer. 2008;47:614–624. doi: 10.1002/gcc.20567. [DOI] [PubMed] [Google Scholar]
- Chia IV, Costantini F. Mouse Axin and Axin2/conductin proteins are functionally equivalent in vivo. Molecular and Cellular Biology. 2005;25:4371–4376. doi: 10.1128/MCB.25.11.4371-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, Major MB, Hwang ST, Rimm DL, Moon RT. Activated Wnt/Beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1193–1198. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chon E, Thompson V, Schmid S, Stein TJ. Activation of the canonical wnt/beta-catenin signalling pathway is rare in canine malignant melanoma tissue and cell lines. Journal of Comparative Pathology. 2013;148:178–187. doi: 10.1016/j.jcpa.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dank G, Rassnick KM, Sokolovsky Y, Garrett LD, Post GS, Kitchell BE, Sellon RK, Kleiter M, Northrup N, Segev G. Use of adjuvant carboplatin for treatment of dogs with oral malignant melanoma following surgical excision. Veterinary and Comparative Oncology. 2014;12:78–84. doi: 10.1111/j.1476-5829.2012.00338.x. [DOI] [PubMed] [Google Scholar]
- Dastjerdi FV, Zeynali B, Tafreshi AP, Shahraz A, Chavoshi MS, Najafabadi IK, Vardanjani MM, Atashi A, Soleimani M. Inhibition of GSK-3beta enhances neural differentiation in unrestricted somatic stem cells. Cell Biology International. 2012;36:967–972. doi: 10.1042/CBI20110541. [DOI] [PubMed] [Google Scholar]
- Freeman KP, Hahn KA, Harris FD, King GK. Treatment of dogs with oral melanoma by hypofractionated radiation therapy and platinum-based chemotherapy (1987–1997) Journal of Veterinary Internal Medicine. 2003;17:96–101. doi: 10.1892/0891-6640(2003)017<0096:todwom>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Grosenbaugh DA, Leard AT, Bergman PJ, Klein MK, Meleo K, Susaneck S, Hess PR, Jankowski MK, Jones PD, Liebman NF, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. American Journal of Veterinary Research. 2011;72:1631–1638. doi: 10.2460/ajvr.72.12.1631. [DOI] [PubMed] [Google Scholar]
- Harvey HJ, MacEwen EG, Braun D, Patnaik AK, Withrow SJ, Jongeward S. Prognostic criteria for dogs with oral melanoma. Journal of the American Veterinary Medical Association. 1981;178:580–582. [PubMed] [Google Scholar]
- Huang KT, Huang YH, Li P, He B, Chen ZK, Yu X, Chen JO, Zhang QY, Shi QY, Shan YF. The correlation between TSC2 and GSK3 levels, and outcomes of patients with hepatocellular carcinoma treated by hepatectomy. Hepatology Research. 2013 doi: 10.1111/hepr.12256. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Current Opinion in Genetics and Development. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Ito K, Kobayashi M, Kuroki S, Sasaki Y, Iwata T, Mori K, Kuroki T, Ozawa Y, Tetsuka M, Nakagawa T, et al. The proteosome inhibitor bortezomib inhibits the growth of canine malignant melanoma cells in vitro and in vivo. The Veterinary Journal. 2013;198:577–582. doi: 10.1016/j.tvjl.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Frend JN, Costantini F. Wnt/β-catenin/ TCF signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Molecular and Cellular Biology. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nature Reviews. Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- Kosovsky JK, Matthiesen DT, Marretta SM, Patnaik AK. Results of partial mandibulectomy for the treatment of oral tumors in 142 dogs. Veterinary Surgery. 1991;20:397–401. doi: 10.1111/j.1532-950x.1991.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Kotliarova S, Pastorino S, Kovell LC, Kotliarov Y, Song H, Zhang W, Bailey R, Maric D, Zenklusen JC, Lee J, et al. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-kappaB, and glucose regulation. Cancer Research. 2008;68:6643–6651. doi: 10.1158/0008-5472.CAN-08-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Activation of AXIN2 expression by β-catenin-T cell factor: A feedback repressor pathway regulating Wnt signaling. Journal of Biological Chemistry. 2002;277:21657–21665. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- Liu L, Nam S, Tian Y, Yang F, Wu J, Wang Y, Scuto A, Polychonopoulos P, Magiatis P, Skaltsounis L, et al. 6-Bromoindirubin-3′-oxime inhibits JAK/STAT3 signaling and induces apoptosis of human melanoma cells. Cancer Research. 2011;71:3972–3979. doi: 10.1158/0008-5472.CAN-10-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten UWM, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/Axin2 in colorectal and liver tumors. Molecular and Cellular Biology. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Developmental Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEwen EG, Patnaik AK, Harvey HJ, Hayes AA, Matus R. Canine oral melanoma: Comparison of surgery versus surgery plus Corynebacterium parvum. Cancer Investigation. 1986;4:397–402. doi: 10.3109/07357908609017520. [DOI] [PubMed] [Google Scholar]
- Madhunapantula SV, Sharma A, Gowda R, Robertson GP. Identification of glycogen synthase kinase 3 alpha as a therapeutic target in melanoma. Pigment Cell and Melanoma Research. 2013;26:886–899. doi: 10.1111/pcmr.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L, Skaltsounis AL, Magiatis P, Polychonopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivaniou A, Dajani R, et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chemistry and Biology. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Nicolaou KA, Liapis V, Evdokiou A, Constantinou C, Magiatis P, Skaltsounis AL, Koumas L, Costeas PA, Constantinou AI. Induction of discrete apoptotic pathways by bromo-substituted indirubin derivatives in invasive breast cancer cells. Biochemical and Biophysical Research Communications. 2012;425:76–82. doi: 10.1016/j.bbrc.2012.07.053. [DOI] [PubMed] [Google Scholar]
- O’Connell MP, Weeraratna AT. Hear the Wnt Ror: How melanoma cells adjust to changes in Wnt. Pigmented Cell and Melanoma Research. 2009;22:724–739. doi: 10.1111/j.1755-148X.2009.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottnod JM, Smedley RC, Walshaw R, Hauptman JG, Kiupel M, Obradovich JE. A retrospective analysis of the efficacy of Oncept vaccine for the adjunct treatment of canine oral malignant melanoma. Veterinary and Comparative Oncology. 2013;11:219–229. doi: 10.1111/vco.12057. [DOI] [PubMed] [Google Scholar]
- Quintayo MA, Munro AF, Thomas J, Kunkler IH, Jack W, Kerr GR, Dixon JM, Chetty U, Bartlett JM. GSK3 and cyclin D1 expression predicts outcome in early breast cancer patients. Breast Cancer Research and Treatment. 2012;136:161–168. doi: 10.1007/s10549-012-2229-8. [DOI] [PubMed] [Google Scholar]
- Ramos-Vara JA, Beissenherz ME, Miller MA, Johnson GC, Pace LW, Fard A, Kottler SJ. Retrospective study of 338 canine oral melanomas with clinical, histologic, and immunohistochemical review of 129 cases. Veterinary Pathology. 2000;37:597–608. doi: 10.1354/vp.37-6-597. [DOI] [PubMed] [Google Scholar]
- Richter A, Murua Escobar H, Gunther K, Soller JT, Winkler S, Nolte I, Bullerdiek J. RAS gene hot-spot mutations in canine neoplasias. Journal of Heredity. 2005;96:764–765. doi: 10.1093/jhered/esi121. [DOI] [PubMed] [Google Scholar]
- Salim T, Sjolander A, Sand-Dejmek J. Nuclear expression of glycogen synthase kinase-3beta and lack of membranous beta-catenin is correlated with poor survival in colon cancer. International Journal of Cancer. 2013;133:807–815. doi: 10.1002/ijc.28074. [DOI] [PubMed] [Google Scholar]
- Shelly S, Chien MB, Yip B, Kent MS, Theon AP, McCallan JL, London CA. Exon 15 BRAF mutations are uncommon in canine oral malignant melanomas. Mammalian Genome. 2005;16:211–217. doi: 10.1007/s00335-004-2441-x. [DOI] [PubMed] [Google Scholar]
- Smedley RC, Spangler WL, Esplin DG, Kitchell BE, Bergman PJ, Ho H-Y, Bergin IL, Kiupel M. Prognostic markers for canine melanocytic neoplasms: A comparative review of the literature and goals for future investigation. Veterinary Pathology. 2011;48:54–72. doi: 10.1177/0300985810390717. [DOI] [PubMed] [Google Scholar]
- Smith SH, Goldschmidt MH, McManus PM. A comparative review of melanocytic neoplasms. Veterinary Pathology. 2002;39:651–678. doi: 10.1354/vp.39-6-651. [DOI] [PubMed] [Google Scholar]
- Tang QL, Xie XB, Wang J, Chen Q, Han AJ, Zou CY, Yin JQ, Liu DW, Liang Y, Zhao ZQ, et al. Glycogen synthase kinase-3beta, NF-kappaB signaling, and tumorigenesis of human osteosarcoma. Journal of the National Cancer Institute. 2012;104:749–763. doi: 10.1093/jnci/djs210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todoroff RJ, Brodey RS. Oral and pharyngeal neoplasia in the dog: A retrospective survey of 361 cases. Journal of the American Veterinary Medical Association. 1979;175:567–571. [PubMed] [Google Scholar]
- Tsai KH, Hsien HH, Chen LM, Ting WJ, Yang YS, Kuo CH, Tsai CH, Tsai FJ, Tsai HJ, Huang CY. Rhubarb inhibits hepatocellular carcinoma cell metastasis via GSK-3-beta activation to enhance protein degradation and attenuate nuclear translocation of beta-catenin. Food Chemistry. 2013;138:278–285. doi: 10.1016/j.foodchem.2012.10.038. [DOI] [PubMed] [Google Scholar]
- Wallace J, Matthiesen DT, Patnaik AK. Hemimaxillectomy for the treatment of oral tumors in 69 dogs. Veterinary Surgery. 1992;21:337–341. doi: 10.1111/j.1532-950x.1992.tb01707.x. [DOI] [PubMed] [Google Scholar]
- Yuan JS, Wang D, Stewart CN. Statistical methods for efficiency adjusted real-time PCR quantification. Biotechnology Journal. 2008;3:112–123. doi: 10.1002/biot.200700169. [DOI] [PubMed] [Google Scholar]