Abstract
Purpose
Pre-clinical studies combining the proteasome inhibitor bortezomib with anthracyclines have shown enhanced anti-tumor activity. We therefore conducted a phase I trial of bortezomib and pegylated liposomal doxorubicin (PLD) in patients with refractory solid tumors.
Methods
Patients received bortezomib, 0.9-1.5 mg/m2, on days 1, 4, 8, and 11 of every 21-day cycle, along with PLD, 30 mg/m2, on day 4. The goals were to determine the dose limiting toxicity (DLT) and maximum tolerated dose (MTD), and to investigate pharmacokinetic and pharmacodynamic interactions of the combination.
Results
A total of 37 patients with 4 median prior therapies were treated. Frequent grade 1-2 toxicities included fatigue, nausea, thrombocytopenia, anemia, neutropenia, constipation, myalgias, and peripheral neuropathy. DLTs included grade 3 nausea and vomiting in 1/6 patients receiving bortezomib at 1.2 mg/m2, and grade 3 nausea, vomiting, and diarrhea in 1/6 patients receiving bortezomib at 1.5 mg/m2. Grade 3 toxicities in later cycles included hand-foot syndrome, thrombocytopenia, anemia, neutropenia, nausea, diarrhea, and abdominal pain. Because of frequent dose-delays, dose-reductions, and gastrointestinal toxicity at the 1.4 and 1.5 mg/m2 levels, bortezomib at 1.3 mg/m2 and PLD at 30 mg/m2 are recommended for further testing. Among 19 patients with breast cancer, four had evidence of a clinical benefit. Pharmacokinetic and pharmacodynamic studies did not show any significant interactions between the two drugs.
Conclusions
A regimen of bortezomib, 1.3 mg/m2 on days 1, 4, 8, and 11 with PLD, 30 mg/m2, on day 4 of a 21-day cycle, was safe in this study, and merits further investigation.
Keywords: phase I, proteasome inhibition, bortezomib, pegylated liposomal doxorubicin, breast cancer
Introduction
Bortezomib (VELCADE®; Millennium Pharmaceuticals, Inc. and Johnson & Johnson Pharmaceuticals Research & Development, L.L.C.) is a dipeptide boronic acid derivative that specifically inhibits the chymotrypsin-like activity of the proteasome (1), a large multi-catalytic proteinase complex responsible for intracellular proteolysis. Proteasome blockade has anti-neoplastic effects through inhibition of several pathways, including growth signaling through the p44/42 mitogen-activated protein kinase (MAPK), cell cycling through stabilization of cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1, survival signaling through Bcl-2 and nuclear factor kappa-B (NF-κB), and angiogenesis. Bortezomib has shown anti-tumor activity in a wide variety of preclinical models both in vitro and in vivo. In clinical trials, single-agent bortezomib has been effective against hematologic malignancies, most notably multiple myeloma (2, 3), for which bortezomib initially received regulatory approval, and several subtypes of non-Hodgkin lymphoma (4-6). Bortezomib has been approved by the FDA both for the treatment of multiple myeloma patients after at least one prior therapy and for the treatment of mantle cell lymphoma patients after at least one prior therapy. Some activity has also been seen in solid tumors, including prostate cancer (7), non-small cell lung cancer (8), renal cell carcinoma (9), and ovarian cancer (10). The most common regimen in hematological malignancies uses bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 of a 21-day cycle, while the maximum tolerated dose (MTD) in solid tumor patients has been defined at 1.50 or 1.56 mg/m2 (10, 11). Common toxicities include gastrointestinal symptoms, fatigue, thrombocytopenia, and sensory neuropathy.
Modulation of proteasome function has been shown to enhance chemosensitivity, and to overcome chemoresistance. By inducing phosphorylation and cleavage of Bcl-2, preventing chemotherapy-mediated activation of NF-κB, and inhibiting normal maturation of P-glycoprotein, proteasome inhibitors have been shown to have additive to synergistic activity in combination with standard chemotherapeutics such as CPT-11, gemcitabine, and taxanes (12, 13). Bortezomib also suppresses DNA damage repair pathways (14), thereby sensitizing tumor cells to DNA damaging agents like anthracyclines. Pegylated liposomal doxorubicin (PLD) has documented activity against a number of tumor types including breast cancer and ovarian cancer. In a phase II study of PLD at 45-60 mg/m2 given every 3 to 4 weeks to treat anthracycline-naïve breast cancer, the overall response rate was 31% (15). Response rates are lower in patients with anthracycline pretreated breast cancer (16), but cumulative dosing of PLD may be less cardiotoxic than parent doxorubicin (17). Preclinical studies in a number of model systems have shown that doxorubicin and bortezomib have synergistic activity, and can overcome prior anthracycline resistance in vitro (18, 19). Further, doxorubicin can suppress proteasome inhibitor-mediated induction of anti-apoptotic factors, such as MAPK phosphatase-1 (19). Finally, the combination of bortezomib with pegylated liposomal doxorubicin (PLD) has been shown to have enhanced activity in vivo in a model of human breast cancer (19).
Here we report the results of a phase I trial of bortezomib and PLD, which was designed to determine the maximum tolerated dose (MTD) of bortezomib when given with a fixed dose of PLD. Additional study objectives were to explore the possibility that there were pharmacokinetic and pharmacodynamic interactions between the two agents. We have previously reported results of a study of this combination in patients with hematologic malignancies (20). Patients with solid tumors were evaluated separately with the hypothesis that toxicities of the regimen, specifically myelosuppression and neuropathy, might be different given differences in disease involvement and prior therapies between the two groups. In the present study we show that bortezomib, 1.3 mg/m2 on days 1, 4, 8, and 11, along with PLD at 30 mg/m2 on day 4, can be safely administered to patients with solid tumors on an every 21-day schedule. Moreover, interesting evidence of anti-tumor activity in patients with advanced breast carcinoma was seen, suggesting this regimen holds promise and should be investigated further in this patient population.
Patients and Methods
Eligibility
Patients with histologically or cytologically confirmed solid tumor malignancies refractory to at least one conventional therapy, or for whom no standard therapy existed, were candidates for this study. Eligibility criteria included age >18 years; Karnofsky performance status >60%; a life expectancy of ≥8 weeks; no major surgery, radiotherapy, or chemotherapy within 21 days of study entry; adequate hematopoietic (hemoglobin >8.0 g/dL, ANC >1500/μL, and platelets >50,000/μL), hepatic (total bilirubin <1.2 mg/dL and transaminases <2.5 times the upper limits of normal), and renal function (creatinine <2.5 mg/dL); adequate cardiovascular function as defined by no evidence of ischemia on electrocardiography (ECG) and a left ventricular ejection fraction (LVEF) >45%; not pregnant or nursing and amenable to using appropriate contraception; and no other coexisting medical problems of sufficient severity to limit full compliance with the study or which could cause undue risk. Patients were ineligible if they had a prior cumulative exposure to doxorubicin >400 mg/m2, or hypersensitivity to PLD, or had uncontrolled active infections, or were known to be human immunodeficiency virus sero-positive, or have active viral hepatitis. All patients gave written, informed consent according to federal and institutional guidelines before treatment.
Trial Design
This was a phase I trial in which bortezomib was escalated from a starting dose of 0.90 mg/m2 as an intravenous bolus on days 1, 4, 8, and 11 of each 3-week cycle, while PLD was held constant at 30 mg/m2 as an intravenous infusion on day 4. A modified Fibonacci escalation was used, with bortezomib dose steps of 0.90, 1.05, 1.20, 1.30, 1.40, and 1.50 mg/m2.
A standard “3+3” dose escalation scheme was employed in which a cohort of 3 patients was entered sequentially, and if none developed a dose limiting toxicitiy (DLT) then the next cohort was enrolled at the next higher bortezomib dose level while maintaining the same PLD dose. All patients in a given cohort were required to have completed one 3-week cycle of therapy before the next cohort was started. If one of the three patients in a cohort had a DLT, 3 additional patients were enrolled at that dose level. Among the 3 additional patients enrolled in a cohort, if no DLTs occurred escalation to the next dose level proceeded. If 2 of 3 to 6 patients in a cohort had a DLT, the dose level exceeded MTD, which was defined as the highest dose level at which the incidence of DLTs was < 33%. In this trial, dose delays and dose reductions, which precluded therapy with assigned drug doses, also impacted the final assessment of a recommended dose, as discussed below. Additional patients were accrued once the recommended dose had been identified to confirm safety and obtain additional experience.
The NCI Common Toxicity Criteria Version 2.0 was used to characterize toxicity. Patients were evaluated weekly. DLT was defined on the first cycle as a ≥grade 3 non-hematological toxicity and/or ≥grade 4 hematological toxicity with the following exceptions: nausea, vomiting, and diarrhea were only considered DLTs if they did not respond to antiemetics and/or anti-diarrheals, recurrent grade 2 or higher hand-foot syndrome (HFS; formerly palmar-plantar erythrodysesthesia) was considered a DLT, grade 4 neutropenia was a DLT only if accompanied by fever or lasting >5 days, and a 2 week or greater dose delay was considered a DLT.
Additionally, all patients had a pre-study assessment of left ventricular ejection fraction, and those patients who had total anthracycline exposure of greater than 300 mg/m2 had serial assessments every 4 cycles thereafter.
Response Criteria
Tumor assessments were performed every 2 cycles, and response was evaluated using the RECIST criteria (21).
Drug Administration
Bortezomib was provided as a sterile, lyophilized powder in vials with mannitol, which was reconstituted with normal saline to a drug concentration of 1 mg/mL, and administered by intravenous push over 3-5 seconds on treatment days. PLD from commercial stock was prepared as per the package insert and administered as a 60-90 minute infusion one-hour after bortezomib administration. Day 4 was chosen to allow evaluation of proteasome inhibition on days 1 and 4 in the presence of bortezomib alone, and on days 8 and 11 with both drugs present, allowing each patient to serve as their own control. Treatment days could be changed by up to 24-hours providing there was a ≥72-hour span between consecutive bortezomib doses.
Pharmacodynamics and Pharmacokinetics
Blood samples were collected at baseline and 1 hour after bortezomib for measurement of 20S proteasome activity during cycle 1. Since bortezomib rapidly exits the intravascular compartment, standard pharmacokinetic parameters do not adequately guide dosing, and a pharmacodynamic assay measuring the percentage proteasome inhibition was used to provide a more relevant characterization (22). PLD pharmacokinetic studies were performed from blood samples collected at baseline, and at 1, 24, and 96 hours, and 7, 14, and 18 days after PLD administration. Doxorubicin released from the liposomal preparation was evaluated by high-performance liquid chromatography (20, 23 and 25). Compartmental and non-compartmental analysis was conducted using WinNonlin® Professional software, version-3.2 (Pharsight Corporation; Mountain View, CA).
Results
Patient Characteristics
Thirty seven patients (median age 54), 29 of whom were women and 19 of whom had breast cancer (Table 1) were enrolled and treated between January 2002 and February 2006 and treated concurrently with a separate cohort of patients with hematologic malignancies who were on a different arm of this trial(20). Most of the patients were heavily pretreated, and the median number of prior therapies was four. Six dose levels were evaluated (Table 2), and a total of 117 cycles of bortezomib/PLD therapy were administered, with a median of two cycles per patient (range 1-10 cycles).
Table 1.
Patient Characteristics
| No. of patients | 37 |
| Sex | |
| Female | 29 |
| Male | 8 |
| Age, years | |
| Mean | 54 |
| Range | 35-75 |
| Race | |
| African American | 6 |
| Caucasian | 29 |
| Hispanic | 1 |
| Other | 1 |
| Diagnoses | |
| Breast Cancer | 19 |
| Lung Cancer | 4 |
| TCC Bladder | 3 |
| Head and Neck | 3 |
| Adrenocortical | 1 |
| Sarcoma | 1 |
| Colorectal | 2 |
| Primary Peritoneal | 1 |
| Ovarian | 1 |
| Kidney | 1 |
| Uterine Carcinosarcoma | 1 |
| Karnofsky performance status | |
| 100 | 11 |
| 90-80 | 17 |
| 70-60 | 9 |
| Prior therapy | |
| Chemotherapy | 37 |
| Anthracycline | 20 |
| Median number of regimens (range) | 4 (1-11) |
| Radiation therapy | 27 |
Table 2.
Dose Escalation and DLTs
| Bortezomib Dose level (mg/m2) | Number evaluable pts | Number DLT | First cycle dose delay | Dose reduction |
|---|---|---|---|---|
| 0.9 | 3 | |||
| 1.05 | 3 | |||
| 1.2 | 6 | 1 NV | 1 | |
| 1.3 | 10 | |||
| 1.4 | 6 | 3 | 3 | |
| 1.5 | 6 | 1 NV D | 3 | 3 |
DLT= Dose limiting toxicity, N = nausea, V = vomiting, D= diarrhea
Adverse Events
Thirty four (92%) patients completed at least one cycle and were evaluable for toxicity, and the most frequent adverse events included grade 1-2 fatigue, nausea, thrombocytopenia, anemia, neutropenia, constipation, myalgias, and peripheral neuropathy (Table 3). DLTs in cycle 1 (Table 2) were nausea and vomiting in 1 of 6 patients treated with bortezomib at 1.2 mg/m2, and nausea, vomiting, and diarrhea in 1 of 6 patients treated with bortezomib at 1.5 mg/m2. Other treatment-related grade 3 toxicities seen in later cycles included HFS, thrombocytopenia, anemia, neutropenia, nausea and diarrhea, and abdominal pain. Grade 3 and 4 adverse events regardless of attribution seen by dose level are shown in Table 4, and included 4 episodes each of anemia, thrombocytopenia and neutropenia, as well as transaminitis (4), nausea (4), constipation (3), diarrhea (3), fatigue (3), peripheral neuropathy (3) and myalgia (1), vomiting (3) and 5 episodes of thrombosis (discussed below). Hematologic toxicity was in general only mild to moderate, with a median nadir ANC of 2600 (range 700-9100) and a median nadir platelet count of 127,000 (range 23-365) across all dose levels. There was no dose-related trend but myelosuppression was most severe in the highest dose level, with a median nadir ANC of 1800 (range 600-4000) and median nadir platelets 86, 000 (range 26,000-168,000). Thrombocytopenia of moderate severity occurred at bortezomib dose levels of 1.2 mg/m2 and above.
Table 3.
Most Frequent Adverse Events (Occurring in >10% cycles) (N=117)
| Adverse Event | Number (%) cycles affected, any grade (N=117) | Number (% ) cycles grade 3 | Number (%) cycles grade 4 | Number (%) patients affected, any grade (N = 37) |
|---|---|---|---|---|
| Fatigue | 92 (79) | 2 | 1 | 32 (86) |
| Nausea | 73 (62) | 4 | 0 | 30 (81) |
| Thrombocytopenia | 46(39) | 8 | 0 | 18 (49) |
| Anemia | 37 (32) | 4 | 1 | 19 (51) |
| Constipation | 35 (30) | 4 | 0 | 17 (46) |
| Peripheral Neuropathy | 33 (28) | 3 | 0 | 17 (6) |
| Neutropenia | 31 (26) | 6 | 1 | 13 (35) |
| Myalgia | 24 (21) | 2 | 0 | 10 (27) |
| Lymphopenia | 24 (21) | 7 | 0 | 9 (24) |
| Diarrhea | 20 (17) | 3 | 0 | 11 (30) |
| Anorexia | 15 (13) | 0 | 0 | 10 (27) |
| Headache | 14 (12) | 1 | 0 | 13 (35) |
| Dyspnea | 13 (11) | 2 | 0 | 10 (27) |
| Rash | 13 (11) | 1 | 0 | 8 (22) |
| Reflux | 13 (11) | 0 | 0 | 8 (22) |
| Palmar-Plantar | 12 (10) | 1 | 0 | 7 (19) |
| Erythrodysesthesia/Han d-Foot Syndrome | ||||
| Insomnia | 12 (10) | 0 | 0 | 5 (14) |
| Vomiting | 12 (10) | 3 | 0 | 8(22) |
| Abdominal pain | 12 (10) | 1 | 0 | 6( 16) |
| Fever | 10(8) | 1 | 0 | 6 (16) |
| Transaminitis | 10 (8) | 4 | 0 | 7 (19) |
Table 4.
Grade 3-4 Adverse Events Occurring in at Least Two Patients by Dose Level
| Adverse_Event | .90 (n=3) | 1.05 (n=3) | 1.20 (n=6) | 1.30 (n=9) | 1.40 (n=6) | 1.50 (n=6) |
|---|---|---|---|---|---|---|
| Anemia | 2 | 1 | 1 | |||
| Elevated LFTs | 2 | 2 | ||||
| Lymphopenia | 1 | 2 | 1 | |||
| Nausea | 1 | 3 | ||||
| Neutropenia | 1 | 1 | 2 | |||
| Thrombocytopenia | 1 | 1 | 2 | |||
| Constipation | 3 | |||||
| Diarrhea | 3 | |||||
| Fatigue | 1 | 1 | 1 | |||
| Peripheral neuropathy | 1 | 2 | ||||
| Vomiting | 1 | 2 | ||||
| Coagulopathy | 1 | 1 | ||||
| DVT (LE) | 1 | 1 | ||||
| Dyspnea | 2 | |||||
| Hypoxia | 2 | |||||
| Myalgia | 1 | 1 | ||||
| Pulmonary emboli | 1 | 1 |
N number of patients evaluable for toxicity.
All cycles. Adverse events regardless of attribution
LFT= liver function tests, DVT= deep vein thrombosis , LE= lower extremity
Peripheral neuropathy and myalgia were observed in 28% and 20% of cycles, respectively. Patients affected typically described an aching burning pain in their lower extremities which was constant. These symptoms, particularly the myalgia, appeared to be related to cumulative bortezomib dose, occurring primarily in patients treated with doses of at least 1.2 mg/m2 (15 of 17 patients affected) who had received 2-4 cycles, with severity increasing with subsequent cycles. These symptoms were managed with NSAIDs, opioids, gabapentin, and pyridoxine with variable success.
Five patients developed venous thromboses while on study, including two each with pulmonary emboli and deep vein thrombosis, and one with superior mesenteric vein thrombosis. In all cases, the investigators felt that the thrombotic events were related to the underlying disease and other risk factors. One patient who had previously received 300 mg/m2 doxorubicin as adjuvant treatment of breast cancer three years prior had an asymptomatic drop in ejection fraction to 35% after 5 cycles of therapy, which improved to 45% without intervention within 2 weeks of discontinuing treatment. Three other patients met criteria for serial evaluations of LVEF during the study, and none experienced a significant decline below baseline.
According to the initial protocol definition of MTD, bortezomib at 1.50 mg/m2 and PLD at 30 mg/m2 met these criteria. Further bortezomib dose escalation was not pursued since this would have exceeded both the single-agent MTD of 1.5 mg/m2 (10), and the 1.3 mg/m2 dose which had been approved for myeloma. Furthermore, among six patients receiving bortezomib at 1.5 mg/m2, three had grade 3 gastrointestinal toxicity during subsequent cycles, and three required first cycle delays for grade 2 neutropenia. Therefore, this level was considered higher than tolerable, and additional patients were enrolled at the next lower dose levels. Among six patients receiving bortezomib at 1.4 mg/m2, three required first cycle dose delays and two needed dose reductions by cycle 3. Due to the frequent need for dose-delays and dose-reductions, and gastrointestinal toxicity in later cycles at the 1.4 and 1.5 mg/m2 levels, bortezomib at 1.30 mg/m2 and PLD at 30 mg/m2 were chosen for further testing.
Pharmacokinetics and Pharmacodynamics
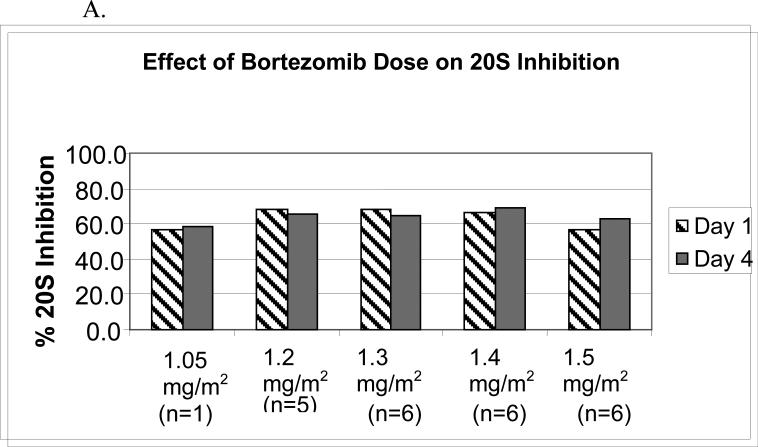
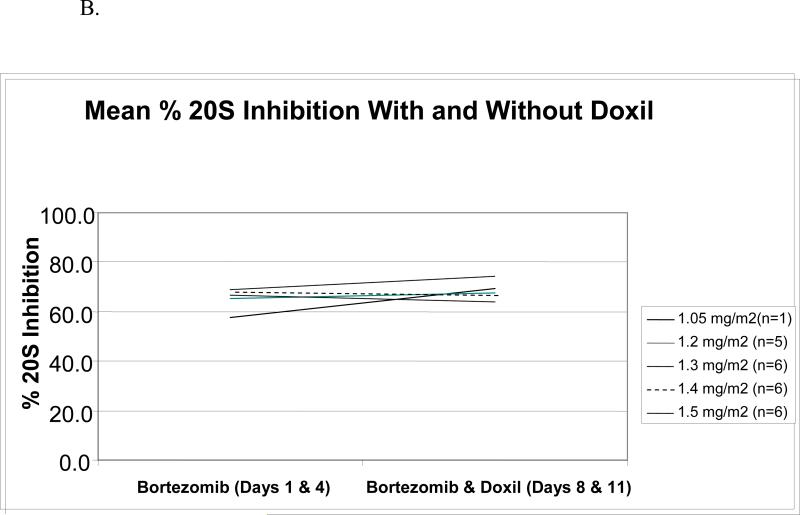
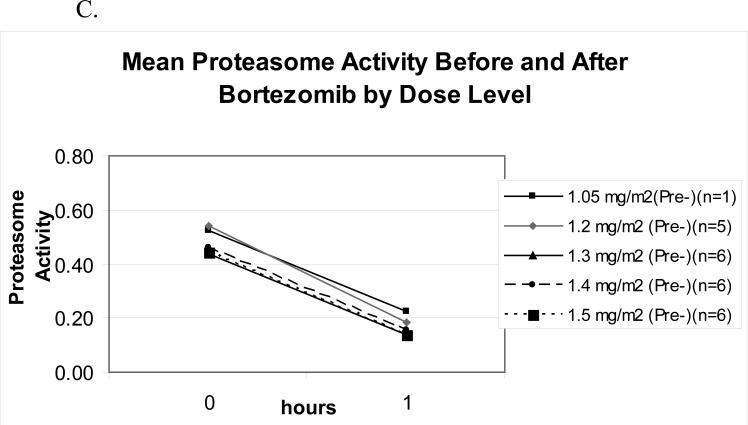
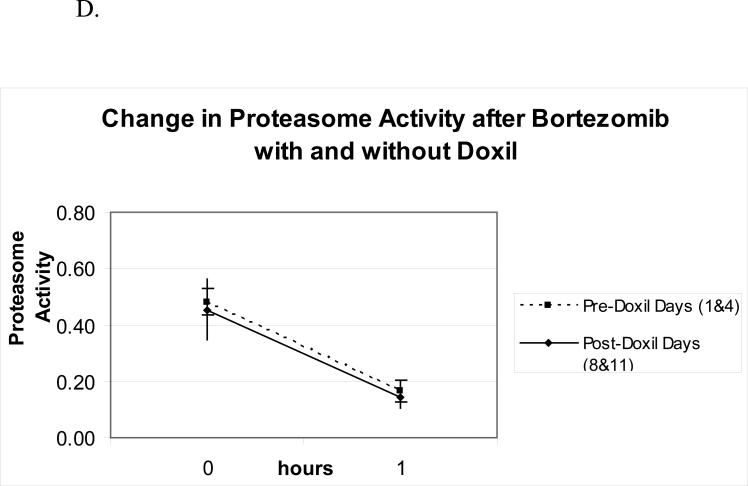
Bortezomib pharmacodynamics was evaluated using an ex vivo assay of the 20S-proteasome (22) during the first cycle of therapy in 24 patients. The mean percent inhibition 1-hour after each bortezomib dose compared with the pre-treatment baseline (Figure 1A) was comparable across dose levels from 1.05 to 1.50 mg/m2, and no significant difference was noted between days 1 and 4. To evaluate whether PLD would impact upon bortezomib-induced effects, proteasome inhibition was compared on days 1 and 4, in the presence only of bortezomib, with days 8 and 11, when bortezomib and PLD were present (Figure 1B). Across all dose levels, mean proteasome inhibition on days 8 and 11, 67.8%, was not different than that measured on days 1 and 4, 67.0%, (p=0.65). The effect of bortezomib and PLD on the specific activity of the chymotryptic proteasome protease was also evaluated. Specific activity decreased with bortezomib (Figure 1C), with baseline and 1-hour post-therapy activities being comparable across the 1.05-1.50-mg/m2/dose range. Finally, the mean specific activity at baseline and 1 hour after dosing was studied on days 1 and 4, and compared with days 8 and 11 (Figure 1D). This activity declined in both situations by approximately the same amount, 0.30 on days 1 and 4, versus 0.26 on days 8 and 11. Thus, the bortezomib-induced decline of the specific activity of the proteasome did not depend on whether PLD was present.
Figure 1.
Pharmacodynamics of bortezomib(B) and PLD. (A) Inhibition of the chymotryptic activity of the 20S proteasome by B is shown as a function of the administered dose level (in mg/m2). The mean percentage inhibition 1 hour after each dose compared to the pretreatment baseline is shown for days 1 and 4. All data presented are from the first cycle of therapy. (B) The mean 20S proteasome inhibition one hour after each dose B alone on days 1 and 4 is compared to mean inhibition on days 8 and 11, when both B and PLD were present. (C) Specific activity of the chymotrypsin like proteasome protease is shown at baseline and one hour after bortezomib treatment on day 1 as a mean for each dose level. The units for specific activity are picomoles of fluorescent chromophore released per second per milligram of total protein. (D) Mean proteasome activity is shown at baseline and one hour after dosing with either bortezomib alone (days 1 and 4) or bortezomib in the presence of PLD (days 8 and 11).
Pharmacokinetic parameters of PLD in the presence of bortezomib were determined by detection of doxorubicin released from pegylated liposomes (23, 24) in 32 patients. The peak plasma concentration of doxorubicin after single dose administration, area under the concentration-time curve, total plasma body clearance, volume of distribution, and half-life were determined at each dose level. For the entire cohort, the median half life (t1/2) for PLD was 69.75 hours (range 33.77-110.54). Maximum plasma concentration (Cmax) was 20.5 μg/mL (range 13.53-42.23), area under the concentration–time curve (AUC) was 2138.5 (905-5147.8), and clearance 26.25 mL/hr (9.79-62.32).
Responses
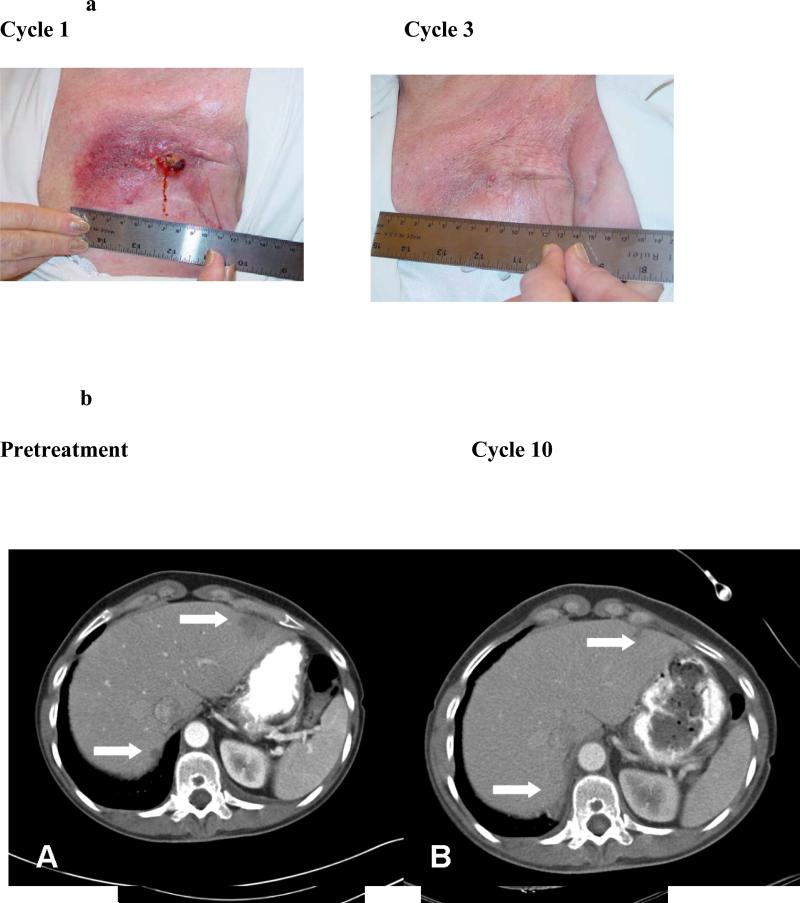
Among the 19 patients with breast cancer, one achieved a near complete remission of cutaneous disease, a second had a partial response of liver metastases (Figure 2), a third experienced resolution of a large malignant effusion and stable adenopathy for 5 cycles, and a fourth attained stable disease in liver metastases for 5 months. The patient with a partial response in liver metastases remained on study for 11 months, but eventually discontinued because of fatigue and logistical constraints. She subsequently progressed through several other treatments, and when bortezomib was approved she was retreated with the combination and again recaptured a response/clinical benefit. This patient as well as the other patient with partial response were treated on dose level 6 (1.5 mg/m2) but both required dose reduction to 1.3 mg/m2 for toxicity. The two other responders were treated at 1.05 mg/m2 and 1.4 mg/m2 levels respectively. Three additional patients with other tumor types in this heavily pre-treated solid tumor population had stable disease for greater than 4 cycles, including one each with renal cell carcinoma, adrenal cortical carcinoma, and non-small cell lung cancer.
Figure 2.
Figure 2a. A patient with cutaneous metastases had dramatic and rapid response shown here from cycle 1 to cycle 3 with time to progression over 4 months.
Figure 2b. A patient with breast cancer hepatic metastases had partial response in her hepatic disease with time to progression 11 months.
Discussion
Bortezomib was the first proteasome inhibitor to enter clinical development and has been approved for use in multiple myeloma and mantle cell non-Hodgkin lymphoma. Preclinical studies have shown augmentation of activity when bortezomib was administered with anthracyclines. Therefore, this phase I study was undertaken to evaluate the maximum tolerated dose of bortezomib given on a day 1, 4, 8, 11 schedule in combination with PLD every 3 weeks in patients with solid tumors. According to the protocol-specified definition, bortezomib at 1.50 mg/m2 with PLD met the criteria for MTD. However, extensive additional information is now available from other studies of bortezomib and the dose of 1.3 mg/m2 on this schedule is now approved in multiple myeloma. To define a regimen that could be administered with a lower likelihood of dose delays and dose reductions, the recommended dose for phase II trials of the combination is bortezomib 1.3 mg/m2 and PLD 30 mg/m2 day 4 of a 21 day cycle.
In this study the most frequent toxicities were fatigue, nausea, myelosuppression, peripheral neuropathy and diarrhea. This is similar to the toxicity profile reported for the single agent in other studies (10, 11, 25). However, neutropenia was more severe and more frequent in our study, likely due to the concomitant PLD, or to differences in the patient populations and prior treatments. HFS was seen in this combination study but has not been reported with the single agent bortezomib to our knowledge. This also is likely attributable to the PLD use. It appears that hematologic toxicity and gastrointestinal toxicity may be dose-related, particularly in the highest three dose levels, and that HFS, neuropathy and myalgia are related to cumulative dose.
In the present study peripheral neuropathy affected 17 of 37 or 46% of patients and complicated 28% of cycles. This incidence is somewhat higher than that seen and reported previously. Combined data from two recent trials in myeloma patients (26) has shown treatment emergent peripheral neuropathy in 37% of patients treated at 1.3 mg/m2. Perhaps we have a higher incidence in this study because twelve of the 37 patients on the study were treated at doses higher that 1.3. Furthermore, 18 of 37 had prior treatment with a taxane or platinum agent, whereas 36% had previous platinum in the myeloma studies and none had taxanes. Dose reduction guidelines for neuropathy now exist for bortezomib treatment (26) that may help in managing patients who develop this toxicity.
We have previously reported our phase I trial of this combination in patients with hematologic malignancies (20) and found a similar toxicity profile despite the differences in underlying disease and prior treatments. Furthermore, subsequent evaluation of this combination in patients with myeloma has shown marked efficacy, and phase III evaluation has shown a better response rate, response quality, TTP, PFS, and OS in patients receiving Doxil/Velcade compared to standard therapy .(27)
The preliminary evidence of anti-breast cancer activity seen in this phase I study is promising and intriguing. A phase II trial to determine the efficacy of this combination in metastatic breast cancer has begun accrual. As described previously there is promising preclinical data and biologic rationale to evaluate this combination in breast cancer (19). However single agent PLD has shown only modest activity in breast cancer (15) and bortezomib as a single agent has not shown significant activity in patients with breast cancer (28). Of interest, two of the four most significant responses seen in our study were in women who had not previously been treated with anthracyclines. Therefore, it is unclear whether the potential efficacy of the combination is mediated through direct activity or modulation of anthracycline resistance.
A number of other combinations of bortezomib and chemotherapy are currently being investigated in clinical trials in patients with solid tumors. Preliminary results from an ongoing study of bortezomib and docetaxel in patients with anthracycline pre-treated breast cancer have been presented showing 6 of 9 patients with partial responses and an MTD had not yet been reached (29). By contrast, Messersmith and colleagues have completed a phase I study of bortezomib with docetaxel using a different schedule and an MTD was defined at the relatively low dose of 0.8 mg/m2 bortezomib in combination with 25 mg/m2 docetaxel (30). Aghajanian and colleagues have conducted a phase I study of bortezomib combined with carboplatin in patients with ovarian cancer, and found that diarrhea, constipation and neuropathy were dose limiting at the 1.5 mg/m2 dose level. Like ours, her recommended dose for further study was also 1.3 mg/m2 bortezomib in combination with a carboplatin area under the curve of 5. Carboplatin had no effect on bortezomib pharmacodynamics in this study.
Recently, Ma and colleagues have published their investigation of bortezomib combined with paclitaxel and carboplatin on two different schedules. In this study the sequence in which bortezomib was given on days 1, 4, and 8 with paclitaxel and carboplatin on day 2 seemed to be better tolerated and more effective that the sequence in which the chemotherapy was given the day before the bortezomib (31). However, in the Messersmith study above, in which the day 1 chemotherapy and day 2 bortezomib dosing schedule also resulted in a lower MTD than expected, docetaxel pharmacokinetics were performed at two time points and the parameters were not altered by the presence of bortezomib on day 5 (30). In our study the bortezomib and PLD were given together on day 4 with the PLD given one hour after the bortezomib. Doxorubicin pharmacokinetic parameters were not significantly different than reported single agent values. Further trials assessing sequence effect are underway, and additionally other schedules are under investigation (32).
Doxorubicin kinetics were assessed using a limited sampling scheme and parameters were not significantly different than published values for PLD alone (17). Furthermore, there was no significant trend detected for a change in these parameters with increasing bortezomib dose . These findings suggest that the presence of bortezomib does not alter the pharmacokinetics of PLD. Because bortezomib rapidly exits the intravascular compartment, the pharmacodynamic assay for 20S inhibition was evaluated rather than standard pharmacokinetic parameters. The percent proteasome inhibition across dose levels evaluated in this study (67%) is consistent with that reported in other studies of single agent bortezomib (10), and does not appear to be impacted by concomitant PLD treatment, as shown in Figure 1. Furthermore, change in specific activity was no different with and without PLD as shown by comparing the slopes in Figure 1D. Therefore we conclude that concomitant doxorubicin is not likely to alter the pharmacodynamic effect of bortezomib.
In conclusion, we have found that in this population of heavily pretreated patients with solid tumors, the combination of bortezomib at 1.3 mg/m2 days 1, 4, 8, 11 and PLD 30 mg/m2 day 4 every 3 weeks is tolerable and worthy of further study. Frequent toxicities included fatigue nausea, myelosuppression, diarrhea, and peripheral neuropathy/myalgia. Dose limiting toxicities were nausea, vomiting, and diarrhea. One patient had a reversible decline in ejection fraction. Studies are ongoing to better define the nature and optimal treatment of cumulative toxicities such as neuropathy. Future studies of this combination should be attentive to potential risk for thrombosis. We found no pharmacokinetic or pharmacodynamic interaction between the drugs. Finally, evidence of activity in metastatic breast cancer has prompted a phase II trial which is now ongoing.
Condensed Abstract.
In this phase I study in 37 patients with refractory solid tumors, a regimen of bortezomib, 1.3 mg/m2 on days 1, 4, 8, and 11 with PLD, 30 mg/m2, on day 4 of a 21-day cycle, was safe and merits further investigation. No pharmacokinetic or pharmacodynamic interactions were appreciated and activity was seen in patients with breast cancer.
Acknowledgements
The authors would like to thank Beth Humes for research nursing, Susan Natoli for regulatory assistance, Anandhi Johri, Mary Jo Lehman, Henry Bell, and Paul Jones for help with data collection and management, and most importantly the patients who participated in this study and their families for their courage and contribution to the evaluation of new cancer therapies.
Supported in part by grants from the following: Millennium Pharmaceuticals, Inc., General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health(RR00046), National Cancer Institute SPORE in Breast Cancer (5-P50-CA58223-09A1 H.S. Earp), National Inst. of Health (K23-RR16536 ECD), Leukemia & Lymphoma Society (6096-07 RZO), and National Cancer Institute (RO1 CA102278 RZO).
References
- 1.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59(11):2615–22. [PubMed] [Google Scholar]
- 2.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 3.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–17. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor OA, Wright J, Moskowitz C, Muzzy J, MacGregor-Cortelli B, Stubblefield M, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23(4):676–84. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 5.Goy A, Younes A, McLaughlin P, Pro B, Romaguera JE, Hagemeister F, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2005;23(4):667–75. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 6.Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24(30):4867–74. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 7.Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X, et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol. 2004;22(11):2108–21. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 8.Fanucchi MP, Fossella FV, Belt R, Natale R, Fidias P, Carbone DP, et al. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2006;24(31):5025–33. doi: 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- 9.Kondagunta GV, Drucker B, Schwartz L, Bacik J, Marion S, Russo P, et al. Phase II trial of bortezomib for patients with advanced renal cell carcinoma. J Clin Oncol. 2004;22(18):3720–5. doi: 10.1200/JCO.2004.10.155. [DOI] [PubMed] [Google Scholar]
- 10.Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8(8):2505–11. [PubMed] [Google Scholar]
- 11.Dy GK, Thomas JP, Wilding G, Bruzek L, Mandrekar S, Erlichman C, et al. A phase I and pharmacologic trial of two schedules of the proteasome inhibitor, PS- 341 (bortezomib, velcade), in patients with advanced cancer. Clin Cancer Res. 2005;11(9):3410–6. doi: 10.1158/1078-0432.CCR-04-2068. [DOI] [PubMed] [Google Scholar]
- 12.Voorhees PM, Orlowski RZ. The proteasome and proteasome inhibitors in cancer therapy. Annu Rev Pharmacol Toxicol. 2006;46:189–213. doi: 10.1146/annurev.pharmtox.46.120604.141300. [DOI] [PubMed] [Google Scholar]
- 13.Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23(3):630–9. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Hideshima T, Mitsiades C, Akiyama M, Hayashi T, Chauhan D, Richardson P, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003;101(4):1530–4. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 15.Ranson MR, Carmichael J, O'Byrne K, Stewart S, Smith D, Howell A. Treatment of advanced breast cancer with sterically stabilized liposomal doxorubicin: results of a multicenter phase II trial. J Clin Oncol. 1997;15(10):3185–91. doi: 10.1200/JCO.1997.15.10.3185. [DOI] [PubMed] [Google Scholar]
- 16.Rivera E, Valero V, Esteva FJ, Syrewicz L, Cristofanilli M, Rahman Z, et al. Lack of activity of stealth liposomal doxorubicin in the treatment of patients with anthracycline-resistant breast cancer. Cancer Chemother Pharmacol. 2002;49(4):299–302. doi: 10.1007/s00280-001-0405-3. [DOI] [PubMed] [Google Scholar]
- 17.Lyass O, Uziely B, Ben-Yosef R, Tzemach D, Heshing NI, Lotem M, et al. Correlation of toxicity with pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in metastatic breast carcinoma. Cancer. 2000;89(5):1037–47. doi: 10.1002/1097-0142(20000901)89:5<1037::aid-cncr13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Mitsiades N, Mitsiades CS, Richardson PG, Poulaki V, Tai YT, Chauhan D, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101(6):2377–80. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 19.Small GW, Shi YY, Edmund NA, Somasundaram S, Moore DT, Orlowski RZ. Evidence that mitogen-activated protein kinase phosphatase-1 induction by proteasome inhibitors plays an antiapoptotic role. Mol Pharmacol. 2004;66(6):1478–90. doi: 10.1124/mol.104.003400. [DOI] [PubMed] [Google Scholar]
- 20.Orlowski RZ, Voorhees PM, Garcia RA, Hall MD, Kudrik FJ, Allred T, et al. Phase 1 trial of the proteasome inhibitor bortezomib and pegylated liposomal doxorubicin in patients with advanced hematologic malignancies. Blood. 2005;105(8):3058–65. doi: 10.1182/blood-2004-07-2911. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Lightcap ES, McCormack TA, Pien CS, Chau V, Adams J, Elliott PJ. Proteasome inhibition measurements: clinical application. Clin Chem. 2000;46(5):673–83. [PubMed] [Google Scholar]
- 23.Gabizon A, Chisin R, Amselem S, Druckmann S, Cohen R, Goren D, et al. Pharmacokinetic and imaging studies in patients receiving a formulation of liposome-associated adriamycin. Br J Cancer. 1991;64(6):1125–32. doi: 10.1038/bjc.1991.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54(4):987–92. [PubMed] [Google Scholar]
- 25.Caravita T, de Fabritiis P, Palumbo A, Amadori S, Boccadoro M. Bortezomib: efficacy comparisons in solid tumors and hematologic malignancies. Nat Clin Pract Oncol. 2006;3(7):374–87. doi: 10.1038/ncponc0555. [DOI] [PubMed] [Google Scholar]
- 26.Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24(19):3113–20. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 27.Orlowski RZ, Nagler A, Sonneveld P, Blade J, Hajek R, Spencer A, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25(25):3892–901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 28.Yang CH, Gonzalez-Angulo AM, Reuben JM, Booser DJ, Pusztai L, Krishnamurthy S, et al. Bortezomib (VELCADE) in metastatic breast cancer: pharmacodynamics, biological effects, and prediction of clinical benefits. Ann Oncol. 2006;17(5):813–7. doi: 10.1093/annonc/mdj131. [DOI] [PubMed] [Google Scholar]
- 29.Cardoso F, Ross JS, Picart MJ, Sotiriou C, Durbecq V. Targeting the ubiquitin-proteasome pathway in breast cancer. Clin Breast Cancer. 2004;5(2):148–57. doi: 10.3816/cbc.2004.n.020. [DOI] [PubMed] [Google Scholar]
- 30.Messersmith WA, Baker SD, Lassiter L, Sullivan RA, Dinh K, Almuete VI, et al. Phase I trial of bortezomib in combination with docetaxel in patients with advanced solid tumors. Clin Cancer Res. 2006;12(4):1270–5. doi: 10.1158/1078-0432.CCR-05-1942. [DOI] [PubMed] [Google Scholar]
- 31.Ma C, Mandrekar SJ, Alberts SR, Croghan GA, Jatoi A, Reid JM, et al. A phase I and pharmacologic study of sequences of the proteasome inhibitor, bortezomib (PS-341, Velcade), in combination with paclitaxel and carboplatin in patients with advanced malignancies. Cancer Chemother Pharmacol. 2007;59(2):207–15. doi: 10.1007/s00280-006-0259-9. [DOI] [PubMed] [Google Scholar]
- 32.Adjei AA. Sequencing bortezomib with chemotherapy and targeted agents. Clin Lung Cancer. 2005;7(Suppl 2):S56–8. doi: 10.3816/clc.2005.s.009. [DOI] [PubMed] [Google Scholar]